Abstract
With the aging of the baby-boom generation and increases in life expectancy, the American population is growing older. Aging is associated with adverse changes in glucose tolerance and increased risk of diabetes; the increasing prevalence of diabetes among older adults suggests a clear need for effective diabetes prevention approaches for this population. The purpose of paper is to review what is known about changes in glucose tolerance with advancing age and the potential utility of resistance training (RT) as an intervention to prevent diabetes among middle-aged and older adults. Age-related factors contributing to glucose intolerance, which may be improved with RT, include improvements in insulin signaling defects, reductions in tumor necrosis factor-α, increases in adiponectin and insulin-like growth factor-1 concentrations, and reductions in total and abdominal visceral fat. Current RT recommendations and future areas for investigation are presented.
1. Introduction
With the aging of the baby boom population and an increased life expectancy, individuals aged 65 years or older are the fastest growing segment of our population [1]. Increases in the number of individuals aged 65+ years will increase demands on health care and health care costs, which could lead to inadequate public resources and less care for the aged [1]. Chronic conditions such as diabetes exert a profound economic impact on our nation; this disease and its associated comorbidities are a major cause of disability and death [2]. In 2007, the total estimated cost of diabetes was $174 billion, which included $116 billion in medical costs and $58 billion in reduced productivity [3].
Total diabetes prevalence (undiagnosed and diagnosed) is currently estimated to be 14% of the U.S adult population [4] and is highest in those aged ≥65 years [2]. Prediabetes, that is, impaired fasting glucose (IFG; 100 mg/dl (5.6 mmol/l)–125 mg/dl (6.9 mmol/l)), or impaired glucose tolerance (IGT; 2-h plasma glucose 140 mg/dl (7.8 mmol/l)–199 mg/dl (11.0 mmol/l)) [5] is also becoming more prevalent in the United States [6]. Individuals with prediabetes are at increased risk for developing diabetes, with the progression of diabetes within 6 years of those with IFG and IGT being 65%, as compared to a 5% progression rate for those with normal blood glucose levels [7]. Recent estimates indicate that by the year 2050, diabetes prevalence could be as high as 33% [4]. The increased prevalence of diabetes among older adults, coupled with the aging of our population, suggests a clear need for effective diabetes prevention strategies. The purpose of the present paper is to review what is currently known about changes in glucose tolerance with advancing age, and the potential utility of resistance training (RT) as an intervention to prevent diabetes among middle-aged and older adults. Current RT recommendations and areas for future investigation are also presented.
2. Aging: Changes in Body Composition and Glucose Tolerance
2.1. Aging and Sarcopenia
Aging brings about a decline in skeletal muscle mass termed sarcopenia [8–10]. Muscle mass declines at a rate of 3–8% each decade after the age of 30 [11]. This loss of muscle mass increases the risk of developing glucose intolerance and diabetes due to the fact that muscle tissue is the primary site of glucose disposal [12–14]. There are many potential causes of sarcopenia including a reduction in muscle cell number through apoptosis, loss of motorneurons, and a reduction in calcium pumping activity. In addition, a decrease in the muscle twitch time and force is experienced, which can be considered a cause or an effect of sarcopenia [9, 15–17]. Increases in inflammatory cytokines and oxidative stress may also contribute to sarcopenia [18]. Other consequences of this decline in muscle mass include reduced muscle strength, reduced resting metabolic rate, reduced lipid oxidative capacity, and increased adiposity (reviewed in [8]). Many clinical studies have shown that increasing lean body mass (primarily muscle mass) parallels the improvements in glucose tolerance seen with resistance training among older adults [19–31]. However, others have suggested that the prevalence of glucose intolerance in older individuals is not a direct reflection of one's lean body mass, but a result of age-associated increases in abdominal fat [32–34]. Although lean mass may not be the most robust predictor of glucose tolerance, the results of numerous clinical trials suggest that increases in lean body mass with RT are associated with improvements in glucose tolerance [19–31]. Therefore, increasing lean mass regardless of baseline levels should improve glucose tolerance and insulin-resistance, which may be an important strategy to combat the age-related increases in insulin-resistance and glucose intolerance.
2.2. Aging and Body Fat Distribution
Along with reductions in lean mass, older individuals often experience increases in adipose tissue [35–37]. Aging is strongly associated with increases in body weight and body fatness [38, 39]. Based on the 2007-2008 National Health and Nutrition Examination Survey (NHANES), 78.4% of men and 68.6% of woman ≥60 years were considered overweight or obese (BMI ≥ 25 kg/m2). This represents the highest prevalence of overweight or obesity across all age groups [40]. This could partially be due to reductions in physical activity; for example, older adults average 37% fewer steps per day when compared to younger adults, and perform significantly less moderate to vigorous physical activity [41]. Older adults often do not achieve the recommended amount of physical activity (i.e., ≥30 minutes of moderate physical activity on five or more days/week) proposed by organizations including the World Health Organization, Center for Disease Control and Prevention, Health Canada, and the Department of Health and Ageing [41].
Body fat accumulation is associated with an increased risk of premature mortality and morbidity [39], as well as hyperinsulinemia and glucose intolerance [38, 42]. Older individuals also demonstrate changes in body fat distribution, with increasing levels of upper body fat [35–37]. This increase in upper body fat (specifically abdominal visceral fat) has been linked to glucose intolerance and diabetes [43–45], and abdominal visceral fat is an independent predictor of glucose intolerance [34, 45]. This adipose tissue depot is sensitive to lipolytic stimuli, and in obese states may lead to increased circulating free fatty acid (FFA) concentrations [46, 47]. Visceral fat lipolysis may be responsible for 5–10% of circulating FFAs in lean individuals, while this value may increase to 20–25% in obese individuals [47, 48]. However, upper body, nonvisceral fat is the primary contributor to FFA concentrations [48]. Increased FFA concentrations have been implicated in the development of insulin-resistance and metabolic inflexibility [47–50].
3. Aging and Glucose Intolerance: Potential Contributing Factors
Although factors such as a reduction in lean body mass, physical inactivity, obesity, and changes in fat distribution may contribute to glucose intolerance, age appears to be an independent determinant of impaired glucose tolerance [42, 51, 52].
3.1. Insulin Signaling within Skeletal Muscle
Insulin's effects on peripheral tissues (i.e., skeletal muscle, adipose tissue) involve a complex framework of signaling pathways that result in the translocation of GLUT4 transporters to the cell surface, which are responsible for the transport of glucose across the plasma membrane into the target cell [53]. An alteration in any of the related pathways reduces insulin's effectiveness and leads to the insulin-resistance and glucose intolerance associated with advancing age. The insulin signaling process is complex and not fully understood (reviewed in [53, 54]). Both diabetes and age-associated declines in glucose tolerance are hallmarked by a decreased uptake of glucose by peripheral tissues, primarily skeletal muscle. The age-associated reduction in glucose uptake is not due to impaired insulin binding, but instead to a defect in the postreceptor intracellular insulin signaling pathway [53, 55–57]. This defect has not been fully elucidated; however, a reduction in the number of insulin-stimulated glucose transport units occurs with aging [56]. Thus, fewer GLUT-4 transporters and/or postreceptor defects in the insulin signaling cascade results in insulin-resistance. Exercise-induced, contraction-mediated GLUT4 translocation to the muscle membrane is independent of insulin and occurs via an alternative mechanism (reviewed in [58]). Importantly, older adults with diminished glucose tolerance do not demonstrate a decline in exercise-induced contractile-mediated GLUT4 translocation [59].
