Abstract
The effectiveness of lifestyle interventions within secondary prevention of coronary heart disease (CHD) remains unclear. This systematic review aimed to determine their effectiveness and included randomized controlled trials of lifestyle interventions, in primary care or community settings, with a minimum follow-up of three months, published since 1990. 21 trials with 10,799 patients were included; the interventions were multifactorial (10), educational (4), psychological (3), dietary (1), organisational (2), and exercise (1). The overall results for modifiable risk factors suggested improvements in dietary and exercise outcomes but no overall effect on smoking outcomes. In trials that examined mortality and morbidity, significant benefits were reported for total mortality (in 4 of 6 trials; overall risk ratio (RR) 0.75 (95% confidence intervals (CI) 0.65, 0.87)), cardiovascular mortality (3 of 8 trials; overall RR 0.63 (95% CI 0.47, 0.84)), and nonfatal cardiac events (5 of 9 trials; overall RR 0.68 (95% CI 0.55, 0.84)). The heterogeneity between trials and generally poor quality of trials make any concrete conclusions difficult. However, the beneficial effects observed in this review are encouraging and should stimulate further research.
1. Introduction
The World Health Organisation has stated that, since 1990, more people worldwide have died from coronary heart disease (CHD) than any other cause [1]. Further, they reported that 80% to 90% of people dying from CHD had one or more major risk factors associated with lifestyle.
In the UK, more than 90,000 deaths per year are due to CHD, and although death rates are falling they are still among the highest in western Europe [2].
Cardiac rehabilitation (CR) programmes were initiated in the 1960s when the benefits of mobilisation and physical activity (PA) following lengthy hospital stays for CHD became known [3]. Since then, secondary prevention has become an essential aspect of care of the patient with CHD [4]. Research has shown that lifestyle change, including PA, a healthy diet, and smoking cessation, alters the course of CHD [5–7], and so disease prevention measures have been designed to focus on a range of lifestyle factors. Indeed, cardiac rehabilitation and secondary prevention programmes have developed from focusing on exercise alone to becoming multidisciplinary and encompassing baseline patient assessments, nutritional counselling, risk factor management (i.e., lipids, hypertension, weight, diabetes, and smoking), psychosocial and vocational counselling, and PA advice and exercise training, in addition to the appropriate use of cardioprotective drugs [4].
Multidisciplinary measures are advocated by governments around the world, and in the UK the National Institute for Clinical Excellence (NICE) set out a series of guidelines in 2007 for care of patients who had had a myocardial infarction (MI) [8]. The guidelines covered secondary prevention in primary and secondary care and were not focused solely on lifestyle interventions. They did, however, incorporate PA, diet, smoking, and drug therapy and were based on systematic reviews of the best available evidence. Priority recommendations, considered to have the most important effect on patient care and outcomes, included that on discharge from hospital every MI patient should have had a confirmed diagnosis of acute MI, results of investigations, future management plans, and advice on secondary prevention. Also, NICE highlighted the importance of advice being given regarding regular PA in the form of 20–30 minutes of exercise per day to the point of slight breathlessness. Patients should also be advised to stop smoking, eat a Mediterranean style diet rich in fibre, fruit, vegetables, and fish, and follow a treatment regime with a combination of ACE (angiotensin-converting enzyme) inhibitors, aspirin, beta-blockers, and statins.
However, despite the evidence that positive lifestyle changes bring about improved outcomes, results from a number of secondary prevention initiatives have been disappointing. In a systematic review of multidisciplinary secondary prevention programmes McAlister et al. [9] reported that although some beneficial impact was achieved on processes of care, morbidity, and mortality, questions remained regarding the duration and frequency of interventions and the best combination of disciplines within an intervention.
The EUROASPIRE (European Action on Secondary and Primary Prevention by Intervention to Reduce Events) surveys by the European Society of Cardiology have shown that the adoption of cardiovascular disease prevention measures as part of daily clinical practice was wholly inadequate [10] and that unhealthy lifestyle trends are continuing. The authors commented on the difficulty experienced by adults in changing behaviour despite having a life threatening disease and that continued professional support was imperative if this was to be achieved.
Few previous reviews of secondary prevention interventions have been published. McAlister et al. [9] carried out a systematic review of RCTs of secondary prevention interventions, published in 2001, and analysed 12 studies. Jolliffe et al. [11] analysed only exercise interventions in secondary prevention, and Rees et al. [12] reviewed psychological interventions. To our knowledge no comprehensive systematic review has been undertaken since 2001 of the effects of diet, exercise, and other lifestyle factors in the secondary prevention of CHD. We therefore performed a systematic review of randomised controlled trials to determine the effectiveness of lifestyle interventions for the secondary prevention of CHD.
2. Methods
This was a systematic review carried out using Cochrane Collaboration methodology [13].
2.1. Participants
We included male and female adults of all ages (aged 18+) with a diagnosis of CHD. Patients included those who had experienced a myocardial infarction (MI), coronary artery bypass graft (CABG), or percutaneous transluminal coronary angioplasty (PTCA) and those with angina pectoris and coronary artery disease defined by angiography. For the purposes of this review we excluded patients who had had a heart transplant, heart valve surgery, or heart failure, unless it was clearly specified that the cause related to CHD.
2.2. Interventions
We have included interventions with a lifestyle and/or behaviour change focus designed for the secondary prevention of CHD, incorporating one or a combination of exercise and diet. Interventions may be categorized as follows.
Dietary.
Exercise.
Psychological.
Educational.
Multifactorial.
Organisational (e.g., case management).
2.3. Exclusions
We have excluded from this review studies which were not randomized controlled trials, focused on primary prevention, involved patients with multiple diseases and/or outcomes which were related to diseases other than CHD, involved patients in a hospital in-patient setting, focused on drug therapy, had short (less than three months) or no follow-up, or reported outcomes which were not the focus of this review (see “Types of outcome measures” section) such as depression, cost-effectiveness, or service delivery.
2.4. Study Duration
Trials were included in this review if they reported a minimum postintervention follow-up of three months to allow some change to take place.
2.5. Settings
For the purposes of this review, interventions have been considered to have been delivered in primary care according to the definition of primary care as stated by the Committee on the Future of Primary Care at the Institute of Medicine in the United States: “Primary care is the provision of integrated, accessible healthcare services by clinicians who are accountable for addressing a large majority of personal healthcare needs, developing a sustained partnership with patients, and practicing in the context of family and community” [14]. Interventions are delivered in primary care by clinicians who are “generally considered to be physicians, nurse practitioners and physical assistants” and “a broader array of individuals in a primary care team” [14]. In the care of CHD patients, the primary care team may include general practitioners, practice nurses, community pharmacists, community and public health nurses, dieticians, occupational therapists, and physiotherapists.
Interventions have been included in the review if they have been delivered in primary care or community settings by primary care clinicians or clinicians whose normal roles may be within secondary care, for example, community hospitals, or tertiary care, for example, general hospitals.
We have excluded from this review studies of interventions undertaken primarily within secondary or tertiary care settings by clinicians or care teams whose relationship with patients is not long term or ongoing.
2.6. Comparators
In the included studies, the comparators are “normal care” or “usual care,” meaning standard clinical care or standard care given by a general practitioner (GP).
2.7. Types of Outcome Measures
All or any number of the following.
Primary Outcomes
All-cause mortality.
Cardiac mortality.
Nonfatal cardiac events.
Hospital admissions (cardiac related/and all-cause if available).
Secondary Outcomes
Diet (e.g., measured by fibre, fruit, and veg quantity).
Exercise (e.g., frequency, duration).
Blood pressure (BP); systolic BP (SBP); diastolic BP (DBP).
Blood lipid levels (high density lipoprotein cholesterol (HDL-chol), low density lipoprotein cholesterol (LDL-chol)).
Smoking behavior.
Health related quality of life (QOL).
Self efficacy.
Medication adherence.
Only outcomes measured using a validated instrument were included.
2.8. Search Methods for Identification of Studies
We searched electronic databases Medline, Cinahl, and Embase for English language randomized trials in humans published since 1990. The search method for Medline is detailed below; it was modified as appropriate for Cinahl and Embase. Reference lists of review articles were also searched.
2.9. Medline Search Method
Selecting an Advanced Ovid search, the keyword “lifestyle” was inserted and the box ticked to select “Map term to subject heading.” After selecting “Search” a new page was presented entitled “Mapping Display.” Options indicating the Subject Headings “Life Style” and “lifestyle.mp. search as keyword” were chosen and “Continue” selected. This allowed further keywords to be entered.
The same process was followed for the keywords “exercise,” “physical activity,” and “diet.” In each case only the keyword as a subject heading and as a keyword were selected on the Mapping Display page.
A fifth search was commanded by using all four keywords above separated by the Boolean operator “OR.” This was done by selecting “click to expand” the “Search History” box, ticking each of the four boxes relating to the keywords, and selecting “Combine selections with OR.”
A sixth search term “secondary prevention” was added as a keyword in same way as above. A seventh “coronary heart disease” was inserted, then its abbreviation “CHD” and four conditions related to it and their abbreviations, where appropriate, all as separate keywords: “myocardial infarction,” “MI,” “coronary artery bypass graft,” “CABG,” “percutaneous transluminal coronary angioplasty,” “PTCA,” and “angina pectoris.” This group incorporating coronary heart disease and related conditions involved a total of nine separate searches, and they were together inserted as a 16th search, with each term separated by “OR.” This was done, as above, by expanding the search history, selecting the relevant boxes beside keywords and selecting “Combine selections with OR.”
The three components of the search were put together in the following way.
On the search history page, search five was selected by clicking on the box beside it (Lifestyle.mp. or Life Style/OR Exercise.mp. or Exercise/OR Physical activity.mp. OR Diet/or diet.mp.). Search six was then selected (Secondary prevention.mp. or Secondary Prevention/), and search 16 (Coronary heart disease.mp. or Coronary Disease/OR CHD.mp. OR Myocardial infarction.mp. or Myocardial Infarction/OR MI.mp OR Coronary Artery Bypass/or coronary artery bypass graft.mp. OR CABG.mp. OR Percutaneous transluminal coronary angioplasty.mp. or Angioplasty, Transluminal, Percutaneous Coronary/OR PTCA.mp. OR Angina pectoris.mp. or Angina Pectoris/). The option “Combine selections with AND” was selected.
This gave search 17. Search 17 was selected by ticking the box, and limits were imposed in an effort to narrow the search. Below the search results, within the “Limits” options, “English language,” “Humans,” and “Publication Year-1990 to Current” were selected. “Additional Limits” was then selected, search 17 was selected, and in the “Age Groups” options “All Adult (19 plus years)” was selected. No other limits were imposed. Then, “Limit A Search” was selected.
To refine the search further, terms relating to a randomized controlled trial study design were inserted. The terms entered were randomized controlled trial.mp. or Randomized Controlled Trial/, controlled clinical trial.mp. or Controlled Clinical Trial/, random allocation.mp. or Random Allocation/, double blind method.mp. or Double-Blind Method/, single blind method.mp. or Single-Blind Method. Each of these terms was combined with “OR,” giving search 24.
