Abstract
Complete sequences of 9.5-kb pPCP1 plasmids in three Yersinia pestis strains from the former Soviet Union (FSU) were determined and compared with those of pPCP1 plasmids in three well-characterized, non-FSU Y. pestis strains (KIM, CO92, and 91001). Two of the FSU plasmids were from strains C2614 and C2944, isolated from plague foci in Russia, and one plasmid was from strain C790 from Kyrgyzstan. Sequence analyses identified four sequence types among the six plasmids. The pPCP1 plasmids in the FSU strains were most genetically related to the pPCP1 plasmid in the KIM strain and least related to the pPCP1 plasmid in Y. pestis 91001. The FSU strains generally had larger pPCP1 plasmid copy numbers compared to strain CO92. Expression of the plasmid's pla gene was significantly (P ≤ .05) higher in strain C2944 than in strain CO92. Given pla's role in Y. pestis virulence, this difference may have important implications for the strain's virulence.
1. Introduction
Yersinia pestis, the causative agent of Black Death, is a highly virulent bacterium responsible for an estimated 200 million human deaths throughout recorded history. The bacterium is believed to have evolved from the much less virulent Y. pseudotuberculosis relatively recently on the evolutionary scale, approximately 1,500–20,000 years ago [1]. In most of the developed world, the genetic organization, virulence mechanisms, and life cycle of Y. pestis have been extensively studied using a few Y. pestis strains [2], some of whose genomes have been fully sequenced [3, 4]. In contrast, there remains a striking paucity of data concerning the genetic organization, virulence traits, prevalence, and epidemiology of Y. pestis in other parts of the world, including the Republic of Georgia and other republics of the former Soviet Union (FSU) [5–7].
The evolution of Y. pestis, and much of its virulence, is due to its acquisition of plasmids. Three plasmids (pCD1, pFra, and pPCP1) are typically present in all biovars of Y. pestis although additional plasmids, many of which are cryptic, have been reported [8–11] to be present in several worldwide strains of Y. pestis. pCD1 (also designated pCad, pLcr, pVW, and pYV) is a 68 to 75 kb plasmid found in all three currently recognized pathogenic species of Yersinia: Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica [9, 12]. It contains genes encoding several essential virulence determinants, including the highly conserved low-calcium response stimulus protein (LCRS), which has both regulatory and antihost functions and the Yersinia outer membrane proteins (Yops).
The pFra plasmid (also designated pYT and pMT1) is unique to Y. pestis. It is typically ca. 100-kb, but its size can vary drastically among various strains, ranging from 60 kb in the deleted version of the Dodson strain [13] to 280-kb in other strains [14]. The plasmid's role in Y. pestis virulence is not fully understood, but it is known to contain genes that encode two putative virulence factors: (i) the F1 protective antigen associated with increased resistance to phagocytosis by monocytes and (ii) the Y. pestis murine toxin (YMT), a phospholipase D encoded by ymt, whose intracellular activity protects Y. pestis from digestion in the flea gut; thus, facilitating the bacterium's ability to colonize the flea's midgut and to increase its arthropod-borne transmission [15].
pPCP1 (also designated pPla, pYP, and pPst) is another plasmid unique to Y. pestis and has a size of approximately 9.6 kb. In addition to possessing a few regulatory genes and genes encoding “hypothetical proteins,” it contains pla, pst, and pim, which encode a plasminogen activator (PLA) protease, the bacteriocin pesticin, and a pesticin immunity protein, respectively [9]. Its size and genetic organization are usually similar among various strains of Y. pestis although some isolates of the bacterium may lack the plasmid or may contain a multimer of the plasmid [8, 14, 16]. pPCP1 is an important virulence determinant in Y. pestis because it contains pla, the gene that encodes the PLA protease virulence factor. However, despite the importance of pPCP1 and other plasmids for Y. pestis virulence, there is a lack of information about the genetic structure of the plasmids in FSU strains. During the studies reported in this communication, we (i) determined the complete sequences of pPCP1 plasmids in three Y. pestis strains isolated from the FSU and examined in a recent study [7], (ii) compared their nucleotide sequences with those of pPCP1 plasmids in three well-characterized, non-FSU strains, and (iii) determined the relative pPCP1 plasmid copy number of three FSU Y. pestis strains.
2. Materials and Methods
2.1. Bacterial Strains
pPCP1 plasmids were extracted from bacteria in a recentlycharacterized FSU Y. pestis strain collection [7]. The pPCP1 plasmid from strain CO92 was used as a control. Multiple small specimens of each strain were stored at −80°C in 70% Luria-Bertani (LB) broth supplemented with 30% glycerol (v/v), and each aliquot was used only once before being discarded.
2.2. Multilocus Variable Number Tandem Repeat (MLVA) Analysis
Our MLVA analysis used a simple 7-polymorphic marker (ms01, ms04, ms06, ms07, ms46, ms62, and ms70) protocol previously described by Pourcel et al. 2004 [17]. The primers used for PCR amplification are listed in Table 1 (supplementary material). The PCR reactions were performed with aliquots of purified chromosomal DNA (50 μL containing 1 to 2 ng) and Choice-Taq Blue DNA polymerase (Denville Scientific, Metuchen, NJ). The sequential reaction conditions were (i) 96°C for 5 minutes, (ii) 34 cycles of denaturation (96°C, 20 seconds), (iii) annealing (54°C, 30 seconds), (iv) elongation (72°C, 1 minute), and (v) a final extension step for 5 minutes at 65°C. The PCR products were purified with a Qia Quick 96-well Plate Kit (QIAGEN, Valencia, CA), and the purified PCR products were sequenced in both directions with a BigDye 200 Terminator Cycle Sequencing Kit and a 201 ABI 3730xl 202 DNA analyzer (Applied Biosystems, Foster City, CA). The individual sequences were analyzed with a Tandem Repeat Finder (TRF) program (http://tandem.bu.edu/trf/trf.html) and the number of repeats for each marker was determined.