3.2. Aging and Pancreatic Beta Cell Function
Insulin secretion decreases at a rate of 0.7% per year with advancing age, and is accelerated twofold in individuals with glucose intolerance [60]; yet it is uncertain the extent to which reduced insulin secretion is due to β-cell dysfunction or reduced β-cell mass [60]. Individuals with glucose intolerance demonstrate a 50% reduction in β-cell mass [61], which may be attributed to increased β-cell apoptosis. The aging of β-cells appears to decrease proliferation and increase sensitivity to hyperglycemia-induced apoptosis [62]. Diminished β-cell function has been reported among individuals with glucose intolerance, which decreases as fasting plasma glucose concentrations increase [63]. Therefore, a combination of β-cell dysfunction and β-cell apoptosis may contribute to age-related declines in glucose tolerance.
3.3. Aging and Mitochondrial Function
A reduction in mitochondrial function may also contribute to age-related declines in glucose uptake [64–67], possibly arising from increases in mitochondrial DNA deletions and mutations [67, 68]. This may lead to a 40% decrease in mitochondrial oxidative metabolism in older adults compared to younger individuals [66]. Specifically, cytochrome c oxidase gene expression and enzyme activity are reduced in aged skeletal muscle [67]. This mitochondrial dysfunction contributes to the decline in physical fitness and oxidative capacity older adults may experience [67, 69]. Insulin resistance is related to increased plasma FFA concentrations and enhanced FFA influx into skeletal muscle [66, 70–72]; decreased mitochondrial oxidative capacity may cause intramyocellular accumulation of fatty acid metabolites such as fatty acyl coenzyme-A, diacylglycerol, and ceramide to accumulate and produce insulin-resistance through serine kinase activation [65, 66, 72]. Serine kinases impede insulin signaling by reducing IRS phosphorylation [64, 65, 72] which leads to a decline in insulin-stimulated GLUT4 translocation and impaired skeletal muscle glucose uptake [64, 65].
3.4. Aging: Adiponectin, Tumor Necrosis Factor Alpha, and Insulin-Like Growth Factor-1
Two strong correlates of aging and insulin-resistance include adiponectin and tumor necrosis factor alpha (TNF-α), with low concentrations of adiponectin and high concentrations of TNF-α being linked to insulin-resistance [43, 73–75]. Both may also play a role in body fat distribution [37, 43, 44, 76, 77] and sarcopenia [18]. Adiponectin is secreted by adipose tissue (i.e., an adipokine) and is a key modulator of insulin sensitivity [43, 75, 78]). Low plasma adiponectin concentrations are associated with insulin-resistance, diabetes, obesity, body fat percentage, body fat distribution, and BMI [37, 43, 76, 79–82]. Adiponectin is believed to activate 5′-AMP-activated protein kinase (AMPK), which activates insulin-independent glucose uptake by the muscle, downregulates gluconeogenic enzymes and increases muscle fatty acid oxidation [83].
Tumor necrosis factor alpha (TNF-α) is an inflammatory cytokine secreted by adipose tissue, macrophages, and other cells, which appears to influence insulin-resistance. Elevated TNF-α concentrations are linked to obesity and insulin-resistance, while obese mice lacking TNF-α are protected from insulin-resistance [84]. Inflammatory pathways that impair insulin signaling at the level of IRS proteins are activated in the presence of TNF-α [73, 84]. TNF-α is correlated with body fat distribution [77] and sarcopenia [18] which may also lead to insulin-resistance among individuals with elevated TNF-α concentrations.
Unlike adiponectin and TNF-α, insulin-like growth factor-I (ILGF-I) is not secreted by adipose tissue, but instead a peptide hormone which possesses insulin-like properties such as the promotion of glucose uptake by peripheral tissues [85, 86]. Insulin-like growth factor-I concentrations decline with age, and is associated with the age-related changes in body composition by both increasing fat mass and decreasing muscle mass [87–89], thus potentially being a modulator of insulin-resistance. Administration of recombinant ILGF-I improves glucose uptake in those with insulin-resistance and type 2 diabetes. Other factors may be involved in the role of ILGF-I and glucose metabolism including binding proteins, hybrid receptors, and growth hormone secretion [90].
4. Resistance Training: Influence on Insulin Resistance
The diabetes prevention program (DPP) demonstrated that lifestyle modification reduces the development of diabetes by focusing on weight loss, increased physical activity, and dietary modification. Lifestyle modification decreased the incidence of type 2 diabetes by 58%, as compared to the 31% among individuals taking metformin [91]. The physical activity component of the DPP recommended that individuals accumulate 150 minutes/week of moderate physical activity. The DPP stressed brisk walking as the physical activity of choice, but also lists aerobic dance, skating, bicycle riding, and swimming as options [91]. In support of the DPP's recommendations for aerobic training (AT), regular AT improves glucose control and insulin sensitivity [92, 93]. The American Diabetes Association (ADA) recommends that individuals with diabetes perform at least 150 minutes of moderate-intensity AT per week [94]. However, factors such as obesity, arthritis, low back pain, and physical disabilities affecting many older adults may preclude this population from regularly performing AT [95–97]. Environmental factors such as unsafe neighborhoods or streets also may discourage engagement in many types of aerobic activity [97]. Therefore, alternative approaches for increasing physical activity among older adults should be considered.
Resistance training is one such alternative that can be safe and effective for older adults, including the elderly [95, 98–102]. The ADA encourages individuals with type 2 diabetes to perform resistance exercise three times a week targeting all major muscle groups, progressing to three sets of 8–10 repetitions at high intensity [103]. By using machines that provide external resistance with controlled movements, even those confined to a wheel chair or a walker can perform some types of RT. Though older adults demonstrate reduced overall muscle protein synthesis (MPS) relative to younger adults after a bout of resistance training [104], clinical trials investigating RT interventions among older adults have shown improvements in insulin-resistance and sarcopenia, by increasing lean body mass [19–31].
To identify published research relevant to the focus of this paper, a literature search was conducted using the PubMed search engine, developed by the US National Library of Medicine of the National Institutes of Health, without restrictions on publication date. Additional inclusion criteria were as follows: randomized controlled trial study design, studies conducted in middle-aged and older adults, study duration greater than one month. Intervention studies which met inclusion criteria are described in Table 1. Of the RT intervention studies reviewed, most reported improvements in glucose uptake, and reduced diabetes risk (i.e., 4 of 5 interventions report beneficial effects of RT on diabetes-related outcomes). Intensity appears to influence the magnitude of improvement in these outcomes; high intensity RT (defined as training loads above 75% one-repetition maximum (RM) [105]) produces greater improvements than RT performed at a moderate or low intensity (training loads between 50%–74% of one RM and below 50% one RM, respectively [105]) [102, 106]. Although AT has been an accepted (see DPP [91]) and recommended (ADA [94, 103]) exercise intervention to improve glucose metabolism, some investigations of the combined effects of RT and AT conclude that RT + AT exercise programs enhance diabetes related outcomes [23, 30], while others have suggested that RT-alone programs have benefits comparable to that of AT-alone programs [22, 107–109]. Evidence to support one mode of training (RT versus AT) over the other is limited and should be further investigated before conclusions can be made as to the superiority of one form of exercise over the other.
Table 1.