Selecting search 18 and search 24, and combining them with “AND” gave the final selection. The Medline search strategy is detailed in the Appendix.
2.10. Data Analysis
From the searching, titles and abstracts were screened by two reviewers (MC, JC), and potentially relevant references were retrieved. The two reviewers (MC, JC) then independently selected trials to be included in the review. Reference lists of relevant systematic reviews were also screened for potential papers to include.
After the final selection of trials was agreed upon, data including study characteristics, outcome measures and results were extracted.
In addition, the quality of trials was assessed in relation to randomization method, loss to follow up, intention to treat analyses, and blinding measures.
Dichotomous outcomes for each study have been expressed as risk ratios, where appropriate, and 95% confidence intervals (CIs). Meta-analyses were performed using random effects models, and data were presented as forest plots using Revman software [15]. We were only able to perform meta-analysis on three outcomes—total mortality, cardiovascular mortality, and nonfatal cardiac events.
Continuous variables have been expressed as the difference between intervention and control groups at study completion and the standard deviation difference where reported.
3. Results
3.1. Study Selection and Evaluation
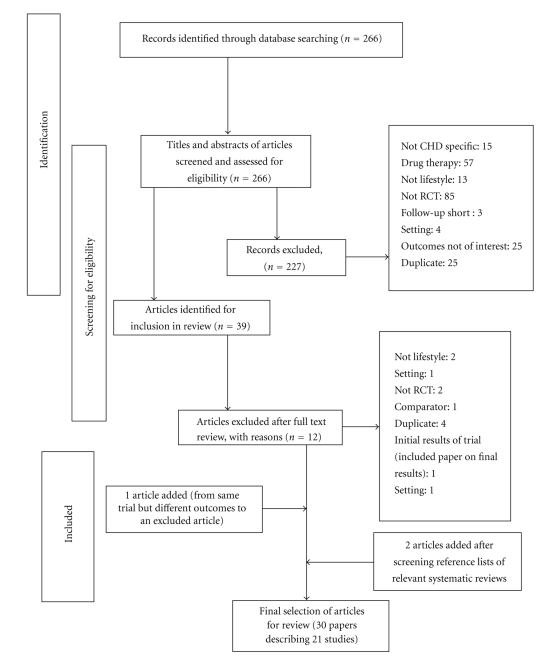
Figure 1 shows the selection, screening, and identification of studies for this review. Of the 266 papers originally identified through searching the three electronic databases, we identified 39 that were potentially eligible for inclusion, and full text versions of these studies were retrieved and assessed. Twelve of the 39 papers were excluded because they included diseases other than CHD, were drug trials, did not focus on lifestyle, were not randomized controlled trials, had a short follow-up period, were conducted within a hospital in-patient setting, had outcomes which were not of interest, or were retrieved more than once from the three search engines. Two papers were added after screening reference lists of relevant systematic reviews, and one trial was added which reported different outcomes of an identical study which emerged in our searches (Figure 1).
Figure 1.
Screening and selection of studies of interventions for secondary prevention of coronary heart disease.
3.2. Description of Studies
Table 1 shows summary data of 30 papers from the 21 randomised controlled trials (RCTs) found to be eligible for this review [16–45]. We have presented them in the six categories described in the “Methods” section (exercise, dietary, psychological, educational, multifactorial, and organisational). The category which had the largest number of trials was multifactorial (10 in total). We also found one which focused on exercise only, one dietary intervention, three which had a psychological approach, four educational, and two organisational.
Table 1.
Studies (all RCTs) included in analysis of secondary prevention programmes in coronary heart disease.
| Source | Study population | Mean age (years) | % Men | Outcomes | Follow-up | Intervention |
|---|---|---|---|---|---|---|
| Exercise | ||||||
| Astengo et al. [16] (2010), Sweden | 62 patients with stable angina who had percutaneous coronary intervention (PCI). | Intervention (I) group (n = 32): 62 (SD 7); Control (C) group (n = 29): 65 (8). |
I: 79% C: 76% |
PA (cycle ergometer test), glucose, and lipid metabolism. | At completion of 8-month intervention. | 8-month intervention: I group patients exercised at home on a bicycle ergometer for ≥30 minutes on ≥5 days/week of 250 day (eight months) study period; resistance exercises; monthly motivational meetings with physiotherapist. |
| Dietary | ||||||
| De Lorgeril et al. [32] (1996), France | 605 subjects <70 years who had survived a MI within 6 months of enrolment. | I (n = 302): 53.5. C (n = 303): 53.5. |
I: 89.4%; C: 92.1% |
All-cause and cardiovascular (CV) mortality, nonfatal CV events, diet (24 hour diet recall, Food Frequency Questionnaire). | 27 months. De Lorgeril et al. [33] (1999): 46 months |
5-year intervention: initial 1-hour advice session from research cardiologist and dietician to adopt Mediterranean type diet of more bread, root and green vegetables, more fish, less meat, fruit every day and butter and cream replaced with margarine; patients seen at 8 weeks from baseline and annually thereafter. |
| Psychological | ||||||
| Lewin et al. [29] (2002), UK | 142 patients with angina diagnosed within previous 12 months randomised to Angina Plan or educational session. | I (n = 68): 66.7 (9.4); C (n = 74): 67.6 (9). |
I: 57%; C: 62%. |
Frequency of angina attacks, physical limitations (Seattle Angina questionnaire), anxiety, depression, use of drugs. | At completion of 6-month intervention. | 6-month intervention: nurse-led Angina Plan: 70 page “workbook” and audio-taped relaxation programme introduced to patient and partner during a 30–40 minute structured interview; nurse sought to correct any misunderstanding of illness. Risk factors discussed; how to reduce these through goal setting and pacing; patients asked to practice relaxation using the tape each day. Nurse contacted patient by phone at end of weeks 1, 4, 8, and 12 and praised the achievement of reaching goals, discussed extending goals. |
| Lisspers et al. [26] (1999), Sweden | 87 patients with at least 1 significant coronary stenosis suitable for PCI and at least 1 additional clinically insignificant coronary lesion. | I (n = 46): 53 (7); C (n = 41): 53 (7). |
I: 80%; C: 88%. |
CV mortality, nonfatal CV events, diet (questionnaire), PA (questionnaire), smoking (questionnaire), QOL (questionnaire:AP-QLQ). | At completion of 12-month intervention. Lisspers et al. [27] (2005): 24, 36 and 60 months from baseline. |
12-month intervention: nurse-led. 4-week residential stay at intervention unit: intense group and individual health education and training; stress management, diet, exercise, smoking; followed by 11-month structured maintenance phase; regular contact with specially assigned nurse through mail and phone calls. Personal lifestyle goals set, diaries kept of everyday lifestyle behaviour. |
| Salminen et al. [25] (2005), Finland | 227 patients with CHD. | I (n = 118): males 72.5 (5.3), females 75.5 (6.6); C (n = 109): males 72.6 (5.5), females 75.3 (6.5). |
I: 49%; C: 50%. |
Diet (patient interviews), PA (self report), smoking (patient interviews), BP, total, LDL and HDL cholesterol. | At completion of 16-month intervention. | 16-month intervention: nurse-led. 16 lectures: 90–120 minutes each; prevention of CHD, diet and weight control, exercising, financial concerns. 8 group discussions: small groups, 8 meetings 90–120 minutes each; treatment of elevated serum lipids, healthy eating, CHD risk factors. 6 light exercise sessions: 60–90 minutes each; walking, gymnastics, relaxation. 3 social activities: picnic at national park, visit to spa, 24-hour cruise. |
| Educational | ||||||
| Carlsson et al. [42, 43] (1997), Sweden | 50–70 years with acute MI, CABG or PTCA less than 2 weeks before study. | Carlsson A [42]: 121 AMI patients: I = 61; C = 60; mean age 62.1.56 CABG patients: I = 27; C = 29; mean age 61.5. Carlsson B [42]: 142 AMI patients: I = 75; C = 67; mean age 62.0. 63 CABG patients: I = 31; C = 32; mean age 61.3. Carlsson C [43]: 168 patients with AMI: I (n = 87): 62.2; C (n = 81): 61.9. |
A: AMI patients: 75%; CABG patients: 84%. B: AMI patients: 77%; CABG patients: 84%. C: 75% |
Diet (questionnaire), PA (questionnaire), smoking (questionnaire), total, LDL and HDL cholesterol, use of drugs. | At completion of 1-year intervention. | 3-month nurse-led education programme: individual and group counselling: 9 hours/patient: 1.5 hours smoking cessation, 5.5 hours diet, 2 hours PA. Exercise training: 2/3 times/week for 10–12 weeks, 40 minutes PA including interval training with cycling and jogging. Education continued by nurse for 1 year. Individual exercise schedules. |
| Cupples and McKnight [22] (1994), UK | 688 patients who had had angina for ≥6 months. | I (n = 342): mean age 62.7 (7.1). C (n = 346): 63.6 (6.8). |
I: 59.4% C: 59.2% |
All-cause and CV mortality, diet (Department of Health and Social Services), PA (patient interviews), smoking (patient interviews), BP, cholesterol, QOL, use of drugs. | At completion of 2-year intervention. Cupples and McKnight [23] (1999): 5 years from baseline. |
Patients given practical advice relating to CV risk factors and reviewed by health visitors at four monthly intervals for two years. |
| Heller et al. [44] (1993), Australia | 450 subjects admitted to hospital with suspected MI. | I (n = 213): 59 (8); C (n = 237): 58 (8). |
I: 76% C: 68% |
Nonfatal CV events, hospital admissions, diet (fat intake), PA (questionnaire), total cholesterol, QOL (Oldridge et al. 1989), use of drugs. | At completion of 6-month intervention. | 6-month intervention: GP-delivered educational intervention. Initial letter to GP on benefits of aspirin and beta-blockers plus first of 3 posted packages for patient. Package 1: Step 1 of “Facts on Fat” kit; quiz, patient target for fat reduction; walking programme and smoking cessation advice. Package 2: Steps 2 and 3; questions on previous week's PA. Package 3: Steps 4 and 5; information on local walking groups. Monthly newsletters posted over next 4 months containing recipes, dietary and PA information, and National Heart Foundation booklet. Two telephone calls attempted, patients urged to telephone if requiring information. |
| Southard et al. [31] (2003), USA | 104 subjects with CHD, congestive heart failure or both. | I (n = 53): 61.8 (10.6); C (n = 51): 62.8 (10.6). |
I: 68% C: 82% |
Nonfatal CV events, diet (MEDFICTS dietary survey), PA (min/wk), BP, total, LDL and HDL cholesterol, triglycerides. | At completion of 6-month intervention. | 6-month intervention: Internet based educational programme for nurse case managers to provide risk factor management training and advice. Patients accessed internet programme at least once a week for 30 minutes, communicating with case manager via website's internal email system, completing educational modules (with interactive multiple choice self-tests), entering data (at any time) to monitor progress. Optional discussions with other participants, rewards given (worth $0.50 to $1.50) for active participation on website. Dietician available to analyse 24 hour diet recalls. Case managers and dietician also available via telephone and post if necessary. |
| Multifactorial | ||||||
| Allen et al. [30] (2002), USA | 228 patients with hypercholesterolaemia who had CABG or PCI. | I (n = 115): 61.1 (10.3); C (n = 113): 59.6 (9.6). |
