Abstract
Perinatal hypoxic-ischemic encephalopathy (HIE) is an important cause of brain injury in the newborn and can result in long-term devastating consequences. Perinatal hypoxia is a vital cause of long-term neurologic complications varying from mild behavioural deficits to severe seizure, mental retardation, and/or cerebral palsy in the newborn. In the mammalian developing brain, ongoing research into pathophysiological mechanism of neuronal injury and therapeutic strategy after perinatal hypoxia is still limited. With the advent of promising therapy of hypothermia in HIE, this paper reviews the pathophysiology of HIE and the future potential neuroprotective strategies for clinical potential for hypoxia sufferers.
1. Introduction
Perinatal hypoxic-ischemic encephalopathy (HIE) occurs in one to three per 1000 live full-term births [1]. Of affected newborns, 15%–20% of affected newborns will die in the postnatal period, and an additional 25% will develop severe and permanent neuropsychological sequelae, including mental retardation, visual motor or visual perceptive dysfunction, increased hyperactivity, cerebral palsy, and epilepsy [2]. The outcomes of HIE are devastating and permanent, making it a major burden for the patient, the family, and society. It is critical to identify and develop therapeutic strategies to reduce brain injury in newborns with HIE. The underlying pathophysiology of perinatal HIE is difficult to study in the human, thus the neonatal rat model for HI brain injury has been developed to model this human condition. Much of what we know is derived from studies conducted in animal models. Rodents were the most frequently used animals in HIE research, followed by piglets and sheep [3].
2. Gestational and Chronological Age
The neuropathological features of perinatal HIE vary considerably with the gestational age of the infant, the nature of the insult, and the intervention types. Studies of the effect of hypoxemia on brain energy metabolism in the immature rat brain have delineated a particular window of vulnerability, characterized by greater vulnerability in the second postnatal week, comparable to the human brain at term than in the first postnatal week, comparable to the human premature brain. In addition, based on the evidence of more resistance of cerebral energy metabolisms and longer duration of survival in the immature than in the adult brains submitted to asphyxia insults, there is a long-held general notion that the perinatal brain is more tolerant to asphyxia than the adult brain [4, 5]. However, neuropathological studies indicate that many critical neuronal groups are more vulnerable to HI injury in the immature animals, particularly related to enhanced density and function of excitatory amino acid receptors and enhanced vulnerability to attack by reactive oxygen species (ROS) and reactive nitrogen species (RNS) [5].
2.1. Major Neuropathology
The development of brain injury after HI insult is an evolving process imitated during the acute insult and extending into a reperfusion phase. The principle pathogenetic mechanism underlying neurological damage in HIE resulting from hypoxemia/ischemia or both is deprivation of glucose and oxygen supply which causes a primary energy failure and initiates a cascade of biochemical events leading to cell dysfunction and ultimately to cell death [6, 7]. A consequent reperfusion injury often deteriorates the brain metabolism by increasing the oxidative stress damage. Particular roles for increase in extracellular glutamate, excessive activation of glutamate receptors (excitotoxicity), increase in cytosolic calcium (Ca2+), and generation of free radicals are emphasized.
The temporal aspects of the changes in glucose and energy metabolism after HI insult have been identified and include primary energy failure and secondary energy failure [8–13]. Immediately after HI insults, primary energy failure, depletion of oxygen precludes oxidative phosphorylation (decrease in high-energy phosphorylated compounds such as ATP and phosphocreatine) and results in a switch to anaerobic metabolism, causing accumulation of lactate and the associated H+. The accumulation of lactate and the associated H+ initially is beneficial for adoption to oxygen deprivation, but later, with progression of lactate formation, it has deleterious effects on (1) impairment of vascular autoregulation, a potential for advanced ischemic injury, (2) inhibition of phosphofructokinase activity by low pH, and (3) a biochemical cascade leading to cellular injury [8]. The occurrence of secondary energy failure varies according to species and nature of the insult with onset at appropriately 8~16 hours and a nadir at 24~48 hours. High-energy phosphate levels recovered to baseline levels in 2~3 hours after reperfusion and reoxygenation, and a second decline in high-energy phosphate was pronounced at the next 48 hours [9, 11, 12].
