Abstract
Stress exerts complex effects on the brain and periphery, dependent on the temporal profile and intensity of the stressor. The consequences of a stressful event can also be determined by other characteristics of the stressor, such as whether it is predictable and controllable. While the traditional view has focused primarily on the negative effects of stress on a variety of somatic systems, emerging data support the idea that certain forms of stress can enhance cellular function. Here we review the current literature on the hypothalamic-pituitary-adrenal (HPA) axis regulation by wheel running, a voluntary and controllable stressor with a distinct temporal profile. While running indeed activates a number of systems related to the stress response, other mechanisms exist to reduce the reactivity to this stressor, with possible crosstalk between running and other forms of stress.
Keywords: stress, wheel running, glucocorticoid, hippocampus, hypothalamus
2. INTRODUCTION
Stress plays a critical role in human interaction and physiology. This role can be considered through the lens of effects on the individual, or based on the intrinsic characteristics of the stressor itself. The intrinsic properties that influence the stress response include predictability and controllability. A stressor is 'controllable' if the animal can generate some behavioral response to initiate or terminate the stimulus; likewise, a stressor is predictable if it occurs at intervals that are regularly spaced over time or associated with a specific environmental cue. The distinct effects of controllable and uncontrollable stressors have been recognized for nearly forty years, since the initial demonstration of learned helplessness by Seligman and colleagues (Seligman and Beagley, 1975). However, much of the work on predictability and controllability has focused on a particular stressor, inescapable shock. In this article, we review recent evidence that some of the same dimensions governing the response to involuntary shock will also apply to a voluntary stressor – wheel running.
Rodents, including mice, rats, squirrels, hamsters, and even rabbits, will all voluntarily run in a wheel apparatus (Kennedy et al., 1994; Refinetti, 2007; Freeman and Zucker, 2000). The amount of voluntary running varies based on the species (Kennedy et al., 1994), genotype (Lightfoot et al., 2004), and gender (Lightfoot et al., 2004) of the experimental model, but it is invariable that animals given access to a wheel will run, at least initially. The amount of voluntary running varies based on the age of the animals, with young animals showing the highest levels of wheel running (Valentinuzzi et al., 1997). Older animals may show an overall decrease in the amount of voluntary running, and sometimes will display both a decrease in the amount of running and a shift in the time when running occurs (Valentinuzzi et al., 1997; Kopp et al., 2006; Antoniadis et al., 2000). Rats will also bar-press for wheel access, which suggests that they are motivated to engage in this behavior (Iversen, 1993). These indicators are significant because they demonstrate that, unlike uncontrollable stressors such as inescapable shock, running is a rewarding, voluntary form of stress for rodents.
Running, like other stressors, will activate the sympathetic nervous system resulting in epinephrine production, as well as the hypothalamic-pituitary-adrenal (HPA) axis resulting in glucocorticoid production (Makatsori et al., 2003; Naylor et al., 2005; Droste et al., 2003; Dahlqvist et al., 2003; Persson et al., 2004; Girard and Garland, 2002; Lancel et al., 2003; Fediuc et al., 2006; Droste et al., 2006; Droste et al., 2007). This is due to the increased demand for energy in somatic tissues. Because epinephrine will increase cellular production of ATP, and glucocorticoids will promote gluconeogenesis in the liver, it is logical that running would recruit systems that evolved to support the ‘fight-or-flight’ response. But how does the brain differentiate between activation of these systems due to running – a voluntary, controllable stressor – and other, involuntary forms of stress? Here, we review evidence demonstrating that voluntary wheel running activates the neuroendocrine stress axes, and clarify issues surrounding the time course and mechanisms of these effects. In addition, we review evidence for and against interactions between exercise, and other forms of stress.
2.1 Terminology: A framework for defining interactions between stressors
Hans Selye, the father of stress research, outlined a number of terms to define interactions between different stressors (Seyle, 1976). He described changes in the response to the same stressor over time with chronic exposure using the term 'homotypic stress.' This term will be used in the current review with respect to changes in the stress response to running over time. Selye also coined the term 'heterotypic stress' to describe the response of an individual with previous stressor experience to a novel form of stress. An example of heterotypic stress that will be relevant here would be the response of an animal engaging in chronic wheel-running to a different stressor, such as restraint. Adopting these terms is key to understanding the complex interactions that exist between different stressors presented repeatedly over time.
3. EXERCISE ACTIVATES STRESS-RELATED SYSTEMS IN THE BRAIN
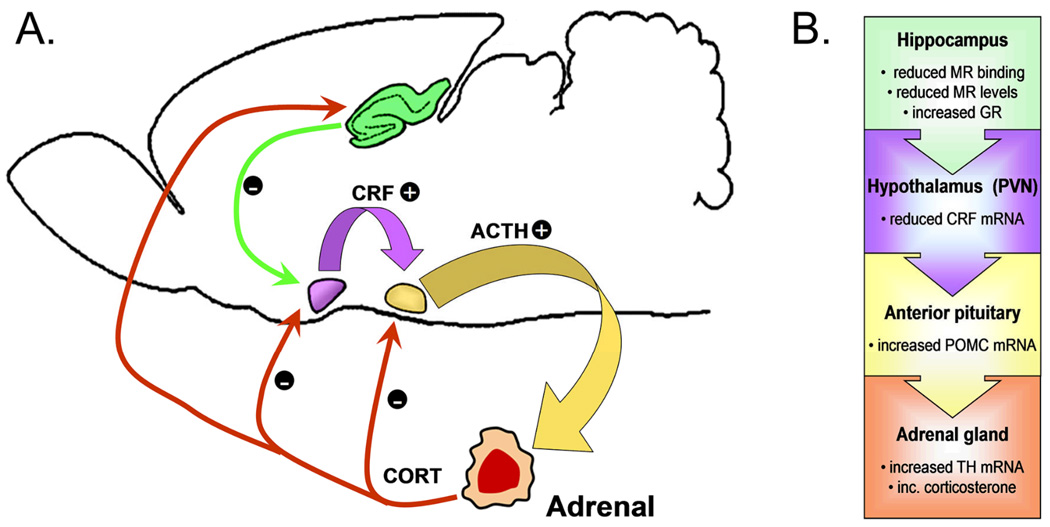
The canonical view of the HPA axis involves its activation by an internal or external stimulus, which is recognized as a threat to homeostasis by higher-order cognitive areas. These threat-recognition regions – including the amygdala, prefrontal cortex, and hippocampus – send excitatory projections to the paraventricular nucleus of the hypothalamus, causing the synthesis and release of corticotrophin-releasing factor (CRF), which acts at the level of the pituitary to induce production of adrenocorticotropic hormone (ACTH). ACTH then signals the adrenal gland to release glucocorticoids (corticosterone in rodents and cortisol in humans), initiating a negative feedback system in which each level induces its own shutoff. It should also be noted that the adrenal glucocorticoids play a role in reducing activation at all levels of the HPA axis, in addition to each factor’s negative feedback upon its own synthesis (Figure 1A).