2.3. PCR Amplification of the pla Gene and Plasmid Extraction
Well-isolated colonies of each strain were obtained by streaking and incubating (28°C) the bacteria on brain heart infusion (BHI) agar. After 48 hours, 5 mL aliquots of BHI broth were inoculated with well-isolated colonies of the strains and incubated at 28°C for 24 hours with agitation (200 rpm). DNA was extracted from the bacteria in 3 mL aliquots from each broth culture, and the pla, pim, and pst genes were PCR-amplified using primers plaF1, plaR1, pimF, pimR pstF, and pstR (Supplementary Material Table 1 available at doi:10.1155/2010/760819), which amplified 470-, 201-, and 197-bp regions of the respective genes. The cultures which were PCR-positive for all three genes were streaked on BHI agar and incubated at 28°C for 24 hours. Aliquots of 100 mL BHI were inoculated with one loopful of culture from the streak plates. After incubation with agitation (200 rpm) at 28°C for 24 hours the bacteria were collected by centrifugation, and plasmid DNA was extracted with a QIA filter Plasmid Midi Kit (QIAGEN) according to the manufacturer's instructions, and the DNA specimens were characterized by agarose gel (1%, w/v) electrophoresis. The reproducibility of the data was confirmed by repeating the experiments at least twice for each strain.
2.4. Sequence Comparison and Confirmation pMT1-pCD1 Chimera Plasmid
To determine the nature of atypical plasmid seen in C790, we obtained 454 sequence of the plasmid and genomic DNA and compared this sequence to the respective plasmids of CO92 genome (Sozhamannan laboratory, unpublished results). To confirm the atypical plasmid as a chimera of pMT1 and pCD1, PCR primers sulk560 (ACTCACGCAGCGTATCTTCC, pMT1) and sulk561 (ATTCTCTGTCGTTCGGCTTG, pCD1) were designed to amplify across the junction by PCR.
2.5. Sequencing the pPCP1 Plasmids
The nucleotide sequences of the extracted pPCP1 plasmids were determined by the primer walking approach. The primers used for sequencing are listed in Table 1 (Supplementary Material). The plasmids were sequenced, in both directions, with a BigDye 200 Terminator Cycle Sequencing Kit and a 201 ABI 3730xl 202 DNA analyzer (Applied Biosystems). Contigs were assembled with Phred [18] and Phrap (available at http://www.washington.edu/) programs. The contigs were viewed with the Consed program [19], and the resulting DNA sequences were trimmed by removing low-quality nucleotide sequences from the end. The sequences were aligned by the Sequencher 4.7 program. The final sequences were compared with those of previously characterized pPCP1 plasmids in three non-FSU Y. pestis strains (KIM, CO92, and 91001). The latter sequences were available from the Institute of Genomic Research (TIGR) database (http://www.tigr.org/). The genetic relatedness among the plasmids was determined by the neighbor joining tree method, using the BLOSUM62 of EMBL-EBI ClustalW2 program (http://www.ebi.ac.uk/).
2.6. Quantitative PCR of DNA and RNA
For extracting genomic DNA and total RNA, cultures were grown over night in 5 ml BHI broth at 28°C shaking at 200 rpm after inoculating fresh well isolated colony from a BHI agar plate. For total RNA extraction 5 ml of fresh BHI media was inoculated with 500 μl of overnight culture and incubated shaking (200 rpm) at 28°C for 4 h.
Total DNA was extracted with a Genomic DNA Purification Kit (Promega, Madison, WI), and the DNA concentration in the extracts was adjusted to 100 ng/μL. Primers for the pla gene (plaF2 and plaR2, Supplementary Material Table 1) were used to estimate the copy number of the pPCP1 plasmid, and glnF and glnR primers (Supplementary Material Table 1) for the chromosomal, single-copy glnA gene were used as the control. A standard curve (for concentrations ranging from 104 copies/μL to 1011copies/μL) was constructed in order to estimate the copy number of pla. An iQ Syber Green Kit (BioRad, Hercules, CA) was used for real-time PCR (RT-PCR), and the ratio of the pla starting quantity to glnA starting quantity was calculated and compared among the strains. The experiment was repeated two times and the average values were estimated.
Total RNA was extracted with an RNEasy Mini Kit (QIAGEN), and contaminating DNA was removed by passing the extracts through a gel matrix containing bound RNase-free DNase (QIAGEN). The RNA concentration in the extracts was adjusted to approximately 50 ng/μL, and 100 ng aliquots in 25 μL reaction mixtures were reverse-transcribed with an iScript cDNA Synthesis Kit (BioRad). RT-PCR was performed with reaction mixtures (25 μL) containing a template composed of 2 μL of the cDNA preparations. The relative expression of pla to the expression of the reference gene (glnA) was determined using the 2−ΔΔC T method [20]. Statistical analyses of each set of the pla expression data were performed separately, with the GraphPad InStat (version 3.05) program (GraphPad Software, San Diego, CA). An unpaired t-test was used to determine whether the differences observed in pla expression among the four strains analyzed (C790, C2614, C2944, and CO92) were statistically significant. A P-value <.05 indicated a statistically significant difference between the results.