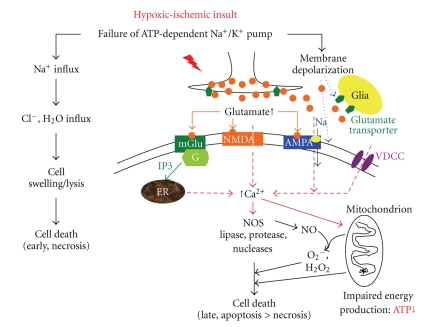
An initial decrease in high-energy phosphate triggers a series of additional mechanisms, beginning with a failure of the ATP-dependent Na+-K+ pump. Transcellular ion pump failure results in the intracellular accumulation of Na+, Ca2+, and water (cytotoxic edema) followed by membrane depolarization, excessive release of excitatory neurotransmitters, specifically glutamate, increase of cytosolic Ca2+, activation of phospholipase, and generation of free radicals. During the past 2 decades, remarkable studies have demonstrated the critical role for glutamate as the mediators of neuronal death in the HI insult [14–16]. Glutamate is the predominant excitatory amino acid neurotransmitter in the brain and has 3 major types of ionotropic receptors, NMDA, AMPA, and KA, as well as a group of G-protein-linked metabotropic glutamate receptor, existing in most neurons and glia possess. Normally, glutamate ionotropic receptors work cooperatively in stabilization of synapses and display a sequential participation in activity-dependent neuronal plasticity and neuronal excitation for normal tasks such as learning and memory. However, their vital role and enhanced function in the perinatal period also make neurons more vulnerable to excitotoxicity. Extracellular glutamate concentrations increase manifold with HI insults [17–19], and specific glutamate receptor channel blockers ameliorate brain injury in HIE [20]. The ontogeny of glutamate, transient dense expression of NMDA receptors and GluR2-deficient AMPA receptors, is relevant to the vulnerability of immature brain regions to excitotoxic cell death in HIE [21, 22]. The presence of a GluR2 subunit renders the AMPA receptors impermeable to calcium. The approximate time of peak sensitivity of excitotoxicity in rats is 6 days for NMDA and 9~10 days for AMPA, appropriate to human prematurity and term newborn, respectively [23, 24]. Moreover, the topography of glutamate synapses, early expression of glutamate receptors in human hippocampus, cerebral cortex, and deep nuclear structures, is similar to regions vulnerable to HI injury in the newborn [18, 19, 25]. The increase in extracellular glutamate concentration and activation of glutamate receptors after hypoxia-ischemia triggers excitotoxic cascade. There is an increase in cytosolic Ca2+ by influx through open NMDA and calcium permeable AMPA receptor channels and other voltage-dependent Ca2+ channels, and the release of calcium from intracellular stores. The deleterious effects of increased cytosolic calcium include the activation of neuronal nitric oxide synthase (nNOS) to form nitric oxide, generation of free radicals, and degradation of cellular lipids by activation of phospholipases, of cellular proteins by activation of protease, and of cellular DNA by activation of nucleases, as well as accentuation of mitochondrial injuries [26–28]. Mitochondrial outer membrane permeabilization, in turn, elicits mitochondrial release of cytochrome C, activation cleavage of caspases 9 and 3, and apoptosis-inducing factor (AIF), leading to apoptosis [29]. The combined effects of cellular energy failure, acidosis, glutamate release, intracellular Ca2+ accumulation, lipid peroxidation, and nitric oxide neurotoxicity serve to disrupt essential components of the cell with its ultimate death. The mechanism of neuronal cell death after hypoxia-ischemia includes neuronal necrosis and apoptosis, depending principally on the severity of the insult and the maturational state of the cell. There is a continuum of necrosis and apoptosis, and often the early cell death appears necrotic and later cell death appears apoptotic [30, 31]. Initial decrease in high-energy phosphates will result in impairment of ATP-dependent Na+-K+ pump, which in the severe insult causes an acute influx of Na+, Cl−, and water with consequent cell swelling, cell lysis, and thus early cell death by necrosis whereas in less severe insult causes membrane depolarization followed by a cascade of excitotoxicity and oxidative stress leading to a delayed cell death, principally apoptosis. Apoptosis is more prevalent as a mode of death in the perinatal brain, and both caspase-dependent and caspase-independent mechanisms of apoptotic cell death have been recognized (Figure 1).
Figure 1.
