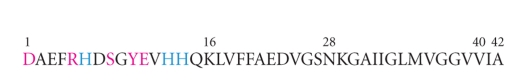Abstract
Zinc, the most abundant trace metal in the brain, has numerous functions, both in health and in disease. Zinc is released into the synaptic cleft of glutamatergic neurons alongside glutamate from where it interacts and modulates NMDA and AMPA receptors. In addition, zinc has multifactorial functions in Alzheimer's disease (AD). Zinc is critical in the enzymatic nonamyloidogenic processing of the amyloid precursor protein (APP) and in the enzymatic degradation of the amyloid-β (Aβ) peptide. Zinc binds to Aβ promoting its aggregation into neurotoxic species, and disruption of zinc homeostasis in the brain results in synaptic and memory deficits. Thus, zinc dyshomeostasis may have a critical role to play in the pathogenesis of AD, and the chelation of zinc is a potential therapeutic approach.
1. Introduction
Alzheimer's disease (AD) is the most prevalent form of dementia, which affects more than 37 million people worldwide, with an estimated cost of $422 billion in 2009 [1, 2]. Moreover, the incidence of the illness and the prospect of an aging population will result in rising social and economic demands. AD is characterised by the presence of extracellular amyloid plaques and intracellular neurofibrillary tangles within the afflicted brain, which cause neuronal loss in the neocortex, hippocampus, and basal forebrain, leading to progressive cognitive and behavioural decline [3]. Zinc, in addition to copper and iron, has been shown to be involved in AD. Here, we review the current literature relating to neuronal zinc metabolism and the way in which zinc can modulate normal brain activity. We discuss also the contribution of zinc to the formation, aggregation, and degradation of the amyloid-β (Aβ) peptide and the contribution of zinc to the pathogenesis of AD.
2. Physiological Role of Zinc in the Brain
As the most abundant trace metal in the brain, zinc is found tightly associated with numerous proteins conferring either structural or catalytic properties upon them [4]. However, a significant amount of loosely bound, chelatable zinc can be found sequestered in presynaptic vesicles forming a subpopulation of “zinc enriched” (ZEN) neurones [5, 6], which co-release zinc with the neurotransmitter glutamate upon excitation. The majority of these “gluzincergic” neurones [7] have their cell bodies located in either the cerebral cortex or the limbic structures of the forebrain [8], and so an extensive network uniting limbic and cerebrocortical functions is created [9]. This connection between zinc and glutamatergic neurotransmission allows the ion to modulate the overall excitability of the brain and also influence synaptic plasticity [10].
The identification of synaptic zinc was first made by McLardy over fifty years ago who identified that a band of zinc dithizonate staining correlated with hippocampal mossy fibre axons [7]. Since then, many more cerebrocortical pathways have been identified which contain zinc-rich synaptic vesicles; indeed nearly 50% of the glutamatergic synapses are actually “gluzincergic” in some parts of the cerebral cortex. Significantly, only small amounts of chelatable zinc can be determined in glutamatergic pathways which originate outside the cerebral cortex or limbic systems.
Despite this extensive network of zinc-containing neurons, little is known about how zinc homeostasis is maintained within the neuron. There are two families of zinc transporters: the ZnT family, which act to decrease intracellular zinc concentrations by exporting zinc from the cytoplasm to the lumen of organelles or the extracellular space, and the ZIP family, which import the metal from the extracellular space or organellar lumen into the cytoplasm [11]. Whilst many of the transporters have particular distribution patterns, only ZnT3 expression is restricted to the brain and the testis [12]. It is located in the vesicular membrane [13] and is necessary to transport zinc from the cytoplasm into the synaptic vesicle of the neuron. The vesicular concentration of zinc correlates with the amount of ZnT3 present [14]. Targeted disruption of ZnT3 in a mouse model resulted in a complete lack of chelatable zinc [15].
A number of approaches have been taken to confirm that zinc is coreleased with glutamate from the presynaptic bouton during neuronal excitation: imaging of zinc in boutons before and after stimulation [16], analytical detection of zinc released into perfusates [17], and direct imaging of released zinc using fluorescent extracellular probes [18, 19]. This latter approach has provided the most definitive results. An early study employed a reporter construct whose fluorescence properties changed upon zinc binding. Stimulation of organotypic cultures from rat hippocampus produced a cloud of green fluorescence as the released zinc bound an apometalloenzyme confirming the release of zinc from the culture [17]. A later study by Quinta-Ferreira and colleagues [20] demonstrated a release of zinc with each pulse of an action potential. Whilst there is now no doubt that zinc is released during synaptic activity, there is little consensus on the amount or duration of its existence in the synaptic cleft [21].
Following an intense burst of neuronal activity, the release of glutamate and postsynaptic membrane depolarisation open a variety of zinc-permeable ion channels which contribute to removing the ion from the extracellular fluid. These include N-methyl-D-aspartate (NMDA) channels and calcium permeable α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)/kainate channels. The consequences of zinc acting on these receptors are diverse and demonstrate the significance zinc has in modulating fast excitatory glutamatergic transmission. Zinc can act to either enhance or depress synaptic activity with varying degrees of potency [21].
The most studied interaction is zinc with NMDA receptors (NMDAR). Initially, zinc was thought to selectively inhibit NMDAR-mediated neuronal activity by inducing a voltage-independent noncompetitive inhibition that decreased the probability of the channel being open [22–24]. A voltage-dependent inhibition of NMDAR could be observed at higher concentrations of zinc (>20 μM) and was believed to be due to binding of the cation within the channel pore [25]. With the cloning of NMDAR subunits, it was confirmed that zinc could cause both voltage-independent and voltage-dependent inhibition [26]. The exceptional sensitivity of the GluN2A subunit towards zinc suggests that even contaminating ions found in routine laboratory solutions are sufficient to cause inhibition [27]. Significantly, despite being responsible for inhibitory effects at NMDAR, it has also been shown that NMDAR activation may provide a route of influx for zinc contributing to toxic effects of exposure [28] (Figure 1).
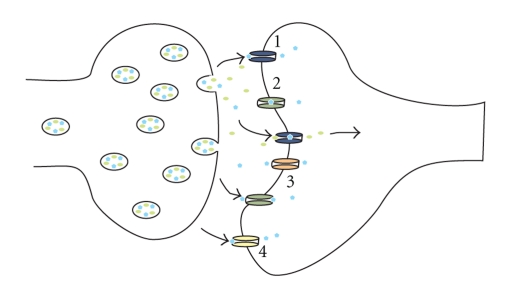
Figure 1.
Multiple mechanisms for zinc uptake following synaptic release. Zinc and glutamate are released from a “gluzincergic” synapse during neurotransmission. The actions of glutamate, alongside membrane depolarisation, open a number of zinc-permeable channels on the postsynaptic membrane which clear the ion from the extracellular fluid. (1) AMPA receptors; (2) NMDA receptors; (3) voltage-gated calcium channels; (4) TRPM7. (blue pentagons) zinc ion and (green ellipses) glutamate molecule. For simplicity, zinc-permeable channels are only shown on the post-synaptic membrane.
