Abstract
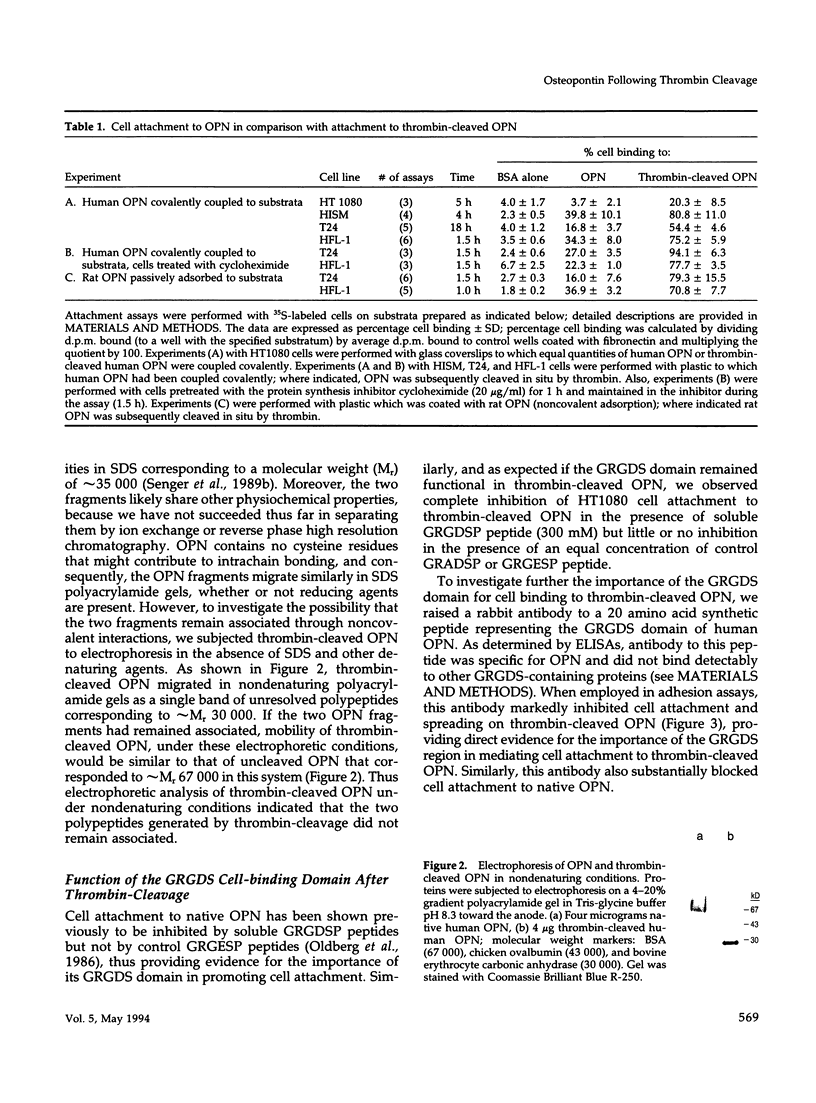
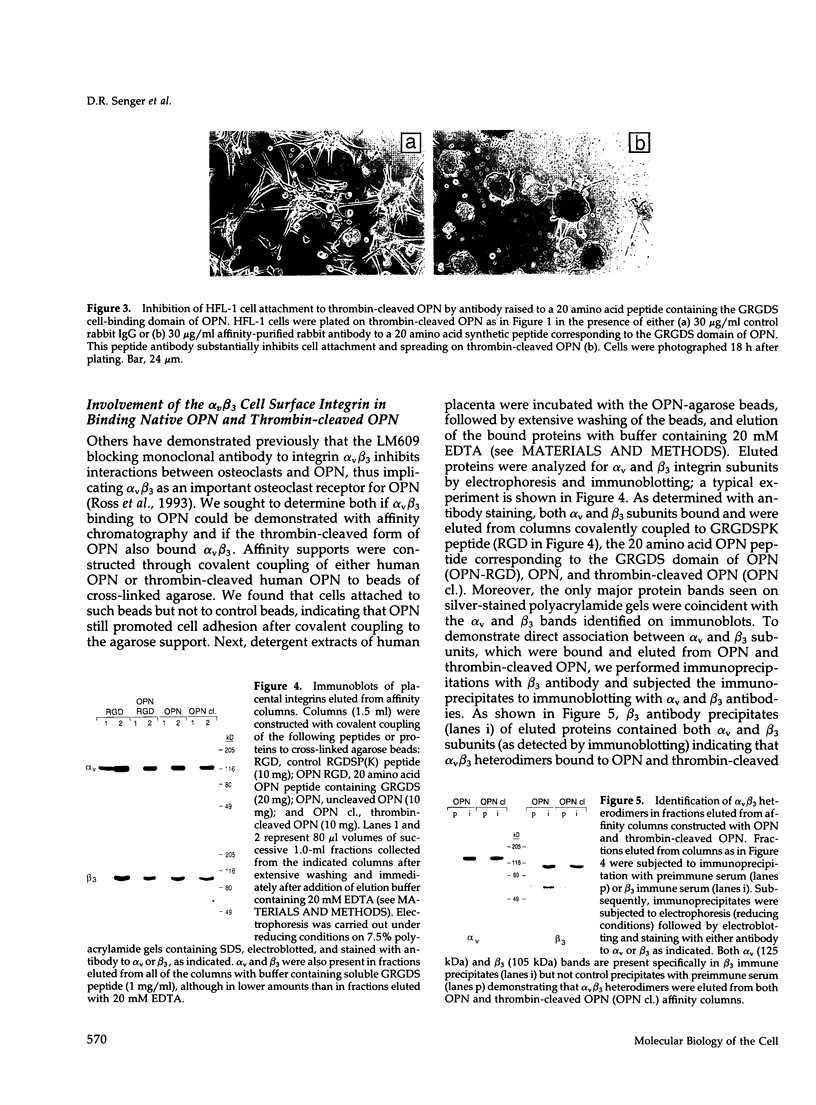
Osteopontin (OPN) is a secreted adhesive glycoprotein with a functional glycine-arginine-glycine-aspartate-serine (GRGDS) cell-binding domain. An interesting feature of OPN structure is the presence of a thrombin-cleavage site in close proximity to the GRGDS region. Cleavage of OPN by thrombin is likely to be of physiological importance, because cleavage of blood plasma OPN occurs naturally after activation of the blood coagulation pathway. To investigate functional consequences of OPN cleavage by thrombin, cell attachment and spreading assays were performed with uncleaved and cleaved forms of OPN. For all cell lines examined, thrombin-cleaved OPN promoted markedly greater cell attachment and spreading than uncleaved OPN. Cell attachment and spreading on thrombin-cleaved OPN was inhibited both by the soluble GRGDS peptides and an OPN-specific antibody raised to the GRGDS domain of OPN, thus implicating the GRGDS region in mediating the increased cell attachment and spreading observed on thrombin-cleaved OPN. Because the GRGDS sequence in OPN is only six residues from the thrombin-cleavage site, the data suggest that possibility that thrombin cleavage allows greater accessibility of the GRGDS domain to cell surface receptors. To investigate receptors that recognize uncleaved and thrombin-cleaved OPN, affinity chromatography was performed on placental extracts; the cell surface integrin alpha v beta 3 bound to columns constructed either with native or thrombin-cleaved OPN and was selectively eluted from each with soluble GRGDS peptide and EDTA. Moreover, adhesion assays performed in the presence of alpha v beta 3 blocking monoclonal antibody LM609 identified alpha v beta 3 as a major functional receptor for thrombin-cleaved OPN. Several lines of evidence suggest that cleavage of OPN by thrombin occurs in vivo, such as in tumors and at sites of tissue injury, and adhesion assay data presented here indicate that such cleavage is important in the regulation of OPN function.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown L. F., Berse B., Van de Water L., Papadopoulos-Sergiou A., Perruzzi C. A., Manseau E. J., Dvorak H. F., Senger D. R. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell. 1992 Oct;3(10):1169–1180. doi: 10.1091/mbc.3.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers A. F., Hota C., Prince C. W. Adhesion of metastatic, ras-transformed NIH 3T3 cells to osteopontin, fibronectin, and laminin. Cancer Res. 1993 Feb 1;53(3):701–706. [PubMed] [Google Scholar]