Proposed pathogenesis of hypoxic-ischemic encephalopathy. The central roles for ATP depletion, membrane depolarization, glutamate-mediated excitotoxicty, and voltage-dependent and glutamate-activated Ca2+ channels are apparent. An initial decrease in high-energy phosphates can result in an acute influx of Na+, Cl−, and water with consequent cell death (necrosis) in the severe insult, whereas in less severe insult, it causes membrane depolarization followed by a cascade of excitotoxicity and oxidative stress leading to a delayed cell death, principally apoptosis. Persistent membrane depolarization results excessive presynaptic glutamate release, reversal of glutamate transport in glia and neural terminals, and activation of NMDA and immature (GluR2 deficiency) AMPA receptors with profound Ca2+ influx with a series of Ca2+-mediated cascades to cell death. The deleterious effects of cytosolic Ca2+ are multiple, including degradation of cellular lipids by activation of phospholipase and of cellular DNA by activation of nucleases and enhancement of generation of free radicals and nitric oxide (NO) by increase of nitric oxide synthase (NOS) [7, 8, 26]. AMPA: α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate; ER: endoplasmic reticulum; mGlu: metabotropic glutamate; NMDA: N-methyl-D-aspartic acid; NOS: nitric oxide synthase; VDCC: voltage-dependent calcium channels
Experimental studies indicate that the first observable change in the neuron is cytoplasmic vacuolation, caused by mitochondrial swelling, occurring within 5~30 minutes after the onset of hypoxia, and the differentiating oligodendrocytes exhibit approximately the same sensitivity to glucose and oxygen deprivation as do neurons. In the immature and mature brain, the order of cellular elements vulnerable to hypoxia-ischemia is neuron > oligodendroglia > astrocyte > microglia [32]. Yue et al. demonstrated that apoptotic neuronal death predominated among immature neurons, whereas necrotic cell death predominated among mature neurons [33]. The feature of neuropathology varies according to the developing age as hypoxia-ischemia insult. Three major regional patterns of selective neuronal necrosis in newborns with HIE, especially the term newborns, diffuse disease, cerebral-deep nuclear with prominent involvement of cerebral neocortex, hippocampus, and basal ganglia-thalamus, and deep nuclear-brain stem disease [34, 35]. The principal form of hypoxia-ischemic brain injury in the immature brain involves cerebral white matter, causing periventricular leukomalacia (PVL), and the data indicate an implication of a particular maturation-dependent intrinsic vulnerability of premyelinating oligodendrocytes (pre-OLs) to both endogenous and exogenous reactive oxygen species [35–37]. The immature brain has a propensity for ischemia to cerebral white matter because of the presence of (1) vascular end zones and border zones in that region and (2) impairment of cerebrovascular autoregulation [7]. Ness et al. noted a transition of cell death in white matter from early necrotic deaths to hybrid cell deaths to classical apoptosis between 4 and 24 h of recovery from hypoxia-ischemia [38]. The delayed time course of apoptosis in pre-OLs supports the feasibility of interventions to improve clinical outcomes for newborns surviving birth asphyxia.
3. Potential Therapies for Neonatal Hypoxic-Ischemic Encephalopathy
3.1. Supportive Intensive Care
Perinatal HIE is a major cause of death and disability worldwide which has been limited to supportive intensive care. It includes correction of hemodynamic and pulmonary disturbances (hypotension, metabolic acidosis, and maintenance of adequate ventilation), correction of metabolic disturbances of glucose, calcium, magnesium, and electrolytes, treatment of seizures if present, and monitoring for other organ system dysfunctions, such as acute renal failure. Maintenance of adequate ventilation and adequate perfusion is a central aspect of supportive care. Oxygen deprivation may lead to disturbance of cerebrovascular autoregulation with consequence of a pressure-passive cerebral circulation and increase additional neuronal and white matter injury. Severe hyperoxia in the first hours of life may contribute to increased oxidative stress, deleterious to long-term neurological consequences [39, 40], and the guideline of the 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations (CoSTR) in the International Liaison Committee on Resuscitation (ILCOR) recommends that, for term babies, it is better to begin resuscitation with room air rather than 100% oxygen [41]. In addition, the maintenance of adequate perfusion to brain, more related to adequate arterial blood pressure than intracranial pressure, is important for prevention of additional ischemic injury.