Whilst it is thought that zinc exposure generally attenuates NMDAR-mediated neurotoxicity, zinc has been shown to potentiate AMPAR-mediated toxicity at suggested physiological concentrations (50 μM). Originally, it was proposed that the toxic effect was due to zinc influx via voltage-gated calcium channels [29], with Lin et al. [30] showing that desensitisation of AMPAR would explain such an enhancement. Subsequently, it has been demonstrated that zinc can carry currents directly via AMPAR, mostly via the calcium-permeable subtype [31] (Figure 1). At high supraphysiological doses (1 mM), zinc has been shown to inhibit AMPAR [21, 32]. A few studies have also looked directly at zinc-mediated inhibition of voltage-gated calcium channels, as their proximity to vesicular release sites on the presynaptic membrane suggests they could interact [33, 34]. Most recently, neurotoxicity has been attributed to transient receptor potential metastatin 7 (TRPM7) channel activation resulting in increased intracellular zinc [35] (Figure 1).
The significance of synaptically released zinc centres on the amount that is released into the synaptic cleft upon excitation. Some authors argue that the amount of zinc (10–100 μM) released following an action potential arriving at the glutamatergic bouton is high enough to bring about the voltage-dependent inhibition of NMDAR and that there would be no spillover onto neighbouring cells [36, 37]. Other authors disagree, suggesting that the zinc concentration would be sufficient to affect nearby cells [38]. It has also been demonstrated that the zinc level (low nM) is only sufficient to block the voltage-independent component of NMDAR activity [39]. Alternatively, it could be that there is little or no diffusible zinc released, supporting the notion that zinc behaves in a “tonic” mode. Kay and Tóth [40] proposed that zinc is exocytosed from the presynaptic membrane and that instead of diffusing into the extracellular space it remains tightly bound to an as-yet unidentified presynaptic component. This would create a “veneer” [41] of zinc ions which would build up with synaptic activity or erode with quiescence to modulate plasticity.
Thus, the implication is that there are three different groups of zinc signals. First, “synaptic zinc” acts as a conventional neurotransmitter, is contained within presynaptic vesicles, and is released upon excitation and binds to a variety of receptors on the postsynaptic membrane. The downstream consequence of receptor binding is one of tonic modulation of glutamatergic excitatory synapses. The second type is similar to calcium signalling and occurs in conjunction with synaptic zinc signalling. A transmembrane flux of “synaptic zinc” from the extracellular space passes through post-synaptic zinc-permeable ion channels. The third is “intracellular zinc signalling” whereby existing intracellular stores are released [7]. This class is difficult to define and as yet has not been identified in neurons but has been demonstrated in mast cells [42].
Therefore, zinc can be classified as an endogenous modulator of synaptic transmission. It is found in synaptic vesicles, released upon excitation, and has multiple synaptic targets. The significant inhibitory effect of zinc on NMDAR, alongside the crucial function of NMDAR in both neurophysiology and pathophysiology, advocates a vital role for zinc in both healthy and diseased brains [21].
3. Role of Zinc in APP Processing
The most prominent lesions in the brain of AD sufferers are the amyloid or “senile” plaques, which predominantly consist of Aβ peptides derived from the proteolytic processing of the amyloid precursor protein (APP). APP is an ubiquitously expressed glycosylated transmembrane protein with a large N-terminal extracellular domain, a single hydrophobic transmembrane domain, and a small C-terminal cytoplasmic domain. A specific and saturable binding site for zinc (KD = 750 nM) has been reported in the cysteine-rich region on the ectodomain of APP [44, 45]. It is hypothesised that zinc could have a role in sustaining the adhesiveness of APP during cell-cell and cell-matrix interactions [46, 47]. APP can be processed by one of two pathways: the amyloidogenic pathway, leading to the production of Aβ, and the non-amyloidogenic pathway (Figure 2(a)), reviewed in [48].
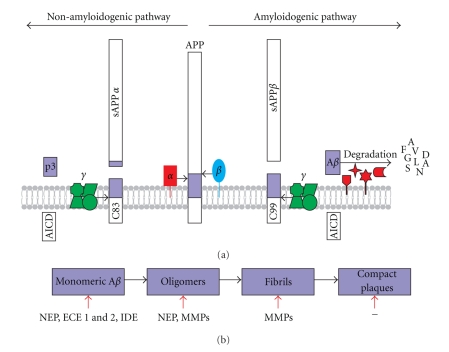
Figure 2.
Zinc metalloprotease activity in APP processing. (a) In the amyloidogenic pathway, the transmembrane APP is cleaved first by β-secretase (β) to form the soluble sAPPβ and the membrane-bound C99, which in turn is cleaved by γ-secretase (γ) to form AICD and the amyloidogenic Aβ peptides. The Aβ peptides are degraded by a number of zinc metalloproteases including NEP and IDE. In the nonamyloidogenic pathway, APP is cleaved first by the α-secretase (α) (zinc metalloprotease) to form the soluble sAPPα and the membrane bound C83, which is then cleaved by γ-secretase (γ) to form p3 and AICD. Zinc metalloproteases depicted in red. Representative amino acids from the degradation of Aβ are shown in single letter code. (b) Action of zinc metalloproteases on monomeric and aggregated forms of Aβ (modified from [43]). Aβ: Amyloid β; AICD: APP intracellular domain; APP: Amyloid precursor protein; ECE: Endothelin-converting enzyme; IDE: Insulin degrading enzyme; MMP: Matrix metalloprotease; NEP: Neprilysin.
In the amyloidogenic pathway, APP is sequentially cleaved by the aspartyl protease, β-site APP-cleaving enzyme 1 (BACE1) forming the secreted APPβ (sAPPβ) fragment and a membrane bound C-terminal fragment of 99 amino acids (C99). The C99 fragment is then further processed by the γ-secretase complex into APP intracellular domain (AICD) and Aβ peptides, predominantly 40 and 42 amino acids in length. It is these aggregation-prone Aβ peptides which form oligomeric and fibrillar structures which deposit in the brain and over time cause AD. The γ-secretase complex comprises four components: presenilin (PS) 1 or 2, nicastrin (Nct), presenilin enhancer 2 (PEN-2), and anterior pharynx defective-1 (Aph-1) [49]. sAPPβ is involved in the pruning of synapses during the development of central and peripheral neurons [50] and AICD is known to be a transcription factor for several genes, including the upregulation of the Aβ-degrading enzyme, neprilysin (NEP) [51].
The predominant APP-processing pathway in healthy brain is the nonamyloidogenic pathway where APP is cleaved by the α-secretase within the Aβ region forming the secreted APPα (sAPPα) fragment and the membrane-bound C-terminal fragment of 83 amino acids (C83) (Figure 2(a)). C83 is subsequently cleaved by the γ-secretase complex generating AICD and p3 (Figure 2(a)). α-secretase activity is attributed to the disintegrin and metalloprotease (ADAM) family of zinc metalloproteases (reviewed in [48, 52]). The ADAMs, along with the matrix metalloproteases (MMPs), are members of the metzincin clan of metalloproteases as they have a long zinc-binding consensus sequence HEBXHXBGBXH (H, histidine, zinc ligand; E, catalytic glutamate; G, glycine; B, bulky apolar residue; X, any amino acid) which contains three zinc ligands [53]. Structurally, the catalytic domain is globular and divided into two subdomains, with the active site cleft running between the two [54]. The defining feature of the metzincins is the conserved methionine residue which creates a 1,4-β-turn (Met-turn), creating the catalytic cleft [53]. The catalytic zinc atom sits at the bottom of the groove between the subdomains, with the subsites in the groove determining specificity for particular amino acid sequences in the substrate (reviewed in [52]). A number of enzymes in this family, namely, ADAM9, 10, 17 (also known as TNF-α converting enzyme, TACE) and 19, are known to exert α-secretase activity, but it is unclear which enzyme or enzymes are responsible for the α-cleavage of APP in vivo [55–58]. ADAM10 appears to be the predominant enzyme as overexpression of functional ADAM10 in AD transgenic mice led to an increase in sAPPα and reduced Aβ production, plaque deposition, and cognitive deficits [59]. Although fibroblasts from ADAM10−/− mice showed no deficiency in α-secretase activity, probably due to compensation by ADAM17 [60], a recent detailed study has provided strong evidence that ADAM10 is the physiologically relevant constitutive α-secretase in primary neurons [61].