Randomized controlled trials >1 month in duration investigating the effect of resistance training on diabetes-related outcomes among nondiabetic middle-aged and older adults†.
| Source | Study design | Study duration | RT protocol | Study population | Primary findings |
|---|---|---|---|---|---|
| Traditional Weight Training only* | |||||
| Iglay et al. [27] | RCT (n = 36): RT + 0.9/g/kg/d protein intake, n = 16 RT + 1.2 g/kg/d, n = 16 |
3 months | 3x week, 8 machine exercises, 2 sets 8 reps + one set to voluntary fatigue at high intensity | Healthy individuals, aged 60–62 yrs. | ↓ glucose OGTT AUC 25–28% with RT, no differences between diet groups. |
| Onambélé-Pearson et al. [110] | RCT (n = 30): LI (~40% 1RM), n = 18 HI (~80% 1RM), n = 12 |
3 months | 3x week, 6 exercises using therabands, progressing from 8–11 reps and 2–4 sets, different intensity groups: HI versus LI | Sedentary individuals, aged 55–80 yrs. | ↑ fasting plasma glucose (4.8 ± 0.19 to 5.51 ± 0.08 mmol/L) in HI group, no change in plasma glucose for LI, no change in plasma insulin for either group. |
| Zachwieja et al. [31] | RCT (n = 15): RT + GH injections, n = 6 RT only, n = 9 |
4 months | 4x week, 9 machine exercises, 4 sets, 4–10 reps at high intensity. | Healthy men, aged 64–75 yrs. | ↑ in glucose disappearance rate (3.0 ± 0.3 to 4.0 ± 0.4 mg/100 mL/min minimal model of glucose kinetics, IVGTT) with RT only |
| RT + AT (either alone or combined) | |||||
| Ahmadizad et al. [79] | RCT (n = 24): AT, n = 8 RT, n = 8 Control, n = 8 |
3 months | 3x week, circuit weight training, 11 machine exercises, 4 sets, 12 reps, at moderate intensity with 30 sec. rest between exercises. | Healthy men, aged 35–48 yrs. | ↓ HOMA-IR 35.7 and 38.5% after AT and RT respectfully; no differences between groups. |
| Smutok et al. [109] | RCT (n = 37): RT, n = 14 AT, n = 13 Control, n = 10 |
4.5 months | 3x week, 11 machine exercises, 2 sets, 12–15 reps at moderate intensity. | Men at risk for CHD with either abnormal glucose tolerance, dyslipidemia, or hypertension, aged 41–59 yrs. | ↓ plasma glucose at 60, 90, and 120 minutes after glucose ingestion with RT; ↓ plasma glucose at 90 and 120 min after glucose ingestion with AT. ↓ fasting glucose with RT, no changes with AT. Insulin OGTT AUC ↓ 24% for AT and 21% for RT, no changes in control. |
*Traditional Weight Training= any muscle strengthening exercises using resistance training machines/equipment, free weights (e.g., dumbbell, barbell) or therabands.
†Abbreviations used: AT: Aerobic training, AUC: Area under curve, GH: Growth Hormone, HI: High intensity, HOMA: Homeostasis model assessment, IVGTT: Intravenous glucose tolerance test, IR: Insulin Resistance, LI: Low intensity, OGTT: Oral glucose tolerance test, Reps: Repetitions, RCT: Randomized controlled trial, RT: Resistance training.
Two RT modes were used in the five investigations included in Table 1. Four interventions utilized weight-training machines [27, 31, 79, 109] while one used therabands [110]. Interestingly, all four studies using a weight-training machine protocol reported improvements in diabetes-related measures, whereas the RT intervention utilizing therabands did not lead to differences between exercise and control groups. The number of studies in this area is limited, yet these findings suggest that RT mode may be an important issue with regard to improvements in glucose metabolism.
Although Table 1 only includes studies investigating chronic RT effects, others have investigated glucose metabolism with acute bouts of RT, and reported conflicting results. Black et al. found that a single RT session performed at either low or high intensity, using either a multiple set or single set protocol, improved 24-hour postexercise insulin sensitivity measured via fasting plasma glucose [106]. Conversely, Jimenez et al. assessed insulin sensitivity using the euglycemic-hyperinsulinemic-clamp technique preexercise, and 12 and 36 hours postexercise, and reported no differences between control and exercise groups [111]. Methodological difference may have contributed to the conflicting findings (i.e., RT protocol, outcome measures, study population). Thus it remains uncertain the extent to which improvements in glucose/insulin metabolism with RT could be attributed to an acute exercise bout versus a result of chronic training.
4.1. Resistance Training: Changes in Insulin Signaling, Adiponectin, TNF-α, and ILGF-1
Resistance-trained muscle has shown increased rates of insulin-stimulated glucose uptake and transport [112, 113]. This has been attributed to the fact that RT increases aspects of the insulin signaling cascade that result in the upregulation of this pathway. Increases in the protein content of the insulin receptor and kinase activity (PIP-3, Akt/PKB, aPKC) are evident in resistance-trained muscle, even without increases in lean mass, and may enhance glucose uptake [96, 112–114]. Akt/PKB, insulin receptor protein, and glycogen synthase activity are increased with RT, all of which are downstream targets in the insulin signaling cascade that may be important in the translocation of GLUT-4 receptors and skeletal muscle glucose uptake [96]. These changes in the insulin signaling cascade are observed even without increases in lean mass [96]. In addition to (or possibly as a result of) the increased activity of the insulin signaling cascade, an increase in GLUT-4 protein concentration has also been observed with RT in humans [96] and rodents [112–114]. Thus, the two possible insulin signaling defects that result in insulin-resistance (decreased number of GLUT-4 transporters and/or post receptor default in the insulin signaling cascade resulting in less GLUT-4 translocation) appear to be improved with RT. Increased insulin signaling activity, along with increases in GLUT-4 protein expression, may lead to increased GLUT-4 translocation thereby increasing glucose transport and reducing insulin-resistance.
Improvements in adiponectin concentrations have been reported with weight loss [115–117], aerobic exercise [78, 80–82, 118] and RT [119, 120]. Since low adiponectin concentrations are associated with obesity, interventions often include weight loss to promote increases in adiponectin. However, some exercise interventions report increases in serum adiponectin concentrations without changes in body weight [20], although others do not [121]. There is also conflicting data on the influence of RT on adiponectin concentrations; some have reported no change [79, 116, 122] while others have reported increases [119, 120]. Methodological differences (i.e., RT intensity, measurement of total versus low/high molecular weight adiponectin) may explain conflicting findings. With regard to TNF-α, high intensity RT appears to reduce TNF-α concentrations and improve insulin sensitivity [20, 123, 124], even when fat mass is unchanged [20].
There is conflicting data on the influence of RT on ILGF-I concentrations. Borst et al. reported that 25 weeks of 1 or 3-set resistance training increased ILGF-I in healthy adults aged 25 to 50 [125]; however, this was not observed in a subsequent study by the same group using adults aged 60–85 years and high and low-intensity resistance training [126]. Conversely, others have reported significant increases in ILGF-I with resistance training in the elderly [99, 127]. These studies concluded that despite atrophy and ultrastructural damage, elders respond to RT with significant increases in musculoskeletal remodeling, cross-sectional area and elevated IGF-I levels [99, 127]. Increases in ILGF-I concentration are also associated with increases in lean mass, indicating that ILGF-I may be important in addressing age-related sarcopenia and insulin-resistance. Although more research is needed in this area, it appears that ILGF-I concentrations can be increased in older adults to augment glucose uptake and improve insulin-resistance.
4.2. Resistance Training and Body Fat Distribution
Body fat distribution may play a major role in the development of insulin-resistance, particularly abdominal fat [33, 42]. Resistance training reduces abdominal fat, including visceral fat, among individuals with diabetes [116, 128, 129]. Both low intensity RT three times per week [128], and high intensity RT twice per week [116] improve insulin-resistance and reduce body fat mass. Strength training-induced changes in abdominal visceral fat were also reported without significant weight loss [129]. Thus, RT alone may reduce abdominal and visceral fat, which is known to increase with advancing age and influence insulin-resistance.