3.2. Control of Seizures
The presence of seizure, practically occurring within the first hours, predicates a poor outcome of HIE. Therefore, antiepileptic drugs (AEDs) are among the medications most commonly used in perinatal HIE. The energy metabolism can be compromised by the hyperactive neurons, and both acute energy deprivation after HI insult and seizures are implicated in excitotoxicity. Thus, the therapeutic value of AEDs may include not only control of seizure activity but also potentially the benefit for the compromised cellular energy metabolism. Studies about perinatal HIE showed a beneficial effect of pretreatment with barbiturates [42, 43]. Phenobarbital remains the preferred drug for the treatment of seizures in neonates with HIE [7]. It is still a controversial issue whether phenobarbital treatment should be administered before the seizure attacks. Close observation with use of continuous EEG to identify seizures is optimal for management of the asphyxiated infants.
3.3. Neuroprotective Strategies
At present, no individual neuroprotective agents have been proven safe and effective for the protection of neonates from neurological sequels after HI insults. The insight into the biochemical and cellular mechanisms of neuronal injury with perinatal HIE helps to provide interventions to interrupt those deleterious cascades, principally focusing on the potential effects of free radical scavengers, such as N-acetylcysteine (NAC) and allopurinol, magnesium, glutamate receptor blockers, erythropoietin (Epo), and hypothermia. NAC is a free radical scavenger and has been demonstrated to minimize hypoxia-ischemia-induced brain injury in various acute models [44–46]. Combination therapy of NAC and systemic hypothermia improves infarct volume, myelin expression after focal HI injury [44]. Treatment of allopurinol, a xanthine oxidase inhibitor and free radical scavenger, also exerts benefit on reduction of cerebral edema and neuropathological damage after neonatal HIE [47]. Epo, the major haemopoietic growth factor, is now considered to have beneficial effects in various nervous system disorders based on the effects of prevention of metabolic compromise, neuronal and vascular degeneration, and inflammatory cell activation [48–50]. Gonzalez et al. demonstrated that the treatment of Epo preserves hemispheric brain volume after an occlusive cerebral injury in P10 rats [48]. Other experimental studies concerning the potential value of magnesium sulfate reveal that blockers of glutamate receptors have conflicting results. However, the most potent and promising intervention to prevent energy depletion is hypothermia. Experimentally, reducing body temperature to 3~5°C below the normal level reduces cerebral injuries, including a decrease in brain energy utilization, reduction of infarct size, and amelioration of neuronal cell loss and hippocampal structure, and improves neurological outcomes after asphyxia [51–54]. The beneficial effects of mild hypothermia occur at multiple sites in the cascade to cell death. Hypothermia must be commenced before the onset of delayed energy failure and particularly excitatory features, such as seizures [7]. Early induction of hypothermia in human infants who had perinatal HIE improves survival and reduces the rate of disability of those survivors [41, 55–58]. The guideline of ILCOR CoSTR 2010 recommends therapeutic hypothermia as a standard practice for term to near term infants with moderate to severe HIE [41].
4. Conclusion
Hypoxia-ischemia in the perinatal period is a major cause of neonatal death and long-term disability. There are advances in research of cellular processes and molecular mechanisms underlying HIE over the last 2 decades. Hypothermia is the only treatment effective in neonatal HIE at present. Combined therapy of hypothermia and other neuroprotective strategies, focusing on prevention of acute injuries, increase of therapeutic time window, and enhancement of neural repair, is expected to improve the neurological outcomes of HIE.
Conflict of Interests
The authors declare that there are no conflict of interests.