4. Role of Zinc in Aβ Degradation
In a healthy brain, the relatively small amount of Aβ-constitutively being produced is rendered safe by Aβ degrading enzymes. The steady state levels of Aβ synthesis and clearance in cerebrospinal fluid (CSF) are 7.6% and 8.3% per hour, respectively [62]. A large number of candidate Aβ-degrading enzymes have been identified, with the majority being zinc metalloproteases. These include NEP, insulin-degrading enzyme (IDE), endothelin-converting enzyme (ECE) 1 and 2, MMP2, 3, and 9, and PreP (reviewed in [43]). NEP, ECE1 and 2, and MMPs have the conserved zinc-binding motif HEXXH (H, histidine, zinc ligand; E, catalytic glutamate; X, any amino acid), and IDE and its homolog PreP are both inverzincins, as they contain the inverted zinc-binding motif HXXEH.
NEP appears to be the dominant Aβ protease [63–66], and is capable of degrading monomeric and oligomeric Aβ [63, 67] (Figure 2(b)). NEP knockout mice have significantly elevated Aβ levels [68, 69] and more importantly, human postmortem tissue analysis suggests that NEP expression is inversely related to the extent of AD pathology [70–72]. IDE is predominantly a cytosolic enzyme but has been found extracellularly [73] and can only degrade monomeric Aβ and not oligomers (Figure 2(b)), although it did lower the net production of oligomers by cultured cells [74]. Even though there are conflicting data regarding IDE and AD [75], IDE knockout mice have elevated levels of endogenous Aβ [76, 77]. There are two isoforms of ECE, 1 and 2, both of which have similar catalytic activity, with ECE 1 being the predominant isoform within the brain [65, 78]. However, both ECEs are primarily localised to endothelial cells but have been detected in neurons and glial [78]. The MMPs, specifically MMP2 and MMP9, degrade fibrillar Aβ [79], whereas PreP degrades monomeric Aβ. However, PreP is confined to mitochondria, so it would therefore have limited action on the majority of Aβ which is extracellular [80].
5. Zinc Binding to Aβ
High concentrations of zinc (up to 1 mM) have also been found within amyloid plaques [81], which is thought to have been released from glutamatergic synapses [82]. Aggregation of the Aβ peptides can be rapidly induced in the presence of zinc ions under physiological conditions in vitro [83, 84] and studies on AD tissues show that zinc colocalised with Aβ deposits [85]. The metal binding site for zinc on Aβ is the same as the copper binding site and lies within the N-terminal hydrophilic region, the first 16 residues [86] (Figure 3). The majority of studies on zinc binding to Aβ have utilised truncated peptides of 16 (Aβ1–16) and 28 (Aβ1–28) amino acids, as the binding region is situated within these sequences [86]. Aβ1–16 does not aggregate under moderate conditions [87–90], making it an ideal model system, and Aβ1–28 can undergo aggregation and fibril formation, but at significantly reduced rates, to the full-length peptides [91]. Zinc binds to Aβ in a 1 : 1 stoichiometry with a mononuclear binding site [84, 86–90, 92–94]. NMR studies have shown that all three histidine residues (residues 6, 13 and 14; see Figure 3) are involved in zinc binding [87, 88, 90, 93, 95], with confirmation from mutational studies [96, 97]. Other possible ligands have also been reported as zinc coordination classically involves four to six ligands [91]. The candidate ligands are Asp1 [91, 93], Arg5 [94], Ser 8 [93], Glu11 [90], and Tyr10 [95] (Figure 3) however, Tyr10 has been ruled out [89] and Arg5 has been deemed highly unlikely [91]. The carboxyl side chain of Glu11 is a zinc ligand [88, 90] however, Asp1 is considered the most attractive zinc ligand, either through its N-terminal amino group and/or its side-chain carboxylate group [87, 88, 91, 93]. Raman spectroscopy has shown zinc binding to the Nτ site of histidine side chains in senile plaques taken from AD brains [98], but it is unclear if zinc only binds to free Aβ peptides that subsequently aggregate or whether zinc binds to Aβ in preformed plaques.
Figure 3.
Aβ peptide sequence with potential zinc binding sites highlighted. Histidine residues are shown in blue and other potential zinc-binding sites are shown in pink.
The reported apparent binding constants (aKd) of zinc to Aβ peptides vary from 1 to 300 μM (reviewed in [91]). The published aKd values vary greatly due to the in vitro conditions (e.g., buffer composition, pH), Aβ fragment, and experimental method. The high aKd (20–300 μM) values come from tyrosine fluorescence experiments however, even these are contentious and hard to reproduce [99–101], and any change in tyrosine fluorescence could be due to Aβ aggregation rather than zinc binding [101]. Discounting the tyrosine fluorescence measurements, the most likely apparent aKd value for zinc binding to Aβ peptides is a range of 1–20 μM [91]. The binding affinity of zinc for preformed Aβ fibrils is approximately the same as of the peptides with a aKd of 1–20 μM [89, 101].
6. Role of Zinc in Alzheimer's Disease
Numerous studies have looked to address whether zinc levels change with AD progression. It has been shown that there is a significant increase in serum [102] and hippocampal [5] zinc in AD patients compared to age-matched controls. Jiménez-Jiménez et al. [103] demonstrated a significant decrease in CSF zinc but could find no difference in serum zinc levels between AD and age-matched controls. A decrease in serum zinc has also been reported, though it is possible that some of the AD patients included in one study were malnourished [104, 105]. Overall, there is currently no consensus on what happens to zinc concentrations in AD subjects though much of the discrepancy could be put down to differences in patient allocation, sample type, postmortem interval, or type of analysis used.
Alternatively, a redistribution of zinc could be sufficient to promote disease progression. Lovell and coworkers [106, 107] have mapped the expression levels of a number of the ZnT zinc transporters in AD. ZnT-1, 4, and 6 were all found to show increased expression in early stages of disease, though ZnT-1 expression was decreased during mild cognitive impairment [106, 107]. Although it is unknown whether increased expression necessarily correlates with increased activity, these changes in transporter level could result in modified subcellular zinc concentrations. An increase in ZnT6 would lead to an increase in zinc in the TGN [108] which could reduce α-secretase activity [83].
It is well established that amyloid plaques contain increased concentrations of copper, iron, and zinc [98, 109]. Whilst copper and iron appear to be primarily responsible for the toxicity of Aβ via oxidative-stress-type mechanisms [109, 110], zinc has a crucial role in Aβ aggregation which is the most well-established contribution that zinc may have in AD pathogenesis. Whilst the concentration of zinc required for fibrillisation to occur is contentious, with concentrations differing by 100-fold being suggested, zinc is an unequivocal partner in the process [83, 111, 112]. In 2006, Dong and co-workers were able to show that zinc could control the rate of self-assembly of the Aβ peptides and go on to regulate the amyloid morphology via specific coordination sites [113]. Furthermore, it has been demonstrated that zinc can spontaneously coordinate both intra-and inter-molecular bridging between two peptides to promote Aβ aggregation [114] and that synaptic zinc promotes Aβ oligomer formation and their accumulation at excitatory synapses [115].
