Abstract
The distinctive relations between biological activity and isotopic effect recorded in biomarkers (e.g., carbon and sulfur isotope ratios) have allowed scientists to suggest that life originated on this planet nearly 3.8 billion years ago. The existence of life on other planets may be similarly identified by geochemical biomarkers, including the oxygen isotope ratio of phosphate (δ18Op) presented here. At low near-surface temperatures, the exchange of oxygen isotopes between phosphate and water requires enzymatic catalysis. Because enzymes are indicative of cellular activity, the demonstration of enzyme-catalyzed PO4–H2O exchange is indicative of the presence of life. Results of laboratory experiments are presented that clearly show that δ18OP values of inorganic phosphate can be used to detect enzymatic activity and microbial metabolism of phosphate. Applications of δ18Op as a biomarker are presented for two Earth environments relevant to the search for extraterrestrial life: a shallow groundwater reservoir and a marine hydrothermal vent system. With the development of in situ analytical techniques and future planned sample return strategies, δ18Op may provide an important biosignature of the presence of life in extraterrestrial systems such as that on Mars.
The basic ingredients required for life include a source of chemical building blocks, energy, and liquid water (1). Mars has possessed all of these attributes in its history and may even have extensive reservoirs of subsurface liquid water today (2, 3). Phosphorus is essential to life on Earth and, therefore, an obvious candidate for a chemical tracer of the presence of life in extraterrestrial systems. Results from recent Mars Pathfinder surveys show relatively high concentrations of P (≈0.4 wt % P2O5) in Mars surface sediments that are thought to be derived from dissolution of apatite by aqueous fluids percolating through the surface layers of Mars and later enriched by evaporation.¶ It has been suggested that anomalies in phosphorus concentrations and P/Th ratios in sediments might be useful tracers of extinct life on Mars (4). The occurrence of phosphate, particularly in association with organic carbon, has been interpreted as the remains of once living organisms in ancient sedimentary strata (5, 6). Here, we demonstrate that the unique chemical and isotopic properties of PO4 make oxygen isotope ratios of phosphate (δ18OP) an ideal signature of the presence of enzymatic activity and, thus, life.
Stable isotope ratios can provide diagnostic signatures of biological activity because of the large and characteristic isotopic fractionations associated with many metabolic reactions (e.g., bacterial sulfate reduction and photosynthesis). As a result, the systematics of stable isotope fractionation for key bioelements (C, N, and S) have been studied extensively, and the isotopic ratios of these elements have been widely used as biosignatures in both terrestrial and extraterrestrial samples. An analogous isotopic biomarker system is not available for P, because, unlike C, N, and S, it has only one stable isotope. Phosphorus is also distinct from other major bioelements in that it occurs primarily in one oxidation state (+5), and in one major form, orthophosphate (PO4). δ18OP values have been used primarily as a paleotemperature proxy recorded in biogenic apatite minerals found in teeth, bones, fish scales, and shells (7). The PO4 radical is fairly inert to chemical redox reactions and to oxygen isotope exchange at low temperature (<80°C).
Enzymes are required for catalysis of oxygen isotope exchange between PO4 and water at low temperature. They are often invoked as the cause of diagenetic alteration of phosphate minerals leading to the loss of integrity of original δ18OP values. Detailed studies of enzyme-catalyzed PO4-H2O exchange reactions (8–10) demonstrate that one can exploit this feature to assess δ18OP values in a fundamentally different way: as an indicator of the presence of enzymatic activity and, hence, living organisms. Few investigators have examined oxygen isotope compositions of dissolved phosphate and nonbiogenic phosphate in modern sediments/soils, and, to our knowledge, no one has attempted to make use of the unique dependence of PO4–H2O oxygen isotope exchange on enzyme activity specifically as a biomarker. Here, we demonstrate the application of δ18Op as a biomarker in two terrestrial systems that serve as analogues for extraterrestrial planetary environments highly targeted in the search for life: a groundwater aquifer, and a marine hydrothermal vent system. Continued advances toward in situ sampling and analytical techniques, as well as sample-return missions planned for Mars, make δ18O analysis of phosphate and water in extraterrestrial materials plausible in the near future.
Method Development
Analytical Methods.
Oxygen isotope ratios of PO4 reported in this study were determined by conversion of inorganic PO4 extracted from groundwater or sediments into silver phosphate. The silver phosphate was then converted to CO2 after the method of O'Neil et al. (11) or by conventional fluorination by using ClF3. Water samples were analyzed by the standard method of CO2–H2O equilibration (12). Isotopic analyses were carried out in the stable isotope facilities at the University of Michigan (ISOLAB) and Yale University. Precision of the isotopic analyses was ±0.1‰ (1σ) for water and ±0.2‰ for phosphate.