2.7. Nucleotide Sequence Accession Numbers
Complete sequences of the three pCP1 plasmids described in this study (extracted from FSU strains C790, C2944, and C2614) have been deposited in GenBank, under accession numbers BankIt1374063 C790 HM807366, BankIt1374063 C2614 HM807367, BankIt1374063 C2944 HM807368, and the partial sequence of C790 chimera plasmid of pMT1 and pCD1 BankIt1408393 Chimera HQ612242.
3. Results and Discussion
3.1. Genetic Relatedness of FSU Y. pestis Strains
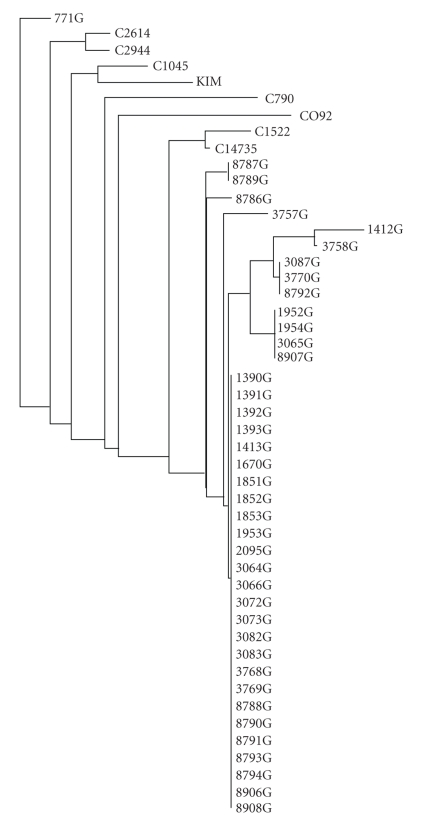
A recent publication [7] describing the forty-six FSU Y. pestis strains isolated from the Republic of Georgia and surrounding countries used biochemical profiling, pulsed field gel electrophoresis (PFGE), and multilocus sequence typing (MLST) to determine the genetic relationships between Georgian Y. pestis strains and Y. pestis strains from neighboring countries and other parts of the world. It was found that the Georgian Y. pestis strains were of clonal origin and that PFGE discriminated the Y. pestis strains better than did MLST. In the present study, we expanded this investigation by analyzing the same strain collection with MLVA, which has been reported to be well-suited for differentiating various bacterial pathogens, including Y. pestis [17, 21, 22]. The results of the overall MLVA analysis were in agreement with those of the PFGE and MLST analyses: MLVA grouped the Georgian strains in three major clusters (Figure 1 and Supplementary Material Table 2), except for strain 771G which was a clear outlier; this strain also clustered differently during previous studies [7]. Further, in agreement with the reports mentioned above, we found that MLVA differentiated the strains with greater sensitivity than PFGE and MLST. Specifically, MLVA was able to discriminate between Georgian Y. pestis strains that were grouped in a single cluster by MLST and PFGE in the previous study by [7]; for example, Y. pestis strains 8787G, 3757G, and 1412G were unresolved by PFGE typing, but they were differentiated by MLVA (Figure 1), which suggests that MLVA is better suited for specific identification of FSU Y. pestis strains than PFGE or MLST.
Figure 1.
Neighbor-joining tree generated from MLVA data based on 7 loci.
3.2. Prevalence of the pPCP1 Plasmid in FSU Strains
Some of the Y. pestis strains isolated from the FSU, including those isolated in the Transcaucasian and Daghestan Mountains; that is, two natural foci of plague adjacent to the areas from which our Y. pestis strains were isolated [7], have been reported not to contain the pPCP1 plasmid [14]. Many, but not all of these strains, are thought to belong to the so-called Pestoides biovar of rhamnose-fermenting Y. pestis strains commonly found in enzootic hosts, but not usually associated with human infections. Also, Yersinia plasmids are unstable and may be easily lost during prolonged storage and passaging. The Y. pestis strains in our collection were isolated during 1966–1997 [7], and they were regularly passaged during their storage, which could have further facilitated plasmid loss. Therefore, we first screened the FSU strains of Y. pestis by pPCP1-specific PCR (using the primers listed in Supplementary Material Table 1), in order to identify the strains in which the plasmid was still present. Only three of the forty-six FSU strains we examined (C2614, C2944, and C790) gave strong specific positive PCR signals for all the three genes (pla, pim, and pst) known to be contained in the pPCP1 plasmid. Also, the buffer control and Y. enterocolitica strain ATCC 9610 (both negative controls) did not yield the PCR amplicons. The results of the pPCP1-targeted PCR screening study suggested that many of the FSU strains in our collection do not contain the pPCP1 plasmid, an observation that was further verified by direct plasmid extraction and other approaches described below. Even though in our previous report [7] we did report all the strains isolated from Republic of Georgia to posses pla gene, in this study, we find only 3 strains to posses the plasmid. We are still investigating the possible cause for this discrepancy and certainly will address in our future publications. Some possible reasons why this could have happened are 1. different batches of strains were used in each of this study, and there could be difference in plasmid composition of strains depending how they were handeled and shipped to us 2. there could have been low level contamination with a pla positive strain/ DNA in our first batch of strains.