Studies with synthetic Aβ showed that chelation chemistry could help solubilise amyloid plaques, with depletion of zinc having a more marked effect on extracting Aβ than depletion of copper [116]. Oral treatment with 5-chloro-7-iodo-8-hydroxyquinoline (Clioquinol CQ) in Tg2576 mice resulted in a 49% reduction in cortical amyloid deposition [117]. Although CQ has a fairly low affinity (nM) for both copper and zinc, it was still able to release the ions from the Aβ binding site [118]. A pilot phase II trial in humans showed a decrease in cognitive decline and a reduction in plasma Aβ1-42 in moderately severe AD compared with placebo control [119]. It has been suggested that although CQ may chelate copper and zinc from metallated Aβ and promote disaggregation, it may not completely holt the aggregation process [120]. A second generation chelator (PBT-2), with improved blood brain barrier penetration, has just completed a phase II clinical trial in early AD with promising results showing good tolerance, a reduction in CSF Aβ and neuropsychological testing [121].
Recently, it has been shown that zinc can also accelerate the aggregation of a Tau peptide under reducing conditions [122]. Zinc inhibited the formation of intramolecular disulphide bonds but promoted intermolecular bonds between key cysteine residues. Furthermore, zinc exposure has been shown to increase the phosphorylation of PI3K and MAPK-dependent pathways which are key players in Tau modifications [123].
The essential requirement for ZnT3 in loading zinc into synaptic vesicles would suggest that this transporter could have a major impact on zinc signalling in the neuron, even regulating cognitive function. Whilst a lack of zinc signalling in brain slices from ZnT3−/− mice confirmed the vesicular origin of the released zinc, the mice failed to express a cognitive phenotype. Initial studies detailed a 20% reduction in total zinc level and a loss of histochemically reactive zinc in the synaptic vesicle; however, there was no impairment of spatial learning, memory, or sensorimotor function [124]. The implication being that the vesicular zinc is not required for cognitive function or that compensatory mechanisms made up for the deficits. However, a follow-up study demonstrated marked differences in learning and memory when an older (6 month) cohort of mice was used [125], suggesting that the lack of effect in the previous study was due to the young age (6–10 weeks) of the mice and highlights the importance of aging (the most significant risk factor) when modelling AD pathology. The results obtained from the older ZnT3−/− cohort established a requirement for zinc in memory function and the maintenance of synaptic health upon aging. Adlard and colleagues proposed that β-amyloid pathology could cause cognitive impairment by trapping zinc within plaques rather than via a directly toxic mechanism [125]. The zinc immobilisation by amyloid would then have similar consequences as a loss of ZnT3 activity with a loss of zinc-dependent synaptic modulation promoting cognitive decline.
An alternative approach to minimising the consequences of released zinc could be to promote mechanisms which enhance reuptake. Recently, it has been shown that the cellular form of the prion protein (PrPC) is an evolutionary descendent of the ZIP family of divalent metal transporters. In particular, ZIPs 5, 6, and 10 were found to have a “prion-like” domain with significant structural similarity. As both PrPC and the ZIPs bind divalent metal ions via histidine-rich motifs contained with N-terminal repeating sequences, this could suggest that PrPC is involved in zinc sensing, scavenging, or transport [126]. In agreement with that possibility, we have shown that PrPC promotes zinc uptake (Watt et al., unpublished). Ensuring efficient clearance of extracellular zinc from the synaptic cleft via PrPC would exploit an existing physiological process. Furthermore, enhancing zinc uptake would help prevent its ability to contribute to the synaptic targeting of Aβ oligomers, thus preserving synaptic function [115] and maintaining the proposed ferroxidase activity of APP [127]. As PrPC levels decrease with age and in sporadic AD [128], it is possible that zinc is cleared less efficiently from the synaptic cleft enhancing aggregation of Aβ and inhibiting APP ferroxidase activity to promote a pro-oxidative environment. This would suggest that preserving PrPC function during AD could provide multifactorial benefits, an inhibition of BACE1 which would reduce Aβ formation [129] and ensure efficient clearance of zinc from the synaptic cleft to prevent aggregation of Aβ peptides, as well as provide protection against oxidative stress [130, 131].
7. Conclusions
It is clear that zinc not only plays critical roles in the structural and functional integrity of many proteins, but that it also modulates the activity of glutamatergic synapses and indeed may act as a neurotransmitter in its own right. Several of the enzymes involved in processing APP and Aβ are zinc metalloproteases, with an essential requirement for zinc in their catalytic activity. Zinc binds to Aβ, promoting its aggregation and thereby modulating its neurotoxicity. Although zinc dyshomeostasis may contribute to the development of AD, further work is required to clarify the molecular and cellular mechanisms affected by zinc under both normal and disease situations.
Acknowledgments
The authors gratefully acknowledge the financial support of the Medical Research Council. N. T. Watt and I. J. Whitehouse contributed equally to this work.
References
- 1.Mount C, Downton C. Alzheimer disease: progress or profit? Nature Medicine. 2006;12(7):780–784. doi: 10.1038/nm0706-780. [DOI] [PubMed] [Google Scholar]
- 2.Wimo A, Winblad B, Jönsson L. The worldwide societal costs of dementia: estimates for 2009. Alzheimer’s and Dementia. 2010;6(2):98–103. doi: 10.1016/j.jalz.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiological Reviews. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 4.Hooper NM. The biological roles of zinc and families of zinc metalloproteases. In: Hooper NM, editor. Zinc Metalloproteases in Health and Disease. London, UK: Talor & Francis; 1996. pp. 1–21. [Google Scholar]
- 5.Danscher G, Jensen KB, Frederickson CJ, et al. Increased amount of zinc in the hippocampus and amygdala of Alzheimer’s diseased brains: a proton-induced X-ray emission spectroscopic analysis of cryostat sections from autopsy material. Journal of Neuroscience Methods. 1997;76(1):53–59. doi: 10.1016/s0165-0270(97)00079-4. [DOI] [PubMed] [Google Scholar]
- 6.Frederickson CJ, Danscher G. Zinc-containin neurons in hippocampus and related CNS structures. Progress in Brain Research. 1990;83:71–84. doi: 10.1016/s0079-6123(08)61242-x. [DOI] [PubMed] [Google Scholar]
- 7.Frederickson CJ, Bush AI. Synaptically released zinc: physiological functions and pathological effects. BioMetals. 2001;14(3-4):353–366. doi: 10.1023/a:1012934207456. [DOI] [PubMed] [Google Scholar]
- 8.Slomianka L, Danscher G, Frederickson CJ. Labeling of the neurons of origin of zinc-containing pathways by intraperitoneal injections of sodium selenite. Neuroscience. 1990;38(3):843–854. doi: 10.1016/0306-4522(90)90076-g. [DOI] [PubMed] [Google Scholar]
