Abstract
The role of mammary tumor epithelial cell (MEC) NF-κB in tumor progression in vivo is unknown as murine NF-κB components and kinases are either required for murine survival or interfere with normal mammary gland development. As NF-κB inhibitors block both tumor-associated macrophages (TAM) and MEC NF-κB, the importance of MEC NF-κB to tumor progression in vivo remained to be determined. Herein, an MEC-targeted inducible transgenic inhibitor of NF-κB (IκBαSR) was developed in ErbB2 mammary oncomice. Inducible suppression of NF-κB in the adult mammary epithelium delayed the onset and number of new tumors. Within similar sized breast tumors, TAM and tumor neoangiogenesis was reduced. Co-culture experiments demonstrated MEC NF-κB enhanced TAM recruitment. Genome wide expression and proteomic analysis demonstrated IκBαSR inhibited tumor stem cell pathways. IκBαSR inhibited breast tumor stem cell markers in transgenic tumors, reduced stem cell expansion in vitro, and repressed expression of Nanog and Sox2 in vivo and in vitro. Mammary epithelial cell NF-κB contributes to mammary tumorigenesis. As we show NF-κB contributes to expansion of breast tumor stem cells and heterotypic signals that enhance TAM and vasculogenesis, these processes may contribute to NF-κB dependent mammary tumorigenesis.
Keywords: ErbB2, NF-κB, inducible transgenics, Ponasterone A
INTRODUCTION
NF-κB proteins, which play a key role in regulating the inflammatory response (1), participate in two intersecting signal pathways. The canonical NF-κB pathway proteins (RelA, c-Rel and p50), form dimers with similar DNA binding specificity (2). The dimers are held in an inactive cytoplasmic complexes with proteins of the IκB (Inhibitor of NF-κB) family. IκB kinases (IKK) phosphorylate IκB bound to NF-κB, directing the IκB to ubiquitin-dependent degradation (3) thereby releasing NF-κB dimers to enter the nucleus. The alternate pathway utilizes the RelB/p52 dimer, which is regulated primarily by the IKKα and interacts with a mostly distinct subset of target genes (4). NF-κB family members are overexpressed or activated in breast cancer cell lines, primary human breast tumors and a range of other malignancies (5, 6). Inhibition of NF-κB in vivo enhanced squamous cell tumor growth (7, 8) and enhanced the development of hepatocellular carcinoma (9). In contrast, the inactivation of IKKβ in intestinal epithelial cells reduced colonic tumor formation in response to chemical pro-carcinogens (10).
Efforts to determine the specific role of mammary epithelial cell vs inflammatory cell NF-κB in tumor progression have been confounded by three key principles. Firstly, most NF-κB signaling proteins are essential for completing embryogenesis or post-natal mammary growth and development, precluding the use of genetically-deleted mouse models for exploring NF-κB function in mammary tumorigenesis in vivo (11). The NF-κB proteins (RelA, p50) and the IκB proteins (IκBα) are expressed during normal murine postnatal mammary gland morphogenesis (12). Transplantation experiments, using tissue donated by mice deficient in NF-κB activity due to IκBα overexpression, support a role for NF-κB in mammary epithelial proliferation and branching during normal mammary development (13). Analysis of the alternate pathway of NF-κB signaling demonstrated IKKα and the Rel-B/p52 complex contribute to mammary gland development and IKKαA/A mice, have defective development of terminal alveolar breast buds (14).
Secondly, NF-κB is expressed widely in multiple distinct cell types. These distinct cell types (inflammatory cells, blood vessels, adipocytes, breast epithelial cells) may each contribute independently to the tumor phenotype. The role of tumor epithelial cell NF-κB in breast tumor progression in vivo in the immune-competent state is poorly understood. The inflammatory response governing interactions between tumor cells and infiltrating immune cells in the tumor microenvironment can either promote or inhibit tumor development (1). Thus, dissecting the role of NF-κB in the mammary epithelial cell requires MEC cell-type-specific regulation in immune-competent animals. Thirdly, NF-κB activity may contribute differently during distinct phases of tumorigenesis. Thus, NF-κB may alter its function to either inhibit or promote differentiation or tumorigenesis during development or tumor progression. Temporarily controlled genetic regulation in the fully developed mammary gland is therefore essential for such analysis.
Herein, we determined the role of NF-κB in ErbB2 mammary tumorigenesis in vivo by developing an animal model that permits inducible suppression of NF-κB activity, targeted to the mammary gland. Transgenic mice were generated encoding an ecdysone-regulated stabilized IκBα protein (IκBα SuperRepressor: IκBαSR), in which serine residues 32 and 36 of IκBα have been changed to alanine and are therefore unavailable to IKKα-mediated phosphorylation and degradation. The three cointegrated transgenes allowed temporally controlled suppression of NF-κB activity in the normally developed mammary gland. These triple transgenics were crossed to mammary-targeted ErbB2 to determine the requirement for NF-κB activity in ErbB2-mediated mammary tumorigenesis in vivo.
Using spatial and temporal control of mammary epithelial cell NF-κB in vivo we demonstrate that mammary epithelial cell NF-κB promotes tumor onset, growth, angiogenesis and the infiltration of TAM. Inhibition of NF-κB correlated with reduction in epithelial mesenchymal transition (EMT), a reduction in breast tumor stem cell expansion and a reduction in a heterotypic signal in which MEC recruited TAM.