3.3. Plasmid Extraction and Copy Numbers of pPCP1
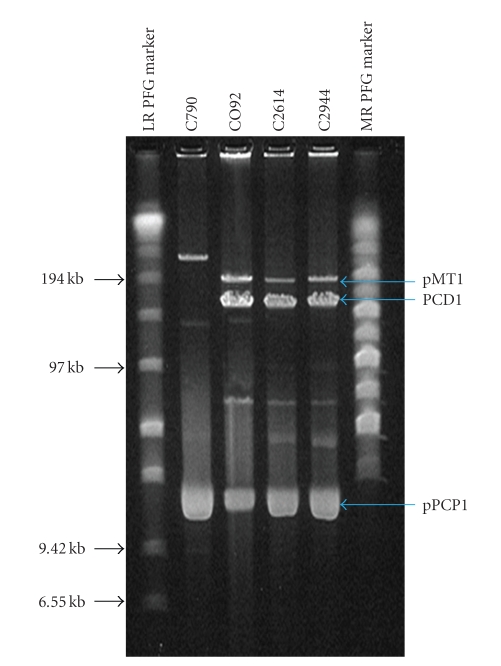
Efforts were made to extract the pPCP1 plasmid from all of the forty-six FSU Y. pestis strains in the collection, including three strains that gave positive signals for pla, pim, and pst. The pPCP1 containing, non-FSU strain CO92 was used as the positive control during plasmid isolation. Plasmid DNA was obtained from all three PCR-positive strains, but not from the forty-three Y. pestis strains that were pla, pim, and pst PCR negative, which supports our PCR screening data and indicates that PCR-based screening is useful for detecting the presence of the pPCP1 plasmid in Y. pestis strains. The presence of the pPCP1 plasmid appeared to be limited to the C2614-C2944 MLVA cluster and closely related clusters (Figure 1). It is possible that the strains in those clusters are inherently more stable in maintaining their plasmid composition; however, it is also possible that the strains in the most common cluster never contained that plasmid. Because of the known laboratory passage histories of these strains, resolution of this question may be resolved by ongoing efforts in the FSU to discover new epizootic strains. Moreover, the dearth of plasmid-positive strains does not allow for rigorous analysis of any possible association between plasmid-containing strains and their distribution among various MLVA groups or clusters. The plasmid yield from the three PCR-positive FSU strains and from CO92 that varied even though the strains were grown under identical conditions and their concentrations (CFU/mL) prior to plasmid extraction were about the same: approximately 250 to 300 μg of plasmid DNA was consistently obtained from Y. pestis strain C790 compared to around 350 to 450 μg from strains C2614, C2944, and CO92. Interestingly, FSU strain C790 lacked the 70-kb pPCD1 plasmid and its pMT1 plasmid was larger than the typical 96-kb pPMT1 plasmid (Figure 2). When 454 whole-genome sequence (WGS) output of C790 was compared to CO92 plasmid sequences, the alignment indicated that the atypical plasmid is in fact a chimera of pMTI and pCD1 with one junction being at NC_003131.1, position 65224 and NC_003134.1, position 73221. Individual sequencing reads that spanned this junction were clearly evident, and this conclusion was verified by PCR amplification of the expected 659 pb PCR product in C790 and but not in CO92 (Figure 4). We also notice a faint band in strain C2944 suggesting it may also have a chimera plasmid in low abundance along with typical pCD1 and pMT1 plasmids. The idea of the atypical plasmid being a chimera plasmid is supported by the fact that both lcrV (encoded on pCD1) and caf1 genes were detected by PCR in this strain [7]. The second junction was not identified by WGS. Because of the tendency of bioinformatic assembly programs to collapse large repeats, we believe that the second junction is likely to lie in one of the large, highly repetitive IS100 elements that are found on all three plasmids and the chromosome. Identification of the second junction point, the full description of the chimera and its effect on pathogenesis will be the subject of future studies. Chimeric plasmids resulting from recombination between homologous sequences of Y. pestis plasmids have been documented; for example, the deep-rooted Angola strain contains a dimeric pPCP1 plasmid that is integrated at an IS100 element in tandem repeats into the pMT1 plasmid [23].
Figure 2.
Pulsed field gel electrophoresis of total plasmid content on 1% agarose gel. Lane 1: low range PFG marker, lane 2: C790, lane 3: CO92, lane 4: C2614, lane 5: C2944 and lane 6: medium range PFG marker.
Figure 4.
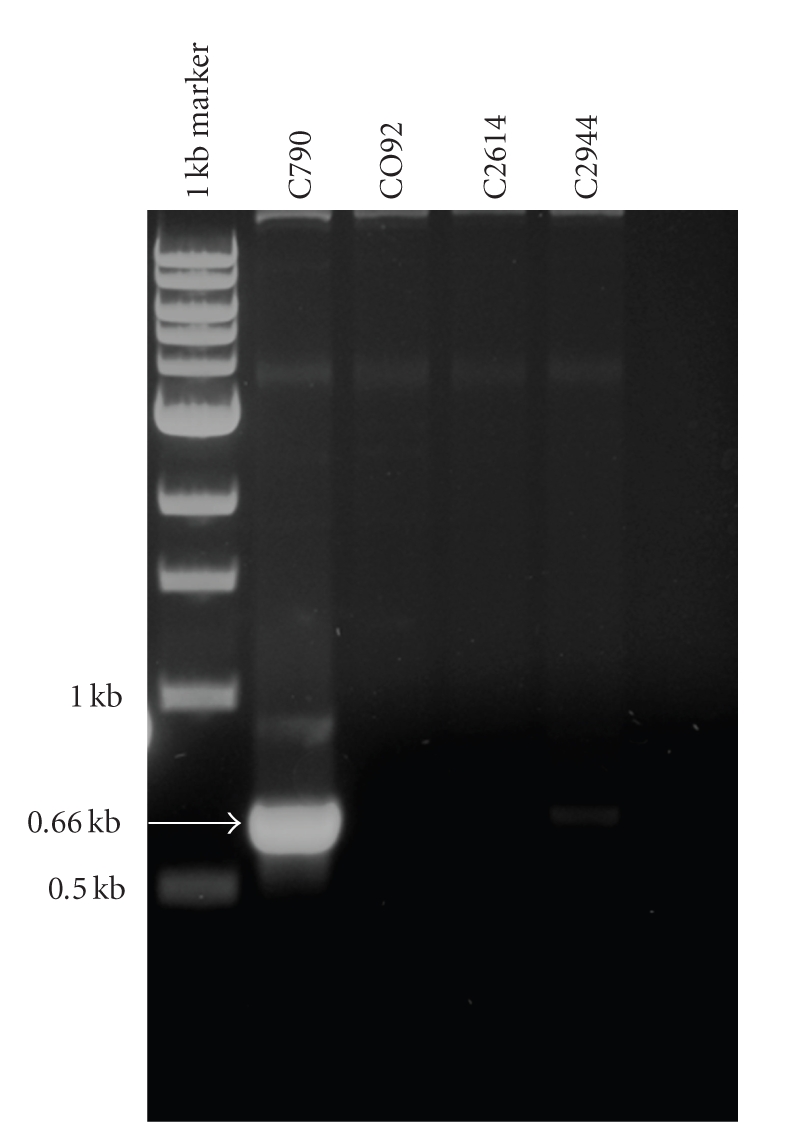
PCR amplification one of the two junctions between pMT1 and pCD1 in strain C790 which has pMT1-pCD1 chimera plasmid.