- Chang P. L., Prince C. W. 1 alpha,25-dihydroxyvitamin D3 stimulates synthesis and secretion of nonphosphorylated osteopontin (secreted phosphoprotein 1) in mouse JB6 epidermal cells. Cancer Res. 1991 Apr 15;51(8):2144–2150. [PubMed] [Google Scholar]
- Chen J., Singh K., Mukherjee B. B., Sodek J. Developmental expression of osteopontin (OPN) mRNA in rat tissues: evidence for a role for OPN in bone formation and resorption. Matrix. 1993 Mar;13(2):113–123. doi: 10.1016/s0934-8832(11)80070-3. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A. Structure, function and biological properties of integrin alpha v beta 3 on human melanoma cells. Cancer Metastasis Rev. 1991 May;10(1):3–10. doi: 10.1007/BF00046839. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Smith J. H., Denhardt D. T. Osteopontin, a transformation-associated cell adhesion phosphoprotein, is induced by 12-O-tetradecanoylphorbol 13-acetate in mouse epidermis. J Biol Chem. 1989 Jun 5;264(16):9682–9689. [PubMed] [Google Scholar]
- Denhardt D. T., Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993 Dec;7(15):1475–1482. [PubMed] [Google Scholar]
- Dvorak H. F., Nagy J. A., Berse B., Brown L. F., Yeo K. T., Yeo T. K., Dvorak A. M., van de Water L., Sioussat T. M., Senger D. R. Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann N Y Acad Sci. 1992 Dec 4;667:101–111. doi: 10.1111/j.1749-6632.1992.tb51603.x. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Gailit J., Ruoslahti E. Regulation of the fibronectin receptor affinity by divalent cations. J Biol Chem. 1988 Sep 15;263(26):12927–12932. [PubMed] [Google Scholar]
- Giachelli C. M., Bae N., Almeida M., Denhardt D. T., Alpers C. E., Schwartz S. M. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993 Oct;92(4):1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Kiefer M. C., Bauer D. M., Barr P. J. The cDNA and derived amino acid sequence for human osteopontin. Nucleic Acids Res. 1989 Apr 25;17(8):3306–3306. doi: 10.1093/nar/17.8.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T., Zhang Q., Wrana J. L., Ber R., Aubin J. E., Butler W. T., Sodek J. Multiple forms of SppI (secreted phosphoprotein, osteopontin) synthesized by normal and transformed rat bone cell populations: regulation by TGF-beta. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1453–1459. doi: 10.1016/0006-291x(89)90837-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mark M. P., Prince C. W., Gay S., Austin R. L., Butler W. T. 44-kDal bone phosphoprotein (osteopontin) antigenicity at ectopic sites in newborn rats: kidney and nervous tissues. Cell Tissue Res. 1988 Jan;251(1):23–30. doi: 10.1007/BF00215443. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y., Setoguchi M., Yoshida S., Higuchi Y., Akizuki S., Yamamoto S. The mouse osteopontin gene. Expression in monocytic lineages and complete nucleotide sequence. J Biol Chem. 1990 Aug 25;265(24):14432–14438. [PubMed] [Google Scholar]