- 9.Koh JY. Endogenous zinc in neurological diseases. Journal of Clinical Neurology. 2005;1:121–133. doi: 10.3988/jcn.2005.1.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Tian Y, Yang Z, Zhang T. Zinc ion as modulator effects on excitability and synaptic transmission in hippocampal CA1 neurons in Wistar rats. Neuroscience Research. 2010;68(3):167–175. doi: 10.1016/j.neures.2010.07.2030. [DOI] [PubMed] [Google Scholar]
- 11.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annual Review of Nutrition. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 12.McMahon RJ, Cousins RJ. Mammalian zinc transporters. Journal of Nutrition. 1998;128(4):667–670. doi: 10.1093/jn/128.4.667. [DOI] [PubMed] [Google Scholar]
- 13.Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(25):14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linkous DH, Flinn JM, Koh JY, et al. Evidence that the ZNT3 protein controls the total amount of elemental zinc in synaptic vesicles. Journal of Histochemistry and Cytochemistry. 2008;56(1):3–6. doi: 10.1369/jhc.6A7035.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(4):1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Hough CJ, Suh SW, Sarvey JM, Frederickson CJ. Rapid translocation of Zn2+ from presynaptic terminals into postsynaptic hippocampal neurons after physiological stimulation. Journal of Neurophysiology. 2001;86(5):2597–2604. doi: 10.1152/jn.2001.86.5.2597. [DOI] [PubMed] [Google Scholar]
- 17.Thompson RB, Whetsell WO, Jr., Maliwal BP, Fierke CA, Frederickson CJ. Fluorescence microscopy of stimulated Zn(II) release from organotypic cultures of mammalian hippocampus using a carbonic anhydrase-based biosensor system. Journal of Neuroscience Methods. 2000;96(1):35–45. doi: 10.1016/s0165-0270(99)00183-1. [DOI] [PubMed] [Google Scholar]
- 18.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nature Reviews Neuroscience. 2005;6(6):449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 19.Bastian C, Li YV. Fluorescence imaging study of extracellular zinc at the hippocampal mossy fiber synapse. Neuroscience Letters. 2007;419(2):119–124. doi: 10.1016/j.neulet.2007.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinta-Ferreira ME, Matias CM, Arif M, Dionísio JC. Measurement of presynaptic zinc changes in hippocampal mossy fibers. Brain Research. 2004;1026(1):1–10. doi: 10.1016/j.brainres.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 21.Paoletti P, Vergnano AM, Barbour B, Casado M. Zinc at glutamatergic synapses. Neuroscience. 2009;158(1):126–136. doi: 10.1016/j.neuroscience.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 22.Peters S, Koh J, Choi DW. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987;236(4801):589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- 23.Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- 24.Christine CW, Choi DW. Effect of zinc on NMDA receptor-mediated channel currents in cortical neurons. Journal of Neuroscience. 1990;10(1):108–116. doi: 10.1523/JNEUROSCI.10-01-00108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legendre P, Westbrook GL. The inhibition of single N-methyl-D-aspartate-activated channels by zinc ions on cultured rat neurones. Journal of Physiology. 1990;429:429–449. doi: 10.1113/jphysiol.1990.sp018266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N, Moshaver A, Raymond LA. Differential sensitivity of recombinant N-methyl-D-aspartate receptor subtypes to zinc inhibition. Molecular Pharmacology. 1997;51(6):1015–1023. doi: 10.1124/mol.51.6.1015. [DOI] [PubMed] [Google Scholar]
- 27.Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. Journal of Neuroscience. 1997;17(15):5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koh JY. Zinc toxicity on cultured cortical neurons: involvement of N-methyl-D-aspartate receptors. Neuroscience. 1994;60(4):1049–1057. doi: 10.1016/0306-4522(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 29.Weiss JH, Hartley DM, Koh JY, Choi DW. AMPA receptor activation potentiates zinc neurotoxicity. Neuron. 1993;10(1):43–49. doi: 10.1016/0896-6273(93)90240-r. [DOI] [PubMed] [Google Scholar]
- 30.Lin DD, Cohen AS, Coulter DA. Zinc-induced augmentation of excitatory synaptic currents and glutamate receptor responses in hippocampal CA3 neurons. Journal of Neurophysiology. 2001;85(3):1185–1196. doi: 10.1152/jn.2001.85.3.1185. [DOI] [PubMed] [Google Scholar]
- 31.Jia Y, Jeng JM, Sensi SL, Weiss JH. Zn2+ currents are mediated by calcium-permeable AMPA/kainate channels in cultured murine hippocampal neurones. Journal of Physiology. 2002;543(1):35–48. doi: 10.1113/jphysiol.2002.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathie A, Sutton GL, Clarke CE, Veale EL. Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacology and Therapeutics. 2006;111(3):567–583. doi: 10.1016/j.pharmthera.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Magistretti J, Castelli L, Taglietti V, Tanzi F. Dual effect of Zn2+ on multiple types of voltage-dependent Ca currents in rat palaeocortical neurons. Neuroscience. 2003;117(2):249–264. doi: 10.1016/s0306-4522(02)00865-5. [DOI] [PubMed] [Google Scholar]
- 34.Sun HS, Hui K, Lee DWK, Feng ZP. Zn2+ sensitivity of high- and low-voltage activated calcium channels. Biophysical Journal. 2007;93(4):1175–1183. doi: 10.1529/biophysj.106.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue K, Branigan D, Xiong ZG. Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. The Journal of Biological Chemistry. 2010;285(10):7430–7439. doi: 10.1074/jbc.M109.040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molnár P, Nadler JV. Synaptically-released zinc inhibits N-methyl-D-aspartate receptor activation at recurrent mossy fiber synapses. Brain Research. 2001;910(1-2):205–207. doi: 10.1016/s0006-8993(01)02720-2. [DOI] [PubMed] [Google Scholar]
- 37.Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26(1):187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- 38.Ueno S, Tsukamoto M, Hirano T, et al. Mossy fiber Zn2+ spillover modulates heterosynaptic N-methyl-D-aspartate receptor activity in hippocampal CA3 circuits. Journal of Cell Biology. 2002;158(2):215–220. doi: 10.1083/jcb.200204066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu YM, Taverna FA, Tu R, et al. Endogenous Zn2+ is required for the induction of long-term potentiation at rat hippocampal mossy fiber-CA3 synapses. Synapse. 2000;38(2):187–197. doi: 10.1002/1098-2396(200011)38:2<187::AID-SYN10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 40.Kay AR, Tóth K. Influence of location of a fluorescent zinc probe in brain slices on its response to synaptic activation. Journal of Neurophysiology. 2006;95(3):1949–1956. doi: 10.1152/jn.00959.2005. [DOI] [PubMed] [Google Scholar]
- 41.Kay AR. Evidence for chelatable zinc in the extracellular space of the hippocampus, but little evidence for synaptic release of Zn. Journal of Neuroscience. 2003;23(17):6847–6855. doi: 10.1523/JNEUROSCI.23-17-06847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamasaki S, Sakata-Sogawa K, Hasegawa A, et al. Zinc is a novel intracellular second messenger. Journal of Cell Biology. 2007;177(4):637–645. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leissring MA. The AβCs of Aβ-cleaving proteases. The Journal of Biological Chemistry. 2008;283(44):29645–29649. doi: 10.1074/jbc.R800022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bush AI, Multhaup G, Moir RD, et al. A novel zinc(II) binding site modulates the function of the βA4 amyloid protein precursor of Alzheimer’s disease. The Journal of Biological Chemistry. 1993;268(22):16109–16112. [PubMed] [Google Scholar]