Figure 1.
Exercise exerts different effects at each level of the HPA axis. (A), Schematic diagram of the rodent HPA axis. The paraventricular nucleus of the hypothalamus (PVN, shown in purple) integrates information from cortical and subcortical regions to initiate activation of the HPA axis by releasing corticotrophin-releasing factor (CRF). CRF acts on the anterior pituitary (shown in yellow) to induce production of adrenocorticotrophic hormone (ACTH). ACTH is released into the bloodstream, where it induces synthesis and release of corticosterone in the adrenal gland. Corticosterone negatively regulates further release of CRF and ACTH; additionally, corticosterone acts on the hippocampus (green) to induce activity among neurons that project to the PVN. Hippocampal projections to the PVN (green arrow) promote HPA axis shutoff. (B) In the hippocampus, wheel running has been shown to reduce mineralocorticoid receptor (MR) binding and expression in mice, and to enhance levels of the glucocorticoid receptor (GR) in rats. At the level of the PVN, running has been shown to decrease CRF mRNA, which would result in reduced activation of the anterior pituitary. In contrast, the anterior pituitary responds to exercise with increases in mRNA for pro-opiomelanocortin (POMC), which would lead to increased adrenocorticotropic hormone signaling to the adrenal glands. Lastly, the adrenal gland responds to exercise with increases in tyrosine hydroxylase mRNA, and increased levels of corticosterone.
In the case of running, the paradigm is shifted slightly. Running poses a threat to homeostasis, without presenting any psychological indicators of threat or fear. In fact, wheel running is an appetitive phenomenon, and rodents will readily learn to bar press for wheel access (Iversen, 1993) and will also develop conditioned place preference to environments previously paired with a running wheel (Belke and Wagner, 2005). The ability to differentiate between harmless and harmful threats to homeostasis is a critical factor in determining susceptibility to neuropsychiatric disorders, such as post-traumatic stress disorder and depression. Research in rodent models can help to elucidate possible pathways that allow the brain to make these distinctions.
Species differences exist between rats and mice in the effects of stress on the hypothalamus; specifically, the relationship between CRF receptor expression and ambient CRF levels is different between the two species (Makino et al., 2005). Research in mouse models supports an attenuated stress response in runners, based on reduced mRNA for CRF in the paraventricular nucleus (Fig. 1; Droste et al., 2003; Droste et al., 2006). This does not hold true for rats, which show no evidence for alterations in CRF mRNA following similar periods of running (Droste et al., 2007). However, little is known about the effects of running on CRF receptors in this and other stress-responsive areas of the brain. Since changes in levels of CRF can influence changes in the expression of CRF receptors, determining their expression will provide additional information about whether the decrease in ligand is functionally significant.
In the pituitary, running has been associated with increased levels of proopiomelanocortin (POMC), a precursor for the synthesis of ACTH (Makatsori et al., 2003). This would suggest increased HPA axis drive, rather than an attenuated stress response. Across multiple brain regions and tissues, glucocorticoids will down-regulate the expression of their own receptors. However, although running increases circulating glucocorticoids, no changes in glucocorticoid receptor levels in the pituitary have been reported (Fediuc et al., 2006; Droste et al., 2007). Taken together with the absence of any change in levels of the Type II glucocorticoid receptor, which is predominantly involved in HPA axis shutoff, these results indicate increased activation at the level of the anterior pituitary (Fig. 1).
The hippocampus is an additional target for central modulation of the HPA axis. Hippocampal CA1 and dentate gyrus neurons express both the mineralocorticoid receptor (MR), which binds corticosterone with high affinity and is tonically occupied; and the glucocorticoid receptor (GR), which is occupied during stress and at certain phases in the circadian cycle. These receptors are dynamically regulated by both positive and negative stressors. In mice, running reduces the affinity of hippocampal MR for corticosterone, without altering the expression of either receptor population (Droste et al., 2003). Rats exhibit subfield-specific increases in GR mRNA, without any change in levels of MR (Droste et al., 2007). The regulation of hippocampal GR in rats may be duration-dependent, since studies using shorter (4 weeks) durations of running reported downregulation of GR, while studies using longer durations (8 weeks) reported no change (Zheng et al., 2006).
Typically, neurons in the hippocampus of animals exposed to chronically high levels of circulating glucocorticoids will retract their dendrites, and show reductions in the density of dendritic spines (Woolley et al., 1990). However, in the brains of runners, dendritic branching in hippocampal neurons has actually been shown to increase, as has the density of dendritic spines (Eadie et al., 2005; Redila and Christie, 2006; Stranahan et al., 2007). This occurs despite high levels of circulating corticosterone (Stranahan et al., 2006). These morphological alterations are indicative of enhanced synaptic function, which could play a role in facilitating hippocampal negative feedback on the HPA axis.
4. INTERACTIONS BETWEEN EXERCISE AND SOCIAL STRESS
Most studies examining the effects of wheel running will house animals one per cage to facilitate quantification of individual activity levels. However, in some species and strains, social isolation can activate the HPA axis, making the animals more prone to the deleterious effects of stress (Baldwin et al., 1995; Ruis et al., 1999; Dronjak et al., 2004; Kiyokawa et al., 2004). Sedentary animals exposed to social isolation during adulthood exhibit elevated basal corticosterone levels, and reduced hippocampal GR expression (Dronjak et al., 2004). Suppression of corticosterone release with the synthetic glucocorticoid dexamethasone is stronger in socially housed animals, indicative of better HPA axis feedback (Ruis et al., 1999). Socially isolated animals also show increased activation of the immediate early gene cfos in the PVN (Kiyokawa et al., 2004). Given these relationships, it is not surprising that running might exert differential effects on the HPA axis in socially isolated animals (Figure 2).