Quantitative PCR (qPCR) was used to verify the difference in the pPCP1 plasmid's copy numbers among the three FSU strains (C790, C2614, and C2944) and CO92, by comparing the pla/glnA gene ratios among them as there is only one copy of glnA in each cell [3]. The mean of three independent qPCR reactions (Table 1) showed that the highest copy number of pla in strain C2944, in which the pla/glnA ratio of 18.16 ± 2.99 suggested that there were approximately eighteen copies of the pla gene (and of the pPCP1 plasmid) in that strain. The next highest copy number was detected in Y. pestis strain C790, in which the pla/glnA ratio was 15.01 ± 1.14, followed by strain C2614 which had a ratio of 13.21 ± 0.64. Strain CO92, the virulent non-FSU strain that was used for comparison, had a pla/glnA ratio of only 6.48 ± 2.07.
Table 1.
Ratio of pla/glnA gene copy numbers determined by qPCR and pla gene expression by the Livak method.
| Strains | pla/glnA | Relative pla gene expression |
|---|---|---|
| C790 | 15.01 ± 1.14 | 1.43 ± 1.03 |
| C2614 | 13.21 ± 0.64 | 2.24 ± 1.29 |
| C2944 | 18.16 ± 2.99 | 3.15 ± 1.31 |
| CO92 | 6.48 ± 2.07 | 1.00 |
Although the absolute copy numbers of the pPCP1 plasmids are difficult to calculate based on the qPCR results (and the validity of the above qPCR-determined copy numbers must be interpreted with caution), the combined data suggest that the three PCR-positive FSU strains contain a larger number of the pPCP1 plasmid than does the non-FSU Y. pestis CO92 strain. Specifically, when compared to strain CO92, the FSU strains consistently had: (i) stronger PCR amplification signals and (ii) higher copy numbers when examined by qPCR. The underlying mechanism(s) for the difference in the copy numbers we observed is difficult to explain at the present time given the rop gene is identical in all the 4 strains. However, and as discussed below, it may have some important implications for the strains' relative virulence.
3.4. Genetic Organization of the FSU's pPCP1 Plasmids
The pPCP1 plasmids in the three PCR-positive FSU strains were approximately 9.61-kb in size and had a G + C content of 45.3%, which is slightly lower than the overall G + C content (47.6%) of the KIM and CO92 strains' chromosomes [3, 4] but is similar to that of the previously characterized pPCP1 plasmid [24]. The plasmids' predicted genes encode a putative (i) transposase (1.02 kb), (ii) ATP-binding protein (782 bp) that together form the insertion sequence IS100 along with the inverted repeats, (iii) replication regulation protein (the 195 bp rop), (iv) transcriptional regulator (the 426 bp pim), (v) pesticin (the 1,074 bp pst), (vi) plasminogen activator (PLA) protease (the 939 bp pla), (vii) transcriptional regulator, and (viii and ix) two hypothetical proteins (Figure 3). Six of the genes-those (encoding the transposase, ATP-binding protein, replication regulation protein, transcriptional regulator, PLA protease, and one of the two hypothetical proteins are transcribed in the same direction, and the remaining three genes (those encoding the transcriptional regulator, pesticin, and the second hypothetical protein) are transcribed in the opposite direction (Figure 3).
Figure 3.
Structural comparison of the pPCP1 plasmids in the FSU's Y. pestis strains and a previously characterized strain Y. pestis CO92. (a) Schematic linear diagram of the pPCP1 plasmid in strain CO92, which shows the locations of variable regions no. 1 and no. 2 present in the FSU strains' pPCP1 plasmids. (b) Sequence variations in variable regions no. 1 and no. 2 in the pPCP1 plasmids examined during our studies. (c) Schematic composition of three pCP1 plasmids in the FSU's Y. pestis strains, and their genetic relatedness (based on neighbor joining tree analysis) to the three pCP1 plasmids from non-FSU strains.
The genetic organization of the pPCP1 plasmids in the three FSU strains was similar to that of the pPCP1 plasmids in the previously characterized non-FSU strains KIM and CO92, and, to a somewhat lesser degree, strain 91001 [9, 25]. Similar to the FSU pPCP1 plasmids, the pPCP1 plasmids in strains CO92 and KIM also contain nine predicted genes the pPCP1 plasmid in strain 91001 contains ten predicted genes, consisting of the same nine genes plus one additional gene encoding a hypothetical protein; (http://www.tigr.org/). All of the FSU pPCP1 plasmids contain insertion sequence IS100, which has been found in all Y. pestis strains and all serotype I strains of Y. pseudotuberculosis, and is homologous to IS21, IS232 and IS640 [26, 27].