I: 70%; C: 73%. |
Diet (Block Health Habits and History), PA (Aerobics Centre questionnaire), total, HDL, and LDL cholesterol, triglycerides. | At completion of 1-year intervention. | 1-year intervention: nurse case management: plan devised 4–6 weeks after hospital discharge including lifestyle counselling and review of drug therapy. Follow-up telephone calls to reinforce counselling and adjust drug therapy (each patient contacted average 7 times during follow-up year); ongoing plan sent regularly to doctor. Diet advice: <30% of total energy as fat, <7% saturated fat, <200 mg per day cholesterol; PA: participation in moderate intensity home-based exercise programme; referral to CR; smoking cessation advice and relapse prevention. |
| Campbell et al. [34] (1998)A (Heart) | 1343 patients with CHD. At 1 yr: I = 593; C = 580. |
66 | 58.2% | Diet (Dietary Instrument for Nutrition Education (DINE) questionnaire), PA (Health Practitioners Index Questionnaire), smoking (Health Practitioners Index Questionnaire), BP, lipid management, aspirin management. | At completion of 1-year intervention. | 1-year intervention: Nurse-led clinics to promote medical and lifestyle aspects of secondary prevention. Clinics: 4 stages: (1) Review of symptoms to identify poor control and refer accordingly. (2) Review of drug treatment; encourage aspirin use. (3) BP and lipid assessment. (4) Assessment of exercise, diet, smoking, and behaviour changes suggested. Follow-up visits every 2–6 months; 20 minutes. |
| Murchie et al. [36] (2003) A: at 4-year follow-up: I = 564; C = 534. |
I: 65.4 (8.2); C: 65.7 (8.6). |
As above | As above plus: All-cause mortality, CV events. | 4 years from baseline. | As above. | |
| Campbell et al. [35] (1998) B: as Campbell A |
As Campbell A | As Campbell A | QOL (Short Form (SF) 36 questionnaire) | At completion of 1-year intervention. | As Campbell A | |
| Murchie et al. [37] (2004) B: as Murchie A |
As Murchie A | As Murchie A | QOL (SF 36) | 4 years from baseline | ||
| Delaney et al. [38] (2008): at 10-year follow-up 531 of 1343 original cohort had died. | All-cause mortality and coronary events (nonfatal MIs and coronary deaths). | 10 years from baseline. | ||||
| Giallauria et al. [18] (2009), Italy | 52 patients with acute myocardial infarction (AMI). | I (n = 26): 58.2 (7.8); C (n = 26): 57.4 (9.7). |
I: 85%; C: 85%. |
Nonfatal CV events, PA (cycle ergometer test), BP, total, LDL and HDL cholesterol, triglycerides. | At completion of 2-year intervention. | 2-year intervention: educational and behavioural; individual and group. Each patient given booklet on exercise, diet and smoking cessation, and ideal targets. Monthly hospital visits: dietary advice, reinforcement of healthy lifestyles, exercise training session to 60–70% of VO2 peak. |
| Gianuzzi et al. [21] (2008), Italy | 3241 patients with recent MI (within past 3 months). | 57.9 (9.2) | 86.3% | All-cause and CV mortality, nonfatal CV events, diet (knowledge/habits), PA (questionnaire), smoking (questionnaire), BP, total, HDL and LDL cholesterol, self/stress management, use of drugs. | At completion of 3-year intervention; Data collected at 6 months, 1, 2 and 3 years. | 3-year intervention: multifactorial, continued educational and behavioural; monthly sessions from months 1 to 6, then every 6 months for 3 years. Each session: 30 minutes supervised aerobic exercise; lifestyle and risk factor counselling lasting at least 1hour; reinforcement of preventive interventions. Booklet on how to deal with exercise, diet, smoking cessation, and stress management. Targets: cease smoking, adopt Mediterranean diet, increase PA to at least 3hours/week at 60–75% of mean max heart rate, maintain BMI of <25, BP 140/85, total cholesterol <200 mg/dL, LDL chol <100 mg/dL, blood glucose <110 mg/dL. Drug treatments positively recommended. |
| Hamalainen et al. [45] (1995), Finland | 375 subjects with MI. | I (n = 188): mean age men 53.4, women 58.8. C (n = 187): mean age men 53.0, women 58.4. |
I: 80% C: 80% |
All-cause and CV mortality, PA (cycle ergometer test), smoking (patient interviews), BP, cholesterol, triglycerides. | 15 years from baseline. | 3-year intervention: optimal medical care, physical activation, antismoking, dietary, psychosocial counselling led by social worker, psychologist, dietician, physiotherapist, and doctors. Intervention most intensive for 3 months after AMI, close contacts with team maintained over 3 years. |
| Murphy et al. [17] (2009), Northern Ireland and Republic of Ireland | 903 subjects with CHD recruited from 48 general practices. | I (n = 444): 68.5 (9.3); C (n = 459): 66.5 (9.9). |
I: 70%; C: 70%. |
BP, total cholesterol, hospital admissions, QOL (SF 12), diet (DINE questionnaire), PA (Godin questionnaire), smoking (Slan National Survey of Health and Lifestyles in Ireland). | At completion of 18-month intervention. | GP and nurse-led tailored care plans for practices: training in prescribing and behaviour change, administrative support, quarterly newsletter; Tailored care plans for patients: motivational interviewing, goal setting, target setting for lifestyle change, info booklet given to each patient, progress reviewed every 4 months. (Social cognitive theory used to develop training in behaviour change, design patient info booklet, and inform development of tailored patient care plans.) |
| Ornish et al. [41] (1998), USA | 48 patients with moderate to severe CHD. | I (n = 20): 57.4 (6.4); C (n = 15): 61.8 (7.5). |
I: 100% C: 80% |
CV mortality, nonfatal CV events, hospital admissions, diet (diaries), PA (questionnaire on type, frequency, duration), BP, total, LDL and HDL cholesterol, triglycerides, apolipoproteins. | At completion of 5-year intervention. | 5-year intervention: week long educational residential stay at hotel (Ornish et al., 1990).Group support meetings, 4 hours twice a week. Diet: low fat vegetarian, for at least a year: fruit, vegetables, grains, legumes, soybean products; no animal products except egg white and 1 cup/day of nonfat milk or yoghurt. Stress management techniques; advised at least 1 hour/day; audiocassette tape to assist. Exercise: individual prescription according to baseline treadmill test results, mainly walking; at least 3 hours/week, 30 minutes per session. Clinical psychologist-led group discussions: social support to encourage adherence. |
| Redfern et al. [19] (2008) A, Australia | 144 acute coronary syndrome (ACS) survivors not accessing standard cardiac rehabilitation (CR). | I (n = 72): 62 (1.6); C (n = 72): 67 (1.3). |
I: 74%; C: 75%. |
PA (Physical Activity Readiness Questionnaire (PARQ)), smoking (self report/Airmet Scientific Micro-smokanalyser), BP, total cholesterol. | At completion of 3-month intervention. Redfern et al. [20] (2009) B: 12 months from baseline |
GP-led behaviour change intervention; 1 hour initial consultation, 3 months of 5 phone calls (Redfern et al., 2006) for risk factor education, assertiveness training and assessment of lifestyle goals. Mandatory cholesterol lowering module, including healthy eating and pharmacological advice, and choice of 2 other modules including BP lowering, smoking cessation, and PA; choice of management options for risk factors including doctor-directed, such as a PA “script” from GP, hospital programme, for example, exercise class, individual programme, or self-help. |
| Vestfold Heartcare Study Group [28] (2003), Norway | 197 subjects with acute MI, hospitalisation for unstable angina, PCI, or CABG. | I (n = 98): 54 (8); C (n = 99): 55 (8). |
I: 81%; C: 83%. |
Hospital admissions, diet (FFQ), PA (self-report/diaries), smoking (self-report), BP, total and HDL cholesterol, QOL (SF 36), use of drugs. | At completion of 2-year intervention. | 2-year intervention: 6-week “heart school” in hospital: PA with physiotherapist: 15 minutes warm-up, 20 minutes walking, 10 minutes cool-down, 10 minutes stretching; advised to exercise on their own every day. Education sessions: twice a week, 2 hours each; dietary advice, smoking cessation, PA counselling, risk factor management, psychosocial management, medication, stress reduction; individual counselling. Followed by 9 weeks' organised PA twice a week at gym supervised by physiotherapist; level increased to jogging; group meetings every 3rd month throughout 2 year follow-up. |
| Wallner et al. [39] (1999), Austria | 60 patients <70 years with angiographically documented CAD and stable angina pectoris; recruited after successful elective PTCA. | I (n = 28): 58 (8). C (n = 32): 60 (7) |
I: 89%; C: 69%. |
Nonfatal CV events, diet (7-day weighted food records), PA (Minnesota Leisure Time questionnaire), BP, LDL and HDL cholesterol. | Mean 26 months (range 18–31) after baseline. | 12-month intervention: nutritionist-led. All patients at baseline: dietary and lifestyle advice: cholesterol 100–150 mg/day, fibre ≥25 g/day, PA ≥3 times/week for 30 minutes, smoking cessation. I group: 1-hour dietary advice sessions with nutritionist weekly during first month, every 2 weeks until month 3 then monthly until end of intervention. |
| Organisational | ||||||
| Jolly et al. [40] (1999), UK | 597 patients; 422 with MI and 175 with new diagnosis of angina. | I (n = 277): 63 (10); C (n = 320): 64 (10). |
I: 68%; C: 74%. |
Total cholesterol, BP, PA (questionnaire, walking test), smoking (questionnaire), BMI. | At completion of 1-year intervention. | Led by 3 specialist cardiac liaison nurses responsible for coordinating follow-up care, especially transfer of responsibility for care between hospital and GP. Liaison nurses provided support to practice staff by phone and visits to practice every 3–6 months. Practice nurses encouraged to attend training on behaviour change based on stages of change model. Each patient was given a patient held record, which prompted and guided follow-up (at approximately 4 to 6 month intervals). |
| Munoz et al. [24] (2007), Spain | 983 subjects with MI, angina, or ischaemia within previous 6 years. | I (n = 515): 64.2 (9.8); C (n = 468): 63.6 (10.3). |
I: 76.1%; C: 73.2%. |
Total mortality, CV mortality, nonfatal CV events, PA (self report), BP, total, LDL and HDL cholesterol, QOL (SF 12), use of drugs. | At completion of 3-year intervention or until an endpoint occurred. | 3-year intervention: Postal reminders to see GP every 3 months during 3-year follow-up. GPs strictly followed most recent guidelines on CV prevention, provide patients with healthy lifestyle advice including Mediterranean diet, PA and smoking cessation; adjusted treatments. |
I: intervention; C: control
In all the trials except where stated, patients randomised to the control group received usual care, which was not defined by every study but usually meant standard clinical care or standard care given by a GP.