MATERIALS AND METHODS
Cell culture, retroviral infection, luciferase reporter assay
The 293T, NAFA, ErbB2-induced mammary tumor cell lines and mouse embryo fibroblasts (MEFs) were cultured as described (15, 16). MCF10A, MCF10A-NeuT cells were cultured in DMEM/F-12 50/50 (Cellgro, Mediatech Inc., Herndon, VA) as recommended by the American Type Culture Collection. Primary murine mammary epithelial cell (MEC) was collected from transgenic mice and cultured as previously described (17). To make the MSCV-IκBαSR-IRES-GFP retroviral vector, an EcoRI fragment coding for IκBαSR (18) was subcloned into the MSCV-IRES-GFP vector (19) at the EcoRI site upstream of the IRES driving expression of GFP (20). Viral supernatants were prepared and target cells were infected as previously described (20). The expression vector plasmid, pVgRXR was obtained from Invitrogen (Carlsbad, CA). The Ponasterone A inducible pBPSTR1-VgEcR/RXRα-EGRE3-IκBαSR vector is an all in one retrovirus based on the pBPSTR1 system. VgEcR and RXRα were subcloned into pBPSTR1 together with EGRE3-IκBαSR. The NF-κB-responsive reporter construct 3xRel-luc, was as previously described (21). Transfections were performed with Superfect transfection reagent (Qiagen, Valencia, CA) or Lipotectamine 2000 Transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacture's protocols. The relative firefly luciferase activities were calculated by normalizing transfection efficiency according to the Renilla luciferase activities. TNF-α was obtained from Sigma (St. Louis, MO). Statistical analyses were performed using the Student t test.
Colony formation, cellular proliferation, DNA synthesis, cell-cycle, apoptosis
Colony formation assay, [3H] thymidine (TdR) incorporation and cell proliferation analysis determined using MTT were previously described (22). Flow cytometry was used for cell-cycle analysis followed the standard protocol (22). Apoptotic cells were assessed using the Annexin V-FITC apoptosis detection kit (Roche, Grenzacherstrasse, Switzerland).
Immunohistochemistry and Angiogenesis
Immunohistochemical staining was performed using the EnVision+ System-HRP following manufacturers guidelines. Slides were made from paraffin-embedded murine mammary tissue from MMTV-ErbB2 and MMTV-ErbB2/IκBαSR mice (N=5/group). The following primary antibodies were used [(CD31 (CM 303A, BIOCARE MEDICAL, Concord CA) (1/50); CD34 (ab8158, abCam, Cambridge, MA) (1/50); vWF (A0082, DAKO, Carpinteria, CA) (1/500); VEGF (sc-507, Santa Cruz, CA) (1/250); VCAM-1 (sc-8304, Santa Cruz, CA) (1/250); CD44 (#550538, BD Pharmingen, Franklin Lakes, NJ) (1/50); F4/80 (ab6640, abCam, Cambridge, MA) (1/700); FLAG-IκBαSR (A9594, Sigma, St. Louis MO) (1/100)]. The signal was visualized using a Cyanine 3 (Cy-3) labeled Tyramide amplification system (Perkin Elmer Life Sciences, INC., Boston, MA). Sections were mounted in aqueous medium containing DAPI as a nuclear counterstain (Vector LABS). A negative control was performed to ensure the specificity of fluorescent immunostaining by replacing primary antibody with a nonimmune rabbit IgG. Microvascular density (MVD) of the tumors was determined as previously described (23).
Ponasterone A-inducible IκBαSR transgenic mice
Animal experiments were approved by the Georgetown University and Thomas Jefferson University animal use committees. The transgenic lines carrying the EGRE3-IκBαSR transgene were crossed with animals expressing mammary gland-targeted MMTV-pVgEcR, CMV-RXRα and EGRE3-β-Gal mice (15, 17) to create Ponasterone A-Inducible IκBαSR (PISA) mice. These lines were then crossed with MMTV-ErbB2 mice (16) to create PISA/ErbB2 mice. Transgenic tumor analysis is described in supplemental data.
Microarray and cluster analysis
Microarrays were performed as previously described (24). Arrays were normalized using robust multiarray analysis (RMA), and P-value of 0.05 was applied as statistical criteria for differential expressed genes. Expression profiles are displayed using Treeview (25). Classification and clustering for pathway level analysis employed ASSESS (Analysis of Sample Set Enrichment Scores) (26).
Bone marrow macrophage isolation and NAFA co-culture assay
Bone marrow cells obtained from tibiae of 5-8 week old female FVB mice were cultured in 24-well plates (3×105 cells/0.5 ml per well) in DMEM containing 10% FBS and 50ng/ml CSF-1 for 4 days (27, 28). Accordingly, almost all the adherent cells should express macrophage-specific antigens Mac-1/CD11b, Moma-2 and F4/80 (28). The macrophages were enriched and selected according to manufacturers guidelines (Miltenyi Biotec Inc., CA).
In the NAFA-macrophage co-culture studies, 3×105 NAFA/GFP or NAFA/IκBSR cells were seeded on coverslips in the bottom of a 24 well plate overnight. The next day, CD11b+ F4/80+ macrophages (3×104 macrophages/transwell) were placed inside the 24-well plate (29). The cells were co-cultured in phenol red free DMEM containing 5% charcoal stripped serum and 5ng/ml CSF-1 for 5 days. After 5 days of co-culture, the cells on the coverslips were then fixed with 4% paraformaldehyde for 20 minutes and permeabilized using ice cold 0.2% Triton-X in PBS for 2 minutes on ice. After washing, the fixed and permeabilized cells were stained for F4/80 (CI:A3-1, Abcam, Cambridge, MA) with a DAPI nuclear stain and the macrophages were counted to determine how many had migrated to the NAFA cells on the coverslips. Images of the F4/80 positive macrophages were captured on a confocal microscope at 40× magnification.
Mammosphere formation and FACS analysis of stem cell surface markers
Mammosphere formation assays were conducted as previously described (30). Immunostaining of cell surface markers by FACS analysis for breast cancer stem cells was based on prior publications (31).