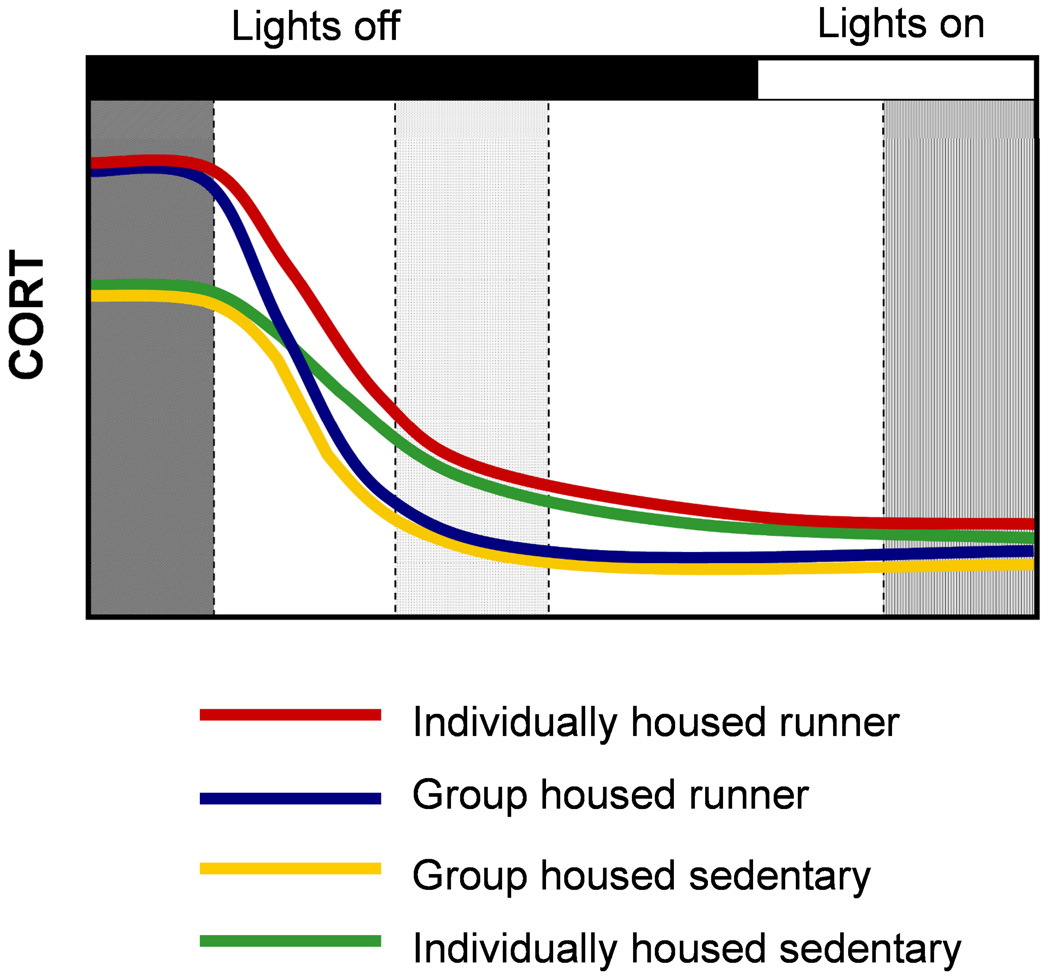
Figure 2.
Circadian-phase dependent modulation of corticosterone levels by social isolation in runners. (Left gray region), At the onset of lights-out, we reported greater elevations in corticosterone in runners, irrespective of housing conditions (Stranahan et al., 2006). (Middle gray region), Four hours after the onset of the dark period, we observed that although all the animals had lower corticosterone levels, the group-housed animals exhibit a larger reduction than individually housed animals, in both runners and sedentary controls. (Right gray region), No differences in serum corticosterone levels were observed in samples taken four hours after the onset of the light period. This figure represents graphically the findings (described in Stranahan et al., 2006).
Running-induced elevations in corticosterone levels are prolonged in socially isolated Sprague-Dawley rats, and socially isolated runners also exhibit larger increases in corticosterone levels following restraint stress (Stranahan et al., 2006). This suggests that social isolation can influence the response of runners to both a homotypic (running) and a heterotypic (restraint) stressor. However, several caveats should be considered when generalizing across models: Sprague-Dawley rats will not form dominance hierarchies unless competition for food, water, and mates is present (Kozorovitskiy and Gould, 2004). Given the docile nature of this model, it is not surprising that social isolation stress would obscure the positive effects of running. In contrast, male C57BL/6 mice will form dominance hierarchies readily, even in the absence of competition for food or mates (Roubertoux et al., 2005). Because of the aggressiveness of this model, it is possible that they would respond differently to social isolation, fostering a distinct time course for the effects of running in individually housed animals.
Social defeat stress is also modulated by physical activity; runners show larger increases in corticosterone following social defeat than sedentary animals (Lancel et al., 2003). This study also examined the protective effects of running against stress-induced alterations in rapid-eye-movement (REM) sleep patterns. Sedentary mice exposed to social defeat in the resident intruder paradigm typically exhibit reductions in REM sleep (Lancel et al., 2003), and sleep deprivation has been linked to increases in circulating corticosterone (Mirescu et al., 2006). Social-defeat-induced reductions in REM sleep are mitigated by voluntary running, but this is not mediated by reduced corticosterone, since runners actually show increased reactivity to the stressor (Lancel et al., 2003). It remains undetermined whether another factor, namely social isolation, might also play a role in promoting the running-induced enhancement of the glucocorticoid response to social defeat.
5. GENDER DIFFERENCES IN THE STRESS RESPONSE TO EXERCISE
Males and females differ in their responses to stressful stimuli. Specifically, the glucocorticoid response to stress predominates in regulating HPA axis plasticity in males, while the central consequences of stress in females are mediated by reproductive hormones (Shors, 1998). Exercise activates the HPA axis in both male and female animals, but in female mice, the amount of voluntary running (and the HPA axis response to running activity) varies across the estrus cycle (Ogawa et al., 2003). During proestrus, when levels of estrogen are highest, levels of voluntary running are also at their peak. During other phases of the ovarian cycle, when levels of estrogen are lower, levels of voluntary running are also lower (Wollnik and Turek, 1988).
Basal levels of corticosterone in female animals also exhibit a similar pattern with respect to stage of estrus. In addition to having elevated basal levels of corticosterone during proestrus, stress-induced elevations in corticosterone are also prolonged during this period (Viau and Meaney, 1991). The central consequences of exercise are also influenced by estrogen in female animals; specifically, the exercise-induced induction of hippocampal BDNF was strongest in ovariectomized females administered exogenous estrogen, and weakest in ovariectomized females without estrogen replacement (Berchtold et al., 2001). Given this relationship, it would not be surprising to note that the time course of HPA axis activation by exercise would be different in males and females, and also within females based on stage of estrus.