All oxygen isotope data are reported in the standard delta notation in per mil (‰) relative to the SMOW (standard mean ocean water) international reference standard. The fractionation factor, α, between PO4 (p) and water (w) is defined as αp-w = (1 + δ18OP/1,000)/(1 + δ18OW/1,000).
Microbial growth and phosphate metabolism studies were carried out as described in the legends of associated figures and data tables.
Calibration of the δ18OP Biomarker.
To employ this potential biomarker, we must first consider the processes recorded by inorganic PO4 dissolved in natural waters and associated with sediments. Reactions of PO4 in natural waters, sediments, and soils are largely under biological control because of the importance of P as a nutrient for microorganisms and plants. There are very few studies of the oxygen isotope systematics of dissolved PO4 and P cycling in natural waters and sediments (13, ‖). However, oxygen isotope compositions of PO4 precipitated by living organisms in the form of biogenic apatite minerals (teeth, shells, and bone) have been studied extensively (e.g., refs. 14–16). Biogenic phosphate is equilibrated with body fluids of an organism as a result of intracellular enzymatic reactions (e.g., ATP utilization and phosphorylation/dephosphorylation). The temperature dependence of oxygen isotope fractionation between apatite and water has been determined both empirically and in laboratory experiments (17–20). The most widely cited relation is the empirical equation of Longinelli and Nuti, t (°C) = 111.4 − 4.3[δ18OP − δ18OW], which is generally assumed to reflect equilibrium isotopic fractionation (17).
In contrast to biogenic phosphates, where the exchange of PO4 with body fluids and subsequent precipitation as apatite occur in a closed system, the metabolism of P in natural waters is mediated largely by microorganisms in a relatively open system. This phenomenon occurs because microbial metabolism of P compounds (inorganic and organic) involves the action of extracellular phosphate-scavenging enzymes and intracellular–extracellular PO4 exchange processes (ion transport proteins), resulting in the release of inorganic PO4 into the extracellular environment (21). Many of these enzymatic reactions involving PO4 are dominated by hydrolytic cleavage reactions that facilitate the incorporation of water (from the medium) into PO4. These reactions promote significant PO4–H2O oxygen isotope exchange that is reflected in the δ18O value of dissolved PO4. Biochemists have exploited this property for decades in elegant studies of phosphoenzyme reaction kinetics and mechanisms by using heavily labeled (e.g., >90% 18O) water and P compounds (22, 23). The capacity of δ18OP as a biomarker at natural 18O abundance levels also results from its ability to record enzymatic activity, and from the lack of significant PO4–H2O oxygen isotope exchange by other abiotic geochemical processes involving P. For example, the precipitation of dissolved phosphate as apatite, important during sediment diagenesis, does not involve exchange of PO4 oxygen isotopes and results in only small dissolved PO4-apatite fractionations (≈1‰; R.E.B. and Y. Liang, unpublished data).
The low dissolved PO4 concentrations found in most natural systems, coupled with intense biological recycling and turnover characteristic of P (24), should ensure complete oxygen isotope exchange between dissolved PO4 and ambient water. Thus, oxygen isotope ratios of dissolved PO4 in natural waters and associated with sediments should record the effects of microbial P metabolism and microbial enzyme activity at the ambient environmental water and temperature conditions.
In summary, δ18OP values can be used to detect enzymatic activity in two ways: (i) by recording exchange between PO4 and ambient water, and (ii) by recording ambient temperature. Both result from the process of PO4–H2O oxygen isotope exchange. At low near-surface temperatures, PO4–H2O exchange is catalyzed by the action of enzymes. Results from detailed laboratory studies of oxygen isotope fractionation associated with microbial metabolism of P compounds and enzyme catalyzed PO4–H2O exchange reactions provide a context for the interpretation of δ18OP values and for the detection of enzymatic activity in natural systems.
Relation between δ18OP, δ18OW, and Temperature.