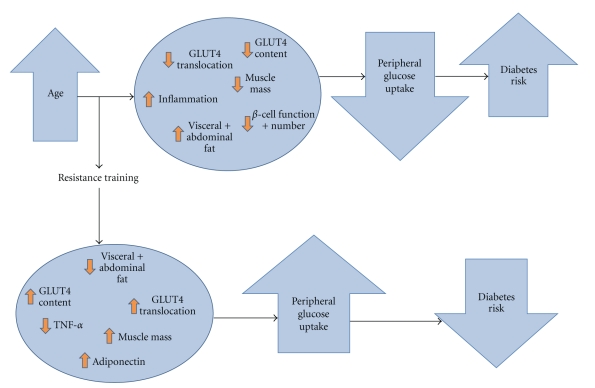
An overview of how RT may influence age-related physiological changes impacting diabetes risk is presented graphically in Figure 1.
Figure 1.
Age-related physiological changes and diabetes risk: Potential influence of RT.
5. Current Recommendations: Aging, Resistance Training, and Diabetes Prevention
Major health organizations such as the American College of Sports Medicine (ACSM), American Heart Association (AHA), and the American Geriatrics Society (AGS) have issued recommendations regarding RT for older or diabetic individuals. As stated previously, the ADA encourages individuals with type 2 diabetes to perform resistance exercise three times per week targeting all major muscle groups, and progressing to three sets of 8–10 repetitions at high intensity [103]. According to the ACSM, older adults should engage in RT at least twice per week. These sessions should include 8–10 exercises of 8–12 repetitions, involving the major muscle groups, done at a moderate to vigorous intensity [130]. Similarly, ACSM's position stand on exercise prescription for diabetes care recommends that individuals engage in RT at least twice per week, with 8–10 exercise involving the major muscle groups to be performed with at least one set of 10–15 repetitions. This position stand recognizes that increased intensity of exercise or adding additional sets may produce greater benefits, but may not be appropriate for some individuals [131]. Both ACSM position stands advocate progressive RT, with increases in resistance as the individual progresses through the program [130, 131]. The AGS recommends 2-3 days per week of RT with 10–15 repetitions at low intensity, 8–10 at moderate intensity, or 6–8 at high intensity [132]. The AHA recommends that older adults engage in resistance training 2-3 nonconsecutive days per week doing one set of 10–15 repetitions at low intensity, and also recognizes that multiple set regimens performed at higher intensities and frequencies (>2 days a week) may provide greater benefits [133]. These recommendations are similar to protocols used in many clinical trials investigating the effect of RT on diabetes-related outcomes among older adults (see Table 1). Two of the four trials included in Table 1 used a high intensity protocol, while 2 used moderate intensities and one had high intensity and low intensity groups. All of the studies used multiple set protocols. Frequency of training was most commonly three days per week (n = 4), while one study used a 4 day per week protocol.
Some studies have addressed the issue of RT intensity and volume on insulin sensitivity. High-intensity protocols show significant increases in insulin sensitivity as compared with moderate intensity protocols [106], and single set protocols may be less effective than multiple set protocols in lowering fasting blood glucose concentrations [106]. A meta analysis concluded that high intensity protocols were more effective than low intensity protocols at increasing strength in older adults [102]. Higher volume interventions are also associated with greater increases in lean body mass in older individuals [134] as well as young men [135]. This suggests the possibility of a dose-response relationship, such that improvements in strength and insulin-resistance are increased as RT intensity and volume increase. Additionally, others have reported that twice weekly RT at low intensity but high volume (three sets of ten repetitions) improved insulin-resistance [136]. Recently, RT interventions stressing volitional fatigue (i.e., the point at which the exercise could not be completed with proper technique) have been conducted [137, 138]; more work is needed to determine if this RT approach is beneficial with respect to blood glucose control and insulin-resistance.
Taken together, existing recommendations and these research studies suggest that high volume and high intensity RT may produce greater improvements in muscle mass gains, insulin-resistance and glucose tolerance; however, it would be prudent for sedentary older diabetic or prediabetic individuals to begin an RT program at low intensity (rate of perceived exertion of ~5-6) and low volume (1 set per exercise, 10–12 reps) twice weekly, and if time and fitness are sufficient, progressively increasing intensity, volume, and frequency [130, 131, 133].
5.1. Future Directions
Research suggests that RT may play a role in improving the age-related increases in insulin-resistance, and prevent the onset of diabetes. Major health organizations have recognized the benefits of RT. However, according to the CDC, only 13% of men and less than 10% of woman aged ≥65 yrs reported engaging in strength training at least two days per week [139]. Possible reasons for low rates of adoption and minimal adherence may include barriers such as the perceived complexity and knowledge needed to perform RT, misinformation of expected RT outcomes (e.g., excessive or undesirable hypertrophy), and the emphasis many public health programs and clinicians place on AT rather than RT. Once effective RT interventions are identified, the translational capabilities of intervention approaches should be investigated. Adherence, simplicity, and cost effectiveness are important for RT interventions to be successful in real-world settings.
Differences in traditional RT versus circuit weight training have not been addressed, as well as differences in protocols using free weights and those using machine weights. It is possible that certain RT approaches lead to greater rates of adoption, adherence and greater cost effectiveness among older, insulin resistant individuals.
Dietary and weight loss interventions in conjunction with RT should be investigated to determine the optimal approach for diabetes prevention with advancing age. For example, the role dietary protein intake may play in reversing insulin-resistance and improving glucose control should be studied more in depth, as high protein diets improve glucose control in individuals with type 2 diabetes when compared to those on a low protein diet [140, 141]. Additionally, a positive relationship between protein intake and change in whole body fat-free mass has been observed after pooling RT studies investigating protein intake in adults aged 50–80 [142]. Based upon these findings, it has been suggested that the RDA for protein intake (0.8 g/kg) is inadequate for older adults who engage in RT [142]. With the possibility that high protein diets can be beneficial to those with impairments in glucose metabolism as well as older adults engaging in RT, the synergistic effect of RT and high protein diets on glucose tolerance warrants further investigation.
Finally, additional work should be done to address mechanisms for RT-induced improvements in insulin-resistance and glucose tolerance. The specific effects of RT on insulin signaling are uncertain, and the effect of RT on pancreatic β-cell function/mass and mitochondrial dysfunction are unknown. It is also possible that other inflammatory markers not yet identified may influence sarcopenia and the response to RT among older adults. Although some work has been done addressing the effect of RT on visceral adipose tissue [76, 77], direct effects on FFA concentrations and gluconeogenesis are uncertain. By continuing to identify the mechanisms by which RT improves insulin-resistance, and by determining optimal combinations of RT with other lifestyle factors to prevent diabetes, interventions can be developed which optimize reduction in diabetes risk with advancing age.
In conclusion, it appears RT may be an effective intervention approach for middle-aged and older adults to counteract age-associated declines in insulin sensitivity and to prevent the onset of type 2 diabetes. Older adults who engage in RT may see benefits with respect to improvements in body composition, body fat distribution, inflammatory markers, and blood glucose homeostasis. Future research investigating mechanisms, optimal RT protocol, and intervention approaches with high translation potential are needed to enhance knowledge in this area, and to increase public awareness and adoption of RT.
Acknowledgments
This work was supported in part by K01KD075424 (to Brenda M. Davy) and R01DK082383 (to Brenda M. Davy and Richard A. Winett).