3.5. Genetic Analysis and Comparison of the pPCP1 Plasmids
We compared the nucleotide sequences of the pPCP1 plasmids in the three FSU strains to the available sequences of the pPCP1 plasmids in the four, non-FSU strains. The sequences of the pPCP1 plasmids in all of the seven strains were similar except for a few nucleotide changes, which confirm earlier observations [9, 14] about the general consistency of the size and genetic organization of the pPCP1 plasmid in Y. pestis. Based on the minor sequence differences we detected, five sequence types (STs) were identified among the plasmids we analyzed (Figure 3): ST1 contained three plasmids, two of which were isolated from the FSU strains C2614 and C2944, and the third plasmid from the KIM strain. ST2 had 1 plasmid from a strain isolated from China Z176003. ST3 had 1 plasmid from Krygyztan strain C790. The pPCP1 plasmids in Y. pestis strains CO92 and 91001 had distinct STs (ST4 and ST5, resp.). The pPCP1 plasmids in the FSU strains were found to be most genetically related (by neighbor joining tree analysis) to the pPCP1 plasmid in the KIM strain, and least related to the pPCP1 plasmid in strain 91001, which is nonpathogenic for humans [28]. The small variations in plasmid sequences were found in two intergenic regions designated variable region 1 and variable region 2 (Figure 3). Though the sequence variations are minor, variable region 1 is within promoter sequence of both pesticin and hypothetical protein as predicted by BMC promoter predicator (http://searchlauncher.bcm.tmc.edu/seq-search/gene-search.html). At this point, we do not know how this variation will affect promoter binding and transcription.
3.6. Analysis of pla Transcript Levels
The observed differences in pPCP1 copy numbers among the strains prompted us to question whether plasmid copy number correlated with expression levels of plaA transcript in several Y. pestis strains. RNA transcript levels of plaA were determined by RT-PCR, and expression of the chromosomal glnA gene was used as the reference point to calculate the relative gene expression of pla. The results were in general agreement with the qPCR data revealing that pla expression was significantly (P ≤ .05) higher in strain C2944 than in strain CO92. The second highest expression of pla was by strain C2614 (1- to 3.5-fold more than by strain CO92), followed by strain C790 (0.4- to 2.5-fold more than by strain CO92) although the differences were not statistically significant (P ≥ .05). Our data suggest that the three FSU strains containing the pPCP1 plasmid may produce larger amounts of PLA (and in the case of C2944, significantly larger amounts of PLA) than, the best-characterized Western North American CO92 strain.
3.7. pla and Virulence of Y. pestis
Although some wild-type Y. pestis strains lacking the pPCP1 plasmid have been reportedly retained their virulence [29, 30], pPCP1 is believed to play a major role in Y. pestis virulence. In addition to regulatory genes and genes encoding hypothetical proteins, the plasmid contains three genes (pst, pim, and pla) that encode proteins required for several important biochemical activities of Y. pestis. However, at the present time, the PLA protease, a 34.6-kDa, multifunctional plasminogen activator protease capable of degrading fibrin, coagulase, and the complement component C3 [9, 25], is the only well-documented virulence factor encoded by the pPCP1 plasmid [5].
The significance of pla in Y. pestis virulence has been clearly documented in literature [31, 32], and some pla-negative mutants have been reported to have greatly reduced virulence when administered subcutaneously [2]. Various virulence roles for the PLA protease have been proposed [9], including (i) cleaving host fibrin deposits that trap the bacterium, (ii) degrading the host's extracellular membranes, and (iii) inhibition of interleukin-8 production. More recently, pla expression has been reported to be essential for the development of primary pneumonic plague in virulent CO92 strain, but not in pPCP1-deficient Pestoides F, in addition to being required for the bubonic form of plague and increased potential for epidemic spread [30, 31, 33, 34]. Other studies have implicated Pla in resistance to cationic antimicrobial peptides such as cathelicidin [35]. In view of these data, our observation that some FSU strains are capable of producing significantly larger amounts of PLA than does the highly pathogenic CO92 raises interesting questions about the pathogenic potential of these strains. In this context, and to put our findings into further perspective, several reports in the “Soviet” literature suggest that Y. pestis virulence for various laboratory animals, including guinea pigs, varies dramatically depending on its plasmid composition and the plague focus in the Caucasus from which the strains were isolated. For example, Y. pestis strains isolated in the Leninakan focus (in the Shirak Highlands of Armenia) were found to be more virulent in guinea pigs than are strains obtained from the Zanzegur-Karabakh region (southeastern range of the Lesser Caucasus mountains) [36]. Similar observations have been reported for Y. pestis strains isolated in the Armenian Highlands [37], the Dagestan-Highland focus [38], and the Gissar and Talas regions [39]. Data comparing the plasmid compositions, the plasmid copy numbers, and pla-expression in those strains are not available. However, given the differences we observed in the pla-expression levels in various FSU strains, additional studies seem warranted to determine the impact of pPCP1 copy number and PLA production on the virulence of the FSU Y. pestis strains. The resulting data would help to advance understanding of the genetic composition and virulence traits of the Y. pestis strain population in the FSU (including FSU regions from which such data are either very scarce or not available), and they may also aid the development of advanced methods for differentiating highly virulent and less virulent strains of Y. pestis.
Supplementary Material
A. Primers used for, amplifying 7 loci for MLVA, primer walking of pPCP1 plasmids, detecting pPCP1 specific genes, quantitative PCR for plasmid DNA, and RNA.