Extensive oxygen isotope exchange between water and dissolved PO4 is demonstrated in laboratory experiments where bioavailable P compounds (inorganic orthophosphate and organic phosphoesters) were metabolized by microorganisms (Fig. 1; Table 1) or degraded by purified cell-free phosphoenzymes (Fig. 2; Table 2; refs. 8 and 9). This exchange occurs even at dissolved PO4 concentrations that are more than 1,000 times those typical of natural systems. Phosphate–water exchange is reflected in a strong positive correlation between the δ18O value of water and the δ18O value of dissolved PO4 (Figs. 1 and 2). Experiments with bacteria also show that microbial metabolism of P is characterized by equilibrium oxygen isotope fractionations that vary with temperature in a manner similar to that observed for biogenic apatites (Fig. 3; Table 3). Similar results were obtained in experiments on the temperature dependence of PO4–H2O oxygen–isotope exchange catalyzed by cell-free, purified enzymes (R.E.B., J. R. O'Neil, and G. A. Garcia, unpublished data), demonstrating that catalytic molecules that are derived from, or possibly precursors of, complex cells may also leave behind a distinctive geochemical signature of biological activity.
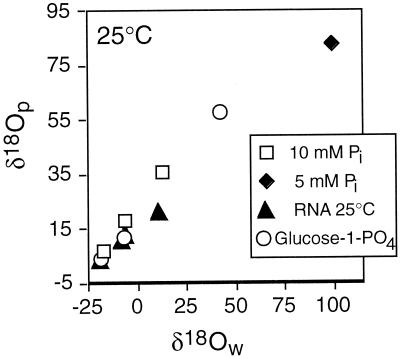
Figure 1.
Microbial P metabolism experiments. Mixed (from soils, rivers) and pure strains of microorganisms [e.g., Klebsiella, Bacillus, Aquaspirillum) were grown on representative bioavailable P compounds (inorganic orthophosphate (Pi), organic P monoesters (glucose-PO4) and diesters (RNA)] to study PO4–H2O oxygen isotope exchange during microbial metabolism of P. Microbial metabolism of P leads to a positive correlation between δ18O of water and δ18O of dissolved PO4 in the medium, for both inorganic and organic P substrates. Plots show representative data from 25°C and include data from Blake et al. (8, 9).
Table 1.
Oxygen isotope compositions of water and dissolved inorganic PO4 derived from various P sources and metabolized by microbial cell cultures at 25°C and plotted in Fig. 1
| P source | δ18Ow | δ18Op |
|---|---|---|
| 5 mM Pi | 99 | 82.6 |
| 10 mM Pi | −17.7 | 7.3 |
| −6.0 | 18.0 | |
| 12.6 | 35.6 | |
| RNA | −6.1 | 13.0 |
| −6.5 | 12.8 | |
| −19.3 | 3.6 | |
| −8.0 | 11.1 | |
| 10.4 | 21.7 | |
| Glucose-1-PO4 | −18.2 | 4.1 |
| −6.7 | 12.1 | |
| 42.2 | 57.9 |
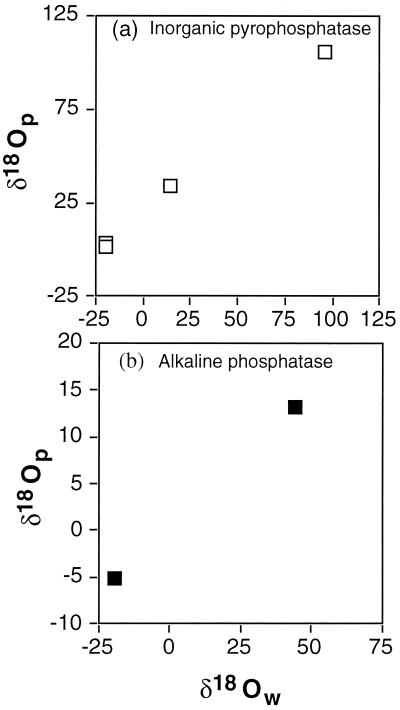
Figure 2.
Purified cell-free enzyme catalyzed PO4–H2O oxygen isotope exchange. Positive correlation between δ18O of dissolved inorganic PO4 and water resulting from PO4–H2O exchange catalyzed by hydrolytic phosphoenzymes inorganic pyrophosphatase (PPiase) and alkaline phosphatase (APase). (a) PPiase exchange experiments at 22°C and 30°C in water with δ18O of −19.7‰ to 96.4‰. The initial δ18Op value of dissolved inorganic PO4 before exchange was 13.5‰. (b) APase exchange experiments in −19.4‰ and 44.1‰ water at 37°C using glycero-2-phosphate as an organic-P substrate (P source).
Table 2.