3.2.1. Study Characteristics
The studies varied greatly in terms of sample size, duration, and intervention elements; however all involved patients of a similar age group (older adults) with CHD.
The sample size of trials ranged from 48 [41] to 3241 [21], and study duration ranged from three months [19, 43] to four [33] and five years [41]. Follow-up analyses varied too, ranging from three months [19] to 15 years [45].
3.2.2. Settings
We found much variation in study setting. Astengo et al. [16] reported a home-based exercise intervention while two studies incorporated initial short residential stays to deliver an intensive educational programme. Lisspers et al. [26] conducted a four-week stay at an intervention unit located in a rural part of northern Sweden, followed by a structured 11-month programme consisting of self-recording of lifestyle behaviours and contact with the patients' personal coaches. It was not clear whether the intervention unit was part of a hospital. Ornish et al. [41] organised a week-long residential stay at a hotel to educate patients on diet, exercise, and stress, followed by twice-weekly group support meetings for five years. The Vestfold Heartcare Study Group [28] conducted an initial six-week “Heart School” at a hospital rehabilitation centre, although it was unclear whether this was residential. Heart School involved physiotherapist-led PA, stress reduction education and lifestyle counselling followed by nine weeks of twice-weekly exercise, and then meetings every three months for two years. No other trial contained a residential element.
3.2.3. Intervention Intensity
Some studies were of intensive interventions. Salminen et al. [25] delivered nurse-led lectures, lasting up to two hours each, once a month for 16 months. In addition, eight group meetings were organised throughout this period, six exercise sessions, and three social events.
Less intensive programmes, in which patients were encouraged to self-manage their lifestyle behaviour change, included those reported by Murphy et al. [17] and Cupples and McKnight [22, 23], in which patients were seen every four months.
Another less intensive intervention, the Lyon Diet Heart Study [32, 33], involved a one-hour education session with a cardiologist and dietician, followed by a repeat visit at eight weeks and annually thereafter.
3.2.4. Professional Involvement
The interventions included in this review involved a range of clinical and other healthcare staff including doctors, nurses, physiotherapists, psychologists, dieticians, and social workers. Seven of the 21 trials were led by nurses, mainly based in primary care. De Lorgeril et al. [32, 33] reported a trial conducted by a cardiologist and dietician. Four studies were GP-led, one conducted by a physiotherapist and one nutritionist-led. Other studies involved a variety of professional input.
3.3. Risk of Bias in Included Studies
The methodological quality of the included studies is presented in Table 2. The quality of the trials, in terms of the method of randomisation, whether groups were similar at baseline, losses to follow up, whether intention to treat analyses were used and blinding of outcome assessors, is as reported in the papers.
Table 2.
Methodological quality of included studies.
| Source | Randomisation method | Groups similar at baseline | Loss to follow up | Intention to treat analyses? | Assessors blind? |
|---|---|---|---|---|---|
| Exercise | |||||
| Astengo et al. [16] (2010) | Unclear. Method of randomisation not stated. Authors state randomised “to training group or control.” |
Yes Only significant difference was fasting glucose level (higher in intervention (I) group than control (C)). |
None. Six patients (9.7%) had major clinical complications and did not complete study. | Unclear | Unclear |
| Dietary | |||||
| De Lorgeril et al. [32, 33] (1996, 1999) | Clear. Consecutive patients with first MI randomised. Inclusion based on modified Zelen design. |
Yes Except: smokers (more in I group). |
Not clear. Rate of withdrawal from follow-up reported: I = 8% C = 7% (De Lorgeril et al. [46], 1994) |
Yes | Yes |
| Psychological | |||||
| Lewin et al. [29] (2002) | Clear. Eligible patients allocated to I or C group by block randomisation, stratifying for age and sex. |
Yes | Not clear. 9 dropouts (6.3%), 1 did not return questionnaires (0.7%), and 2 died (1.4%). |
Yes | Yes |
| Lisspers et al. [26] (1999) | Unclear. Method not stated/described. |
Yes; Except C group: significantly higher proportion taking beta blockers. |
Data missing from exercise tests (4 I patients, 4 Cs), and questionnaires (numbers not stated). 5-year follow-up (Lisspers et al. [27], 2005): data available from 28 intervention patients (39% loss) and 27 control (34% loss). Further detail not reported. |
Unclear | Unclear |
| Salminen et al. [25] (2005) | Unclear. Method not stated/described. Authors stated that patients identified from longitudinal study data and randomly divided into intervention and control groups. |
Yes | None. 12 did not wish to take part in study after randomisation (4.5%); 29 died (10.8%). |
Unclear | Unclear |
| Educational | |||||
| Carlsson et al. [42, 43] (1997, 1998) | Unclear. Carlsson A and B [42]: patients randomised in groups of 20; no further detail on method given. Carlsson C [43]: patients admitted to coronary care unit who fulfilled inclusion criteria were randomised. |
Carlsson A, B, and C: yes | Carlsson A, B, and C: unclear. Carlsson C: 4 (4.9%) of usual care patients lost to follow up; no data given for intervention patients. Authors reported figures for missing data relating to specific variables. |
Carlsson A, B, and C: unclear. | Carlsson A, B, and C: unclear. |
| Cupples and McKnight [22] (1994). | Clear. Patients randomised to one of two groups. A health visitor opened an opaque, sealed, and numbered envelope containing the allocation which had been generated by a computer programme using random permuted blocks. |
Yes | 2 years: I: 25/342 (7.3%). C: 46/346 (13.3%); reasons given 5 years [23]: I: 92/342 (29.9%). C: 109/346 (31.5%); reasons given. |
Yes | Yes |
| Heller et al. [44] (1993) | Clear. Patients allocated to I or C groups according to name of GP. General practices stratified by number of doctors and randomly allocated to I or C. |
Yes I = 213, C = 237: nos. not similar because randomisation was by GP. |
Did not return follow-up questionnaires: I: 45/213 (21.1%) C: 30/237 (12.7%) (no further detail given) |
Unclear | Unclear |
| Southard et al. [31] (2003) | Clear. Patients stratified by ethnic group, CR participation, and acute status, then allocated to I or C groups by computer generated random number. |
Yes | 4 (3.84%); reasons stated. | Yes | Unclear |
| Multifactorial | |||||
| Allen et al. [30] (2002) | Clear. Consecutive patients randomised with computerised schema. |
Yes | Unclear. 158 out of 228 completed follow-up (31% loss); reasons for these losses given. |
Yes | Unclear |
| Campbell et al. [34] (1998) | Clear. Random numbers tables used to centrally randomise patients (by individual after stratification by age, sex, and practice) to I and C groups. |
Yes | Unclear. Available at 1-year analysis: I: 593 (88%) out of original total 673; C: 580 (87%) out of 670; reasons for loss given. | Yes | Unclear |
| Murchie et al. [36] (2003): available at 4-year follow-up analysis: I: 500 (74%); C: 461 (69%); reasons for loss given. | |||||
| Delaney et al. [38] (2008): available at 10-year follow-up: I: 385 (57%); C: 365 (54%). These figures include deaths but loss to follow up was reported as 62 patients (4.6%). | |||||
| Giallauria et al. [18] (2009) | Clear. Patients randomised 1 : 1 using computer generated randomisation. |
Yes | None | Unclear | Unclear |
| Gianuzzi et al. [21] (2008) | Clear. Patients randomised 1-to-1; randomisation centrally determined by fax at coordinating secretariat using computerised algorithm. |
Yes | 154 (4.7%). | Yes | Yes |
| Hamalainen et al. [45] (1995) | Unclear. Consecutive patients hospitalised for AMI allocated to I or C group by stratified randomisation. No further detail on method given. |
Yes | Unclear | Unclear | Unclear |
| Murphy et al. [17] (2009) | Clear. Practices: computer generated random numbers; patients: randomly selected at remote site, sent invitation in sequence from lists in random order. |
Yes Except: different proportions in control and intervention groups admitted to hospital in previous 12 months. This was adjusted for in analysis. |
None | Yes | Yes |
| Ornish et al. [41] (1998) | Unclear. Method stated; not fully described. After angiography patients randomised using invitational design to minimise crossover, ethical concerns, nocebo effects, and dropout. |
Yes Except C group had significantly higher levels of HDL-chol and apolipoprotein A-I. |
20 (71%) intervention patients completed 5-year follow-up; 15 (75%) control patients. | Yes | Yes |
| Redfern et al. [19] (2008) | Clear. Consecutive patients randomised following blinded baseline assessment. Computer generated random allocation sequence was implemented using consecutively numbered envelopes. |
Of 15 demographic and clinical characteristics, groups were similar in 9. Statistically different were European and Asian/African origin, employment status, CVD history, and CABG status. | 1 (uncontactable). (4 withdrew, 3 died). |
Yes | Yes |
| Vestfold Heartcare Study Group [28] (2003) | Clear. Patients randomised by use of preprepared sealed opaque envelopes containing details on group allocation. Patients opened envelopes so that their group allocation was revealed to them without previous knowledge of study investigators. |
Yes | Unclear. Percentages attending follow-up meetings, keeping diaries and adhering to PA levels only given for I group. |
Unclear: where there were missing data at 6 months and 2 years, the last recorded value of the variable from previous visit was used. | Unclear |
| Wallner et al. [39] (1999) | Unclear. Method of randomisation not stated/described. |
Yes Except: statistically significant differences in number with previous MI (I group more), SBP (I group higher), exercise/day (I group more). |
Unclear. Patients randomised: I = 28, C = 32. At 12 months, analyses performed in 25 I patients (89%), 13 C (40%) (only patients with complete data for 12 months were included at baseline) because all patients did not agree to complete lengthy questionnaires. Cardiological follow-up was completed in all patients recruited. |
Yes | Unclear |
| Organisational | |||||
| Jolly et al. [40] (1999) | Clear. Practices randomised (before consent sought) to I or C after stratification by size of practice and distance from district general hospital. |
Yes | 10% in both groups; reasons given. | Yes | Unclear |
| Munoz et al. [24] (2007). | Clear. Primary care facilities allocated to I or C by random sequence generated by computer programme. |
Yes Except: C group higher proportion had previous hypertension, peripheral vascular disease; higher proportion taking beta blockers, ACE inhibitors; higher BP; I group higher HDL-chol. |
11 (1.1%). Also: Withdrew: 28 (2.8%); Unwilling: 2 (0.2%); Died: 59 (6%). |
Yes | Unclear |
I: intervention; C: control.
In seven of the 21 original studies (excluding follow-up papers), the randomisation method was not clearly described. In six of these trials, the method was not stated at all [16, 25, 26, 39, 43, 45]. The majority of the remaining studies described various randomisation techniques including the use of computer-generated random numbers, random numbers tables, and sealed envelopes. Lewin et al. [29] used block randomisation, while Carlsson [42] allocated patients in groups of 20. Murphy et al. [17] used cluster randomisation because their intervention was aimed to alter practitioners' behaviour which could contaminate their interactions with control patients.