References
- 1.Centers for Disease Control and Prevention. Public health and aging: trends in aging—United States and worldwide. The Journal of the American Medical Association. 2003;289(11):1371–1373. [PubMed] [Google Scholar]
- 2.McBean AM, Li S, Gilbertson DT, Collins AJ. Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ethnic groups: whites, blacks, Hispanics, and Asians. Diabetes Care. 2004;27(10):2317–2324. doi: 10.2337/diacare.27.10.2317. [DOI] [PubMed] [Google Scholar]
- 3.Dall T, Mann SE, Zhang Y, et al. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31(3):596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 4.Boyle JP, et al. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population Health Metrics. 2010;8(1):p. 29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Standards of medical care in diabetes—2009. Diabetes Care. 2009;32(supplement 1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norris SL, Kansagara D, Bougatsos C, Fu R. Screening adults for type 2 diabetes: a review of the evidence for the U.S. preventive services task force. Annals of Internal Medicine. 2008;148(11):855–868. doi: 10.7326/0003-4819-148-11-200806030-00008. [DOI] [PubMed] [Google Scholar]
- 7.Barr ELM, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab) Circulation. 2007;116(2):151–157. doi: 10.1161/CIRCULATIONAHA.106.685628. [DOI] [PubMed] [Google Scholar]
- 8.Strasser B, Siebert U, Schobersberger W. Resistance training in the treatment of the metabolic syndrome: a systematic review and meta-analysis of the effect of resistance training on metabolic clustering in patients with abnormal glucose metabolism. Sports Medicine. 2010;40(5):397–415. doi: 10.2165/11531380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. Journal of the Neurological Sciences. 1988;84(2-3):275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 10.Roubenoff R, Castaneda C. Sarcopenia—understanding the dynamics of aging muscle. Journal of the American Medical Association. 2001;286(10):1230–1231. doi: 10.1001/jama.286.10.1230. [DOI] [PubMed] [Google Scholar]
- 11.Percheron G, Hogrel JY, Denot-Ledunois S, et al. Effect of 1-year oral administration of dehydroepiandrosterone to 60- to 80-year-old individuals on muscle function and cross-sectional area: a double-blind placebo-controlled trial. Archives of Internal Medicine. 2003;163(6):720–727. doi: 10.1001/archinte.163.6.720. [DOI] [PubMed] [Google Scholar]
- 12.Dutta C, Hadley EC. The significance of sarcopenia in old age. Journals of Gerontology A, Biological Sciences and Medical Sciences. 1995;50:1–4. doi: 10.1093/gerona/50a.special_issue.1. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ, III, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. Journal of the American Geriatrics Society. 2000;48(6):625–630. [PubMed] [Google Scholar]
- 14.Sayer AA, Syddall HE, Dennison EM, et al. Grip strength and the metabolic syndrome: findings from the Hertfordshire Cohort Study. Quarterly Journal of Medicine. 2007;100(11):707–713. doi: 10.1093/qjmed/hcm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lexell J. Human aging, muscle mass, and fiber type composition. Journals of Gerontology A, Biological Sciences and Medical Sciences. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 16.Vandervoort AA. Aging of the human neuromuscular system. Muscle and Nerve. 2002;25(1):17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 17.Burton LA, Sumukadas D. Optimal management of sarcopenia. Clinical Interventions in Aging. 2010;5:217–228. doi: 10.2147/cia.s11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen GL. Inflammation: roles in aging and sarcopenia. Journal of Parenteral and Enteral Nutrition. 2008;32(6):656–659. doi: 10.1177/0148607108324585. [DOI] [PubMed] [Google Scholar]
- 19.Baldi JC, Snowling N. Resistance training improves glycaemic control in obese type 2 diabetic men. International Journal of Sports Medicine. 2003;24(6):419–423. doi: 10.1055/s-2003-41173. [DOI] [PubMed] [Google Scholar]
- 20.Balducci S, Zanuso S, Nicolucci A, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutrition, Metabolism and Cardiovascular Diseases. 2010;20(8):608–617. doi: 10.1016/j.numecd.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Castaneda C, Layne JE, Munoz-Orians L, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25(12):2335–2341. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- 22.Cauza E, Hanusch-Enserer U, Strasser B, et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Archives of Physical Medicine and Rehabilitation. 2005;86(8):1527–1533. doi: 10.1016/j.apmr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26(11):2977–2982. doi: 10.2337/diacare.26.11.2977. [DOI] [PubMed] [Google Scholar]
- 24.Dunstan DW, Daly RM, Owen N, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25(10):1729–1736. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- 25.Dunstan DW, Vulikh E, Owen N, Jolley D, Shaw J, Zimmet P. Community center-based resistance training for the maintenance of glycemic control in adults with type 2 diabetes. Diabetes Care. 2006;29(12):2586–2591. doi: 10.2337/dc06-1310. [DOI] [PubMed] [Google Scholar]
- 26.Fenicchia LM, Kanaley JA, Azevedo JL, et al. Influence of resistance exercise training on glucose control in women with type 2 diabetes. Metabolism. 2004;53(3):284–289. doi: 10.1016/j.metabol.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Iglay HB, Thyfault JP, Apolzan JW, Campbell WW. Resistance training and dietary protein: effects on glucose tolerance and contents of skeletal muscle insulin signaling proteins in older persons. American Journal of Clinical Nutrition. 2007;85(4):1005–1013. doi: 10.1093/ajcn/85.4.1005. [DOI] [PubMed] [Google Scholar]
- 28.Ishii T, Yamakita T, Sato T, Tanaka S, Fujii S. Resistance training improves insulin sensitivity in NIDDM subjects without altering maximal oxygen uptake. Diabetes Care. 1998;21(8):1353–1355. doi: 10.2337/diacare.21.8.1353. [DOI] [PubMed] [Google Scholar]
- 29.Maiorana A, O’Driscoll G, Goodman C, Taylor R, Green D. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Research and Clinical Practice. 2002;56(2):115–123. doi: 10.1016/s0168-8227(01)00368-0. [DOI] [PubMed] [Google Scholar]
- 30.Sigal RJ, Kenny GP, Boule NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Annals of Internal Medicine. 2007;147(6):357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 31.Zachwieja JJ, Toffolo G, Cobelli C, Bier DM, Yarasheski KE. Resistance exercise and growth hormone administration in older men: effects on insulin sensitivity and secretion during a stable-label intravenous glucose tolerance test. Metabolism. 1996;45(2):254–260. doi: 10.1016/s0026-0495(96)90063-3. [DOI] [PubMed] [Google Scholar]
- 32.Kuk JL, Kilpatrick K, Davidson LE, Hudson R, Ross R. Whole-body skeletal muscle mass is not related to glucose tolerance or insulin sensitivity in overweight and obese men and women. Applied Physiology, Nutrition and Metabolism. 2008;33(4):769–774. doi: 10.1139/H08-060. [DOI] [PubMed] [Google Scholar]
- 33.Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO. Insulin resistance in aging is related to abdominal obesity. Diabetes. 1993;42(2):273–281. [PubMed] [Google Scholar]