Oxygen isotope compositions of water and dissolved inorganic PO4 derived from reaction of organic-P compounds and inorganic PO4 with purified, cell-free phosphatase (hydrolytic) enzymes
| P source | δ18Ow | δ18Op |
|---|---|---|
| Alkaline phosphatase at 37°C | ||
| Glycero-2-PO4 | −19.4 | −5.2 |
| 44.1 | 13.1 | |
| Inorganic pyrophosphatase at 22–30°C | ||
| 20 mM Pi | −19.7 | 3.6 |
| 10 mM Pi | 14.2 | 33.7 |
| 10 mM Pi | −19.5 | 1.8 |
| 10 mM Pi | 96.4 | 105.8 |
Data plotted in Fig. 2.
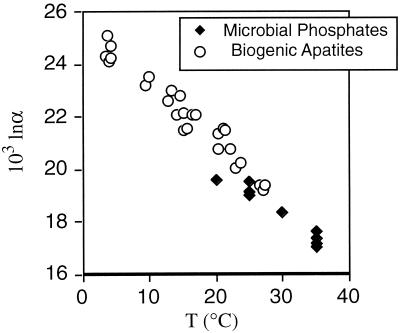
Figure 3.
Temperature dependence of PO4–H2O oxygen isotope exchange accompanying microbial metabolism of P compounds. The PO4–H2O fractionations produced by bacteria agree well with fractionations determined from biogenic apatites. Biogenic apatite data are from Longinelli and Nuti (18) and are the basis for the PO4–H2O thermometry equation: t (°C) = 111.4 − 4.3[δ18OP − δ18OW] (18). Microbial phosphate data from Blake et al. (8, 9).
Table 3.
Oxygen isotope ratios of water and dissolved inorganic phosphate liberated during microbial degradation of organophosphorus compounds (RNA) at 20 to 35°C
| T, °C | δ18Ow | δ18Op | 103 lnα |
|---|---|---|---|
| 20 | −6.3 | 13.4 | 19.63 |
| 25 | −6.5 | 12.7 | 19.14 |
| 25 | −6.1 | 13 | 19.03 |
| 30 | −6.2 | 12.2 | 18.35 |
| 35 | −6.2 | 11.5 | 17.65 |
| 35 | −6.6 | 10.8 | 17.36 |
| 35 | −5.5 | 11.7 | 17.15 |
| 25 | −8 | 11.6 | 19.57 |
| 35 | −0.5 | 16.7 | 17.06 |
Data plotted in Fig. 3.
The above results indicate that microbial metabolism of P should result in oxygen isotope exchange between dissolved PO4 and ambient water, regardless of the nature (organic or inorganic) or initial δ18O value of the P source. Further, such metabolized PO4 should record the temperature of equilibrium exchange according to the same relation used for oxygen isotope thermometry of biogenic apatites. Therefore, in conjunction with measured or inferred temperature and δ18Ow values, the δ18O value of dissolved PO4 and sediment-associated PO4 can be used to detect enzymatic activity and, thus, infer the presence of life.
Application of the δ18OP Biomarker
Dissolved PO4 in Groundwater—Correlation Between δ18Ow and δ18OP.
Oxygen isotope ratios of dissolved PO4 from a shallow glacial outwash aquifer in Cape Cod, MA were measured in a study of microbial turnover of PO4 in groundwater.¶ Dissolved phosphate concentrations (30–108 μM) reach levels that are 1 to 2 orders of magnitude higher than those in typical aquatic and marine systems because of contamination of the ground water by sewage. Phosphate within the contaminant plume is not fully equilibrated with ground water at measured ambient aquifer temperatures (Table 4). Complete metabolic turnover and exchange of the entire phosphate pool with water is probably limited by low dissolved organic carbon concentrations in the aquifer. At dissolved PO4 concentrations more typical of uncontaminated systems (<5 μM), it is expected that the entire phosphate pool will be metabolized and more fully equilibrated with water.
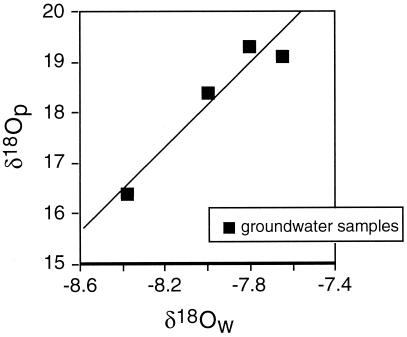
Table 4.