B. Each of the 7 MLVA loci were PCR amplified and the purified PCR product was sequence in both directions.
The Tandem Repeat Finder was used to determine the number of repeats.
Acknowledgments
The research described in this paper was made possible by grant 1R21 AI055660-01A1 (to A. Sulakvelidze) from the National Institute of Allergy and Infectious Diseases, and by financial support provided by the U.S. Defense Threat Reduction Agency (DTRA) projects GG-1 and GG-18 (to A. Sulakvelidze). The research performed at the US Army Edgewood Chemical Biological Center was supported by DTRA project AA06TAS025 (to H. Gibbons). The authors thank Arnold Kreger for his invaluable editorial assistance, Sonya Narodny and Richard Obiso for their helpful suggestions, and the honorable Andrew Weber, Kevin O' Connell, Gavin Braunstein, James Bartholomew, and Jay Valdes for their support of the project.
References
- 1.Achtman M, Zurth K, Morelli G, Torrea G, Guiyoule A, Carniel E. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(24):14043–14048. doi: 10.1073/pnas.96.24.14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry RD, Fetherston JD. Yersinia pestis—etiologic agent of plague. Clinical Microbiology Reviews. 1997;10(1):35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkhill J, Wren BW, Thomson NR, et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature. 2001;413(6855):523–527. doi: 10.1038/35097083. [DOI] [PubMed] [Google Scholar]
- 4.Deng W, Burland V, Plunkett G, et al. Genome sequence of Yersinia pestis KIM. Journal of Bacteriology. 2002;184(16):4601–4611. doi: 10.1128/JB.184.16.4601-4611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anisimov AP, Lindler LE, Pier GB. Intraspecific diversity of Yersinia pestis. Clinical Microbiology Reviews. 2004;17(2):434–464. doi: 10.1128/CMR.17.2.434-464.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowell JL, Zhansarina A, Yockey B, et al. Phenotypic and molecular characterizations of Yersinia pestis isolates from Kazakhstan and adjacent regions. Microbiology. 2007;153(1):169–177. doi: 10.1099/mic.0.29059-0. [DOI] [PubMed] [Google Scholar]
- 7.Revazishvili T, Rajanna C, Bakanidze L, et al. Characterisation of Yersinia pestis isolates from natural foci of plague in the Republic of Georgia, and their relationship to Y. pestis isolates from other countries. Clinical Microbiology and Infection. 2008;14(5):429–436. doi: 10.1111/j.1469-0691.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferber DM, Brubaker RR. Plasmids in Yersinia pestis. Infection and Immunity. 1981;31(2):839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bearden SW, Fetherston JD, Perry RD. Genetic organization of the yersiniabactin biosynthetic region and construction of avirulent mutants in Yersinia pestis. Infection and Immunity. 1997;65(5):1659–1668. doi: 10.1128/iai.65.5.1659-1668.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazanova LP, Maevskii MP, Khabarov AV. An experimental study of the possibility for the preservation of the causative agent of plague in the nest substrate of the long-tailed suslikEksperimental’noe izuchenie vozmozhnosti sokhraneniia vozbuditelia chumy v substrate gnezda dlinnokhvostogo suslika. Meditsinskaia parazitologiia i parazitarnye bolezni. 1997;(4):37–39. [PubMed] [Google Scholar]
- 11.Aragao AI, Seoane AC, Leal TC, Leal NC, Almeida AM. Surveillance of plague in the State of Ceara: 1990–1999. Revista da Sociedade Brasileira de Medicina Tropical. 2002;35(2):143–148. [PubMed] [Google Scholar]
- 12.Sulakvelidze A. Yersiniae other than Y. enterocolitica, Y. pseudotuberculosis, and Y. pestis: the ignored species. Microbes and Infection. 2000;2(5):497–513. doi: 10.1016/s1286-4579(00)00311-7. [DOI] [PubMed] [Google Scholar]
- 13.Perry RD, Pendrak ML, Schuetze P. Identification and cloning of a hemin storage locus involved in the pigementation phenotype of Yersinia pestis. Journal of Bacteriology. 1990;172(10):5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippov AA, Solodovnikov NS, Kookleva LM, Protsenko OA. Plasmid content in Yersinia pestis strains of different origin. FEMS Microbiology Letters. 1990;55(1-2):45–48. doi: 10.1016/0378-1097(90)90165-m. [DOI] [PubMed] [Google Scholar]
- 15.Felek S, Muszyński A, Carlson RW, Tsang TM, Hinnebusch BJ, Krukonis ES. Phosphoglucomutase of Yersinia pestis is required for autoaggregation and polymyxin B resistance. Infection and Immunity. 2010;78(3):1163–1175. doi: 10.1128/IAI.00997-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eppinger M, Worsham PL, Nikolich MP, et al. Genome sequence of the deep-rooted Yersinia pestis strain angola reveals new insights into the evolution and pangenome of the plague bacterium. Journal of Bacteriology. 2010;192(6):1685–1699. doi: 10.1128/JB.01518-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pourcel C, André-Mazeaud F, Neubauer H, Ramisse F, Vergnaud G. Tandem repeats analysis for the high resolution phylogenetic analysis of Yersinia pestis. BMC Microbiology. 2004;4, article 22 doi: 10.1186/1471-2180-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Research. 1998;8(3):175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- 19.Gordon D, Abajian C, Green P. Consed: a graphical tool for sequence finishing. Genome Research. 1998;8(3):195–202. doi: 10.1101/gr.8.3.195. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2T method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Kong F, Jelfs P, Gilbert GL. Extended phage locus typing of Salmonella enterica serovar Typhimurium, using multiplex PCR-based reverse line blot hybridization. Journal of Medical Microbiology. 2008;57(7):827–838. doi: 10.1099/jmm.0.47766-0. [DOI] [PubMed] [Google Scholar]