References
- 1.Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. American Journal of Obstetrics and Gynecology. 2008;199(6):587–595. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 2.Vannucci RC, Perlman JM. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics. 1997;100(6):1004–1014. doi: 10.1542/peds.100.6.1004. [DOI] [PubMed] [Google Scholar]
- 3.Roohey T, Raju TNK, Moustogiannis AN. Animal models for the study of perinatal hypoxic-ischemic encephalopathy: a critical analysis. Early Human Development. 1997;47(2):115–146. doi: 10.1016/s0378-3782(96)01773-2. [DOI] [PubMed] [Google Scholar]
- 4.Painter MJ. Animal models of perinatal asphyxia: contributions, contradictions, clinical relevance. Seminars in Pediatric Neurology. 1995;2(1):37–56. doi: 10.1016/s1071-9091(05)80004-x. [DOI] [PubMed] [Google Scholar]
- 5.Yager JY, Thornhill JA. The effect of age on susceptibility to hypoxic-ischemic brain damage. Neuroscience and Biobehavioral Reviews. 1997;21(2):167–174. doi: 10.1016/s0149-7634(96)00006-1. [DOI] [PubMed] [Google Scholar]
- 6.Perlman JM. Summary proceedings from the neurology group on hypoxic-ischemic encephalopathy. Pediatrics. 2006;117(3):S28–S33. doi: 10.1542/peds.2005-0620E. [DOI] [PubMed] [Google Scholar]
- 7.Volpe JJ. Neurology of the Newborn. 5th edition. 2008. [PubMed] [Google Scholar]
- 8.Johnston MV, Trescher WH, Ishida A, Nakajima W, Zipursky A. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatric Research. 2001;49(6):735–741. doi: 10.1203/00006450-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Lorek A, Takei Y, Cady EB, et al. Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatric Research. 1994;36(6):699–706. doi: 10.1203/00006450-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Kusaka T, Matsuura S, Fujikawa Y, et al. Relationship between cerebral interstitial levels of amino acids and phosphorylation potential during secondary energy failure in hypoxic-ischemic newborn piglets. Pediatric Research. 2004;55(2):273–279. doi: 10.1203/01.PDR.0000102702.39608.82. [DOI] [PubMed] [Google Scholar]
- 11.Penrice J, Lorek A, Cady EB, et al. Proton magnetic resonance spectroscopy of the brain during acute hypoxia-ischemia and delayed cerebral energy failure in the newborn piglet. Pediatric Research. 1997;41(6):795–802. doi: 10.1203/00006450-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Vannucci RC, Towfighi J, Vannucci SJ. Secondary energy failure after cerebral hypoxia-ischemia in the immature rat. Journal of Cerebral Blood Flow and Metabolism. 2004;24(10):1090–1097. doi: 10.1097/01.WCB.0000133250.03953.63. [DOI] [PubMed] [Google Scholar]
- 13.Wyatt JS, Edwards AD, Azzopardi D, Reynolds EOR. Magnetic resonance and near infrared spectroscopy for investigation of perinatal hypoxic-ischaemic brain injury. Archives of Disease in Childhood. 1989;64(7):953–963. doi: 10.1136/adc.64.7_spec_no.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barks JDE, Silverstein FS. Excitatory amino acids contribute to the pathogenesis of perinatal hypoxic-ischemic brain injury. Brain Pathology. 1992;2(3):235–243. doi: 10.1111/j.1750-3639.1992.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 15.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annual Review of Neuroscience. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 16.McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatric Neurology. 2004;30(4):227–235. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Hagberg H, Thornberg E, Blennow M, et al. Excitatory amino acids in the cerebrospinal fluid of asphyxiated infants: relationship to hypoxic-ischemic encephalopathy. Acta Paediatrica. 1993;82(11):925–929. doi: 10.1111/j.1651-2227.1993.tb12601.x. [DOI] [PubMed] [Google Scholar]
- 18.Hattori H, Wasterlain CG. Excitatory amino acids in the developing brain: ontogeny, plasticity, and excitotoxicity. Pediatric Neurology. 1990;6(4):219–228. doi: 10.1016/0887-8994(90)90111-d. [DOI] [PubMed] [Google Scholar]
- 19.Jantzie LL, Cheung PY, Johnson ST, Bigam DL, Todd KG. Cerebral amino acid profiles after hypoxia-reoxygenation and N-acetylcysteine treatment in the newborn piglet. Neonatology. 2010;97(3):195–203. doi: 10.1159/000252972. [DOI] [PubMed] [Google Scholar]
- 20.Hirose K, Chan PH. Blockade of glutamate excitotoxicity and its clinical applications. Neurochemical Research. 1993;18(4):479–483. doi: 10.1007/BF00967252. [DOI] [PubMed] [Google Scholar]