δ18O of dissolved inorganic PO4 and groundwater in the Cape Cod aquifer
| δ18Ow | δ18Op | T, °C |
|---|---|---|
| −8.0 | 18.4 | 9.5 |
| −7.8 | 19.3 | 7.7 |
| −7.7 | 19.1 | 7.7 |
| −8.4 | 16.4 | 8.2 |
| −7.9 | 18.4 | 13.7 |
δ18O values of dissolved PO4 in the groundwater show clear evidence of phosphate metabolism. There is a positive correlation between the δ18O of water and the δ18O of dissolved PO4 (Fig. 4). This correlation suggests that extensive oxygen isotope exchange has occurred between the water and dissolved PO4. Importantly, temperatures measured within the aquifer, approximately 8°C to 14°C, are much too low for the observed PO4–H2O exchange to have occurred by abiotic, nonenzymatic reactions. Therefore, enzymatic activity and metabolism of dissolved inorganic PO4 in the groundwater is indicated.
Figure 4.
Comparison between the δ18O of dissolved PO4 and groundwater from the Cape Cod aquifer. Positive correlation between δ18OP and δ18OW at low temperature (8°C to 14°C) indicates the presence of enzyme catalyzed PO4–H2O oxygen isotope exchange and active microbial metabolism of P within the aquifer.
δ18O of Dissolved Inorganic PO4 Associated with Sediments: Fe Oxide Deposits in a Marine Hydrothermal System.
Currently, it is widely held that life on Earth may have originated in hydrothermal systems (25). Protected from intense UV irradiation by overlying water, the first biomolecules and primitive cellular life may have evolved in deep-sea vent environments by using the energy supplied by hydrothermal activity and reduced forms of sulfur and hydrogen (25). Similar hydrothermal systems may have been active over 3 billion years ago on Mars (26). Phosphate in active and fossil hydrothermal systems may provide evidence of metabolic activity.
Dissolved inorganic PO4 can become strongly associated with sediments, especially iron oxides, via sorption or precipitation processes (27). Hydrothermal iron oxide deposits are produced by oxidation of erupting hydrothermal fluids rich in reduced iron, by ambient oxygenated seawater (27, 28) and in some cases, may involve the action of iron-oxidizing bacteria (29, 30). Iron oxides are very efficient scavengers and natural concentrators of dissolved PO4 (27, 31).
The δ18OP of inorganic PO4 associated with Fe oxide deposits (Fe–PO4) was analyzed from two diffuse flow hydrothermal systems at Larson's Seamounts located near 21°N along the East Pacific Rise (EPR) and from Seamount No. 5, located 86 km east of the EPR near 13°N (refs. 29 and 32, and Table 5). These hydrothermal systems are located off-axis of the main EPR spreading center. One sampling site at Red Seamount was experiencing active venting at the time of sampling whereas the other sites were inactive and comprised fossil hydrothermal deposits.
Table 5.
δ18O values of PO4 associated with hydrothermal Fe-oxide deposits
| Sampling location | δ18Op | T, °C calc.* |
|---|---|---|
| Green Seamount | 25.6 (n = 4) | 1.3 |
| Seamount no. 5 | 25.1 (n = 3) | 3.5 |
| Red Seamount | 11.9 (n = 4) | 60.2 |
| Red Seamount | 23.4 (n = 7) | 10.8 |
Temperatures calculated using the Longinelli and Nuti equation (17).
Evidence for enzymatic activity and microbial metabolism of the PO4 associated with these Fe oxide deposits comes from the temperatures recorded in PO4 oxygen isotope ratios. Hydrothermal systems provide a variable temperature structure that can be used to detect PO4–H2O oxygen isotope exchange in an otherwise constant δ18Ow environment of ambient seawater. Iron oxides at Red Seamount are believed to have formed by precipitation from actively venting hydrothermal fluids. At Green Seamount, the ocherous, Fe oxide sediments are interpreted to be secondary alteration products formed by oxidation of iron sulfide minerals precipitated from hydrothermal fluids (29, 32, 33).
δ18O values of PO4 associated with iron oxide deposits collected from Red Seamount record temperatures that are very warm relative to ambient seawater (2°C–5°C; Table 5). One sample taken from the upper 40 cm of an actively venting orifice yielded a temperature of 10.8°C that closely matches measured vent temperatures of 10°C–15°C (29). δ18Op temperatures were calculated by using the PO4–H2O temperature equation (17) and assume a δ18O of seawater of 0‰. The recording of temperatures as low as 10.8°C in δ18OP values strongly suggests that the PO4 has been metabolized and has undergone oxygen isotope exchange with seawater by enzyme-catalyzed reactions at ambient temperatures.
Temperatures calculated from δ18O values of Fe-PO4 at another site at Red Seamount that was not actively venting at the time of sampling averaged 60°C. This higher temperature suggests the venting of warm temperature fluids at this site in the past. Further evidence for higher temperature processes in the past at Red Seamount is provided by the presence of iron-rich talc deposits and from temperatures calculated from δ18O values of nontronite deposits (32). A temperature of 60°C, however, is still too low to promote significant PO4–H2O oxygen isotope exchange by inorganic/abiotic means. Thus, the warm temperatures recorded in δ18Op values of these Fe-PO4 deposits also suggest the presence of enzymatic activity and microbial metabolism of the PO4.