Females also respond differently to other forms of stress, besides running. Acute shock facilitates hippocampus-dependent trace eyeblink conditioning in males, but females show estrogen-dependent impairment on the task (Wood and Shors, 1998). Given that baseline differences in stress responsivity (Shors, 1998) and patterns of running activity (Girard and Garland, 2002) exist between males and females, gender most likely plays a critical role in determining the time course, and possibly even the mechanism of central differentiation of voluntary versus involuntary stress. A number of other factors exist, such as the relationship between the size of the living area and the amount of wheel running (Reebs and Maillet, 2003), and the amount of force required to turn the wheel (Collier et al., 1975). Methodological constraints abound when the stressor in question involves voluntary activity (summarized in Table 1, for mice, and in Table 2, for rats), but it is clear that running activates the neuroendocrine stress axis in both male and female animals. It remains to be determined whether sexual dimorphisms exist in the exercise-induced modification of the HPA axis.
Table 1.
Effects of Voluntary Running on Adrenal Steroids in the Mouse. All animals were 1 to 4 months old at the start of the experiments presented in the table. Durations were rounded to the nearest week.
| Citation | Strain | Sex | Housing | Duration | Distance | Parameter | Results |
|---|---|---|---|---|---|---|---|
| (Droste et al., 2006) | C57/BL6 | M | individual | 4wk | 5 km/d | basal and novel environment stress-induced CORT |
no change in basal or stress-induced CORT |
| (Adlard and Cotman, 2004) | C57/BL6 | M | individual | 3wk | Not reported | Basal and 10hr post- immobilization stress CORT |
no baseline differences; ↑ CORT 10hr post stress |
| (Droste et al., 2003) | C57/BL6 | M | individual | 4wk | 4 km/d | ACTH, CORT, CBG during different circadian phases |
↓ ACTH at lights-on, ↑ CORT and CBG at lights-off |
| (Lancel et al., 2003) | C57BL6 | M | individual | 4wk | 4 km/d | Basal and social stress- induced CORT and ACTH |
↑ post-stress CORT; no change in ACTH |
| (Girard and Garland, 2002) | Hsd:ICR | F | individual | 8wk | 7 km/d | Basal CORT at different points during the estrus cycle |
↑ basal CORT; effect of estrus stage |
| (van Praag et al., 1999) | C57/BL6 | F | group | 8wk | 5 km/d | Basal CORT | no change in basal CORT |
Table 2.
Effects of Voluntary Running on Adrenal Steroids in the Rat. All animals were 2 to 4 months of age at the start of the studies. Durations were rounded to the nearest week.
| Citation | Strain | Sex | Housing | Duration | Distance | Parameter | Results |
|---|---|---|---|---|---|---|---|
| (Stranahan et al., 2006) | SD | M | individual or group |
2 wk | 2 km/d | basal and post- immobilization stress CORT |
prolonged ↑ in CORT in individually housed runners |
| (Zheng et al., 2006) | SD | M | individual | 5–8 wk | not reported |
basal CORT | ↑ basal CORT |
| (Fediuc et al., 2006) | SD | M | individual | 5 wk | 9 km/d | circadian and post-immobilization stress CORT | circadian phase-dependent ↑ in CORT; no change in stress-induced CORT |
| (Makatsori et al., 2003) | Lewis | M | individual | 3 wk | 5 km/d | basal ACTH and CORT | ↑ basal CORT and ACTH |
| (Campisi et al., 2003) | Fischer F344 | M | individual | 8 wk | 3 km/d | basal and post-shock stress CORT | no change |
| (Naylor et al., 2005) | SHR | F | individual | 1 or 3 wk | 22 km/d | basal CORT | duration-dependent ↑ in basal CORT |
6. COMPARING ACROSS GENOTYPES REVEALS A POSITIVE CORRELATION BETWEEN SERUM CORTICOSTERONE LEVELS AND VOLUNTARY RUNNING
Genetic background plays a pivotal role in modulating stress responsivity in sedentary animals (Jones et al., 1998; Lu et al., 1998; Cabib et al., 1996). Strain differences also exist in the amount of voluntary running (Lightfoot et al., 2004), but it remains to be determined whether strain differences in basal corticosterone levels drive differences in running, or vice versa. By comparing across studies, it would appear that there is a positive correlation between basal corticosterone levels and the amount of voluntary wheel running in mice. C57BL/6ByJ mice exhibit lower levels of wheel running, relative to Balb/CyJ mice (Lightfoot et al., 2004), and also show a smaller increase in corticosterone and ACTH following footshock (Lu et al., 1998). DBA/2J mice also run more than C57BL/6ByJ mice (Lightfoot et al., 2004), and exhibit larger increases in corticosterone following restraint stress (Jones et al., 1998) and passive avoidance training, which involves shock (Cabib et al., 1996). The DBA/2J strain also exhibits higher levels of GR and MR expression in the hippocampus, indicative of greater HPA axis negative feedback, relative to the C57BL/6ByJ (Cabib et al., 1996). A positive correlation between corticosterone levels and voluntary running activity has also been reported in house mice (Hsd:ICR strain) selected for high levels of voluntary running over multiple generations (Malisch et al., 2007).
A similar relationship between resting corticosterone levels and amount of voluntary running exists for rats. Adrenalectomy decreases wheel running activity, and corticosterone replacement restores normal activity patterns (Leshner, 1971). Looking across different strains, the Sprague-Dawley rat has lower basal corticosterone levels than the Spontaneously Hypertensive Rat (SHR; McMurtry and Wexler, 1981), and also runs less (Ferguson and Cada, 2003). This relationship is specific to running wheel activity, because in the open field, the Sprague-Dawley shows greater activity than the SHR (Ferguson and Cada, 2003). The Wistar-Kyoto rat exhibits larger increases in corticosterone following swim stress than the Sprague-Dawley rat (Rittenhouse et al., 2002), and also runs more (Ferguson and Cada, 2003). Another commonly used strain, the Long-Evans rat, exhibits high levels of running activity (Bauer, 1990), and shows larger increases in corticosterone following a chronic mild stress paradigm (Bielajew et al., 2002). Overall, the literature is fairly consistent in supporting a positive correlation between corticosterone levels and voluntary running activity, but it is uncertain whether this is due to increased metabolic efficiency, or central alterations that would increase motivation to run.