- 21.Parnavelas JG, Cavanagh ME. Transient expression of neurotransmitters in the developing neocortex. Trends in Neurosciences. 1988;11(3):92–93. doi: 10.1016/0166-2236(88)90150-6. [DOI] [PubMed] [Google Scholar]
- 22.Represa A, Tremblay E, Ben-Ari Y. Transient increase of NMDA-binding sites in human hippocampus during development. Neuroscience Letters. 1989;99(1-2):61–66. doi: 10.1016/0304-3940(89)90265-6. [DOI] [PubMed] [Google Scholar]
- 23.Ikonomidou C, Mosinger JL, Salles KS, Labruyere J, Olney JW. Sensitivity of the developing rat brain to hypobaric/ischemic damage parallels sensitivity to N-methyl-aspartate neurotoxicity. Journal of Neuroscience. 1989;9(8):2809–2818. doi: 10.1523/JNEUROSCI.09-08-02809.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald JW, Trescher WH, Johnston MV. Susceptibility of brain to AMPA induced excitotoxicity transiently peaks during early postnatal development. Brain Research. 1992;583(1-2):54–70. doi: 10.1016/s0006-8993(10)80009-5. [DOI] [PubMed] [Google Scholar]
- 25.Sakurai SY, Cha JHJ, Penney JB, Young AB. Regional distributiion and properties of [H]MK-801 binding sites determined by quantitative autoradiography in rat brain. Neuroscience. 1991;40(2):533–543. doi: 10.1016/0306-4522(91)90139-f. [DOI] [PubMed] [Google Scholar]
- 26.Ankarcrona M, Dypbukt JM, Bonfoco E, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15(4):961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 27.Ferriero DM, Holtzman DM, Black SM, Sheldon RA. Neonatal mice lacking neuronal nitric oxide synthase are less vulnerable to hypoxic-ischemic injury. Neurobiology of Disease. 1996;3(1):64–71. doi: 10.1006/nbdi.1996.0006. [DOI] [PubMed] [Google Scholar]
- 28.Gilland E, Puka-Sundvall M, Hillered L, Hagberg H. Mitochondrial function and energy metabolism after hypoxia-ischemia in the immature rat brain: involvement of NMDA-receptors. Journal of Cerebral Blood Flow and Metabolism. 1998;18(3):297–304. doi: 10.1097/00004647-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Hagberg H, Mallard C, Rousset Catherine CI, Wang X. Apoptotic mechanisms in the immature Brain: involvement of mitochondria. Journal of Child Neurology. 2009;24(9):1141–1146. doi: 10.1177/0883073809338212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Northington FJ, Ferriero DM, Graham EM, Traystman RJ, Martin LJ. Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiology of Disease. 2001;8(2):207–219. doi: 10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]
- 31.Northington FJ, Zelaya ME, O’Riordan DP, et al. Failure to complete apoptosis following neonatal hypoxia-ischemia manifests as “continuum” phenotype of cell death and occurs with multiple manifestations of mitochondrial dysfunction in rodent forebrain. Neuroscience. 2007;149(4):822–833. doi: 10.1016/j.neuroscience.2007.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salford LG, Plum F, Brierley JB. Graded hypoxia oligemia in rat brain. II. Neuropathological alterations and their implications. Archives of Neurology. 1973;29(4):234–238. doi: 10.1001/archneur.1973.00490280046006. [DOI] [PubMed] [Google Scholar]
- 33.Yue X, Mehmet H, Penrice J, et al. Apoptosis and necrosis in the newborn piglet brain following transient cerebral hypoxia-ischaemia. Neuropathology and Applied Neurobiology. 1997;23(1):16–25. [PubMed] [Google Scholar]
- 34.Ferriero DM. Medical progress: neonatal brain injury. The New England Journal of Medicine. 2004;351(19):1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 35.Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends in Neurosciences. 2009;32(9):496–505. doi: 10.1016/j.tins.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. Journal of Neuroscience. 1998;18(16):6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Back SA, Luo NL, Mallinson RA, et al. Selective vulnerability of preterm white matter to oxidative damage defined by F-isoprostanes. Annals of Neurology. 2005;58(1):108–120. doi: 10.1002/ana.20530. [DOI] [PubMed] [Google Scholar]
- 38.Ness JK, Romanko MJ, Rothstein RP, Wood TL, Levison SW. Perinatal hypoxia-ischemia induces apoptotic and excitotoxic death of periventricular white matter oligodendrocyte progenitors. Developmental Neuroscience. 2001;23(3):203–208. doi: 10.1159/000046144. [DOI] [PubMed] [Google Scholar]