- 34.Usui C, Asaka M, Kawano H, et al. Visceral fat is a strong predictor of insulin resistance regardless of cardiorespiratory fitness in non-diabetic people. Journal of Nutritional Science and Vitaminology. 2010;56(2):109–116. doi: 10.3177/jnsv.56.109. [DOI] [PubMed] [Google Scholar]
- 35.Douchi T, Yamamoto S, Yoshimitsu N, Andoh T, Matsuo T, Nagata Y. Relative contribution of aging and menopause to changes in lean and fat mass in segmental regions. Maturitas. 2002;42(4):301–306. doi: 10.1016/s0378-5122(02)00161-5. [DOI] [PubMed] [Google Scholar]
- 36.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Research Reviews. 2009;8(4):339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Zoico E, Di Francesco V, Mazzali G, et al. Adipocytokines, fat distribution, and insulin resistance in elderly men and women. Journals of Gerontology A, Biological Sciences and Medical Sciences. 2004;59(9):M935–M939. doi: 10.1093/gerona/59.9.m935. [DOI] [PubMed] [Google Scholar]
- 38.Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Medicine. 2000;30(5):327–346. doi: 10.2165/00007256-200030050-00002. [DOI] [PubMed] [Google Scholar]
- 39.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. New England Journal of Medicine. 1998;338(1):1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 40.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. Journal of the American Medical Association. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 41.Davis MG, Fox KR. Physical activity patterns assessed by accelerometry in older people. European Journal of Applied Physiology. 2007;100(5):581–589. doi: 10.1007/s00421-006-0320-8. [DOI] [PubMed] [Google Scholar]
- 42.Coon PJ, Rogus EM, Drinkwater D, Muller DC, Goldberg AP. Role of body fat distribution in the decline in insulin sensitivity and glucose tolerance with age. Journal of Clinical Endocrinology and Metabolism. 1992;75(4):1125–1132. doi: 10.1210/jcem.75.4.1400882. [DOI] [PubMed] [Google Scholar]
- 43.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 44.Mazzali G, Di Francesco V, Zoico E, et al. Interrelations between fat distribution, muscle lipid content, adipocytokines, and insulin resistance: effect of moderate weight loss in older women. American Journal of Clinical Nutrition. 2006;84(5):1193–1199. doi: 10.1093/ajcn/84.5.1193. [DOI] [PubMed] [Google Scholar]
- 45.Sandeep S, Gokulakrishnan K, Velmurugan K, Deepa M, Mohan V. Visceral & subcutaneous abdominal fat in relation to insulin resistance & metabolic syndrome in non-diabetic south Indians. Indian Journal of Medical Research. 2010;131(5):629–635. [PubMed] [Google Scholar]
- 46.Bjorntorp P. ’Portal’ adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10(4):493–496. [PubMed] [Google Scholar]
- 47.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. Journal of Clinical Investigation. 2004;113(11):1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen MD. Is visceral fat involved in the pathogenesis of the metabolic syndrome? Human model. Obesity. 2006;14(supplement 1):20S–24S. doi: 10.1038/oby.2006.278. [DOI] [PubMed] [Google Scholar]
- 49.Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. American Journal of Physiology. 2008;295(5):E1009–E1017. doi: 10.1152/ajpendo.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bock G, Man CD, Campioni M, et al. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 2006;55(12):3536–3549. doi: 10.2337/db06-0319. [DOI] [PubMed] [Google Scholar]
- 51.Katz MS, Lowenthal DT. Influences of age and exercise on glucose metabolism: implications for management of older diabetics. Southern Medical Journal. 1994;87(5):S70–S73. [PubMed] [Google Scholar]
- 52.Shimokata H, Muller DC, Fleg JL, Sorkin J, Ziemba AW, Andres R. Age as independent determinant of glucose tolerance. Diabetes. 1991;40(1):44–51. doi: 10.2337/diab.40.1.44. [DOI] [PubMed] [Google Scholar]
- 53.Brady MJ, Saltiel AR. Closing in on the cause of insulin resistance and type 2 diabetes. Journal of Clinical Investigation. 1999;104(6):675–676. doi: 10.1172/JCI8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klip A. The many ways to regulate glucose transporter 4. Applied Physiology, Nutrition and Metabolism. 2009;34(3):481–487. doi: 10.1139/H09-047. [DOI] [PubMed] [Google Scholar]
- 55.Jackson RA, Blix PM, Matthews JA. Influence of ageing on glucose homeostasis. Journal of Clinical Endocrinology and Metabolism. 1982;55(5):840–848. doi: 10.1210/jcem-55-5-840. [DOI] [PubMed] [Google Scholar]
- 56.Fink RI, Wallace P, Olefsky JM. Effects of aging on glucose-mediated glucose disposal and glucose transport. Journal of Clinical Investigation. 1986;77(6):2034–2041. doi: 10.1172/JCI112533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noth RH, Mazzaferri EL. Age and the endocrine system. Clinics in Geriatric Medicine. 1985;1(1):223–250. [PubMed] [Google Scholar]
- 58.Holloszy JO. Invited review: exercise-induced increase in muscle insulin sensitivity. Journal of Applied Physiology. 2005;99(1):338–343. doi: 10.1152/japplphysiol.00123.2005. [DOI] [PubMed] [Google Scholar]
- 59.Skov-Jensen C, Skovbro M, Flint A, Helge JW, Dela F. Contraction-mediated glucose uptake is increased in men with impaired glucose tolerance. Applied Physiology, Nutrition and Metabolism. 2007;32(1):115–124. doi: 10.1139/h06-098. [DOI] [PubMed] [Google Scholar]
- 60.Szoke E, Shrayyef MZ, Messing S, et al. Effect of aging on glucose homeostasis: accelerated deterioration of β-cell function in individuals with impaired glucose tolerance. Diabetes Care. 2008;31(3):539–543. doi: 10.2337/dc07-1443. [DOI] [PubMed] [Google Scholar]
- 61.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 62.Maedler K, Schumann DM, Schulthess F, et al. Aging correlates with decreased β-cell proliferative capacity and enhanced sensitivity to apoptosis: a potential role for fas and pancreatic duodenal homeobox-1. Diabetes. 2006;55(9):2455–2462. doi: 10.2337/db05-1586. [DOI] [PubMed] [Google Scholar]
- 63.Van Haeften TW, Pimenta W, Mitrakou A, et al. Relative contributions of β-cell function and tissue insulin sensitivity to fasting and postglucose-load glycemia. Metabolism. 2000;49(10):1318–1325. doi: 10.1053/meta.2000.9526. [DOI] [PubMed] [Google Scholar]
- 64.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circulation Research. 2008;102(4):401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abdul-Ghani MA, DeFronzo RA. Mitochondrial dysfunction, insulin resistance, and type 2 diabetes mellitus. Current Diabetes Reports. 2008;8(3):173–178. doi: 10.1007/s11892-008-0030-1. [DOI] [PubMed] [Google Scholar]
- 66.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. Journal of Biological Chemistry. 2000;275(5):3343–3347. doi: 10.1074/jbc.275.5.3343. [DOI] [PubMed] [Google Scholar]
- 68.Cortopassi GA, Shibata D, Soong NW, Arnheim N. A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(16):7370–7374. doi: 10.1073/pnas.89.16.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sial S, Coggan AR, Carroll R, Goodwin J, Klein S. Fat and carbohydrate metabolism during exercise in elderly and young subjects. American Journal of Physiology. 1996;271(6, part 1):E983–E989. doi: 10.1152/ajpendo.1996.271.6.E983. [DOI] [PubMed] [Google Scholar]