Small interfering RNA transfection
Small interfering RNAs (siRNAs) against NF-κB p105 (J-003520-09-0010, NM_003998), NF-κB p65 (J-003533-08-0010, NM_021975), and nontargeting siRNA (D-001810–03) were acquired from Dharmacon RNA Technology (Lafayette, CO) and were solubilized according to manufacturer's protocol. MCF10A-NeuT cells were transfected by the Nucleofector technology using Nucleofector Kit V and program T-024 (Amaxa Biosystems, Gaithersburg, MD). Transfection efficiency was monitored by non-silencing fluorescein labeled siRNA from QIAGEN.
Immunoprecipitation and Western blot
Immunoprecipitation and Western blot analysis was conducted as previously described (22). The antibodies used were directed to p65 (Rockland Immunochemicals Inc, Gilbertsville, PA), p50 (Upstate, Lake Placid, NY), E-cadherin (BD Biosciences, Franklin Lakes, NJ), ZO-1 (33-9100, Zymed, San Francisco, CA), N-Cadherin (3B9, Zymed, San Francisco, CA), Fibronectin (F3648, Sigma, St. Louis, MO), β-Catenin (E-5, Santa Cruz, CA), Vimentin (H-84, Santa Cruz, CA), FLAG (C-21, Santa Cruz, CA), IκBα (C19, Santa Cruz, CA), anti-FLAG M2 Affinity Gel (A2220, Sigma, St. Louis, MO) and the loading control guanine dissociation inhibitor (GDI, RTG Sol. LLC, Gaithersburg, MD), Vinculin (V9131, Sigma, St. Louis, MO) or β-Actin (Upstate, Lake Placid, NY). The appropriate HRP-conjugated secondary antibodies were subsequently applied and immunodetection was conducted using the ECL procedure.
RNA isolation, RT-PCR and quantitative real-time PCR
Total RNA was isolated from NAFA cells infected with IκBαSR inducible system, MMTV-ErbB2 and MMTV-ErbB2/IκBαSR mammary tumors used Trizol (17). One step RT-PCR for IκBαSR transgene expression was performed using a mRNA Selective PCR Kit (Takara Shuzo, Otsu, Japan) according to the manufacturers' instructions. Expression values were normalized to ribosomal protein L19 (RPL-19).
SYBR Green based real-time PCR reactions were performed using QuantiTect SYBR Green PCR kit (Qiagen Inc, Valencia, CA) and Quantitect pre-validated Primer Assays for mouse MMP3, MMP9, PDGF-AA, ICAM1, Arcp30, and 18S rRNA as internal control following manufacturers recommendations on an ABI Prism 7900HT system (Applied Biosystems Inc., Foster City, CA). All primer sequences used in RT-PCR were listed in the material supplemental table.
RESULTS
Transgenic mice express IκBαSR, targeted to mammary epithelial cells
Germ-line deletion of the NF-κB proteins results in embryonic lethality. IκBα-deficient mice die at ~9 days postnatally. A transgenic system, in which IκBαSR expression was targeted to the mammary gland and temporally regulated by ecdysone receptor activation was developed to determine the role of NF-κB in tumorigenesis. A cell line derived from tumors of transgenic mice in which activated ErbB2 was targeted to the mammary gland (NAFA) was used. In the presence of the stably-integrated pVgEcR/RXRα/EGRE3-IκBαSR expression vector, Ponasterone A induced expression of the EGRE3-IκBαSR transgene in NAFA cells as determined by Western blotting for either the FLAG epitope or for IκBαSR (Supplementary Fig. S1A). This led to suppression of NF-κB activity, as measured with the 3xRel-luc luciferase reporter construct (Supplementary Fig. S1B).
Founder lines co-integrating the EGRE3-IκBαSR transgene were established (Supplementary Fig. S1C). Transgenic mice expressing MMTV-VgEcR, RXRα, EGRE3-β-gal, EGRE3-IκBαSR are known as PISA mice (Ponasterone A-inducible IκBαSR). The mouse embryo fibroblasts (MEFs) created from PISA/ErbB2 transgenic mice were transfected with the NF-κB-responsive reporter, 3xRel-luc and treated with Ponasterone A to induce IκBαSR expression (Supplementary Fig. S1D). Ponasterone A induced IκBαSR FLAG epitope expression in the MEFs, as also demonstrated by immunohistochemical staining (Supplementary Fig. S1E). In the absence of Ponasterone A, TNF-α induced NF-κB activity 15-fold at 48 h, which was inhibited ~60% upon induction of the endogenous IκBαSR transgene with Ponasterone A (Supplementary Fig. S1F).
Endogenous NF-κB regulates epithelial cell proliferation and survival
Inducible transgenic IκBαSR expression was confirmed by Western blot analyses of 3 separate MEC cultures (Fig. 1A). Inhibition of NF-κB reduced cellular growth and DNA synthesis by approximately 35% to 50%, as measured by MTT reduction and [3H] thymidine (TdR) incorporation (Fig. 1A, B), but did not significantly influence cell cycle distribution in MEC (data not shown).
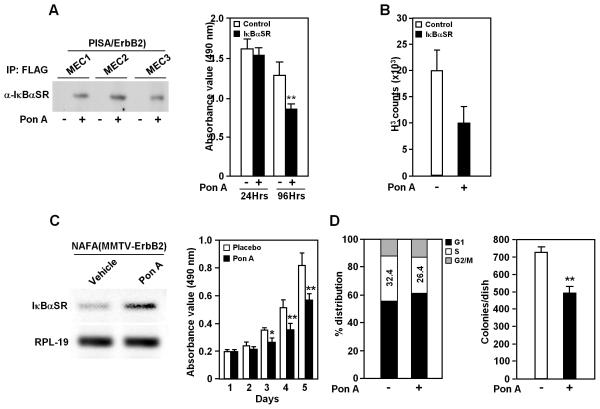
Figure 1. NF-κB enhances DNA synthesis, cellular proliferation or colony formation in mammary epithelial cells (MEC) and NAFA cells carrying Ponasterone A-inducible system.