- 39.Grafe MR, Woodworth KN, Noppens K, Perez-Polo JR. Long-term histological outcome after post-hypoxic treatment with 100% or 40% oxygen in a model of perinatal hypoxic-ischemic brain injury. International Journal of Developmental Neuroscience. 2008;26(1):119–124. doi: 10.1016/j.ijdevneu.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klinger G, Beyene J, Shah P, Perlman M. Do hyperoxaemia and hypocapnia add to the risk of brain injury after intrapartum asphyxia? Archives of Disease in Childhood. 2005;90(1):F49–F52. doi: 10.1136/adc.2003.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perlman JM, Wyllie J, Kattwinkel J, et al. Part 11: neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation. 2010;122(16, supplement 2):S516–S538. doi: 10.1161/CIRCULATIONAHA.110.971127. [DOI] [PubMed] [Google Scholar]
- 42.Evans DJ, Levene MI, Tsakmakis M. Anticonvulsants for preventing mortality and morbidity in full term newborns with perinatal asphyxia. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD001240.pub2. Article ID CD001240. [DOI] [PubMed] [Google Scholar]
- 43.Hall RT, Hall FK, Daily DK. High dose phenobarbital therapy in term newborn with severe perinatal asphyxia: a randomized, prospective study with three-year follow-up. Journal of Pediatrics. 1998;132(2):345–348. doi: 10.1016/s0022-3476(98)70458-5. [DOI] [PubMed] [Google Scholar]
- 44.Jatana M, Singh I, Singh AK, Jenkins D. Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatric Research. 2006;59(5):684–689. doi: 10.1203/01.pdr.0000215045.91122.44. [DOI] [PubMed] [Google Scholar]
- 45.Liu J-Q, Lee T-F, Chen C, Bagim DL, Cheung P-Y. N-acetylcysteine improves hemodynamics and reduces oxidative stress in the brains of newborn piglets with hypoxia-reoxygenation injury. Journal of Neurotrauma. 2010;27(10):1865–1873. doi: 10.1089/neu.2010.1325. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Svedin P, Nie C, et al. N-Acetylcysteine reduces lipopolysaccharide-sensitized hypoxic-ischemic brain injury. Annals of Neurology. 2007;61(3):263–271. doi: 10.1002/ana.21066. [DOI] [PubMed] [Google Scholar]
- 47.Palmer C, Vannucci RC, Towfighi J. Reduction of perinatal hypoxic-ischemic brain damage with allopurinol. Pediatric Research. 1990;27(4):332–336. doi: 10.1203/00006450-199004000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez FF, McQuillen P, Mu D, et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Developmental Neuroscience. 2007;29(4-5):321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- 49.Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: elucidating new cellular targets that broaden therapeutic strategies. Progress in Neurobiology. 2008;85(2):194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. Journal of the American Medical Association. 2005;293(1):90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amess PN, Penrice J, Cady EB, et al. Mild hypothermia after severe transient hypoxia-lschemia reduces the delayed rise in cerebral lactate in the newborn piglet. Pediatric Research. 1997;41(6):803–808. doi: 10.1203/00006450-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Bona E, Hagberg H, Løberg EM, Bågenholm R, Thoresen M. Protective effects of moderate hypothermia after neonatal hypoxia- ischemia: short- and long-term outcome. Pediatric Research. 1998;43(6):738–745. doi: 10.1203/00006450-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Carroll M, Beek O. Protection against hippocampal CA cell loss by post-ischemic hypothermia is dependent on delay of initiation and duration. Metabolic Brain Disease. 1992;7(1):45–50. doi: 10.1007/BF01000440. [DOI] [PubMed] [Google Scholar]
- 54.Sirimanne ES, Blumberg RM, Bossano D, et al. The effect of prolonged modification of cerebral temperature on outcome after hypoxic-ischemic brain injury in the infant rat. Pediatric Research. 1996;39(4):591–597. doi: 10.1203/00006450-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. The New England Journal of Medicine. 2009;361(14):1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 56.Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. British Medical Journal. 2010;340, article c363 doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. The Lancet. 2005;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 58.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. The New England Journal of Medicine. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]