- 22.Kingston JJ, Tuteja U, Kapil M, Murali HS, Batra HV. Genotyping of Indian Yersinia pestis strains by MLVA and repetitive DNA sequence based PCRs. Antonie van Leeuwenhoek, International Journal of General and Molecular Microbiology. 2009;96(3):303–312. doi: 10.1007/s10482-009-9347-2. [DOI] [PubMed] [Google Scholar]
- 23.Eppinger M, Guo Z, Sebastian Y, et al. Draft genome sequences of Yersinia pestis isolates from natural foci of endemic plague in China. Journal of Bacteriology. 2009;191(24):7628–7629. doi: 10.1128/JB.01227-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso JM. Epidemiology and epizootiology of plague : the main place of supervision of wild rodents reservoir in the control of permanent focusesEpidemiologie et epizootioiogie de la peste : le role majeur de la surveillance des populations reservoirs de rongeurs sauvages dans le controle des foyers inveteres. Medecine Tropicale. 1998;58(2):21–24. [PubMed] [Google Scholar]
- 25.Sodeinde OA, Goguen JD. Genetic analysis of the 9.5-kilobase virulence plasmid of Yersinia pestis. Infection and Immunity. 1988;56(10):2743–2748. doi: 10.1128/iai.56.10.2743-2748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helmuth R, Stephan R, Bulling E. R-factor cointegrate formation in Salmonella typhimurium bacteriophage type 201 strains. Journal of Bacteriology. 1981;146(2):444–452. doi: 10.1128/jb.146.2.444-452.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podladchikova ON, Dikhanov GG, Rakin AV, Heesemann J. Nucleotide sequence and structural organization of Yersinia pestis insertion sequence IS100. FEMS Microbiology Letters. 1994;121(3):269–274. doi: 10.1111/j.1574-6968.1994.tb07111.x. [DOI] [PubMed] [Google Scholar]
- 28.Han Y, Zhou D, Pang X, et al. Microarray analysis of temperature-induced transcriptome of Yersinia pestis. Microbiology and Immunology. 2004;48(11):791–805. doi: 10.1111/j.1348-0421.2004.tb03605.x. [DOI] [PubMed] [Google Scholar]
- 29.Aparin GP, Balakhonov SV, Timofeeva LA, Logachev AI. [Numerical analysis of the phenotypic properties and total genomic characteristics of strains of Yersinia pestis related to different subspecies] Zhurnal Mikrobiologii Epidemiologii i Immunobiologii. 1987;(11):16–20. [PubMed] [Google Scholar]
- 30.Welkos SL, Friedlander AM, Davis KJ. Studies on the role of plasminogen activator in systemic infection by virulent Yersinia pestis strain C092. Microbial Pathogenesis. 1997;23(4):211–223. doi: 10.1006/mpat.1997.0154. [DOI] [PubMed] [Google Scholar]
- 31.Lathem WW, Price PA, Miller VL, Goldman WE. A plasminogen-activating protease specifically controls the development of primary pneumonic plague. Science. 2007;315(5811):509–513. doi: 10.1126/science.1137195. [DOI] [PubMed] [Google Scholar]
- 32.Lorange EA, Race BL, Sebbane F, Hinnebusch BJ. Poor vector competence of fleas and the evolution of hypervirulence in Yersinia pestis. Journal of Infectious Diseases. 2005;191(11):1907–1912. doi: 10.1086/429931. [DOI] [PubMed] [Google Scholar]
- 33.Worsham PL, Roy C. Pestoides F, a Yersinia pestis strain lacking plasminogen activator, is virulent by the aerosol route. Advances in Experimental Medicine and Biology. 2003;529:129–131. doi: 10.1007/0-306-48416-1_25. [DOI] [PubMed] [Google Scholar]
- 34.Sebbane F, Jarrett CO, Gardner D, Long D, Hinnebusch BJ. Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(14):5526–5530. doi: 10.1073/pnas.0509544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galván EM, Lasaro MAS, Schifferli DM. Capsular antigen fraction 1 and Pla modulate the susceptibility of Yersinia pestis to pulmonary antimicrobial peptides such as cathelicidin. Infection and Immunity. 2008;76(4):1456–1464. doi: 10.1128/IAI.01197-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elkin YM, Petrov PA. Particularly Dangerous Infections in the Caucasus. Stavropol, Russia: Stavropol' Research Anti-Plague; 1974. Paleogenesis of Transcaucasian highland plague focus in connection with dissimilarity in virulence of Yersinia pestis vole's strains from different landscape-geographical Caucasus localities; pp. 43–45. [Google Scholar]
- 37.Abgaryan GP. Characterization of Some Yersinia Pestis Strains which Were Isolated on Armenian Highland from Common Voles. Saratovl, Russia: All-Union Research Anti-Plague Institute “Microbe”; 1966. [Google Scholar]
- 38.Diatlov AI. Evolutional Aspects in Natural Plague Focality. Stavropol, Russia: Stavropol Bookish Press; 1989. [Google Scholar]
- 39.Sludskii AA. Vole's Type of Natural Plague Foci (Structure and Functioning) Saratovl, Russia: Russian Research Anti-Plague Institute “Microbe”; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Primers used for, amplifying 7 loci for MLVA, primer walking of pPCP1 plasmids, detecting pPCP1 specific genes, quantitative PCR for plasmid DNA, and RNA.
B. Each of the 7 MLVA loci were PCR amplified and the purified PCR product was sequence in both directions.
The Tandem Repeat Finder was used to determine the number of repeats.