Oxygen isotope exchange between PO4 and water may also have occurred deeper within the sediments or in the underlying basaltic basement rocks at higher temperatures, by the action of subsurface bacteria. Subsurface vent microbial habitats have been reported from Loihi Seamount, where thermophilic bacteria with high metabolic activity were observed at temperatures of 60°C (30).
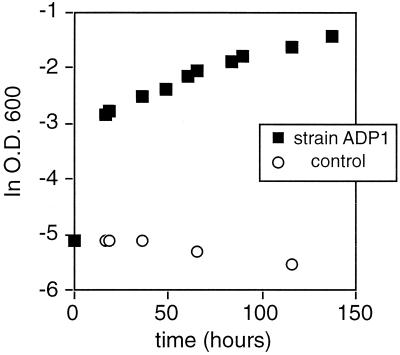
The mechanism proposed for exchange of Fe-PO4 with water is microbial uptake and metabolism of the phosphate. This mechanism requires that PO4 bound to Fe oxides be biologically available. To assess the bioavailability of Fe oxide-associated PO4, separate aliquots of the Fe oxide sediments analyzed for δ18Op were used in microbial growth experiments as a sole source of P. The two strains of bacteria tested in these experiments, Acinetobacter ADP1 and Shewenella MR-1, were able to grow readily on minimal media with Fe-PO4 sediments supplied as the sole source of P (Fig. 5). No dissolved PO4 was detected in the Fe-PO4 growth media initially or over the course of the growth experiments, as determined by periodic extraction and analysis of growth media for dissolved PO4. Under the oxic conditions of the growth experiments and also of natural environments of formation of Fe oxides, PO4 is efficiently scavenged from solution by the Fe oxide sediments.
Figure 5.
Bioavailability of PO4 from Red Seamount hydrothermal Fe oxide deposits. Growth curve measuring changes in optical density of microbial cultures measured as absorbance at 600 nM, during growth of Acinetobacter ADP1 on minimal salts medium M9 with Fe-PO4 sediment as a sole P source at room temperature (≈22°C). Fe-PO4 sediments were rinsed with sterile medium before growth experiments to remove easily desorbed PO4. No PO4 was released to the rinse buffer during this procedure.
δ18O values of Fe-PO4 samples from the inactive fossil deposits at Green Seamount average 25–26‰ and reflect cold temperatures near that of the ambient seawater (Table 5). The absence of recorded warm temperatures in these deposits is also consistent with their mode of origin as secondary products of the oxidation of hydrothermal iron sulfides at ambient seawater temperatures.
Iron oxide deposits from both Red and Green Seamount also show morphological evidence of microbial activity (29, 33). The iron oxides are composed, almost entirely in some cases, of twisted filaments, stalks, and hollow sheaths that resemble the characteristic remains of the iron-oxidizing bacteria Gallionella ferruginea and Leptothrix known from neutral pH freshwater systems (29). Similar microbial structures have been observed in iron oxide deposits from Loihi Seamount (30). High-resolution structural analyses of iron oxyhydroxides produced by bacteria of the Gallionella and Leptothrix genus indicate that these iron biominerals have distinct structural orientations (34). Recent discoveries, however, suggest that novel iron-oxidizing bacteria that deposit iron oxides not in the morphologically distinct stalks and sheaths of Gallionella and Leptothrix, but as amorphous iron oxide coatings, may be responsible for the majority of microbial iron oxidation in near-neutral pH environments such as seawater (35). Despite strong morphological evidence for the presence of Fe-oxidizing bacteria in some hydrothermal sediments, the importance of microbial activity in formation of hydrothermal iron oxides remains the subject of debate. δ18O values of PO4 associated with the microbial iron oxides from Red Seamount provide independent and strong geochemical evidence of metabolic activity within these types of sediments. The association of this PO4 with iron oxides of probable microbial origin makes Fe-oxidizing bacteria the prime candidate for metabolism of the PO4. The exact location and identity of microbes responsible for PO4 metabolism in this system is currently under investigation.