A, Induction of IκBαSR expression by Ponasterone A in MECs (left diagram) and effect on proliferation (N≥16) (right diagram). B, DNA synthesis in MEC was analyzed by 3H-thymidine incorporation (48 h Ponasterone A treatment, N=3). C, Inducible IκBαSR expression in NAFA cells carrying Ponasterone A-inducible system (pBPSTR1-VgEcR/RXRα-EGRE3-IκBαSR) determined by RT-PCR (left diagram) and the effect on proliferation as determined by MTT assay (right diagram). D, Cell cycle distribution was determined using FACS (N≥2), showing decline in proportion of S phase after IκBαSR induction via Ponasterone A exposure for 48 h (left diagram) and reduced colony formation in soft agar after Ponasterone A treatment for 2 weeks (right diagram). (5μM of Ponasterone A was used in culture. * P<0.05; ** P<0.01).
Ponasterone A induced expression of IκBαSR in NAFA cells transfected with the pBPSTR1-VgEcR/RXRα-EGRE3-IκBαSR vector (Fig. 1C). After 5 days of IκBαSR expression, NAFA proliferation was reduced by ~35% (Fig. 1C). The proportion of cells in the DNA synthetic phase of the cell-cycle was reduced by induction of IκBαSR expression via Ponasterone A in NAFA cell lines (Fig. 1D). The NAFA tumor cells formed colonies in soft agar and induction of the IκBαSR transgene reduced colony growth 20~35% (Fig. 1D). IκBαSR suppresses, and thus NF-κB maintains, cell proliferation and colony formation of ErbB2-derived murine mammary tumor cell lines. The trend was mirrored in a PISA/ErbB2 derived tumor cell line (Supplementary Fig. S2)
Endogenous NF-κB activity regulates initiation of ErbB2-induced mammary tumorigenesis
Mammary tumors develop spontaneously in PISA/ErbB2 mice in the absence of IκBαSR transgene induction. To circumvent the requirement for NF-κB in normal ductal development, the multigenic PISA/ErbB2 mice were allowed to develop to maturity, then treated with Ponasterone A or placebo, by pellet implantation from 13 weeks (to study tumor onset) (Supplementary Fig. S3A) or 23 weeks (to measure growth-linked outcomes). In the absence of Ponasterone A induction of IκBαSR, PISA/ErbB2 mice developed multifocal mammary tumors (Supplementary Fig. S3B, C). In contrast, PISA/ErbB2 mice in which IκBαSR had been induced with Ponasterone A, rarely developed tumors by 21 weeks. Tumors that arose in mammary glands of IκBαSR-expressing transgenic mice were typically fewer in number (Supplementary Fig. S3C).
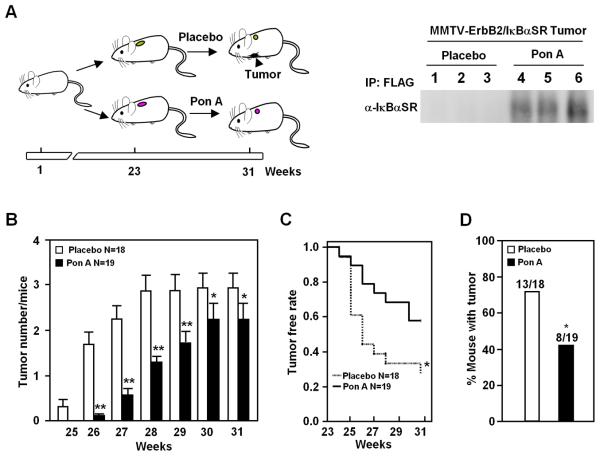
When NF-κB activity was suppressed after 23 weeks of age (Fig. 2A), IκBαSR transgene-expression reduced the average number of tumors developing per mouse significantly over the following 6 weeks (Fig. 2B). Fewer Ponasterone A-treated animals had any tumors after 2 weeks and the difference was significant at 5 weeks (Fig. 2C, D). Together, these studies demonstrate for the first time that in immune-competent transgenic mice mammary NF-κB activity governs the rate of initiation and the number of ErbB2 tumors.
Figure 2. IκBαSR delays ErbB2 induced mammary tumor onset.
A, A group of thirty-seven PISA/ErbB2 mice were treated from 23 weeks of age with Ponasterone A (n = 19) or placebo (n = 18) (left diagram). Inducible IκBαSR expression in transgenic mice mammary tumor treated with Ponasterone A and placebo, displayed by immunoprecipitation with anti-FLAG and detection with anti-IκBα (right diagram). B, Average number of tumors per mouse in Ponasterone A or placebo-exposed groups. C, Kaplan-Meier plots of proportion of animals free of mammary tumor in Ponasterone A and placebo treated groups. D, Percentage of mice in each group free of tumor at 60 days after pellet implantation. (* P< 0.05, ** P<0.01).
NF-κB regulates mammary epithelial cell gene expression of reactive oxygen species (ROS), EMT and stem cells
Gene expression profiles were compared between MMTV-ErbB2 animals and Ponasterone A-treated PISA/ErbB2 (Supplementary Fig. S4). Gene targets of NF-κB were identified with functions in cell survival, cell cycle control, signaling, DNA repair, chromosomal organization, biogenesis, and functions in transcription, cytoskeleton formation, transport and metabolism (Supplementary Fig. S4A; Supplementary Table S1). In order to identify functional pathways regulated by endogenous NF-κB, genome-wide enrichment analysis was conducted using ASSESS. These studies identified pathways inhibited by IκBαSR including NF-κB signaling, ROS production, epithelial mesenchymal transition (EMT), cellular invasion, cellular proliferation, inflammation and vasculogenesis (Supplementary Fig. S4B).