- 70.Perseghin G, Scifo P, De Cobelli F, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a H-C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48(8):1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 71.Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a H NMR spectroscopy study. Diabetologia. 1999;42(1):113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 72.Griffin ME, Marcucci MJ, Cline GW, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C θ and alterations in the insulin signaling cascade. Diabetes. 2000;48(6):1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 73.Nieto-Vazquez I, Fernández-Veledo S, Krämer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Archives of Physiology and Biochemistry. 2008;114(3):183–194. doi: 10.1080/13813450802181047. [DOI] [PubMed] [Google Scholar]
- 74.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Molecular Medicine. 2008;14(11-12):741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pajvani UB, Scherer PE. Adiponectin: systemic contributor to insulin sensitivity. Current Diabetes Reports. 2003;3(3):207–213. doi: 10.1007/s11892-003-0065-2. [DOI] [PubMed] [Google Scholar]
- 76.Kanaya AM, Harris T, Goodpaster BH, Tylavsky F, Cummings SR. Adipocytokines attenuate the association between visceral adiposity and diabetes in older adults. Diabetes Care. 2004;27(6):1375–1380. doi: 10.2337/diacare.27.6.1375. [DOI] [PubMed] [Google Scholar]
- 77.Koster A, Stenholm S, Alley DE, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity. 2010;18(12):2354–2361. doi: 10.1038/oby.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lim S, Sung HC, Jeong INK, et al. Insulin-sensitizing effects of exercise on adiponectin and retinol-binding protein-4 concentrations in young and middle-aged women. Journal of Clinical Endocrinology and Metabolism. 2008;93(6):2263–2268. doi: 10.1210/jc.2007-2028. [DOI] [PubMed] [Google Scholar]
- 79.Ahmadizad S, Haghighi AH, Hamedinia MR. Effects of resistance versus endurance training on serum adiponectin and insulin resistance index. European Journal of Endocrinology. 2007;157(5):625–631. doi: 10.1530/EJE-07-0223. [DOI] [PubMed] [Google Scholar]
- 80.Blüher M, Williams CJ, Klöting N, et al. Gene expression of adiponectin receptors in human visceral and subcutaneous adipose tissue is related to insulin resistance and metabolic parameters and is altered in response to physical training. Diabetes Care. 2007;30(12):3110–3115. doi: 10.2337/dc07-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blüher M, Bullen JW, Lee JH, et al. Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training. Journal of Clinical Endocrinology and Metabolism. 2006;91(6):2310–2316. doi: 10.1210/jc.2005-2556. [DOI] [PubMed] [Google Scholar]
- 82.Kim ES, Im J-A, Kim KC, et al. Improved insulin sensitivity and adiponectin level after exercise training in obese Korean youth. Obesity. 2007;15(12):3023–3030. doi: 10.1038/oby.2007.360. [DOI] [PubMed] [Google Scholar]
- 83.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Medicine. 2002;8(11):1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 84.Hotamisligil GS. Inflammatory pathways and insulin action. International Journal of Obesity. 2003;27(supplement 3):S53–S55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 85.Le Roith D. Insulin-like growth factors. New England Journal of Medicine. 1997;336(9):633–640. doi: 10.1056/NEJM199702273360907. [DOI] [PubMed] [Google Scholar]
- 86.Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. Journal of Biological Chemistry. 1978;253(8):2769–2776. [PubMed] [Google Scholar]
- 87.Benbassat CA, Maki KC, Unterman TG. Circulating levels of insulin-like growth factor (IGF) binding protein- 1 and -3 in aging men: relationships to insulin, glucose, IGF, and dehydroepiandrosterone sulfate levels and anthropometric measures. Journal of Clinical Endocrinology and Metabolism. 1997;82(5):1484–1491. doi: 10.1210/jcem.82.5.3930. [DOI] [PubMed] [Google Scholar]
- 88.Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocrine Reviews. 1993;14(1):20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- 89.Rudman D, Feller AG, Nagraj HS, et al. Effects of human growth hormone in men over 60 years old. New England Journal of Medicine. 1990;323(1):1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- 90.Rajpathak SN, Gunter MJ, Wylie-Rosett J, et al. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes/Metabolism Research and Reviews. 2009;25(1):3–12. doi: 10.1002/dmrr.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. Journal of the American Medical Association. 2001;286(10):1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 93.Dengel DR, Pratley RE, Hagberg JM, Rogus EM, Goldberg AP. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. Journal of Applied Physiology. 1996;81(1):318–325. doi: 10.1152/jappl.1996.81.1.318. [DOI] [PubMed] [Google Scholar]
- 94.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(6):1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 95.Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. New England Journal of Medicine. 1994;330(25):1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 96.Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JFP, Dela F. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes. 2004;53(2):294–305. doi: 10.2337/diabetes.53.2.294. [DOI] [PubMed] [Google Scholar]
- 97.Rantakokko M, Iwarsson S, Hirvensalo M, Leinonen R, Heikkinen E, Rantanen T. Unmet physical activity need in old age. Journal of the American Geriatrics Society. 2010;58(4):707–712. doi: 10.1111/j.1532-5415.2010.02792.x. [DOI] [PubMed] [Google Scholar]
- 98.Seynnes O, Singh MAF, Hue O, Pras P, Legros P, Bernard PL. Physiological and functional responses to low-moderate versus high-intensity progressive resistance training in frail elders. Journals of Gerontology A, Biological Sciences and Medical Sciences. 2004;59(5):503–509. doi: 10.1093/gerona/59.5.m503. [DOI] [PubMed] [Google Scholar]
- 99.Fiatarone Singh MA, Ding W, Manfredi TJ, et al. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. American Journal of Physiology. 1999;277(1, part 1):E135–E143. doi: 10.1152/ajpendo.1999.277.1.E135. [DOI] [PubMed] [Google Scholar]
- 100.Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of progressive resistance training in depressed elders. Journals of Gerontology A, Biological Sciences and Medical Sciences. 1997;52(1):M27–M35. doi: 10.1093/gerona/52a.1.m27. [DOI] [PubMed] [Google Scholar]
- 101.Hurley BF, Redmond RA, Pratley RE, Treuth MS, Rogers MA, Goldberg AP. Effects of strength training on muscle hypertrophy and muscle cell disruption in elder men. International Journal of Sports Medicine. 1995;16(6):378–384. doi: 10.1055/s-2007-973024. [DOI] [PubMed] [Google Scholar]
- 102.Steib S, Schoene D, Pfeifer K. Dose-response relationship of resistance training in older adults: a meta-analysis. Medicine and Science in Sports and Exercise. 2010;42(5):902–914. doi: 10.1249/MSS.0b013e3181c34465. [DOI] [PubMed] [Google Scholar]
- 103.Standards of medical care in diabetes—2006. Diabetes Care. 2006;29(supplement 1):S4–S42. [PubMed] [Google Scholar]
- 104.Kumar V, Selby A, Rankin D, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. Journal of Physiology. 2009;587(1):211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.U.S. Department of Health and Human Sercices, C.f.D.C.a.P., National Center for Chronic Disease Prevention and Health Promotion. U.S. Department of health and Human Services: Physical Activity and health: A Report of the Surgeon General. Atlanta, 1996.