- Nagai T., Yamakawa N., Aota S., Yamada S. S., Akiyama S. K., Olden K., Yamada K. M. Monoclonal antibody characterization of two distant sites required for function of the central cell-binding domain of fibronectin in cell adhesion, cell migration, and matrix assembly. J Cell Biol. 1991 Sep;114(6):1295–1305. doi: 10.1083/jcb.114.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T., Todescan R., Goldberg H. A., Zhang Q., Sodek J. Sulphation of secreted phosphoprotein I (SPPI, osteopontin) is associated with mineralized tissue formation. Biochem Biophys Res Commun. 1989 Nov 30;165(1):234–240. doi: 10.1016/0006-291x(89)91059-0. [DOI] [PubMed] [Google Scholar]
- Nemir M., DeVouge M. W., Mukherjee B. B. Normal rat kidney cells secrete both phosphorylated and nonphosphorylated forms of osteopontin showing different physiological properties. J Biol Chem. 1989 Oct 25;264(30):18202–18208. [PubMed] [Google Scholar]
- Nomura S., Wills A. J., Edwards D. R., Heath J. K., Hogan B. L. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol. 1988 Feb;106(2):441–450. doi: 10.1083/jcb.106.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando R. A., Cheresh D. A. Arginine-glycine-aspartic acid binding leading to molecular stabilization between integrin alpha v beta 3 and its ligand. J Biol Chem. 1991 Oct 15;266(29):19543–19550. [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Argraves S., Suzuki S., Ruoslahti E. Arginine-glycine-aspartic acid adhesion receptors. Methods Enzymol. 1987;144:475–489. doi: 10.1016/0076-6879(87)44196-7. [DOI] [PubMed] [Google Scholar]
- Ross F. P., Chappel J., Alvarez J. I., Sander D., Butler W. T., Farach-Carson M. C., Mintz K. A., Robey P. G., Teitelbaum S. L., Cheresh D. A. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem. 1993 May 5;268(13):9901–9907. [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Gracey C. F., Papadopoulos A., Tenen D. G. Secreted phosphoproteins associated with neoplastic transformation: close homology with plasma proteins cleaved during blood coagulation. Cancer Res. 1988 Oct 15;48(20):5770–5774. [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Papadopoulos A. Elevated expression of secreted phosphoprotein I (osteopontin, 2ar) as a consequence of neoplastic transformation. Anticancer Res. 1989 Sep-Oct;9(5):1291–1299. [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Papadopoulos A., Tenen D. G. Purification of a human milk protein closely similar to tumor-secreted phosphoproteins and osteopontin. Biochim Biophys Acta. 1989 Jun 13;996(1-2):43–48. doi: 10.1016/0167-4838(89)90092-7. [DOI] [PubMed] [Google Scholar]