Mammary epithelial cell NF-κB determines recruitment of tumor-associated macrophages and angiogenesis during ErbB2 tumorigenesis
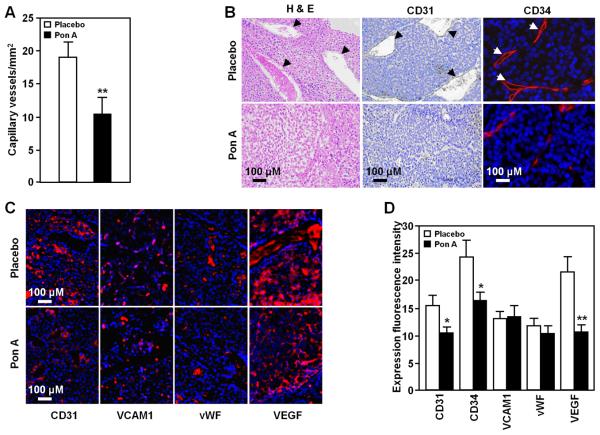
Gene expression array analysis of the transgenic mice mammary tumors (Supplementary Fig. S4) identified a number of genes inhibited by IκBαSR that promote angiogenesis (Table S3). As the growth of tumors beyond 1 to 2 mm requires the induction and maintenance of a blood supply (32), we examined the influence of IκBαSR transgene expression on tumor vascularity in Ponasterone A and placebo-exposed PISA mice. Tumors of transgenic mice expressing IκBαSR demonstrated a ~45% reduction in tumor microvessel density (Fig. 3A). Tumors were less vascular by histomorphometric and immunohistochemical analyses (Fig. 3B). Integrin engagement activates mammary tumor NF-κB in vitro when assayed with 3D matrigel cultures (33). We examined candidate proangiogenic extracellular matrix (ECM) integrin-binding proteins, to determine the mechanism by which NF-κB promoted mammary tumor angiogenesis in vivo. Neither the α1α4 integrin binding protein VCAM1, nor the αvα3 binding ECM protein vWF changed in abundance (Fig. 3C, D). In contrast, VEGF abundance and the endothelium-expressed PECAM-1/CD31 were significantly reduced by IκBαSR expression in vivo.
Figure 3. Reduced vasculogenesis and bone marrow macrophages in PISA/ErbB2 mammary tumors.
Analysis of mammary tumors in PISA mice (N=10), with or without Ponasterone A-induced IκBαSR expression to determine: A, Microvessel density; B, Histopathology by hematoxylin and eosin staining (left), immunostaining to demarcate CD31 (middle), CD34 (right) positive tumor blood vessels and C, immunohistochemical staining for candidate proangiogenic factors; E, Quantitation of immunohistochemical staining for proangiogenic factors, shown as mean + SEM (* P< 0.05, ** P<0.01). All pictures taken using 40x objective.
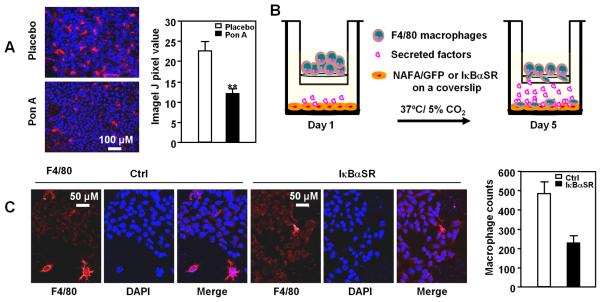
Mouse models with defective CSF1 signaling due to mutation of the CSF1 receptor suggested TAM (Tumor Associated Macrophages) contribute to murine mammary tumor progression by enhancing angiogenesis (34). It has been hypothesized, but never proven, that mammary epithelial tumor induced-NF-κB may contribute to mammary tumor TAM recruitment. To determine whether mammary epithelial cell NF-κB regulates TAM recruitment, we examined the infiltration of bone marrow macrophages assayed using F4/80 immunostaining. IκBαSR expression reduced the proportion of F4/80 staining macrophages within the mammary tumors by ~50% (Fig. 4A). F4/80 expressing macrophages were isolated by fluorescence activated cell sorting and migration was assessed (Fig. 4B). The lower chamber of the Boyden chamber contained equal numbers of either cultured NAFA-IκBαSR or NAFA-GFP control cells. The number of migrating macrophages was reduced ~40% in the presence of NAFA-IκBαSR (Fig. 4C). Thus, NF-κB within MEC contributes heterotypic signals to recruit TAM.
Figure 4. Mammary epithelial cell NF-κB determines recruitment of tumor-associated macrophages.
Analysis of mammary tumors in PISA mice (N=10), with or without Ponasterone A-induced IκBαSR expression to determine: A, Immunohistochemical staining and quantitation for F4/80; B and C, Macrophage migration assays in response to secreted factors of IκBαSR expressing mammary tumors. Schematic representation of protocol in which bone marrow macrophages were differentiated with G-CSF for 5 days then assessed for migration through a transwell toward NAFA/GFP or NAFA/IκBαSR cells seed on the bottom of a Boyden chamber. Macrophages that had migrated to the bottom were stained for F4/80 and counted (C, right diagram).