Implications for the Search for Extraterrestrial Life
Mounting evidence for the possible existence of liquid water in subsurface sediments on Mars, beneath the ice-covered surface of Europa, and most recently, Ganymede and Callisto (36), make application of the δ18OP biomarker tenable. Systems most likely to be targeted in the search for evidence of extraterrestrial life are those that show evidence of liquid water today (e.g., groundwater seeps on Mars or beneath ice on Europa). In the absence of water for direct comparison of dissolved PO4 δ18OP, certain sediment and mineral phases may be used for δ18OP measurement. Specifically, analyses could be made of sediment-associated PO4 (Fe oxide-bound P) and authigenic phosphate minerals formed by precipitation of dissolved PO4 from low-temperature fluids, especially occurrences of such phases in sediments and formations interpreted as lacustrine, fluvial, marine, or hydrothermal in origin. Any phosphate that has been processed by biological activity should have an anomalous δ18OP value when compared with δ18OP of potential PO4 sources, such as basaltic Martian bedrock.
On Earth, the largest occurrence of biogenic and biologically processed PO4 is in marine systems (e.g., phosphorite deposits, marine sediments, and dissolved oceanic P). Compared with high-temperature basaltic and igneous sources of PO4 (5.3‰ to 8‰; refs. 13 and 37), the δ18OP of biogenic marine phosphate has a distinctly heavy signature (≈20‰–25‰; refs. 13 and 17) because of the constant, relatively heavy δ18O value of seawater coupled with the large oxygen isotope fractionations between PO4 and water at low, near-surface temperatures (Fig. 3). Markel (13) identified biological cycling of phosphate in lake sediments based solely on varying δ18OP values of detrital and authigenic apatite mineral phases in the sediments, without requiring the δ18O value of the water. Anomalous δ18OP values in soils have also been attributed to biological activity (37, 38). Thus, δ18OP variations and anomalies in sedimentary and authigenic phosphate phases formed at low temperature are also indicative of biological activity. The relatively high concentrations of P measured in Mars surface sediments by Mars Pathfinder (4),¶ if present as PO4, would easily allow δ18OP analysis.
Conclusions
It has been suggested that life may exist elsewhere in our solar system, in near-surface groundwater on Mars (2) or beneath the ice-covered surface of Europa (39). It is also possible that life evolved 3.5 billion years ago on Mars when hydrothermal systems are believed to have been active (26, 40). Our application of δ18Op as a biomarker demonstrates that δ18Op values can successfully detect enzymatic activity and microbial metabolism in terrestrial systems. These systems serve as important analogues for extraterrestrial environments that are targeted in the search for life because of the possible presence of liquid water. The δ18Op biomarker should provide strong evidence for the presence of life.
Acknowledgments
We thank G. A. Garcia for helpful suggestions in the design of enzyme experiments and J. R. O'Neil and J. P. Greenwood for fruitful discussions and helpful comments. Thanks also go to N. F. Moreira and D. LeBlanc for field sampling support and analysis. This work was supported in part by: National Science Foundation Postdoctoral Fellowship (awarded to R.E.B., 1998); Bateman Postdoctoral Research Award (R.E.B., Yale University, 1998); and National Science Foundation Grant OCE-00-82416.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Dreibus, G., Bruckner, J. & Wanke, H. Lunar Planet. Sci. XXXI, 31, 1127 (abstr.).
Moreira, N. F., Martini, A. M. & Blake, R. E., Northeast Section Meeting of the Geological Society of America, March 2000, New Brunswick, NJ (abstr.).