Differences in hippocampal brain-derived neurotrophic factor (BDNF) levels across strains may also be an a priori determinant of wheel running activity. Sedentary black hooded rats (PVG/OlaHsd) exhibit higher levels of hippocampal BDNF, and also show greater levels of voluntary running, relative to Sprague-Dawley rats (Johnson and Mitchell, 2003). In a separate report, the amount of voluntary running was positively correlated with hippocampal BDNF levels in a rodent model of depression and anxiety (Bjornebekk et al., 2005). In seeming contrast to this relationship, sequestering BDNF through microinjection of TrkB-IgG microbeads into the hippocampus had no effect on the amount of voluntary running (Vaynman et al., 2004). However, this method would sequester some, but not all hippocampal BDNF, and a dose-response relationship between hippocampal BDNF and running activity has yet to be established. In addition, it has yet to be determined whether genotype- and species-specific differences in HPA axis reactivity arise from, or induce baseline differences in central levels of BDNF.
7. INTERACTIONS BETWEEN EXERCISE AND OTHER FORMS OF STRESS
While running exerts subtle effects on basal levels of GR and MR in the hippocampus, more dramatic effects are observed in studies that combine running with other forms of stress. Chronic elevation of glucocorticoids due to negative stress will decrease levels of central GR, impairing HPA axis shutoff, and increasing the duration of the stress response (De Kloet, 2004). Running protects against the decrease of the hippocampal GR observed in animals subjected to chronic mild stress, a paradigm in which a variety of stressors are applied intermittently (Zheng et al., 2006). Levels of BDNF, another factor related to hippocampal plasticity, are maintained in physically active mice following immobilization stress (Adlard and Cotman, 2004). Running also protects against the behavioral and neurochemical consequences of inescapable shock (reviewed by Greenwood and Fleshner, this issue).
Runners exhibit greater elevations in circulating corticosterone levels when subjected to swim stress, relative to sedentary animals (Droste et al., 2007). This amplification of the corticosterone response is the opposite of what Droste et al. observed in the same group of runners subjected to novel environment stress (Droste et al., 2007). The idea of stressor-specific adaptation fits into the framework of homo- and heterotypic stress; the runner adapts to running over time (homotypic stress), and at different points during adaptation to the same stressor, the response to a different stressor (heterotypic stress) might be amplified, or attenuated. Future experiments will be necessary to determine whether exercise modulates the time course of adaptation to a psychological stressor (restraint, or predator odor) differently than that of a physiological stressor (inescapable shock).
8. CENTRAL MECHANISMS UNDERLYING HPA AXIS ADAPTATION TO EXERCISE
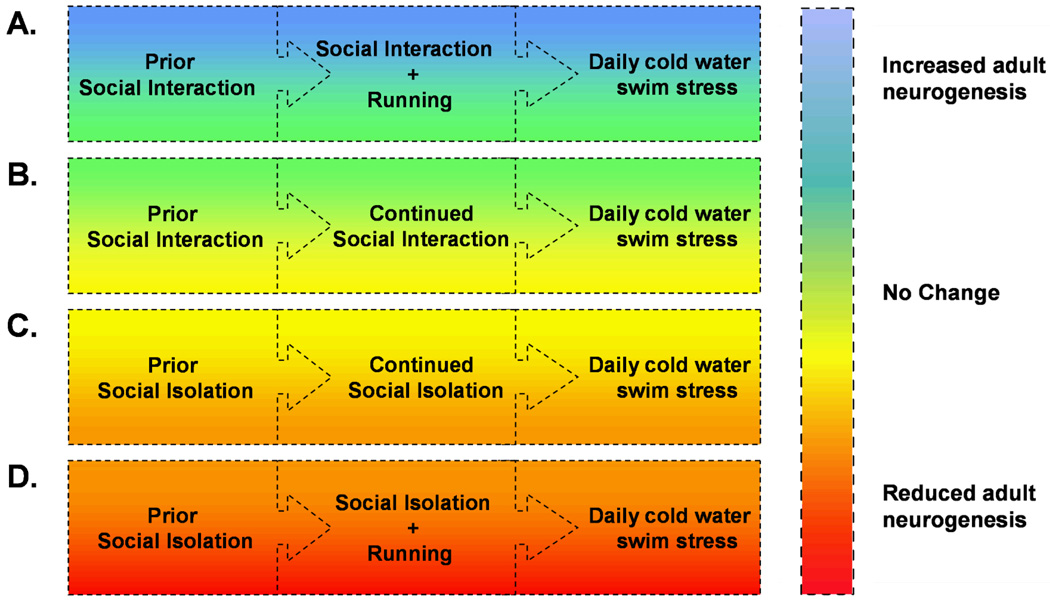
The dentate gyrus of the hippocampus continues to generate new neurons throughout the lifespan of the organism, and this process is thought to play a role in mood regulation and some forms of learning (Leuner et al., 2006). The number of new neurons is increased with voluntary running, but this relationship can be altered with concurrent social isolation stress (Stranahan et al., 2007). Socially isolated animals require longer durations of wheel running to enhance adult neurogenesis. When social isolation and cold water swim stress are combined with running, the number of new dentate granule neurons is actually reduced (Figure 3). These results suggest that social isolation may play a role in modulating the effects of running on the brain. While it is uncertain whether this would generalize to other species that are prone to fighting when housed in groups, these findings highlight the importance of a multilevel analysis of stressor interactions. Other studies have begun to examine possible relationships between running and diet, with respect to hippocampal oxidative stress. Running protects against the diet-induced activation of oxidative stress pathways, and also restores hippocampus-dependent learning (Molteni et al., 2004). Clearly, the interactions between exercise and different physiological and psychological stressors have only begun to be elucidated, and most studies to date have focused primarily on the hippocampus.
Figure 3.
Effects of combined stressors on hippocampal neurogenesis in the Sprague-Dawley rat. (A), Group-housed rats that are maintained in their groups with the addition of a running wheel will show enhancement of neurogenesis, even when concurrently subjected to repeated daily swim stress (Stranahan et al., 2006). (B), Group-housed animals that remain in group housing without exposure to wheel running, while also being subjected to daily swim stress, show no changes in levels of adult neurogenesis. (C), Animals that were previously group-housed, and then moved into individual housing for one week, followed by continual individual housing during the twelve days of exposure to swim stress, show no changes in hippocampal neurogenesis. (D), Rats that were initially group-housed, then moved into individual housing for one week, followed by the introduction of a running wheel in conjunction with daily swim stress, exhibit reduced adult neurogenesis. Because changes in adult neurogenesis have been proposed to play a role in depression (van Praag et al., 1999), these findings are in support of the idea that combinations of positive (physical activity) and negative (social isolation, swim stress) stressors can have disparate effects on the central mechanisms underlying mood regulation.