NF-κB in mammary tumors maintains progenitor cell expansion
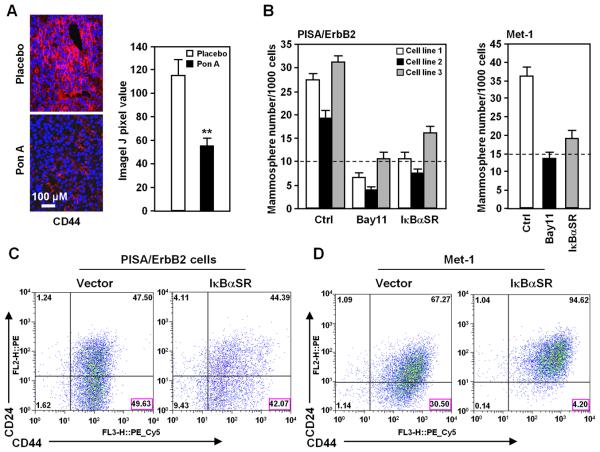
In view of the finding that NF-κB inhibition reduced EMT, we determined the function of NF-κB in regulating stem cell features in transgenic mice. CD44 is a transmembrane cell-surface protein that is essential for the homing and stem cell properties of leukemic stem cells and serves as a useful marker for solid tumor-initiating cells in mammary tumors (35). IκBαSR expression reduced the proportion of CD44 positive cell in PISA/ErbB2 tumor by ~50% (Fig. 5A).
Figure 5. NF-κB promotes breast tumor stem cell marker expression and mammosphere production.
A, Analysis of breast cancer stem cell marker CD44 in mammary tumors of PISA mice (N≥3) with or without IκBαSR induced by Ponasterone A. B, Mammosphere assays (N≥4) were conducted of breast cancer cell lines derived from 3 separate PISA/ErbB2 cell lines (left diagram) and in Met-1 cells (right diagram), and quantitation was conducted after 10 days. Treatment was with the IKKα inhibitor (Bay11-7082, 10μM). C, D, Stem cell surface markers (CD24−/low/CD44+/+) of breast cancer cell line PISA/ErbB2 (C) or Met-1 (D). Cells were transduced with IκBαSR or control vector (N≥3).
A small number of primary breast cancer cells, tumor initiating cell (TIC) or cancer stem cells form secondary tumors (36). TICs form non-adherent mammospheres when cultured under specific conditions in the absence of serum. Inhibition of NF-κB activity reduced mammosphere number by >60% in cell lines derived from PISA/ErbB2 mice and in Met-1 cells (Fig. 5B). Cancer stem cells can be enriched by sorting for CD44+/CD24−/low cells in human and murine breast cancer cells (37, 38). In mouse breast tumor cells, CD44+/CD24− cells are enriched 50- to 100-fold in tumor growth potential in syngeneic transplantation, and are enriched for expression of stem cell associated genes (37). Reduction of endogenous NF-κB through IκBαSR expression reduced the proportion of CD44+/CD24-/low cells by 15% in the cell lines derived from PISA/ErbB2 mice (Fig. 5C) and 75% in a second murine cell line Met1 (Fig. 5D).
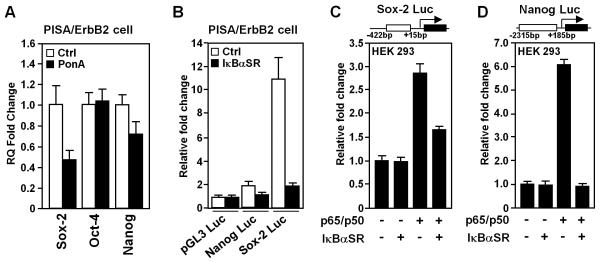
The molecular circuitry governing embryonic stem (ES) cells is active in many tumors, with altered expression of key ES cell regulators (Oct4, Sox2 and Nanog). mRNA from tumors of transgenic mice examined by QT-PCR (Fig. 6A) for candidate ES cell regulators (Sox2, Oct4, Nanog), demonstrated a reduction in Sox2 (~60%) and Nanog (~30%). Expression of IκBαSR reduced Nanog and Sox2 promoter activity in the PISA/ErbB2-derived cell line 50% and 90% respectively (Fig. 6B). p65/p50 induced the Sox2 promoter 3-fold and the Nanog promoter was induced 6-fold. IκBαSR reduced NF-κB dependent activity of the promoter (Fig. 6C, D).
Figure 6. NF-κB in mammary tumors maintains progenitor cell expansion.
A, Quantitative analysis of (QT-PCR) of ES cell marker genes (Sox2, Oct4, Nanog) in mammary tumors of transgenic mice (PISA/ErbB2) with or without Ponasterone A treatment. Data are mean ± SEM of N=6 separate tumors. B, Luciferase reporter gene assays were conducted in PISA/ErbB2 tumor cells. Data are mean luciferase activity ± SEM or N≥3 separate experiments. C, D, Luciferase reporter gene assays were conducted by transient transfection as described in the materials and methods.
DISCUSSION
The current studies include the first in vivo analysis of the molecular genetic pathways regulated by endogenous MEC NF-κB. The PISA mouse provided a valid model for examining mammary tumorigenesis without disturbing antecedent physiological mammary gland development or disturbing other NF-κB regulated functions, such as inflammatory and immune responses in the tumor. This system allowed normal NF-κB activity throughout the animal during development, providing a physiologically valid background to determine the function of NF-κB in the normally-developed adult mammary gland. Local, timed suppression of NF-κB activity in these multiply-transgenic mice was used to examine the significance of MEC NF-κB on tumor progression and local heterotypic signals. Mammary epithelial cell IκBαSR induction delayed tumor appearance and reduced average tumor number. Suppressed tumor formation rate in vivo was reflected in IκBαSR-regulated suppression of growth in PISA mammary epithelial cell lines in vitro. IκBαSR expression induced by ponasterone did not reduce the relative expression of ErbB2 mRNA compared with vehicle control (N=6, 8.59 vs 8.58) by microarray nor abundance by Western blot analysis (Supplementary Fig. S5). Inhibition of MEC NF-κB in vivo reduced the infiltration of F4/80 staining macrophages and reduced tumor vascularity and features of neoangiogenesis. Reconstitution analysis demonstrated endogenous breast tumor epithelial cell NF-κB governs macrophage recruitments. Through selectively inactivating MEC NF-κB in the adult mammary gland we have demonstrated MEC NF-κB governs a heterotypic signal in vivo that controls the initiation of mammary tumor growth, recruits TAM and promotes neoangiogenesis.