Loss to follow up was not reported by some authors. Where it was reported, it was defined differently. Some authors included as losses to follow up deaths and withdrawals due to poor health, while others defined it as people who could not be accounted for or contacted at follow-up. Follow-up as a quality marker relates only to original studies and varied considerably for longer-term follow-up studies as would be expected.
Thirteen of the 21 studies stated that analyses were conducted using intention to treat principles. The Vestfold Heartcare Study Group [28] used the last recorded value of a variable from the previous visit if there were missing data.
Only seven out of 21 studies reported that outcome assessors were blind to group allocation. Overall, the quality of many trials was poor with a majority not having blinded outcome assessors and many not describing the method of randomisation.
3.4. Effects of Interventions
3.4.1. All-Cause Mortality
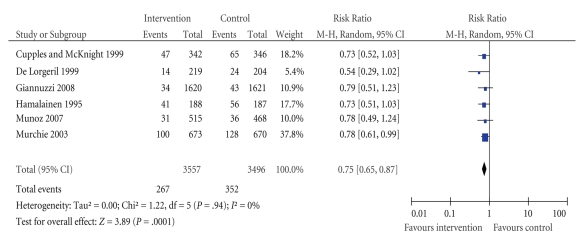
Six studies reported total mortality and are presented in Figure 2. Overall, four studies showed benefits for intervention patients compared to controls in relation to total mortality. Because of the large differences in follow-up periods, we have used data from the studies which range from 3 to 5 years to allow easier comparison of studies. For all interventions except two, this constituted their study endpoint.
Figure 2.
Effect of interventions on all-cause mortality: comparison of intervention versus control groups.
Murchie et al. [36] reported a significant survival effect for intervention patients compared to controls at their 4.7 year follow-up. Within the same study, Delaney et al. [38] reported that, at 10 years, the observed difference was no longer significant between the groups.
Hamalainen et al. [45] reported a significant survival effect for intervention patients compared to controls at their 4.7 year follow-up.
The I2 test for heterogeneity combined with the Chi2 nonsignificant P value suggests that the level of heterogeneity between studies is not important.
3.4.2. Cardiovascular Mortality
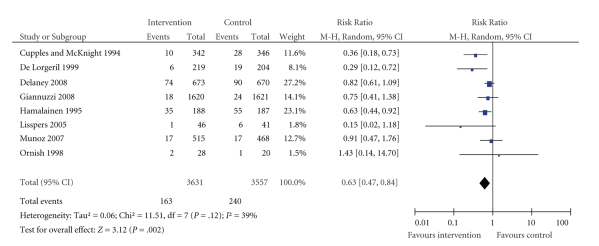
Eight studies reported cardiovascular mortality and are presented in Figure 3. Three studies showed significant survival effects. Data were collected at times ranging from 2 to 5 years. Delaney et al. [38] reported a 10-year follow-up study but we have presented their data collected at 4.7 years to allow for easier comparison with other studies. For the same reason we have used data reported by Hamalainen et al. [45] at three years of follow-up instead of the authors' results at 15 years.
Figure 3.
Effect of interventions on cardiovascular mortality: comparison of intervention versus control groups.
The three studies reporting significant survival effects were Cupples and McKnight [22], two-year follow-up results from an educational intervention, De Lorgeril et al. [33], a Mediterranean diet study with four-year follow-up, and Hamalainen et al. [45], a trial of a multifactorial intervention.
Hamalainen et al. [45], who reported coronary mortality, also reported significant survival effects for intervention patients compared to controls in their 15-year follow-up study. Delaney et al. [38] reported CV and coronary deaths at both 4.7- and 10-year follow-up points. However, the mortality figures were combined with nonfatal MI to calculate a proportional hazard ratio. Murchie et al. [36], a paper from the same study at 4.7 years of follow-up, reported an adjusted hazard ratio of 0.76 for coronary events (coronary deaths plus nonfatal MIs) (0.58 to 1.00, P = .049).
The I2 test for heterogeneity indicated a moderate level of heterogeneity across studies.
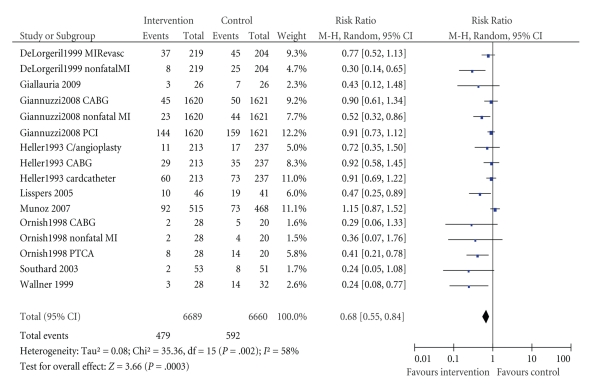
3.4.3. Nonfatal Cardiac Events
This was reported by nine studies. In Figure 4, we have presented data for major nonfatal events (MI, CABG, PCI, coronary angioplasty). Significant benefits for intervention patients compared to controls were shown by five studies. Four studies reported nonfatal events separately [21, 33, 41, 44] while five reported the number of patients who had an event.
Figure 4.
Effect of interventions on nonfatal cardiac events: comparison of intervention versus control groups. MIRevasc = elective myocardial revascularisation, CABG = coronary artery bypass graft, PCI = percutaneous coronary intervention, C/angioplasty = coronary angioplasty, Cardcatheter = cardiac catheter, PTCA = percutaneous transluminal coronary angioplasty.
Lisspers et al. [27] included mortality in “event” results, therefore although they reported figures for various events and mortality they did not report a separate statistic for nonfatal events. Delaney et al. [38] reported coronary events which included coronary deaths and nonfatal MIs; however separate figures for each variable were not reported.
Gianuzzi et al. [21] reported nonfatal stroke, heart failure, and angina data but none of the values was significant.
Lewin et al. [29] reported the frequency of angina attack in number of episodes per week. Intervention group patients had a reduction of three attacks per week compared to a reduction of 0.4 attacks among control subjects (P = .016).
In addition to nonfatal MI and coronary revascularisation, De Lorgeril et al. [33] reported a number of major and minor secondary endpoints. They calculated risk ratios for nonfatal AMI combined with CV deaths, major secondary endpoints (periprocedural infarction, unstable angina, heart failure, stroke, pulmonary embolism, peripheral embolism) combined with primary endpoints, and primary and major secondary endpoints combined with minor secondary endpoints (stable angina, post-PCTA restenosis, and thrombophlebitis). Separate risk ratios for nonfatal CV events were not reported.
In addition to MI, PTCA, and CABG, Ornish et al. [41] reported the frequency, duration, and severity of angina chest pain at one year and five years. One significant value out of three outcomes reported at the two different time points each was reported: chest pain severity (scale 1–7, baseline to one year: intervention group 1.5 (1.5) to 0.7 (1.2); control group 0.6 (0.8) to 1.4 (1.2); P < .001).
The I2 test for heterogeneity indicated that there may be substantial heterogeneity across studies so results of this meta-analysis have to be interpreted with caution.
3.4.4. Hospital Admissions
Five studies reported results relating to hospital admissions. While there was an overall trend to reduced admissions in intervention groups, only one of these studies reported a significant reduction in intervention patients [17]. The data are presented in Table 3.
Table 3.
Impact of interventions on hospital admissions.
| Study | Intervention | Control | Risk ratio |
|---|---|---|---|
| Murphy* | Overall: | P = .03 | |
| Baseline to 18 months: | |||
| 0.3 (0.6) to 0.4 (0.7) |
0.4 (0.8) to 0.5 (1.0) |
||
| CV: | P = .04 | ||
| At 18 months: | |||
| 0.14 (0.50) | 0.23 (0.7) | ||
| Other: | P = .22 | ||
| 0.24 (0.6) | 0.32 (0.7) | ||
| Vestfold** | 20 | 33 | NS (not significant) |
| Ornish*** | 23 | 44 | 0.685 (0.012–13.2), P = .81 |
| Heller**** | 47 | 60 | 0.253***** Diff -0.8% (−10.0–8.4); NS |
| Delaney***** | 7647 | 8642 | P = .998 |
*Mean number of admissions per patient (at 18 months).
**20 admissions related to chest pain without evidence of ischaemia among 11 patients in intervention group, 33 admissions among 14 patients in control group (at 2 years).
***Cardiac hospitalisations (at 5 years).
****Patients with ≥1 hospital readmission (at 6 months).
*****Total number of admissions (at 10 years).
3.4.5. Lifestyle Risk Factors
Data relating to lifestyle risk factors for CHD–diet, PA, and smoking are presented in Table 4.
Table 4.
Impact of interventions on lifestyle risk factors.