- 45.Bush AI, Pettingell WH, De Paradis M, Tanzi RE, Wasco W. The amyloid β-protein precursor and its mammalian homologues. Evidence for a zinc-modulated heparin-binding superfamily. The Journal of Biological Chemistry. 1994;269(43):26618–26621. [PubMed] [Google Scholar]
- 46.Multhaup G, Busha AI, Pollweina P, Masters CL. Interaction between the zinc(II) and the heparin binding site of the Alzheimer's disease βA4 amyloid precursor protein (APP) FEBS Letters. 1994;355(2):151–154. doi: 10.1016/0014-5793(94)01176-1. [DOI] [PubMed] [Google Scholar]
- 47.Multhaup G, Ruppert T, Schlicksupp A, et al. Copper-binding amyloid precursor protein undergoes a site-specific fragmentation in the reduction of hydrogen peroxide. Biochemistry. 1998;37(20):7224–7230. doi: 10.1021/bi980022m. [DOI] [PubMed] [Google Scholar]
- 48.Chow VW, Mattson MP, Wong PC, Gleichmann M. An overview of APP processing enzymes and products. Neuromolecular Medicine. 2010;12(1):1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of γ-secretase activity. Nature Cell Biology. 2003;5(5):486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 50.Nikolaev A, McLaughlin T, O’Leary DDM, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457(7232):981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Słomnicki ŁP, Leśniak W. A putative role of the Amyloid Precursor Protein Intracellular Domain (AICD) in transcription. Acta Neurobiologiae Experimentalis. 2008;68(2):219–228. doi: 10.55782/ane-2008-1691. [DOI] [PubMed] [Google Scholar]
- 52.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Molecular Aspects of Medicine. 2009;29(5):258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bode W, Gomis-Ruth FX, Stockler W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the ‘ metzincins’ . FEBS Letters. 1993;331(1-2):134–140. doi: 10.1016/0014-5793(93)80312-i. [DOI] [PubMed] [Google Scholar]
- 54.Gomis-Rüth FX. Structural aspects of the metzincin clan of metalloendopeptidases. Applied Biochemistry and Biotechnology Part B. 2003;24(2):157–202. doi: 10.1385/MB:24:2:157. [DOI] [PubMed] [Google Scholar]
- 55.Allinson TMJ, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein alpha-secretases. Journal of Neuroscience Research. 2003;74(3):342–352. doi: 10.1002/jnr.10737. [DOI] [PubMed] [Google Scholar]
- 56.Asai M, Hattori C, Szabó B, et al. Putative function of ADAM9, ADAM10, and ADAM17 as APP α-secretase. Biochemical and Biophysical Research Communications. 2003;301(1):231–235. doi: 10.1016/s0006-291x(02)02999-6. [DOI] [PubMed] [Google Scholar]
- 57.Fahrenholz F, Gilbert S, Kojro E, Lammich S, Postina R. α-Secretase activity of the disintegrin metalloprotease ADAM 10: influences of domain structure. Annals of the New York Academy of Sciences. 2000;920:215–222. doi: 10.1111/j.1749-6632.2000.tb06925.x. [DOI] [PubMed] [Google Scholar]
- 58.Tanabe C, Hotoda N, Sasagawa N, Sehara-Fujisawa A, Maruyama K, Ishiura S. ADAM19 is tightly associated with constitutive Alzheimer’s disease APP α-secretase in A172 cells. Biochemical and Biophysical Research Communications. 2007;352(1):111–117. doi: 10.1016/j.bbrc.2006.10.181. [DOI] [PubMed] [Google Scholar]
- 59.Postina R, Schroeder A, Dewachter I, et al. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alheizmer disease mouse model. Journal of Clinical Investigation. 2004;113(10):1456–1464. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartmann D, de Strooper B, Serneels L, et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for α-secretase activity in fibroblasts. Human Molecular Genetics. 2002;11(21):2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- 61.Kuhn P-H, Wang H, Dislich B, et al. ADAM10 is the physiologically relevant, constitutive α-secretase of the amyloid precursor protein in primary neurons. EMBO Journal. 2010;29(17):3020–3032. doi: 10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-β synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nature Medicine. 2006;12(7):856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Amouri SS, Zhu H, Yu J, Marr R, Verma IM, Kindy MS. Neprilysin: an enzyme candidate to slow the progression of Alzheimer’s disease. American Journal of Pathology. 2008;172(5):1342–1354. doi: 10.2353/ajpath.2008.070620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hersh LB, Rodgers DW. Neprilysin and amyloid beta peptide degradation. Current Alzheimer Research. 2008;5(2):225–231. doi: 10.2174/156720508783954703. [DOI] [PubMed] [Google Scholar]
- 65.Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Aβ-degrading enzymes in Alzheimer’s disease. Brain Pathology. 2008;18(2):240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vardy ERLC, Catto AJ, Hooper NM. Proteolytic mechanisms in amyloid-β metabolism: therapeutic implications for Alzheimer’s disease. Trends in Molecular Medicine. 2005;11(10):464–472. doi: 10.1016/j.molmed.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 67.Kanemitsu H, Tomiyama T, Mori H. Human neprilysin is capable of degrading amyloid β peptide not only in the monomeric form but also the pathological oligomeric form. Neuroscience Letters. 2003;350(2):113–116. doi: 10.1016/s0304-3940(03)00898-x. [DOI] [PubMed] [Google Scholar]
- 68.Apelt J, Ach K, Schliebs R. Aging-related down-regulation of neprilysin, a putative β-amyloid-degrading enzyme, in transgenic Tg2576 Alzheimer-like mouse brain is accompanied by an astroglial upregulation in the vicinity of β-amyloid plaques. Neuroscience Letters. 2003;339(3):183–186. doi: 10.1016/s0304-3940(03)00030-2. [DOI] [PubMed] [Google Scholar]
- 69.Farris W, Schütz SG, Cirrito JR, et al. Loss of neprilysin function promotes amyloid plaque formation and causes cerebral amyloid angiopathy. American Journal of Pathology. 2007;171(1):241–251. doi: 10.2353/ajpath.2007.070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akiyama H, Kondo H, Ikeda K, Kato M, McGeer PL. Immunohistochemical localization of neprilysin in the human cerebral cortex: inverse association with vulnerability to amyloid β-protein (Aβ) deposition. Brain Research. 2001;902(2):277–281. doi: 10.1016/s0006-8993(01)02390-3. [DOI] [PubMed] [Google Scholar]
- 71.Eckman EA, Adams SK, Troendle FJ, et al. Regulation of steady-state β-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. The Journal of Biological Chemistry. 2006;281(41):30471–30478. doi: 10.1074/jbc.M605827200. [DOI] [PubMed] [Google Scholar]
- 72.Hellström-Lindahl E, Ravid R, Nordberg A. Age-dependent decline of neprilysin in Alzheimer’s disease and normal brain: inverse correlation with Aβ levels. Neurobiology of Aging. 2008;29(2):210–221. doi: 10.1016/j.neurobiolaging.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 73.Duckworth WC, Bennett RG, Hamel FG. A direct inhibitory effect of insulin on a cytosolic proteolytic complex containing insulin-degrading enzyme and multicatalytic proteinase. The Journal of Biological Chemistry. 1994;269(40):24575–24580. [PubMed] [Google Scholar]