The role of MEC NF-κB in generating angiogenesis and specific heterotypic signals was previously unknown. IκBαSR expression reduced angiogenesis in mammary tumors of PISA/ErbB2 mice. Microarray analysis demonstrated a reduction in expression of gene clusters regulating angiogenesis and a subset of secreted factors capable of regulating neovasculogenesis. Analysis of the mechanisms by which MEC NF-κB regulated angiogenesis identified reduced secretion of VEGF and several other factors (Acrp30, Mip2, Rantes, K6, MMP-3, MMP-9) in the NAFA cell line expressing IκBαSR, corresponding to a reduction in gene expression within the mammary tumor expressing IκBαSR (data not shown). The reduction in angiogenesis may have contributed to the delay in onset and or progression of the ErbB2 tumors.
Herein, MEC NF-κB enhanced the infiltration of TAM. G-CSF is produced in advanced human and murine breast cancers (39). Breast tumor derived CSF-1 increased hematopoietic stem cells and endothelial progenitor cell numbers in the mouse (39). In prior studies investigating the potential role of macrophages in mammary tumor progression, the polyoma middle T antigen was expressed in the murine mammary gland and the animals crossed with mice expressing a mutation of the CSF-1 receptor (34). Tumor progression and angiogenesis was reduced in the mice expressing a mutant CSF-1 receptor. Although CSF-1 receptor is expressed on vascular smooth muscle cells, these studies were consistent with a model in which CSF-1-recruitment of TAM contributes to mammary tumor progression. The mechanisms by which macrophages contribute to breast tumor progression are unclear. Macrophage infiltration correlates with angiogenesis (34) and bone marrow cells recruited through the neuropilin-1 receptor promote neoangiogenesis formation in mice (40).
The current studies provide several lines of evidence that the canonical NF-κB pathway contributes to self renewal of mammary tumor cells in vivo. First, genome wide analysis implicated NF-κB function in regulating genes involved in ES cell function. Secondly, expression of CD44, a transmembrane receptor used as a marker of human breast tumor stem cells (35) was reduced ~50% in the transgenic mouse tumors in vivo upon induction of the IκBαSR transgene. Thirdly, mammosphere production, a recognized surrogate for self renewal, using cell lines derived from the transgenic mice, was inhibited by IκBαSR expression. Fourth, the generation of CD44high/CD24-/low cells, characteristic of human and murine mammary stem cells was reduced by IκBαSR expression (37, 38). The specific mechanism by which NF-κB regulates self-renewal may involve transcriptional repression of the key regulators of ES cell identity (Sox2 and Nanog). Herein IκBαSR was shown to regulate the abundance of Sox2 and Nanog in mammary tumors and to directly regulate their gene expression. Our findings with the canonical NF-κB pathway are consistent with studies of the alternate (IKKα) pathway in breast tumor self-renewal using a kinase knockin model (41). The canonical NF-κB and alternate pathways are often distinct and may be mutually antagonistic. The keratinocyte defect of IKKα−/− mice is NF-κB-independent (42). IKKα has been shown to suppress NF-κB activation by accelerating the turnover of RelA and C-Rel, and inactivation of IKKα, in contrast with inactivation of the canonical NF-κB pathway, can lead to enhanced inflammation (43). An IKKαAA/AA knockin mouse model demonstrated a role for IKKα in normal mammary gland development and mammary epithelial stem cell expansion (14, 41). Importantly, no alteration in NF-κB activity was observed in the WT vs IKKαAA/AA mammary tumors suggesting the IKKα mediated induction of mammary stem cell expansion involves distinct mechanisms from the current studies which specifically inactivated NF-κB signaling. Collectively, these studies are consistent with a role for both the canonical and alternative NF-κB pathways in maintaining tumor stem cells.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by R01CA70896, R01CA75503, R01CA107382, R01CA86072 (R.G.P.) and R01CA120876 (M.P.L). The Kimmel Cancer Center was supported by the NIH Cancer Center Core grant P30CA56036 (R.G.P.). This project is funded in part from the Dr. Ralph and Marian C. Falk Medical Research Trust and a grant from Pennsylvania Department of Health (R.G.P.). The Department specifically disclaims responsibility for any analysis, interpretations or conclusions. We thank Atenssa L. Cheek and Stefanie Pierpoint for the preparation of this manuscript.
Footnotes
There are no conflicts of interest associated with this manuscript.