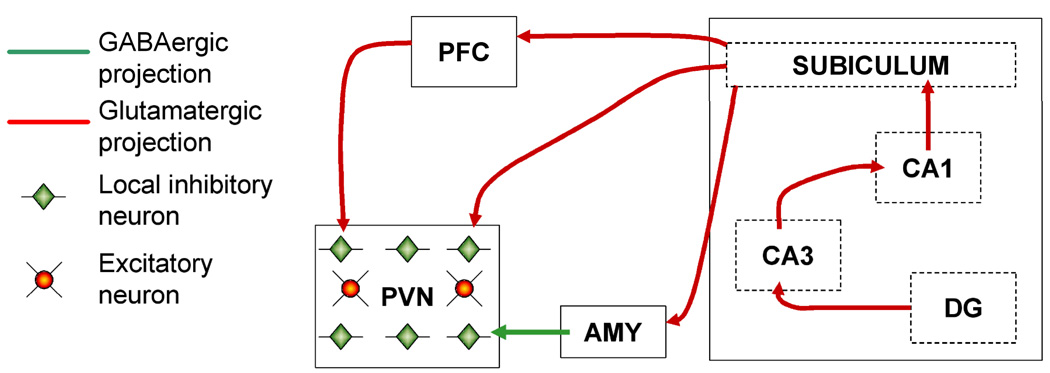
Far less is known about possible interactions between running and stress in other brain regions, such as the prefrontal cortex and amygdala (Figure 4). The medial prefrontal cortex has emerged as an important locus for the detection of stressor controllability (Amat et al., 2005), but it is uncertain whether inactivation of excitatory neurotransmission in the prefrontal cortex would alter the central perception of running as a non-threatening challenge to homeostasis. Activity in the prefrontal cortex has been implicated in the motivation for wheel running activity (Rhodes et al., 2003). In this study, wild type mice and mice that had been selected for high levels of wheel running were permitted to run, and then denied access to the wheel before being sacrificed for regional analysis of Fos immunoreactivity. Wild type runners showed an increase in the number of Fos-immunoreactive cells, while animals from the selected lines showed a larger increase in Fos labeling when wheels were blocked. This would implicate prefrontal cortical activity in the motivation to run, but the role of this region might not necessarily be to drive running activity. In an earlier study, Nonneman and Corman observed increased wheel running following lesions to the prefrontal cortex in rats (Nonneman and Corwin, 1981). Taken together, these data suggest a role for the prefrontal cortex in the inhibition of voluntary wheel running, but the role of the prefrontal cortex in modulating the stress response to exercise remains unexplored.
Figure 4.
Integrating voluntary physical activity into the framework of stress circuitry. Voluntary exercise has been demonstrated using a number of functional and structural assays to enhance the capacity for excitatory synaptic transmission in the hippocampus. Output from hippocampal area CA1 ultimately reaches the paraventricular nucleus of the hypothalamus (PVN), via excitatory projections from the subiculum. These projections terminate on inhibitory neurons in the PVN, thereby reducing HPA axis activity at this level. The hippocampus projects to the prefrontal cortex (PFC) via the subiculum, and the PFC sends excitatory projections to the PVN, also terminating on inhibitory neurons to reduce HPA axis output. Increased HPA axis activation likely occurs through increased activity in the central nucleus of the amygdala with voluntary running (AMY; Burghardt et al., 2006). This region sends inhibitory projections to inhibitory neurons of the PVN, increasing HPA axis drive. The balance between HPA axis activation and inhibition with voluntary running is likely to be shifted, and the consequences of such a shift could determine the response to other, heterotypic stressors.
Inputs from the amygdala to the hypothalamus also play a critical role in HPA axis regulation (Figure 4). Activity in amygdalar projections to the hypothalamus will increase HPA axis activity, while the projections from the prefrontal cortex to the hypothalamus will reduce HPA axis activity (reviewed in Herman et al., 2005). Chronic immobilization stress increases dendritic spine density on neurons in the basolateral amygdala (Mitra et al., 2005), but it remains uncertain whether running might exert similar or different effects on stress-induced growth of new spines in this region. Running reduces the density of Fos-immunopositive cells in the basolateral amygdala, and increases the density of labeled cells in the central nucleus of the amygdala (Burghardt et al., 2006). In addition to altering basal patterns of activation, the sudden inability to engage in wheel running will activate neurons in the amygdala (Rhodes et al., 2003). While it is clear that running increases immediate early gene activation in this region, the informative value of running-induced activity in the amygdala has yet to be determined.
Within the classical components of the HPA axis, still less is known about the role of connections between different hypothalamic nuclei, and their role in HPA axis adaptation to running and other stressors. Wheel running is frequently employed in studies of the function of clock genes governing periodicity in the suprachiasmatic nucleus (SCN) of the hypothalamus. Projections from the SCN to the PVN mediate circadian fluctuations in HPA axis hormones (Tousson and Meissl, 2004), which in turn play a pivotal role in regulating wheel running activity (Leshner, 1971). Rhythmic fluctuations in corticosterone are ‘flattened’ with aging and neuropsychiatric disorders (Magri et al., 2006; Bao et al., 2007). However, the role of hypothalamic interconnections in mediating adaptive stress responses is still being elucidated. A multimodal analysis of these interactions will provide an exciting glimpse into possible avenues for the therapeutic treatment of neurodegenerative and psychiatric disorders.
9. SUMMARY
In humans, regular exercise enhances cognitive function and elevates mood (Kramer and Erickson, 2007). However, theories regarding the cognitive benefits of exercise have yet to be reconciled with the excitation transfer phenomenon, in which physiological activation due to one form of stress can increase the response to a heterotypic stressor. The classical example of excitation transfer is the famous 'Wobbly Bridge Study,' in which subjects rated experimenters as being more physically attractive when testing was conducted under fear-inducing conditions on a wobbly bridge, relative to testing under safe conditions on a more stable bridge. Other studies have examined excitation transfer following exercise in humans, and observed that males will be more aggressive when provoked immediately after exercise (Zillmann et al., 1972). The idea that proximal causes of physiological arousal in humans can be misinterpreted opens the possibility that this phenomenon may occur in other species as well.
In this review, we describe wheel running as a form of voluntary, controllable stress in rodents. This characterization hews closely to Selye's original definition of stress as "the nonspecific response of the body to any demand, whether it is caused by, or results in, pleasant or unpleasant conditions (Seyle, 1976).” Although running is comparable to other forms of stress in terms of the magnitude of the initial elevation in corticosterone, it undoubtedly elicits patterns of neural activity that correspond with 'predictable, controllable, rewarding' activity - in contrast to the regional patterns of activation induced by inescapable shock, which would encode 'unpredictable, uncontrollable, aversive' activity. Comparing the patterns of brain activation following different forms of stress may help to understand the perception of danger vs. safety, with relevance for treating stress-related psychiatric disorders. Moreover, because humans exist in a far less controlled environment than laboratory animals, understanding how competing patterns elicited by different stressors interact may also prove relevant to human disease.