- 74.Vekrellis K, Ye Z, Qiu WQ, et al. Neurons regulate extracellular levels of amyloid β-protein via proteolysis by insulin-degrading enzyme. Journal of Neuroscience. 2000;20(5):1657–1665. doi: 10.1523/JNEUROSCI.20-05-01657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang S, Wang R, Chen L, Bennett DA, Dickson DW, Wang D-S. Expression and functional profiling of neprilysin, insulin-degrading enzyme, and endothelin-converting enzyme in prospectively studied elderly and Alzheimer's brain. Journal of Neurochemistry. 2010;115(1):47–57. doi: 10.1111/j.1471-4159.2010.06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leal MC, Dorfman VB, Gamba AF, et al. Plaque-associated overexpression of insulin-degrading enzyme in the cerebral cortex of aged transgenic Tg2576 mice with Alzheimer pathology. Journal of Neuropathology and Experimental Neurology. 2006;65(10):976–987. doi: 10.1097/01.jnen.0000235853.70092.ba. [DOI] [PubMed] [Google Scholar]
- 77.Miller BC, Eckman EA, Sambamurti K, et al. Amyloid-β peptide levels in brain are inversely correlated with insulysin activity levels in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(10):6221–6226. doi: 10.1073/pnas.1031520100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davenport AP, Kuc RE, Mockridge JW. Endothelin-converting enzyme in the human vasculature: evidence for differential conversion of big endothelin-3 by endothelial and smooth-muscle cells. Journal of Cardiovascular Pharmacology. 1998;31(supplement 1):S1–S3. doi: 10.1097/00005344-199800001-00002. [DOI] [PubMed] [Google Scholar]
- 79.Yin KJ, Cirrito JR, Yan P, et al. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-β peptide catabolism. Journal of Neuroscience. 2006;26(43):10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Falkevall A, Alikhani N, Bhushan S, et al. Degradation of the amyloid β-protein by the novel mitochondrial peptidasome, PreP. The Journal of Biological Chemistry. 2006;281(39):29096–29104. doi: 10.1074/jbc.M602532200. [DOI] [PubMed] [Google Scholar]
- 81.Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer’s disease senile plaques. Journal of the Neurological Sciences. 1998;158(1):47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 82.Lee JY, Cole TB, Palmiter RD, Suh SW, Koh JY. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(11):7705–7710. doi: 10.1073/pnas.092034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bush AI, Pettingell WH, Multhaup G, et al. Rapid induction of Alzheimer Aβ amyloid formation by zinc. Science. 1994;265(5177):1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 84.Lim KH, Kim YK, Chang YT. Investigations of the molecular mechanism of metal-induced Aβ (1–40) amyloidogenesis. Biochemistry. 2007;46(47):13523–13532. doi: 10.1021/bi701112z. [DOI] [PubMed] [Google Scholar]
- 85.Miller LM, Wang Q, Telivala TP, Smith RJ, Lanzirotti A, Miklossy J. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with β-amyloid deposits in Alzheimer’s disease. Journal of Structural Biology. 2006;155(1):30–37. doi: 10.1016/j.jsb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 86.Kozin SA, Zirah S, Rebuffat S, Hui Bon Hoa G, Debey P. Zinc binding to Alzheimer’s Aβ(1-16) peptide results in stable soluble complex. Biochemical and Biophysical Research Communications. 2001;285(4):959–964. doi: 10.1006/bbrc.2001.5284. [DOI] [PubMed] [Google Scholar]
- 87.Mekmouche Y, Coppel Y, Hochgräfe K, et al. Characterization of the ZnII binding to the peptide amyloid-β1−16 linked to Alzheimer’s disease. ChemBioChem. 2005;6(9):1663–1671. doi: 10.1002/cbic.200500057. [DOI] [PubMed] [Google Scholar]
- 88.Syme CD, Viles JH. Solution H NMR investigation of Zn2+ and Cd2+ binding to amyloid-beta peptide (Aβ) of Alzheimer’s disease. Biochimica et Biophysica Acta. 2006;1764(2):246–256. doi: 10.1016/j.bbapap.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 89.Talmard C, Bouzan A, Faller P. Zinc binding to amyloid-β: isothermal titration calorimetry and Zn competition experiments with Zn sensors. Biochemistry. 2007;46(47):13658–13666. doi: 10.1021/bi701355j. [DOI] [PubMed] [Google Scholar]
- 90.Zirah S, Kozin SA, Mazur AK, et al. Structural changes of region 1-16 of the Alzheimer disease amyloid β-peptide upon zinc binding and in vitro aging. The Journal of Biological Chemistry. 2006;281(4):2151–2161. doi: 10.1074/jbc.M504454200. [DOI] [PubMed] [Google Scholar]
- 91.Faller P, Hureau C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-β peptide. Dalton Transactions. 2009;(7):1080–1094. doi: 10.1039/b813398k. [DOI] [PubMed] [Google Scholar]
- 92.Talmard C, Guilloreau L, Coppel Y, Mazarguil H, Faller P. Amyloid-beta peptide forms monomeric complexes with CuII and ZnII prior to aggregation. ChemBioChem. 2007;8(2):163–165. doi: 10.1002/cbic.200600319. [DOI] [PubMed] [Google Scholar]
- 93.Danielsson J, Pierattelli R, Banci L, Gräslund A. High-resolution NMR studies of the zinc-binding site of the Alzheimer’s amyloid β-peptide. FEBS Journal. 2007;274(1):46–59. doi: 10.1111/j.1742-4658.2006.05563.x. [DOI] [PubMed] [Google Scholar]
- 94.Zirah S, Rebuffat S, Kozin SA, et al. Zinc binding properties of the amyloid fragment Aβ(1–16) studied by electrospray-ionization mass spectrometry. International Journal of Mass Spectrometry. 2003;228(2-3):999–1016. [Google Scholar]
- 95.Curtain CC, Ali F, Volitakis I, et al. Alzheimer's disease amyloid-beta binds copper and zinc to generate an allosterically ordered membrane-penetrating structure containing superoxide dismutase-like subunits. The Journal of Biological Chemistry. 2001;276(23):20466–20473. doi: 10.1074/jbc.M100175200. [DOI] [PubMed] [Google Scholar]
- 96.Liu ST, Howlett G, Barrow CJ. Histidine-13 is a crucial residue in the zinc ion-induced aggregation of the Aβ peptide of Alzheimer’s disease. Biochemistry. 1999;38(29):9373–9378. doi: 10.1021/bi990205o. [DOI] [PubMed] [Google Scholar]
- 97.Yang DS, McLaurin J, Qin K, Westaway D, Fraser PE. Examining the zinc binding site of the amyloid-β peptide. European Journal of Biochemistry. 2000;267(22):6692–6698. doi: 10.1046/j.1432-1327.2000.01767.x. [DOI] [PubMed] [Google Scholar]
- 98.Dong J, Atwood CS, Anderson VE, et al. Metal binding and oxidation of amyloid-β within isolated senile plaque cores: raman microscopic evidence. Biochemistry. 2003;42(10):2768–2773. doi: 10.1021/bi0272151. [DOI] [PubMed] [Google Scholar]
- 99.Garzon-Rodriguez W, Yatsimirsky AK, Glabe CG. Binding of Zn(II), Cu(II), and Fe(II) ions to Alzheimer’s Aβ peptide studied by fluorescence. Bioorganic and Medicinal Chemistry Letters. 1999;9(15):2243–2248. doi: 10.1016/s0960-894x(99)00357-1. [DOI] [PubMed] [Google Scholar]
- 100.Ricchelli F, Drago D, Filippi B, Tognon G, Zatta P. Aluminum-triggered structural modifications and aggregation of β-amyloids. Cellular and Molecular Life Sciences. 2005;62(15):1724–1733. doi: 10.1007/s00018-005-5141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tõugu V, Karafin A, Palumaa P. Binding of zinc(II) and copper(II) to the full-length Alzheimer’s amyloid-β peptide. Journal of Neurochemistry. 2008;104(5):1249–1259. doi: 10.1111/j.1471-4159.2007.05061.x. [DOI] [PubMed] [Google Scholar]