REFERENCES
- 1.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–83. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh S, Karin M. Missing Pieces in the NF-κB Puzzle. Cell. 2002;109:S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 3.Thanos D, Maniatis T. NF-κB: A lesson in family values. Cell. 1995;80:529–32. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 4.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–9. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 5.Dejardin E, Bonizzi G, Bellahcene A, Castronovo V, Merville MP, Bours V. Highly-expressed p100/p52 (NFKB2) sequesters other NF-kappa B-related proteins in the cytoplasm of human breast cancer cells. Oncogene. 1995;11:1835–41. [PubMed] [Google Scholar]
- 6.Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, et al. Aberrant nuclear factor-kB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–1960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, et al. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–43. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- 8.Seitz CS, Lin Q, Deng H, Khavari PA. Alterations in NF-kappaB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-kappaB. Proc Natl Acad Sci USA. 1998;95:2307–12. doi: 10.1073/pnas.95.5.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brantley DM, Chen CL, Muraoka RS, Bushdid PB, Bradberry JL, Kittrell F, et al. Nuclear factor-kappaB (NF-kappaB) regulates proliferation and branching in mouse mammary epithelium. Mol Biol Cell. 2001;12:1445–55. doi: 10.1091/mbc.12.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Albanese C, Hulit J, Sakamaki T, Pestell RG. Recent advances in inducible expression in transgenic mice. Semin Cell Dev Biol. 2002;13:129–41. doi: 10.1016/s1084-9521(02)00021-6. [DOI] [PubMed] [Google Scholar]
- 12.Brantley DM, Yull FE, Muraoka RS, Hicks DJ, Cook CM, Kerr LD. Dynamic expression and activity of NF-kappaB during post-natal mammary gland morphogenesis. Mech Dev. 2000;97:149–55. doi: 10.1016/s0925-4773(00)00405-6. [DOI] [PubMed] [Google Scholar]
- 13.Brantley DM, Chen C-L, Muraoka RS, Bushdid PB, Bradberry JL, Kittrell F, et al. Nuclear Factor- kB (NF-kB) Regulates Proliferation and Branching in Mouse Mammary Epithelium. Molecular Biology of the Cell. 2001;12:1445–55. doi: 10.1091/mbc.12.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, et al. IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell. 2001;107:763–75. doi: 10.1016/s0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- 15.Albanese C, Reutens AT, Bouzahzah B, Fu M, D'Amico M, Link T, et al. Sustained mammary gland-directed, ponasterone A-inducible expression in transgenic mice. Faseb J. 2000;14:877–84. doi: 10.1096/fasebj.14.7.877. [DOI] [PubMed] [Google Scholar]
- 16.Lee RJ, Albanese C, Fu M, D'Amico M, Lin B, Watanabe G, et al. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol Cell Biol. 2000;20:672–83. doi: 10.1128/mcb.20.2.672-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakamaki T, Casimiro MC, Ju X, Quong AA, Katiyar S, Liu M, et al. Cyclin D1 determines mitochondrial function in vivo. Mol Cell Biol. 2006;26:5449–69. doi: 10.1128/MCB.02074-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–99. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persons DA, Allay JA, Allay ER, Ashmun RA, Orlic D, Jane SM, et al. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood. 1999;93:488–99. [PubMed] [Google Scholar]
- 20.Neumeister P, Pixley FJ, Xiong Y, Xie H, Wu K, Ashton A, et al. Cyclin D1 governs adhesion and motility of macrophages. Mol Biol Cell. 2003;14:2005–15. doi: 10.1091/mbc.02-07-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joyce D, Bouzahzah B, Fu M, Albanese C, D'Amico M, Steer J, et al. Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-kappaB-dependent pathway. J Biol Chem. 1999;274:25245–9. doi: 10.1074/jbc.274.36.25245. [DOI] [PubMed] [Google Scholar]
- 22.Wu K, Liu M, Li A, Donninger H, Rao M, Jiao X, et al. The Cell Fate Determination Factor DACH1 Inhibits c-Jun Induced Contact-Independent Growth. Mol Biol Cell. 2007;18:755–67. doi: 10.1091/mbc.E06-09-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ju X, Katiyar S, Wang C, Liu M, Jiao X, Li S, et al. Akt1 governs breast cancer progression in vivo. Proc Natl Acad Sci U S A. 2007;104:7438–43. doi: 10.1073/pnas.0605874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–85. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 25.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–8. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edelman E, Porrello A, Guinney J, Balakumaran B, Bild A, Febbo PG, et al. Analysis of sample set enrichment scores: assaying the enrichment of sets of genes for individual samples in genome-wide expression profiles. Bioinformatics. 2006;22:e108–16. doi: 10.1093/bioinformatics/btl231. [DOI] [PubMed] [Google Scholar]
- 27.Itoh K, Udagawa N, Kobayashi K, Suda K, Li X, Takami M, et al. Lipopolysaccharide promotes the survival of osteoclasts via Toll-like receptor 4, but cytokine production of osteoclasts in response to lipopolysaccharide is different from that of macrophages. J Immunol. 2003;170:3688–95. doi: 10.4049/jimmunol.170.7.3688. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–86. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, et al. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol. 2005;175:1197–205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 30.Lindsay J, Jiao X, Sakamaki T, Casimiro MC, Shirley LA, Tran T, Ju X, Liu M, Li Z, Wang C, Katiyar S, Rao M, Allen KG, Glazer RI, Ge C, Stanley P, Lisanti M, Rui H, Pestell RG. ErbB2 induces Notch1 activity and function in breast cancer cells. Clinical and Translational Science. 2008;1(2):107–15. doi: 10.1111/j.1752-8062.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–8. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 32.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 33.Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–16. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–6. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 35.Godar S, Ince TA, Bell GW, Feldser D, Donaher JL, Bergh J, et al. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell. 2008;134:62–73. doi: 10.1016/j.cell.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10:R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chizea JP, Dailey VK, Williams E, Johnson MD, Pestell RG, Ojeifo JO. Endothelial Progenitor Cells Significantly Contribute to Vasculatures in Human and Mouse Breast Tumors. The Open Hematology Journal. 2008;2:30–62. [Google Scholar]
- 40.Zacchigna S, Pattarini L, Zentilin L, Moimas S, Carrer A, Sinigaglia M, et al. Bone marrow cells recruited through the neuropilin-1 receptor promote arterial formation at the sites of adult neoangiogenesis in mice. J Clin Invest. 2008;118:2062–75. doi: 10.1172/JCI32832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao Y, Luo JL, Karin M. IkappaB kinase alpha kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proc Natl Acad Sci U S A. 2007;104:15852–7. doi: 10.1073/pnas.0706728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sil AK, Maeda S, Sano Y, Roop DR, Karin M. IkappaB kinase-alpha acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004;428:660–4. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- 43.Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–43. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.