| Source | Diet | Exercise | Smoking |
|---|---|---|---|
| Exercise | |||
| Astengo et al. [16] (2010) | NR | Maximum workload: P = .02. Self-reported training (days/week): P value for difference between groups <.001. Self reported training (minutes/session): P<.001. Maximum heart rate: NS. |
NR |
| Dietary | |||
| % calories consumed: | NR | NR | |
| Total lipids: P = .002. | |||
| Saturated fats: P = .0001. | |||
| Polyunsaturated fats: P = .0001. | |||
| Oleic: P = .0001. | |||
| De Lorgeril et al. [32] (1996). | Linoleic: P = .0001. | ||
| Linolenic: P = .0001. | |||
| Alcohol: NS. | |||
| Proteins: NS. | |||
| Fibre: P = .004. | |||
| Cholesterol: P = .0001. | |||
| Psychological | |||
| Lewin et al. [29] (2002) | NR | Seattle Angina Questionnaire: physical limitations score for I group reduced; for C group increased: P < .001. | NR |
| Diet knowledge index: P < .0005. | PA frequency: P < .025. Maximum workload: P < .0025. Chest pain ratings during exercise tests: P < .025. |
Self rated smokers P < .01. | |
| Lisspers et al. [26] (1999). | Self rated dietary habits: P < .0005. | ||
| Lisspers et al. [27] (2005) calculated overall lifestyle scores incorporating diet, PA, smoking, and stress: I group significantly higher score than C group at 12, 24, 36, and 60 months. P values not given for individual components of score. | |||
| Salminen et al. [25] (2005) | Type of milk/type of fat consumed: NS (data not reported). | PA frequency: NS (data not reported). | NS (data not reported). |
| Educational | |||
| Carlsson et al. [42, 43] (1997, 1998). | A: NR | A: work capacity: NS (AMI patients: P = .08; CABG patients: P = .75). B: NR. | A: NR |
| B: NR | C: PA: frequency: NS. Stopped | B: NR | |
| C: Concern about food habits: P = .008 | physical training: P = .43; started physical training: P = 0.50. | C: smoking cessation: NS. | |
| Cupples and McKnight [22] (1994). | Intake of poultry (P = .02), green vegetables (P = .002), high fibre food (P = .01), red meat (P = .005), fried food (P = .045), biscuits and sweets (P = .0001), saturated fat (P = .013). | PA frequency: P < .0001. | Smoking cessation rate: NS (P = .82). |
| Cupples and McKnight [23] (1999) | Diet score: difference between groups: NS | PA frequency: P < .05. | Smoking cessation rate: difference between groups: NS. |
| Heller et al. [44] (1993) | Mean fat score: P = .002. | Proportion exercising 3 times weekly: difference between groups NS. | Current smoker: difference in proportions between groups: NS |
| Southard et al. [31] (2003) | MEDFICTS dietary score: NS | Canadian Angina Grade, Duke Activity Status Score: NS. PA duration: NS. |
NR |
| Multifactorial | |||
| 1 year: | MET, hr/wk ≥6 1 year: P = .02. |
NR | |
| Fat: P = .009; | |||
| Allen et al. [30] (2002). | Saturated fat: P = .004; | ||
| Cholesterol: P = .006; | |||
| Fibre: NS. | |||
| Campbell et al. [34] (1998) A | Low fat diet: effect size: OR 1.47, CI 1.10 to 1.96, P = .009. | Moderate PA: effect size: OR 1.67, CI 1.23 to 2.26, P = .001. | Proportion of non-smokers: OR 0.78, P = .322. |
| Giallauria et al. [18] (2009). | NR | VO2peak (increase in oxygen at peak exercise): | NR |
| 3, 12, and 24 months: P < .001. | |||
| VO2AT (anaerobic threshold): 3, 12 and 24 months: P < .001. | |||
| Wattmax : 3, 12 and 24 months: P < .001. | |||
| Gianuzzi et al. [21] (2008) | Dietary score 3.9% higher in I group (P < .001); maintained throughout study. | Mean score: 6 months: P < .01; maintained throughout study. |
At 6 months: P = .02. 3 years: NS (P = .60). |
| NR | Frequency of PA and work capacity: NS (data not reported). | No. cigarettes smoked/day: | |
| 1 year: NS; | |||
| Hamalainen et al. [45] (1995). | 2 years: P = .02; | ||
| 3 years: P = .002; | |||
| Years 6 and 10: NS. | |||
| Murchie et al. [36] (2003) A | NS I group improvements sustained in all areas but at 4 years C group improved and differences no longer significant. |
NS | NS |
| Murphy et al. [17] (2009). | DINE fibre: NS (P = .06) DINE fat: NS (P = .86). |
Godin exercise score: NS (P = 0.67). | Self reported smoker: NS (P = .23). |
| Fat intake, g per day: | Adherence to exercise: | NR | |
| 1 year: P < .001. | |||
| 5 years: P < .001. | |||
| Ornish et al. [41] (1998). | Fat intake, % of energy intake: | ||
| 1 year: P < .001. | 1 year: NS. | ||
| 5 years: P < .001. | |||
| Dietary cholesterol: | |||
| 1 year: P < .001. | |||
| 5 years: P = .002. | 5 years: NS. | ||
| Energy intake: | |||
| 1 year: P = .64. | |||
| 5 years: P = .86. | |||
| Redfern et al. [19] (2008) A Baseline to 3 months | NR | METS/kg/min: P = .01. | Smokers: P < 0.01. |
| Redfern et al. [20] (2009) B | NR | METS/kg/min, P = .001. | NR |
| Vestfold [28] (2003). | 6 months: I patients significantly lower intake saturated and monounsaturated fat, sugar, and cholesterol, higher fibre than C. | PA frequency: 6 months: P < .001. |
6 months: P < .05. 2 years: P < .05. |
| 2 years: I group significantly lower total fat intake, saturated fat and monounsaturated, significantly higher fibre, lower sugar and cholesterol. | 2 years: P < .01. | ||
| Total fat: P = .001; | Kcal/day: P = .001. | NR | |
| SFA: P = .05; | |||
| MUFA: P = .04; | |||
| Carbohydrate: P = .001; | |||
| Wallner et al. [39] (1999). | Fibre: P = .006; | ||
| Cholesterol: P = .03; | |||
| Vitamin C: P = .006; | |||
| Energy intake (kcal), PUFA, protein, vitamin E: NS. | |||
| Organisational | |||
| Jolly et al. [40] (1999) | NR | Fitness test (distance walked in 6 minutes, metres): NS. | Quit rate (proportion who stopped smoking): NS. |
| Munoz et al. [24] (2007) | NR | PA (amount of exercise): both groups increased but difference between groups NS. | NR |
NR: not reported; NS: not significant; I: intervention; C: control.
Table 5 shows a summary of lifestyle risk findings from our included studies. For each of the three areas of diet, exercise, and smoking, we have presented the number of studies reporting each outcome, the number of outcomes, and the numbers of significant and nonsignificant values.
Table 5.
Summary of lifestyle risk findings.
| Outcome | Number of studies with this outcome | Number of outcomes | Number significantly improved | Number of outcomes with no significant difference |
|---|---|---|---|---|
| Exercise | 21 | 37 | 20 | 17 |
| Diet | 15 | 51 | 39 | 12 |
| Smoking | 13 | 20 | 7 | 13 |
Note: we counted Campbell and Murchie as separate studies as the patients in each were not necessarily the same. Other follow-up studies, Cupples, Ornish, Vestfold, and Redfern we counted as one study but counted the outcomes from each time point as different outcomes (hence the 20 outcomes for the 13 studies reporting smoking outcomes).
Diet —
Fifteen studies reported diet as an outcome, with a total of 51 outcomes. Of these, 39 showed significant benefits for intervention patients compared to controls in relation to dietary consumption. These included significant improvement in specific food intake, such as fat, fibre, sugar, and cholesterol [28, 30, 33, 34, 39, 41], diet score, diet knowledge, and habits [21, 27, 44, 47], and for concern about dietary habits [43].
PA —
Twenty-one studies incorporated PA, with 37 outcomes. Of these, 20 showed significant improvements for intervention patients compared to controls. These included significant improvements in maximal workload and VO2peak [16, 18, 27]. Eleven studies used validated questionnaires or patient diaries [17, 20, 23–25, 28, 30, 34, 36, 39, 41]. Four conducted patient interviews or used questionnaires which were not reported as validated [21, 40, 43, 44]. Of these, eight studies reported significant improvements [20, 21, 23, 27, 28, 30, 34, 39].
Smoking —
While all studies reported proportions of the study populations that smoked, only 13 studies reported smoking as an outcome and five of these reported significant reductions in smoking behaviours in the intervention groups [20, 21, 27, 28, 45]. Hamalainen et al. [45] reported a nonsignificant difference between intervention and control groups at one year, but significant at two and three years.
BP —
Thirteen studies reported BP as an outcome, and five reported significant benefits for intervention compared to control patients [18, 20, 34, 39, 45]. Giallauria et al. [18] reported significant improvements in SBP and DBP at 12 months and 24 months. Redfern et al. [20] reported significant difference in SBP among intervention compared to control patients at three months and 12 months. Campbell et al. [34] collected BP data from medical records and classified it as being managed according to British Hypertension Society recommendations if the last recorded measurement was less than 160/90 mmHg or receiving attention. The significant difference between intervention and control groups at one year was no longer observed at four-year follow-up [36].
Wallner et al. [39] found significant improvements in SBP and DBP in intervention patients compared to controls. Hamalainen et al. [45] reported significant benefits for intervention compared to control patients relating to SBP and DBP at one, two, and three years but not at six or 10 years.
Total Cholesterol and/or Lipid Levels —
These outcomes were reported by 19 studies and 12 demonstrated significant benefits for intervention patients. Seven of these 12 studies reported significant improvements in total cholesterol for intervention patients compared to controls [18, 20, 21, 30, 41, 42, 45]. Another study found a significant difference in total cholesterol between female intervention and control groups but not male [25].
Low-Density Lipoprotein Cholesterol (LDL-chol) —
Five studies reported significant improvements in levels of LDL-chol among intervention patients compared to controls [18, 30, 39, 41, 42]. Salminen et al. [25] found significant improvements between female intervention and control groups but not male.
Only one study, that by Giallauria et al. [18], found a significant improvement in levels of high-density lipoprotein cholesterol (HDL-chol) among intervention patients compared to controls.
QOL —
10 studies reported quality of life, and four reported significant benefits for intervention patients—including for “physical activity factor” [26], physical function [28], and emotional [44]. Campbell et al. [35] reported five significant results out of the eight health status domains (physical, social, role physical, role emotional, and pain).
Self Efficacy —
This was reported by only one study. Gianuzzi et al. [21] reported a significantly better score in intervention patients compared to controls relating to self/stress management at six month follow-up and throughout their study.
Medication —
Nine studies reported use of medications, and six reported significant improvements among intervention patients compared to controls.
Lewin et al. [29] reported the number of glyceryl trinitrate pills or puffs of sublingual spray taken per day which were self-reported in a diary by patients. Intervention patients reported a significant reduction in doses per week compared to control group patients.
Campbell et al. [34] reported proportions taking aspirin which was ascertained by postal questionnaire. A significantly greater number of intervention patients at one-year follow-up was taking aspirin compared to controls.
Carlsson [42] found significant differences between intervention and control patients in use of statins and cholestyramine; however it was unclear how this data was collected.
In the study by Cupples and McKnight [22, 23] trained health visitors assessed the use of prophylactic drugs for angina by interviewing patients. At the two- and five-year follow-ups, a significantly higher number of intervention patients were using prophylactic medication than controls.
4. Discussion
We have conducted a systematic review of lifestyle interventions for the secondary prevention of CHD using Cochrane Collaboration methodology. The review indicates that lifestyle interventions have mixed effects with some benefits in relation to total mortality, CV mortality, and nonfatal CV events as well as PA, diet, blood pressure, cholesterol, QOL, and medication adherence. The review was restricted to the period after 1990 because the concept of secondary prevention and methods to promote it are a relatively recent development in healthcare. Nonetheless, we found trials of interventions which were heterogeneous, and this was evidenced by the range of categories into which they could be classified. Few trials evaluated a single component of lifestyle, while many assessed the effects of a complex, multifactorial intervention. Other differences between studies included trial quality, intervention setting, intensity, duration, and length of follow-up.
For all trials the control group was usual medical care. We excluded trials which evaluated the intervention against a different intervention or other programme which could not be defined as usual care.
4.1. Effectiveness of Interventions
Overall, a small number of studies showed a significant reduction in deaths and nonfatal MIs while several reported positive results relating to adherence to lifestyle change. Regarding total mortality, four studies out of the six reporting this outcome showed significant benefits in the relatively short time scale studied (3–5 years). The Lyon Diet Heart Study [33] showed a significant impact on All-cause mortality and fewer deaths from CV causes among intervention patients compared to controls. The protective effect of the intervention was maintained for up to four years. This was a noteworthy result because patients were seen annually, indicating that they were able to maintain the diet for long periods alone and without professional motivation. The other trials showing significant benefits were of an educational intervention [22] and two multifactorial [36, 45]. Only three out of eight studies showed significant results for cardiovascular mortality. These were a dietary intervention [33], educational [22] and multifactorial ones [45], all of which had also shown significant effects on total mortality.