- 102.Rulon LL, Robertson JD, Lovell MA, Deibel MA, Ehmann WD, Markesbery WR. Serum zinc levels and Alzheimer’s disease. Biological Trace Element Research. 2000;75(1–3):79–85. doi: 10.1385/bter:75:1-3:79. [DOI] [PubMed] [Google Scholar]
- 103.Jiménez-Jiménez FJ, Molina JA, Aguilar MV, et al. Cerebrospinal fluid levels of transition metals in patients with Parkinson’s disease. Journal of Neural Transmission. 1998;105(4-5):497–505. doi: 10.1007/s007020050073. [DOI] [PubMed] [Google Scholar]
- 104.Jeandel C, Nicolas MB, Dubois F, Nabet-Belleville F, Penin F, Cuny G. Lipid peroxidation and free radical scavengers in Alzheimer’s disease. Gerontology. 1989;35(5-6):275–282. doi: 10.1159/000213037. [DOI] [PubMed] [Google Scholar]
- 105.Baum L, Chan IHS, Cheung SKK, et al. Serum zinc is decreased in Alzheimer’s disease and serum arsenic correlates positively with cognitive ability. BioMetals. 2010;23(1):173–179. doi: 10.1007/s10534-009-9277-5. [DOI] [PubMed] [Google Scholar]
- 106.Lovell MA, Smith JL, Xiong S, Markesbery WR. Alterations in zinc transporter protein-1 (ZnT-1) in the brain of subjects with mild cognitive impairment, early, and late-stage Alzheimer’s disease. Neurotoxicity Research. 2005;7(4):265–271. doi: 10.1007/BF03033884. [DOI] [PubMed] [Google Scholar]
- 107.Smith JL, Xiong S, Markesbery WR, Lovell MA. Altered expression of zinc transporters-4 and -6 in mild cognitive impairment, early and late Alzheimer’s disease brain. Neuroscience. 2006;140(3):879–888. doi: 10.1016/j.neuroscience.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 108.Lovell MA. A potential role for alterations of zinc and zinc transport proteins in the progression of alzheimer’s disease. Journal of Alzheimer’s Disease. 2009;16(3):471–483. doi: 10.3233/JAD-2009-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith MA, Harris PLR, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(18):9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid β-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radical Biology and Medicine. 2007;43(5):658–677. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Clements A, Allsop D, Walsh DM, Williams CH. Aggregation and metal-binding properties of mutant forms of the amyloid Aβ peptide of Alzheimer’s disease. Journal of Neurochemistry. 1996;66(2):740–747. doi: 10.1046/j.1471-4159.1996.66020740.x. [DOI] [PubMed] [Google Scholar]
- 112.Esler WP, Stimson ER, Jennings JM, Ghilardi JR, Mantyh PW, Maggio JE. Zinc-induced aggregation of human and rat β-amyloid peptides in vitro. Journal of Neurochemistry. 1996;66(2):723–732. doi: 10.1046/j.1471-4159.1996.66020723.x. [DOI] [PubMed] [Google Scholar]
- 113.Dong J, Shokes JE, Scott RA, Lynn DG. Modulating amyloid self-assembly and fibril morphology with Zn(II) Journal of the American Chemical Society. 2006;128(11):3540–3542. doi: 10.1021/ja055973j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miller Y, Ma B, Nussinov R. Zinc ions promote Alzheimer aβ aggregation via population shift of polymorphic states. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(21):9490–9495. doi: 10.1073/pnas.0913114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deshpande A, Kawai H, Metherate R, Glabe CG, Busciglio J. A role for synaptic zinc in activity-dependent aβ oligomer formation and accumulation at excitatory synapses. Journal of Neuroscience. 2009;29(13):4004–4015. doi: 10.1523/JNEUROSCI.5980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cherny RA, Legg JT, McLean CA, et al. Aqueous dissolution of Alzheimer’s disease Aβ amyloid deposits by biometal depletion. The Journal of Biological Chemistry. 1999;274(33):23223–23228. doi: 10.1074/jbc.274.33.23223. [DOI] [PubMed] [Google Scholar]
- 117.Cherny RA, Atwood CS, Xilinas ME, et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron. 2001;30(3):665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 118.Opazo C, Luza S, Villemagne VL, et al. Radioiodinated clioquinol as a biomarker for β-amyloid: Zn2+ complexes in Alzheimer’s disease. Aging Cell. 2006;5(1):69–79. doi: 10.1111/j.1474-9726.2006.00196.x. [DOI] [PubMed] [Google Scholar]
- 119.Ritchie CW, Bush AI, Mackinnon A, et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Archives of Neurology. 2003;60(12):1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 120.Mancino AM, Hindo SS, Kochi A, Lim MH. Effects of clioquinol on metal-triggered amyloid-beta aggregation revisited. Inorganic Chemistry. 2009;48(20):9596–9598. doi: 10.1021/ic9014256. [DOI] [PubMed] [Google Scholar]
- 121.Lannfelt L, Blennow K, Zetterberg H, et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. The Lancet Neurology. 2008;7(9):779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 122.Mo ZY, Zhu YZ, Zhu HL, Fan JB, Chen J, Liang Y. Low micromolar zinc accelerates the fibrillization of human Tau via bridging of Cys-291 and Cys-322. The Journal of Biological Chemistry. 2009;284(50):34648–34657. doi: 10.1074/jbc.M109.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.An WL, Bjorkdahl C, Liu R, Cowburn RF, Winblad B, Pei JJ. Mechanism of zinc-induced phosphorylation of p70 S6 kinase and glycogen synthase kinase 3β in SH-SY5Y neuroblastoma cells. Journal of Neurochemistry. 2005;92(5):1104–1115. doi: 10.1111/j.1471-4159.2004.02948.x. [DOI] [PubMed] [Google Scholar]
- 124.Cole TB, Martyanova A, Palmiter RD. Removing zinc from synaptic vesicles does not impair spatial learning, memory, or sensorimotor functions in the mouse. Brain Research. 2001;891(1-2):253–265. doi: 10.1016/s0006-8993(00)03220-0. [DOI] [PubMed] [Google Scholar]
- 125.Adlard PA, Parncutt JM, Finkelstein DI, Bush AI. Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer’s disease? Journal of Neuroscience. 2010;30(5):1631–1636. doi: 10.1523/JNEUROSCI.5255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schmitt-Ulms G, Ehsani S, Watts JC, Westaway D, Wille H. Evolutionary descent of prion genes from the ZIP family of metal Ion transporters. PLoS ONE. 2009;4(9) doi: 10.1371/journal.pone.0007208. Article ID e7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Duce JA, Tsatsanis A, Cater MA, et al. Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer's disease. Cell. 2010;142(6):857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Whitehouse IJ, Jackson C, Turner AJ, Hooper NM. Prion protein is reduced in aging and in sporadic but not in familial Alzheimer's Disease. The Journal of Alzheimer's Disease. 2010;22:1023–1031. doi: 10.3233/JAD-2010-101071. [DOI] [PubMed] [Google Scholar]
- 129.Parkin ET, Watt NT, Hussain I, et al. Cellular prion protein regulates β-secretase cleavage of the Alzheimer’s amyloid precursor protein. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(26):11062–11067. doi: 10.1073/pnas.0609621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Watt NT, Taylor DR, Gillott A, Thomas DA, Perera WSS, Hooper NM. Reactive oxygen species-mediated β-clevage of the prion protein in the cellular response to oxidative stress. The Journal of Biological Chemistry. 2005;280(43):35914–35921. doi: 10.1074/jbc.M507327200. [DOI] [PubMed] [Google Scholar]
- 131.Haigh CL, Drew SC, Boland MP, et al. Dominant roles of the polybasic proline motif and copper in the PrP23-89-mediated stress protection response. Journal of Cell Science. 2009;122(10):1518–1528. doi: 10.1242/jcs.043604. [DOI] [PubMed] [Google Scholar]