- 106.Black LE, Swan PD, Alvar BA. Effects of intensity and volume on insulin sensitivity during acute bouts of resistance training. Journal of Strength and Conditioning Research. 2010;24(4):1109–1116. doi: 10.1519/JSC.0b013e3181cbab6d. [DOI] [PubMed] [Google Scholar]
- 107.Eriksson J, Tuominen J, Valle T, et al. Aerobic endurance exercise or circuit-type resistance training for individuals with impaired glucose tolerance? Hormone and Metabolic Research. 1998;30(1):37–41. doi: 10.1055/s-2007-978828. [DOI] [PubMed] [Google Scholar]
- 108.Smutok MA, Reece C, Kohhinos PF, et al. Effects of exercise training modality on glucose tolerance in men with abnormal glucose regulation. International Journal of Sports Medicine. 1994;15(6):283–289. doi: 10.1055/s-2007-1021061. [DOI] [PubMed] [Google Scholar]
- 109.Smutok MA, Reece C, Kokkinos PF, et al. Aerobic versus strength training for risk factor intervention in middle- aged men at high risk for coronary heart disease. Metabolism. 1993;42(2):177–184. doi: 10.1016/0026-0495(93)90032-j. [DOI] [PubMed] [Google Scholar]
- 110.Onambélé-Pearson GL, Breen L, Stewart CE. Influence of exercise intensity in older persons with unchanged habitual nutritional intake: skeletal muscle and endocrine adaptations. Age. 2010;32(2):139–153. doi: 10.1007/s11357-010-9141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jimenez C, Santiago M, Sitler M, Boden G, Homko C. Insulin-sensitivity response to a single bout of resistive exercise in type 1 diabetes mellitus. Journal of Sport Rehabilitation. 2009;18(4):564–571. doi: 10.1123/jsr.18.4.564. [DOI] [PubMed] [Google Scholar]
- 112.Yaspelkis BB. Resistance training improves insulin signaling and action in skeletal muscle. Exercise and Sport Sciences Reviews. 2006;34(1):42–46. doi: 10.1097/00003677-200601000-00009. [DOI] [PubMed] [Google Scholar]
- 113.Krisan AD, Collins DE, Crain AM, et al. Resistance training enhances components of the insulin signaling cascade in normal and high-fat-fed rodent skeletal muscle. Journal of Applied Physiology. 2004;96(5):1691–1700. doi: 10.1152/japplphysiol.01054.2003. [DOI] [PubMed] [Google Scholar]
- 114.Yaspelkis BB, III, Singh MK, Trevino B, Krisan AD, Collins DE. Resistance training increases glucose uptake and transport in rat skeletal muscle. Acta Physiologica Scandinavica. 2002;175(4):315–323. doi: 10.1046/j.1365-201X.2002.00998.x. [DOI] [PubMed] [Google Scholar]
- 115.Behre CJ, Gummesson A, Jernås M, et al. Dissociation between adipose tissue expression and serum levels of adiponectin during and after diet-induced weight loss in obese subjects with and without the metabolic syndrome. Metabolism. 2007;56(8):1022–1028. doi: 10.1016/j.metabol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 116.Ibanez J, Izquierdo M, Martínez-Labari C, et al. Resistance training improves cardiovascular risk factors in obese women despite a significative decrease in serum adiponectin levels. Obesity. 2010;18(3):535–541. doi: 10.1038/oby.2009.277. [DOI] [PubMed] [Google Scholar]
- 117.Weiss EP, Racette SB, Villareal DT, et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. American Journal of Clinical Nutrition. 2006;84(5):1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oberbach A, Tönjes A, Klöting N, et al. Effect of a 4 week physical training program on plasma concentrations of inflammatory markers in patients with abnormal glucose tolerance. European Journal of Endocrinology. 2006;154(4):577–585. doi: 10.1530/eje.1.02127. [DOI] [PubMed] [Google Scholar]
- 119.Fatouros IG, Chatzinikolaou A, Tournis S, et al. Intensity of resistance exercise determines adipokine and resting energy expenditure responses in overweight elderly individuals. Diabetes Care. 2009;32(12):2161–2167. doi: 10.2337/dc08-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brooks N, Layne JE, Gordon PL, Roubenoff R, Nelson ME, Castaneda-Sceppa C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. International Journal of Medical Sciences. 2007;4(1):19–27. doi: 10.7150/ijms.4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hulver MW, Zheng D, Tanner CJ, et al. Adiponectin is not altered with exercise training despite enhanced insulin action. American Journal of Physiology. 2002;283(4):E861–E865. doi: 10.1152/ajpendo.00150.2002. [DOI] [PubMed] [Google Scholar]
- 122.Klimcakova E, Polak J, Moro C, et al. Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. Journal of Clinical Endocrinology and Metabolism. 2006;91(12):5107–5112. doi: 10.1210/jc.2006-0382. [DOI] [PubMed] [Google Scholar]
- 123.Greiwe JS, Bo C, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor α in frail elderly humans. FASEB Journal. 2001;15(2):475–482. doi: 10.1096/fj.00-0274com. [DOI] [PubMed] [Google Scholar]
- 124.Phillips MD, Flynn MG, McFarlin BK, Stewart LK, Timmerman KL. Resistance training at eight-repetition maximum reduces the inflammatory milieu in elderly women. Medicine and Science in Sports and Exercise. 2010;42(2):314–325. doi: 10.1249/MSS.0b013e3181b11ab7. [DOI] [PubMed] [Google Scholar]
- 125.Borst SE, De Hoyos DV, Garzarella L, et al. Effects of resistance training on insulin-like growth factor-I and IGF binding proteins. Medicine and Science in Sports and Exercise. 2001;33(4):648–653. doi: 10.1097/00005768-200104000-00021. [DOI] [PubMed] [Google Scholar]
- 126.Borst SE, Vincent KR, Lowenthal DT, Braith RW. Effects of resistance training on insulin-like growth factor and its binding proteins in men and women aged 60 to 85. Journal of the American Geriatrics Society. 2002;50(5):884–888. doi: 10.1046/j.1532-5415.2002.50215.x. [DOI] [PubMed] [Google Scholar]
- 127.Bermon S, Ferrari P, Bernard P, Altare S, Dolisi C. Responses of total and free insulin-like growth factor-I and insulin-like growth factor binding protein-3 after resistance exercise and training in elderly subjects. Acta Physiologica Scandinavica. 1999;165(1):51–56. doi: 10.1046/j.1365-201x.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- 128.Kwon HR, et al. The effects of resistance training on muscle and body fat mass and muscle strength in type 2 diabetic women. Korean Diabetes Journal. 2010;34(2):101–110. doi: 10.4093/kdj.2010.34.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Treuth MS, Hunter GR, Kekes-Szabo T, Weinsier RL, Goran MI, Berland L. Reduction in intra-abdominal adipose tissue after strength training in older women. Journal of Applied Physiology. 1995;78(4):1425–1431. doi: 10.1152/jappl.1995.78.4.1425. [DOI] [PubMed] [Google Scholar]
- 130.Chodzko-Zajko WJ, et al. American college of sports medicine position stand. Exercise and physical activity for older adults. Medicine & Science in Sports & Exercise. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 131.Albright A, Franz M, Hornsby G, Kriska A, Marrero D, Ullrich I. ACSM position stand on exercise and type 2 diabetes. Medicine and Science in Sports and Exercise. 2000;32(7):1345–1360. doi: 10.1097/00005768-200007000-00024. [DOI] [PubMed] [Google Scholar]
- 132.Lundebjerg N. Exercise prescription for older adults with osteoarthritis pain: consensus practice recommendations. Journal of the American Geriatrics Society. 2001;49(6):808–823. doi: 10.1046/j.1532-5415.2001.00496.x. [DOI] [PubMed] [Google Scholar]
- 133.Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116(5):572–584. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 134.Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. doi: 10.1249/MSS.0b013e3181eb6265. Medicine & Science in Sports & Exercise. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Burd NA, Holwerda AM, Selby KC, et al. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. Journal of Physiology. 2010;588(16):3119–3130. doi: 10.1113/jphysiol.2010.192856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kodama S, Shu M, Saito K, et al. Even low-intensity and low-volume exercise training may improve insulin resistance in the elderly. Internal Medicine. 2007;46(14):1071–1077. doi: 10.2169/internalmedicine.46.0096. [DOI] [PubMed] [Google Scholar]
- 137.Burd NA, West DWD, Staples AW, et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One. 2010;5(8):p. 10. doi: 10.1371/journal.pone.0012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Phillips SM, Winett RA. Uncomplicated resistance training and health-related outcomes: evidence for a public health mandate. Current Sports Medicine Reports. 2010;9(4):208–213. doi: 10.1249/JSR.0b013e3181e7da73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Strength Training among adults aged ≥65—United States, 2001, 2001. [PubMed]
- 140.Gannon MC, Nuttall FQ. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes. 2004;53(9):2375–2382. doi: 10.2337/diabetes.53.9.2375. [DOI] [PubMed] [Google Scholar]
- 141.Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. American Journal of Clinical Nutrition. 2003;78(4):734–741. doi: 10.1093/ajcn/78.4.734. [DOI] [PubMed] [Google Scholar]
- 142.Campbell WW, Leidy HJ. Dietary protein and resistance training effects on muscle and body composition in older persons. Journal of the American College of Nutrition. 2007;26(6):696S–703S. doi: 10.1080/07315724.2007.10719650. [DOI] [PubMed] [Google Scholar]