ACKNOWLEDGEMENTS
A.S. is supported by NIH NRSA F31 AG024690-03 through Princeton University, and by the National Institute on Aging Intramural Research Program.
REFERENCES
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124:985–992. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat.Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Antoniadis EA, Ko CH, Ralph MR, McDonald RJ. Circadian rhythms, aging and memory. Behav.Brain Res. 2000;114:221–233. doi: 10.1016/s0166-4328(00)00290-4. [DOI] [PubMed] [Google Scholar]
- Baldwin DR, Wilcox ZC, Baylosis RC. Impact of differential housing on humoral immunity following exposure to an acute stressor in rats. Physiol.Behav. 1995;57:649–653. doi: 10.1016/0031-9384(94)00313-0. [DOI] [PubMed] [Google Scholar]
- Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: Focus on the human hypothalamus. Brain Res.Rev. 2007 doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Bauer MS. Intensity and precision of circadian wheel running in three outbred rat strains. Physiol.Behav. 1990;47:397–401. doi: 10.1016/0031-9384(90)90162-w. [DOI] [PubMed] [Google Scholar]
- Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav.Processes. 2005;68:165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur.J.Neurosci. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- Bielajew C, Konkle AT, Merali Z. The effects of chronic mild stress on male Sprague-Dawley and Long Evans rats: I. Biochemical and physiological analyses. Behav.Brain Res. 2002;136:583–592. doi: 10.1016/s0166-4328(02)00222-x. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Brene S. The antidepressant effect of running is associated with increased hippocampal cell proliferation. Int.J.Neuropsychopharmacol. 2005;8:357–368. doi: 10.1017/S1461145705005122. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Pasumarthi RK, Wilson MA, Fadel J. Alterations in fear conditioning and amygdalar activation following chronic wheel running in rats. Pharmacol.Biochem.Behav. 2006;84:306–312. doi: 10.1016/j.pbb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Cabib S, Castellano C, Patacchioli FR, Cigliana G, Angelucci L, Puglisi-Allegra S. Opposite strain-dependent effects of post-training corticosterone in a passive avoidance task in mice: role of dopamine. Brain Res. 1996;729:110–118. [PubMed] [Google Scholar]
- Campisi J, Leem TH, Greenwood BN, et al. Habitual physical activity facilitates stress-induced HSP72 induction in brain, peripheral, and immune tissues. Am.J.Physiol.Regul.Integr.Comp.Physiol. 2003;284:R520–R530. doi: 10.1152/ajpregu.00513.2002. [DOI] [PubMed] [Google Scholar]
- Collier G, Hirsch E, Levitsky D, Leshner AI. Effort as a dimension of spontaneous activity in rats. J.Comp.Physiol.Psychol. 1975;88:89–96. doi: 10.1037/h0076217. [DOI] [PubMed] [Google Scholar]
- Dahlqvist P, Ronnback A, Risedal A, et al. Effects of postischemic environment on transcription factor and serotonin receptor expression after permanent focal cortical ischemia in rats. Neuroscience. 2003;119:643–652. doi: 10.1016/s0306-4522(03)00195-7. [DOI] [PubMed] [Google Scholar]
- De Kloet ER. Hormones and the stressed brain. Ann.N.Y.Acad.Sci. 2004;1018:1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- Dronjak S, Gavrilovic L, Filipovic D, Radojcic MB. Immobilization and cold stress affect sympatho-adrenomedullary system and pituitary-adrenocortical axis of rats exposed to long-term isolation and crowding. Physiol.Behav. 2004;81:409–415. doi: 10.1016/j.physbeh.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Droste SK, Chandramohan Y, Hill LE, Linthorst AC, Reul JM. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenocortical axis mainly at the adrenal level. Neuroendocrinology. 2007;86:26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- Droste SK, Schweizer MC, Ulbricht S, Reul JM. Long-term voluntary exercise and the mouse hypothalamic-pituitary-adrenocortical axis: impact of concurrent treatment with the antidepressant drug tianeptine. J.Neuroendocrinol. 2006;18:915–925. doi: 10.1111/j.1365-2826.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- Droste SK, Gesing A, Ulbricht S, Muller MB, Linthorst AC, Reul JM. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144:3012–3023. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J.Comp.Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Fediuc S, Campbell JE, Riddell MC. Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. J.Appl.Physiol. 2006;100:1867–1875. doi: 10.1152/japplphysiol.01416.2005. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Cada AM. A longitudinal study of short- and long-term activity levels in male and female spontaneously hypertensive, Wistar-Kyoto, and Sprague-Dawley rats. Behav.Neurosci. 2003;117:271–282. doi: 10.1037/0735-7044.117.2.271. [DOI] [PubMed] [Google Scholar]
- Freeman DA, Zucker I. Temperature-independence of circannual variations in circadian rhythms of golden-mantled ground squirrels. J.Biol.Rhythms. 2000;15:336–343. doi: 10.1177/074873000129001341. [DOI] [PubMed] [Google Scholar]
- Girard I, Garland T., Jr Plasma corticosterone response to acute and chronic voluntary exercise in female house mice. J.Appl.Physiol. 2002;92:1553–1561. doi: 10.1152/japplphysiol.00465.2001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog.Neuropsychopharmacol.Biol.Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Iversen IH. Techniques for establishing schedules with wheel running as reinforcement in rats. J.Exp.Anal.Behav. 1993;60:219–238. doi: 10.1901/jeab.1993.60-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Mitchell GS. Exercise-induced changes in hippocampal brain-derived neurotrophic factor and neurotrophin-3: effects of rat strain. Brain Res. 2003;983:108–114. doi: 10.1016/s0006-8993(03)03039-7. [DOI] [PubMed] [Google Scholar]
- Jones BC, Sarrieau A, Reed CL, Azar MR, Mormede P. Contribution of sex and genetics to neuroendocrine adaptation to stress in mice. Psychoneuroendocrinology. 1998;23:505–517. doi: 10.1016/s0306-4530(98)00014-6. [DOI] [PubMed] [Google Scholar]