Concerning nonfatal CV events, we presented 16 outcomes from nine studies. There were significant improvements in five of these trials, which were of dietary [33], psychological [27], and multifactorial [21, 39, 41] interventions. Of the five studies which reported hospital admissions, only one, Murphy et al. [17], reported significant benefit for intervention patients compared to controls, both for overall and CV admissions.
In terms of risk factors for CHD, these were assessed by greatly differing methods which made comparison difficult. Diet, PA, smoking status, blood pressure, and cholesterol were widely reported, with varying results. In relation to diet, significant results were reported for the dietary intervention, one psychological, three educational, and six multifactorial interventions. Significant results relating to PA were reported for one exercise trial, one educational, two psychological, and seven multifactorial. Of the five studies which showed significant benefits for intervention patients compared to controls in relation to smoking, four were multifactorial and one psychological. All five interventions which showed significant results relating to BP were multifactorial. Concerning cholesterol and lipid levels, the interventions which showed significant results were one psychological, one educational, and eight multifactorial.
In relation to quality of life, one psychological trial reported a significant benefit for intervention patients compared to controls, as did one educational and two multifactorial.
Few trials reported medication intake or adherence. However, as appropriate therapy is a key aspect of secondary prevention of CHD [8], this should surely be given greater consideration. The trials which reported significant results relating to medication were classified as psychological (one), multifactorial (two), and educational (three).
Likewise, self-efficacy, a patient's ability, and confidence to manage their own condition, was only reported in one study. This multifactorial intervention reported a significant outcome for intervention patients compared to controls.
4.2. Relevance of Lifestyle and Risk Factor Modification
Recent evidence has shown the importance of focusing on lifestyle to effect positive changes relating to CHD. Bennett et al. [48] used the IMPACT CHD model to examine the decline in CHD mortality in Ireland between 1985 and 2000, and possible reasons for this. The mortality rate fell during this period by some 47%, representing 3673 fewer observed CHD deaths. The authors found that 48% of this decrease was due to a reduction in major risk factors including smoking and cholesterol levels. Conversely, an upward trend was seen in obesity, diabetes, and PA which were said to contribute an additional 500 deaths in 2000. These results were similar to those found by Palmieri et al. [49], who also used the IMPACT model and investigated the decline in CHD mortality in Italy between 1980 and 2000. They concluded that over half of the mortality fall was due to risk factors, mainly blood pressure and cholesterol, and just under half was due to medical therapies.
The Lyon Diet Heart Study [33] was the first clinical trial evidence in support of the Mediterranean diet, which comprises a high intake of fruit, vegetables, nuts, legumes, and grains. Other recent evidence has been found supporting the Mediterranean diet's protective effect in the secondary prevention of CHD [50–52]. O'Connor et al. [6] examined RCTs of cardiac rehabilitation with exercise and found a moderate reduction of 20% in total and CV mortality after one year, with a reduced risk maintained for three years after infarction. In a recent review of interventions incorporating exercise as part of a cardiac rehabilitation programme, Jolliffe et al. [11] also observed a reduction in total mortality. Exercise-only interventions resulted in a 27% reduction in total mortality and 31% reduction in cardiac mortality while comprehensive rehabilitation reduced mortality to a lesser extent (26%).
The psychological interventions included in this review showed small beneficial effects. This is in keeping with similar findings in a recent review of 36 RCTs of psychological interventions by Rees et al. [12] which showed no evidence of effect on total or cardiac mortality, but found small reductions in anxiety and depression.
We included two organisational interventions in this review, and both reported disappointing results. Jolly et al. [40] investigated a programme to improve communication between hospital and GP practices using liaison nurses. The programme was effective in promoting follow-up care of patients in general practice; however health outcomes were not improved. Munoz et al. [24] examined an intervention involving postal reminders sent to patients to encourage them to visit their GP. Blood pressure and HDL-cholesterol levels were improved, but no effect was observed on mortality or morbidity.
4.3. Limitations of the Review
This review has a number of potential limitations. The relatively small number of studies which included mortality as an outcome, the heterogeneity between trials, and poor quality of reporting all arise from the primary data. Further, imprecise descriptions of the interventions and the limited data have made it difficult to determine the benefits of various components of the interventions. The majority of the population of the interventions was male and relatively low risk; however greater benefit could have been derived from the interventions by higher risk patients who were excluded on the basis of comorbidity.
The broad range of trials included, and the subsequent large number discovered in our search, may have made comparisons difficult. Nonetheless this reflects the current lack of clarity in respect of the optimal components of effective interventions for CHD secondary prevention.
4.4. Implications for Practice
We have found that lifestyle interventions promoting regular PA, a healthy diet, and adherence to medication have beneficial effects among patients with CHD. It is therefore reasonable to promote such a healthy lifestyle to patients similar to those included in the RCTs we investigated—mainly older adults who had suffered a coronary event. As practitioners endeavour to achieve target levels of blood pressure and cholesterol by altering their patients' prescribed medication, the potential value of their advice regarding exercise and diet should not be overlooked.
4.5. Implications for Future Research
Overall, the current evidence suggests that lifestyle interventions have some beneficial effects on total and cardiac mortality, morbidity, and on behaviour change in relation to modifiable cardiac risk factors. Even where little or no effect was observed relating to mortality or morbidity, some trials reported benefits in terms of lifestyle behaviour change. That healthcare education and even small-scale interventions can lead to healthier lifestyle choices, as shown in this review and others, should be an encouragement to professionals in practice. Future studies should be designed carefully, with attention given to aspects of study quality, which we addressed in Table 2. For example, RCTs of a cluster design help to avoid contamination of control patients. Further, outcomes should be matched to intervention elements. In addition, it is important to incorporate concealment of allocation and blinding of outcomes. Maximal follow-up should be ensured and more consideration given to the underlying theoretical foundation for the intervention.
However, the fact that more profound and wide reaching benefits were not seen in our review is surprising considering that all guidelines focus on the importance of lifestyle interventions. Future research should perhaps focus on the components of interventions and what the ideal combination of measures, intervention intensity, and duration should be. In addition, investigations into the barriers to lifestyle change among patients with CHD may shed new light on why some well-designed and executed interventions have not resulted in expected benefits.
Acknowledgments
M. Cupples and J. Cole gratefully acknowledge funding from the Centre of Excellence for Public Health (Northern Ireland), a UKCRC Public Health Research Centre of Excellence, funded by the British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, Research and Development Office for the Northern Ireland Health and Social Services, and the Wellcome Trust, under the auspices of the UK Clinical Research Collaboration.
Appendix
Medline Search Strategy
Lifestyle.mp. or Life Style/
Exercise.mp. or Exercise/
Physical activity.mp.
Diet/ or diet.mp.
1 OR 2 OR 3 OR 4.
Secondary prevention.mp. or Secondary Prevention/
Coronary heart disease.mp. or Coronary Disease/
CHD.mp.
Myocardial infarction.mp. or Myocardial Infarction/
MI.mp.
Coronary Artery Bypass/or coronary artery bypass graft.mp.
CABG.mp.
Percutaneous transluminal coronary angioplasty.mp. or Angioplasty, Transluminal, Percutaneous Coronary/
PTCA.mp.
Angina pectoris.mp. or Angina Pectoris/
7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15.
5 AND 6 AND 16.
Limit search 17 by English language, humans, 1990-current and adults all ages (19 plus).
randomized controlled trial.mp. or Randomized Controlled Trial/
controlled clinical trial.mp. or Controlled Clinical Trial/
random allocation.mp. or Random Allocation/
double blind method.mp. or Double-Blind Method/
single blind method.mp. or Single-Blind Method/
19 OR 20 OR 21 OR 22.
18 AND 24.
References
- 1.Mackay J, Mensah G. The Atlas of Heart Disease and Stroke. Part three: the burden. Deaths from Coronary Heart Disease. World Health Organisation, 2004, http://www.who.int/cardiovascular_diseases/resources/atlas/en/
- 2.British Heart Foundation. Mortality. August 2010, http://www.heartstats.org/topic.asp?id=17.