References
- 1.Jakosky B M. J Geophys Res. 1997;102:23673–23674. [Google Scholar]
- 2.Malin M C, Edgett K. Science. 2000;288:2330–2335. doi: 10.1126/science.288.5475.2330. [DOI] [PubMed] [Google Scholar]
- 3.Leshin L A. Geophys Res Lett. 2000;27:2017–2020. [Google Scholar]
- 4.Weckwerth G, Shcidlowski M. Adv Space Res. 1995;15:185–191. doi: 10.1016/s0273-1177(99)80082-9. [DOI] [PubMed] [Google Scholar]
- 5.Mojzsis S J, Arrhenius G, McKeegan K D, Harrison T M, Nutman A P, Friend C R L. Nature (London) 1996;384:55–59. doi: 10.1038/384055a0. [DOI] [PubMed] [Google Scholar]
- 6.Mojzsis S J, Arrhenius G. J Geophys Res. 1998;103:28495–28511. [Google Scholar]
- 7.Fricke H C, O'Neil J R. Paleont Paleogr Palaeo. 1996;126:91–100. [Google Scholar]
- 8.Blake R E, O'Neil J R, Garcia G A. Geochim Cosmochim Acta. 1997;61:4411–4422. [Google Scholar]
- 9.Blake R E, O'Neil J R, Garcia G A. Am Mineral. 1998;83:1516–1531. [Google Scholar]
- 10.Blake R E, O'Neil J R, Garcia G A. Mineralog Mag. 1998;62A:163–164. [Google Scholar]
- 11.O'Neil J R, Roe L J, Reinhard E, Blake R E. Isr J Earth Sci. 1994;43:203–212. [Google Scholar]
- 12.Cohn M, Urey H C. J Am Chem Soc. 1938;60:679–682. [Google Scholar]
- 13.Markel D, Kolodny Y, Luz B, Nishri A. Isr J Earth Sci. 1994;43:165–178. [Google Scholar]
- 14.Luz B, Kolodny Y. Earth Planet Sci Lett. 1985;75:29–36. [Google Scholar]
- 15.Bryant J D, Froelich P N, Showers W J, Genna B J. Palaios. 1996;11:397–408. [Google Scholar]
- 16.Longinelli A. Geochim Cosmochim Acta. 1984;48:385–390. [Google Scholar]
- 17.Longinelli A, Nuti S. Earth Planet Sci Lett. 1973;19:373–376. [Google Scholar]
- 18.Kolodny Y, Luz B, Navon O. Earth Planet Sci Lett. 1983;64:398–404. [Google Scholar]
- 19.Lecuyer C, Grandjean P, Emig C C. Paleont Paleogr Palaeo. 1996;126:101–108. [Google Scholar]
- 20.Shemesh A, Kolodny Y, Luz B. Geochim Cosmochim Acta. 1988;52:2433–2438. [Google Scholar]
- 21.Maloney P C. BioEssays. 1992;14:757–762. doi: 10.1002/bies.950141106. [DOI] [PubMed] [Google Scholar]
- 22.Cohn M. J Biol Chem. 1953;201:735–750. [PubMed] [Google Scholar]
- 23.Boyer P D. Am Chem Soc Accts Chem Res. 1978;11:295–305. [Google Scholar]
- 24.Benitez-Nelson C R, Buesseler K O. Nature (London) 1999;398:502–505. [Google Scholar]
- 25.Simpson S. Science News. 1999;155:24–26. [Google Scholar]
- 26.Shock E L. J Geophys Res. 1998;102:23687–23694. doi: 10.1029/97je01087. [DOI] [PubMed] [Google Scholar]
- 27.Mills R A, Elderfield H. AGU Geophys Monogr. 1995;91:392–407. [Google Scholar]
- 28.Hannington M D, Jonasson I R. In: Biomineralization Processes, Iron, Manganese. Skinner H C W, Fitzpatrick R W, editors. Cremlingen-Destedt, Germany: Catena Verlag; 1992. pp. 351–370. [Google Scholar]
- 29.Alt J C. Marine Geol. 1988;81:227–239. [Google Scholar]
- 30.Karl D M, McMurty G M, Malahoff A, Garcia M O. Nature (London) 1988;335:532–535. [Google Scholar]
- 31.Froelich P N, Bender M L, Heath G R. Earth Planet Sci Lett. 1977;34:351–359. [Google Scholar]
- 32.Alt J C, Lonsdale P, Haymon R, Muehlenbachs K. Geol Soc Am Bull. 1987;98:157–168. [Google Scholar]
- 33.Alt J C. Econ Geol. 1988;83:1026–1033. [Google Scholar]
- 34.Banfield J F, Welch S A, Zhang H, Ebert T T, Penn R L. Science. 2000;289:751–754. doi: 10.1126/science.289.5480.751. [DOI] [PubMed] [Google Scholar]
- 35.Emerson D, Moyer C. Appl Environ Microbiol. 1997;63:4784–4792. doi: 10.1128/aem.63.12.4784-4792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang K. New York Times. 2000. , Dec. 24, Week in Review, p. 27. [Google Scholar]
- 37.Mizota C, Domon Y, Yoshida N. Geoderma. 1992;53:111–123. [Google Scholar]
- 38.Ayliffe L K, Veeh H H, Chivas A R. Earth Planet Sci Lett. 1992;108:119–129. [Google Scholar]
- 39.Carr M H, Belton M J, Chapman C R, Davies M E, Geissler P, Greenberg R, McEwen A S, Tufts B R, Greeley R, Sullivan R, et al. Nature (London) 1998;391:363–365. doi: 10.1038/34857. [DOI] [PubMed] [Google Scholar]
- 40.Jakosky B M, Shock E L. J Geophys Res Planets. 1998;103:19359–19364. doi: 10.1029/98je01892. [DOI] [PubMed] [Google Scholar]