- Singh K., DeVouge M. W., Mukherjee B. B. Physiological properties and differential glycosylation of phosphorylated and nonphosphorylated forms of osteopontin secreted by normal rat kidney cells. J Biol Chem. 1990 Oct 25;265(30):18696–18701. [PubMed] [Google Scholar]
- Singh R. P., Patarca R., Schwartz J., Singh P., Cantor H. Definition of a specific interaction between the early T lymphocyte activation 1 (Eta-1) protein and murine macrophages in vitro and its effect upon macrophages in vivo. J Exp Med. 1990 Jun 1;171(6):1931–1942. doi: 10.1084/jem.171.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioussat T. M., Dvorak H. F., Brock T. A., Senger D. R. Inhibition of vascular permeability factor (vascular endothelial growth factor) with antipeptide antibodies. Arch Biochem Biophys. 1993 Feb 15;301(1):15–20. doi: 10.1006/abbi.1993.1109. [DOI] [PubMed] [Google Scholar]
- Somerman M. J., Prince C. W., Butler W. T., Foster R. A., Moehring J. M., Sauk J. J. Cell attachment activity of the 44 kilodalton bone phosphoprotein is not restricted to bone cells. Matrix. 1989 Jan;9(1):49–54. doi: 10.1016/s0934-8832(89)80018-6. [DOI] [PubMed] [Google Scholar]
- Swanson G. J., Nomura S., Hogan B. L. Distribution of expression of 2AR (osteopontin) in the embryonic mouse inner ear revealed by in situ hybridisation. Hear Res. 1989 Sep;41(2-3):169–177. doi: 10.1016/0378-5955(89)90008-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana J. L., Zhang Q., Sodek J. Full length cDNA sequence of porcine secreted phosphoprotein-I (SPP-I, osteopontin). Nucleic Acids Res. 1989 Dec 11;17(23):10119–10119. doi: 10.1093/nar/17.23.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Adhesive recognition sequences. J Biol Chem. 1991 Jul 15;266(20):12809–12812. [PubMed] [Google Scholar]
- Yoon K., Buenaga R., Rodan G. A. Tissue specificity and developmental expression of rat osteopontin. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1129–1136. doi: 10.1016/s0006-291x(87)80250-4. [DOI] [PubMed] [Google Scholar]
- Young M. F., Kerr J. M., Termine J. D., Wewer U. M., Wang M. G., McBride O. W., Fisher L. W. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN). Genomics. 1990 Aug;7(4):491–502. doi: 10.1016/0888-7543(90)90191-v. [DOI] [PubMed] [Google Scholar]
- van Dijk S., D'Errico J. A., Somerman M. J., Farach-Carson M. C., Butler W. T. Evidence that a non-RGD domain in rat osteopontin is involved in cell attachment. J Bone Miner Res. 1993 Dec;8(12):1499–1506. doi: 10.1002/jbmr.5650081213. [DOI] [PubMed] [Google Scholar]