- Kennedy GA, Hudson R, Armstrong SM. Circadian wheel running activity rhythms in two strains of domestic rabbit. Physiol.Behav. 1994;55:385–389. doi: 10.1016/0031-9384(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Partner's stress status influences social buffering effects in rats. Behav.Neurosci. 2004;118:798–804. doi: 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- Kopp C, Ressel V, Wigger E, Tobler I. Influence of estrus cycle and ageing on activity patterns in two inbred mouse strains. Behav.Brain Res. 2006;167:165–174. doi: 10.1016/j.bbr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J.Neurosci. 2004;24:6755–6759. doi: 10.1523/JNEUROSCI.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn.Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Lancel M, Droste SK, Sommer S, Reul JM. Influence of regular voluntary exercise on spontaneous and social stress-affected sleep in mice. Eur.J.Neurosci. 2003;17:2171–2179. doi: 10.1046/j.1460-9568.2003.02658.x. [DOI] [PubMed] [Google Scholar]
- Leshner AI. The adrenals and the regulatory nature of running wheel activity. Physiol.Behav. 1971;6:551–558. doi: 10.1016/0031-9384(71)90204-6. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR. Genetic influence on daily wheel running activity level. Physiol.Genomics. 2004;19:270–276. doi: 10.1152/physiolgenomics.00125.2004. [DOI] [PubMed] [Google Scholar]
- Lu ZW, Song C, Ravindran AV, Merali Z, Anisman H. Influence of a psychogenic and a neurogenic stressor on several indices of immune functioning in different strains of mice. Brain Behav.Immun. 1998;12:7–22. doi: 10.1006/brbi.1997.0510. [DOI] [PubMed] [Google Scholar]
- Magri F, Cravello L, Barili L, et al. Stress and dementia: the role of the hypothalamicpituitary-adrenal axis. Aging Clin.Exp.Res. 2006;18:167–170. doi: 10.1007/BF03327435. [DOI] [PubMed] [Google Scholar]
- Makatsori A, Duncko R, Schwendt M, Moncek F, Johansson BB, Jezova D. Voluntary wheel running modulates glutamate receptor subunit gene expression and stress hormone release in Lewis rats. Psychoneuroendocrinology. 2003;28:702–714. doi: 10.1016/s0306-4530(02)00062-8. [DOI] [PubMed] [Google Scholar]
- Makino S, Tanaka Y, Nazarloo HP, Noguchi T, Nishimura K, Hashimoto K. Expression of type 1 corticotropin-releasing hormone (CRH) receptor mRNA in the hypothalamic paraventricular nucleus following restraint stress in CRH-deficient mice. Brain Res. 2005;1048:131–137. doi: 10.1016/j.brainres.2005.04.065. [DOI] [PubMed] [Google Scholar]
- Malisch JL, Saltzman W, Gomes FR, Rezende EL, Jeske DR, Garland T., Jr Baseline and stress-induced plasma corticosterone concentrations of mice selectively bred for high voluntary wheel running. Physiol.Biochem.Zool. 2007;80:146–156. doi: 10.1086/508828. [DOI] [PubMed] [Google Scholar]
- McMurtry JP, Wexler BC. Hypersensitivity of spontaneously hypertensive rats (SHR) to heat, ether, and immobilization. Endocrinology. 1981;108:1730–1736. doi: 10.1210/endo-108-5-1730. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc.Natl.Acad.Sci.U.S.A. 2006;103:19170–19175. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc.Natl.Acad.Sci.U.S.A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Naylor AS, Persson AI, Eriksson PS, Jonsdottir IH, Thorlin T. Extended voluntary running inhibits exercise-induced adult hippocampal progenitor proliferation in the spontaneously hypertensive rat. J.Neurophysiol. 2005;93:2406–2414. doi: 10.1152/jn.01085.2004. [DOI] [PubMed] [Google Scholar]
- Nonneman AJ, Corwin JV. Differential effects of prefrontal cortex ablation in neonatal, juvenile, and young adult rats. J.Comp.Physiol.Psychol. 1981;95:588–602. doi: 10.1037/h0077800. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144:230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- Persson AI, Naylor AS, Jonsdottir IH, Nyberg F, Eriksson PS, Thorlin T. Differential regulation of hippocampal progenitor proliferation by opioid receptor antagonists in running and non-running spontaneously hypertensive rats. Eur.J.Neurosci. 2004;19:1847–1855. doi: 10.1111/j.1460-9568.2004.03268.x. [DOI] [PubMed] [Google Scholar]
- Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Reebs SG, Maillet D. Effect of cage enrichment on the daily use of running wheels by Syrian hamsters. Chronobiol.Int. 2003;20:9–20. doi: 10.1081/cbi-120018329. [DOI] [PubMed] [Google Scholar]
- Refinetti R. Absence of circadian and photoperiodic conservation of energy expenditure in three rodent species. J.Comp.Physiol.[B] 2007;177:309–318. doi: 10.1007/s00360-006-0130-7. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav.Neurosci. 2003;117:1243–1256. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Rittenhouse PA, Lopez-Rubalcava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology. 2002;27:303–318. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- Roubertoux PL, Guillot PV, Mortaud S, et al. Attack behaviors in mice: from factorial structure to quantitative trait loci mapping. Eur.J.Pharmacol. 2005;526:172–185. doi: 10.1016/j.ejphar.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, Buwalda B, et al. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Beagley G. Learned helplessness in the rat. J.Comp.Physiol.Psychol. 1975;88:534–541. doi: 10.1037/h0076430. [DOI] [PubMed] [Google Scholar]
- Seyle H. The Stress of Life. NY: McGraw-Hill; 1976. [Google Scholar]
- Shors TJ. Stress and Sex Effects on Associative Learning: For Better or for Worse. Neuroscientist. 1998;4:353–364. 353. [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007 doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat.Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousson E, Meissl H. Suprachiasmatic nuclei grafts restore the circadian rhythm in the paraventricular nucleus of the hypothalamus. J.Neurosci. 2004;24:2983–2988. doi: 10.1523/JNEUROSCI.5044-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am.J.Physiol. 1997;273:R1957–R1964. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc.Natl.Acad.Sci.U.S.A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur.J.Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Wollnik F, Turek FW. Estrous correlated modulations of circadian and ultradian wheel-running activity rhythms in LEW/Ztm rats. Physiol.Behav. 1988;43:389–396. doi: 10.1016/0031-9384(88)90204-1. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc.Natl.Acad.Sci.U.S.A. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Zheng H, Liu Y, Li W, et al. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav.Brain Res. 2006;168:47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillmann D, Katcher AH, Milavshky B. Excitation transfer from physical exercise to subsequent aggressive behavior. J.Exp.Soc.Psychol. 1972;8:247–259. 247. [Google Scholar]