- 3.Pashkow FJ. Issues in contemporary cardiac rehabilitation: a historical perspective. Journal of the American College of Cardiology. 1993;21(3):822–834. doi: 10.1016/0735-1097(93)90116-i. [DOI] [PubMed] [Google Scholar]
- 4.Leon AS, Franklin BA, Costa F, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (subcommittee on exercise, cardiac rehabilitation, and prevention) and the Council on Nutrition, Physical Activity, and Metabolism (subcommittee on physical activity), in collaboration with the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2005;111(3):369–376. doi: 10.1161/01.CIR.0000151788.08740.5C. [DOI] [PubMed] [Google Scholar]
- 5.Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. Journal of the American Medical Association. 1988;260(7):945–950. [PubMed] [Google Scholar]
- 6.O’Connor GT, Buring JE, Yusuf S, et al. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80(2):234–244. doi: 10.1161/01.cir.80.2.234. [DOI] [PubMed] [Google Scholar]
- 7.Schuler G, Hambrecht R, Schlierf G, et al. Regular physical exercise and low-fat diet: effects on progression of coronary artery disease. Circulation. 1992;86(1):1–11. doi: 10.1161/01.cir.86.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Cooper A, Skinner J, Nherera L, et al. Clinical Guidelines and Evidence Review for Post Myocardial Infarction: Secondary Prevention in Primary and Secondary Care for Patients Following a Myocardial Infarction. London, UK: National Collaborating Centre for Primary Care and Royal College of General Practitioners; 2007. [PubMed] [Google Scholar]
- 9.McAlister FA, Lawson FME, Teo KK, Armstrong PW. Randomised trials of secondary prevention programmes in coronary heart disease: systematic review. British Medical Journal. 2001;323(7319):957–962. doi: 10.1136/bmj.323.7319.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotseva K, Wood D, De Backer G, De Bacquer D, Pyorala K, Keil U. Cardiovascular prevention guidelines in daily practice: a comparison of EUROASPIRE I, II, and III surveys in eight European countries. The Lancet. 2009;373(9667):929–940. doi: 10.1016/S0140-6736(09)60330-5. [DOI] [PubMed] [Google Scholar]
- 11.Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database of Systematic Reviews. 2001;(1) doi: 10.1002/14651858.CD001800. Article ID CD001800. [DOI] [PubMed] [Google Scholar]
- 12.Rees K, Bennett P, West R, Davey SG, Ebrahim S. Psychological interventions for coronary heart disease. Cochrane Database of Systematic Reviews. 2004;(2) doi: 10.1002/14651858.CD002902.pub2. Article ID CD002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration; 2009. [Google Scholar]
- 14.Donaldson MS, Yordy KD, Lohr KN, Vanselow NA. Primary Care: America’s Health in a New Era. Committee on the Future of Primary Care, Division of Health Care Services, Institute of Medicine. Washington, DC, USA: National Academy Press; 1996. [Google Scholar]
- 15.Review Manager (RevMan) Version 5.0. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration; 2008. [Google Scholar]
- 16.Astengo M, Dahl Å, Karlsson T, Mattsson-Hultén L, Wiklund O, Wennerblom B. Physical training after percutaneous coronary intervention in patients with stable angina: effects on working capacity, metabolism, and markers of inflammation. European Journal of Cardiovascular Prevention and Rehabilitation. 2010;17(3):349–354. doi: 10.1097/HJR.0b013e3283336c8d. [DOI] [PubMed] [Google Scholar]
- 17.Murphy AW, Cupples ME, Smith SM, Byrne M, Byrne MC, Newell J. Effect of tailored practice and patient care plans on secondary prevention of heart disease in general practice: cluster randomised controlled trial. British Medical Journal. 2009;339(7731):p. 1186. doi: 10.1136/bmj.b4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giallauria F, Lucci R, D'Agostino M, et al. Two-year multicomprehensive secondary prevention program: favorable effects on cardiovascular functional capacity and coronary risk profile after acute myocardial infarction. Journal of Cardiovascular Medicine. 2009;10(10):772–780. doi: 10.2459/JCM.0b013e32832d55fe. [DOI] [PubMed] [Google Scholar]
- 19.Redfern J, Briffa T, Ellis E, Freedman SB. Patient-centered modular secondary prevention following acute coronary syndrome: a randomized controlled trial. Journal of Cardiopulmonary Rehabilitation and Prevention. 2008;28(2):107–117. doi: 10.1097/01.HCR.0000314204.86805.13. [DOI] [PubMed] [Google Scholar]
- 20.Redfern J, Briffa T, Ellis E, Freedman SB. Choice of secondary prevention improves risk factors after acute coronary syndrome: 1-year follow-up of the CHOICE (Choice of Health Options in prevention of Cardiovascular Events) randomised controlled trial. Heart. 2009;95(6):468–475. doi: 10.1136/hrt.2008.150870. [DOI] [PubMed] [Google Scholar]
- 21.Giannuzzi P, Temporelli PL, Marchioli R, et al. Global secondary prevention strategies to limit event recurrence after myocardial infarction: results of the GOSPEL study, a multicenter, randomized controlled trial from the Italian Cardiac Rehabilitation Network. Archives of Internal Medicine. 2008;168(20):2194–2204. doi: 10.1001/archinte.168.20.2194. [DOI] [PubMed] [Google Scholar]
- 22.Cupples ME, McKnight A. Randomised controlled trial of health promotion in general practice for patients at high cardiovascular risk. British Medical Journal. 1994;309(6960):993–996. doi: 10.1136/bmj.309.6960.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cupples ME, McKnight A. Five year follow up of patients at high cardiovascular risk who took part in randomised controlled trial of health promotion. British Medical Journal. 1999;319(7211):687–688. doi: 10.1136/bmj.319.7211.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munoz MA, Vila J, Cabañero M, et al. Efficacy of an intensive prevention program in coronary patients in primary care, a randomised clinical trial. International Journal of Cardiology. 2007;118(3):312–320. doi: 10.1016/j.ijcard.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Salminen M, Isoaho R, Vahlberg T, Ojanlatva A, Kivelä S-L. Effects of health advocacy, counselling and activation among older coronary heart disease (CHD) patients. Ageing Clinical and Experimental Research. 2005;17:472–478. doi: 10.1007/BF03327414. [DOI] [PubMed] [Google Scholar]
- 26.Lisspers J, Sundin O, Hofman-Bang C, et al. Behavioural effects of a comprehensive multifactorial programme for lifestyle change after percutaneous transluminal coronary angioplasty: a prospective, randomised, controlled trial. Journal of Psychosomatic Research. 1999;46(2):143–154. doi: 10.1016/s0022-3999(98)00074-9. [DOI] [PubMed] [Google Scholar]
- 27.Lisspers J, Sundin O, Hofman-Bang C, et al. Long-term effects of lifestyle behaviour change in coronary artery disease: effects on recurrent coronary events after percutaneous coronary intervention. Health Psychology. 2005;24(1):41–48. doi: 10.1037/0278-6133.24.1.41. [DOI] [PubMed] [Google Scholar]
- 28.The Vestfold Heartcare Study Group. Influence on lifestyle measures and five-year coronary risk by a comprehensive lifestyle intervention programme in patients with coronary heart disease. European Journal of Cardiovascular Prevention and Rehabilitation. 2003;10(6):429–437. doi: 10.1097/01.hjr.0000107024.38316.6a. [DOI] [PubMed] [Google Scholar]
- 29.Lewin RJP, Furze G, Robinson J, et al. A randomised controlled trial of a self-management plan for patients with newly diagnosed angina. British Journal of General Practice. 2002;52(476):194–201. [PMC free article] [PubMed] [Google Scholar]
- 30.Allen JK, Blumenthal RS, Margolis S, Young DR, Miller ER, Kelly K. Nurse case management of hypercholesterolemia in patients with coronary heart disease: results of a randomized clinical trial. American Heart Journal. 2002;144(4):678–686. doi: 10.1067/mhj.2002.124837. [DOI] [PubMed] [Google Scholar]
- 31.Southard BH, Southard DR, Nuckolls J. Clinical trial of an internet-based case management system for secondary prevention of heart disease. Journal of Cardiopulmonary Rehabilitation. 2003;23(5):341–348. doi: 10.1097/00008483-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 32.De Lorgeril M, Salen P, Martin JL, et al. Effect of a mediterranean type of diet on the rate of cardiovascular complications in patients with coronary artery disease. Insights into the cardioprotective effect of certain nutriments. Journal of the American College of Cardiology. 1996;28(5):1103–1108. doi: 10.1016/S0735-1097(96)00280-X. [DOI] [PubMed] [Google Scholar]
- 33.De Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99(6):779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 34.Campbell NC, Ritchie LD, Thain J, Deans HG, Rawles JM, Squair JL. Secondary prevention in coronary heart disease: a randomised trial of nurse led clinics in primary care. Heart. 1998;80(5):447–452. doi: 10.1136/hrt.80.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell NC, Thain J, Deans HG, Ritchie LD, Rawles JM, Squair JL. Secondary prevention clinics for coronary heart disease: randomised trial of effect on health. British Medical Journal. 1998;316(7142):1434–1437. doi: 10.1136/bmj.316.7142.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murchie P, Campbell NC, Ritchie LD, Simpson JA, Thain J. Secondary prevention clinics for coronary heart disease: four year follow up of a randomised controlled trial in primary care. British Medical Journal. 2003;326(7380):84–87. doi: 10.1136/bmj.326.7380.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murchie P, Campbell NC, Ritchie LD, Deans HG, Thain J. Effects of secondary prevention clinics on health status in patients with coronary heart disease: 4 year follow-up of a randomized trial in primary care. Family Practice. 2004;21(5):567–574. doi: 10.1093/fampra/cmh514. [DOI] [PubMed] [Google Scholar]
- 38.Delaney EK, Murchie P, Lee AJ, Ritchie LD, Campbell NC. Secondary prevention clinics for coronary heart disease: a 10-year follow-up of a randomised controlled trial in primary care. Heart. 2008;94(11):1419–1423. doi: 10.1136/hrt.2007.126144. [DOI] [PubMed] [Google Scholar]
- 39.Wallner S, Watzinger N, Lindschinger M, et al. Effects of intensified lifestyle modification on the need for further revascularization after coronary angioplasty. European Journal of Clinical Investigation. 1999;29(5):372–379. doi: 10.1046/j.1365-2362.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- 40.Jolly K, Bradley F, Sharp S, et al. Randomised controlled trial of follow up care in general practice of patients with myocardial infarction and angina. Final results of the Southampton heart integrated care project (SHIP) British Medical Journal. 1999;318(7185):706–711. doi: 10.1136/bmj.318.7185.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. Journal of the American Medical Association. 1998;280(23):2001–2007. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 42.Carlsson R. Serum cholesterol, lifestyle, working capacity and quality of life in patients with Coronary Artery Disease. Experiences from a hospital-based secondary prevention programme. Scandinavian Cardiovascular Journal, Supplement. 1998;32(3, supplement 50):17–20. doi: 10.1080/140174398427956-1. [DOI] [PubMed] [Google Scholar]
- 43.Carlsson R, Lindberg G, Westin L, Israelsson BO. Influence of coronary nursing management follow up on lifestyle after acute myocardial infarction. Heart. 1997;77(3):256–259. doi: 10.1136/hrt.77.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heller RF, Knapp JC, Valenti LA, Dobson AJ. Secondary prevention after acute myocardial infarction. American Journal of Cardiology. 1993;72(11):759–762. doi: 10.1016/0002-9149(93)91058-p. [DOI] [PubMed] [Google Scholar]
- 45.Hamalainen H, Luurila OJ, Kallio V, Knuts LR. Reduction in sudden deaths and coronary mortality in myocardial infarction patients after rehabilitation: 15-year follow-up study. European Heart Journal. 1995;16(12):1839–1844. doi: 10.1093/oxfordjournals.eurheartj.a060837. [DOI] [PubMed] [Google Scholar]
- 46.De Lorgeril M, Renaud S, Mamelle N, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. The Lancet. 1994;343(8911):1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 47.Kohl HW, Blair SN, Paffenbarger RS, Macera CA, Kronenfeld JJ. A mail survey of physical activity habits as related to measured physical fitness. American Journal of Epidemiology. 1988;127(6):1228–1239. doi: 10.1093/oxfordjournals.aje.a114915. [DOI] [PubMed] [Google Scholar]
- 48.Bennett K, Kabir Z, Unal B, et al. Explaining the recent decrease in coronary heart disease mortality rates in Ireland, 1985–2000. Journal of Epidemiology and Community Health. 2006;60(4):322–327. doi: 10.1136/jech.2005.038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmieri L, Bennett K, Giampaoll S, Capewell S. Explaining the decrease in coronary heart disease mortality in Italy between 1980 and 2000. American Journal of Public Health. 2010;100(4):684–692. doi: 10.2105/AJPH.2008.147173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. British Medical Journal. 2008;337, article a1344 doi: 10.1136/bmj.a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chrysohoou C, Panagiotakos DB, Aggelpoulos P, et al. The Mediterranean diet contributes to the preservation of left ventricular systolic function and to the long-term favourable prognosis of patients who have had an acute coronary event. American Journal of Clinical Nutrition. 2010;92(1):47–54. doi: 10.3945/ajcn.2009.28982. [DOI] [PubMed] [Google Scholar]
- 52.Panagiotakos DB, Pitsavos C, Chrysohoou C, Skoumas I, Stefanadis C. Five-year incidence of cardiovascular disease and its predictors in Greece: the ATTICA study. Vascular Medicine. 2008;13(2):113–121. doi: 10.1177/1358863x07087731. [DOI] [PubMed] [Google Scholar]