Abstract
Thyrotropin-releasing hormone (TRH) was found to antagonize pentobarbital-induced sleeping time and hypothermia. While 3 to 100 mg/kg of TRH reduced pentobarbital sleeping time when administered prior to the barbiturate, a dose-response relationship to TRH could not be established. However, doses of 10 to 100 mg/kg of TRH enhanced the lethality of pentobarbital when these compounds were administered simultaneously. Thyrotropin or l-triiodothyronine did not imitate and hypophysectomy did not reduce the effects of TRH, indicating that the pituitary is not essential for its antagonism of pentobarbital Studies of TRH analogs provided further support of this view In addition TRH reduced the sleep and hypothermia produced by thiopental amobarbital, seco-barbital and phenobarbital, and it antagonized the hypothermia and reduced motor activity produced by chloral hydrate, reserpine, chlorpromazine and diazepam Intracisternally administered TRH also reduced pentobarbital sleeping time and hypothermia but melanocyte-stimulating hormone release-inhibiting factor and somatostatin administered by this route did not While reduction of pentobarbital sleeping time by TRH could not be attributed to an affect on monoamine systems or to deamidated TRH, this action was reduced by intracisternally administered atropine suggesting that cholinergic mechanisms may contribute to the effects of TRH. Thus the results provide evidence that TRH acts on brain independent of an effect on the pituitary.
Thyrotropin-releasing hormone (TRH; l-pyroglutamyl-l-histidyl-l-proline amide) has been found to release thyrotropin (Bowers et al., 1971; Hollander et al., 1972) and prolactin from the anterior pituitary (Bowers et al., 1971; Jacobs et al., 1971). In addition to these classical actions on the pituitary, other reports have suggested that TRH may have effects on the central nervous system unrelated to this endocrine function. Evidence for this latter view was first provided in a study demonstrating that TRH enhanced the stimulant properties of l-dihydroxyphenylalanine (l-dopa) in pargyline-treated mice after hypophysectomy (Plotnikoff et al., 1972; 1974a) as well as after thyroidectomy (Plotnikoff et al., 1974b).
TRH has further been reported to antagonize pentobarbital-induced sleep and hypothermia (Prange et al., 1974; Breese et al., 1974a) and to antagonize the narcosis and hypothermia induced by ethanol (Breese et al., 1974a,b). Clinical reports indicate that intravenously administered TRH can alter the symptoms of certain mental disorders (Prange et al., 1972, 1973; Kastin et al., 1972; Tiwary et al., 1973), providing further support that TRH possesses central activity. In the present report, the effects of TRH on the actions of pentobarbital and other centrally acting drugs are detailed to provide further evidence concerning the actions of this tripeptide on the central nervous system.
Methods
General procedures
Male and female Swiss-Webster mice (19–27 g) obtained from Dublin Laboratory (Dublin, Va.) were treated intraperitoneally with various doses of pentobarbital sodium. After administration of pentobarbital sodium, sleeping time was measured as the time from which righting reflex was lost until it was regained. Effects of TRH on sleep and hypothermia were also examined after thiopental sodium, amobarbital sodium, secobarbital sodium and phenobarbital sodium. Other mice received l-triiodothyronine (0.1 and 1 mg/kg) or bovine thyrotropin (10 mg/kg, Armour Pharmaceutical Co., Chicago, Ill.) before receiving pentobarbital sodium. The ability of TRH to antagonize pentobarbital and phenobarbital were also examined in hypohysectomized mice (22 g; Charles River Breeding Laboratories, Inc., Wilmington, Mass.). In another series of experiments, 20 mg/kg of TRH were administered twice daily for 3 days before determining if 1 or 5 mg/kg of TRH would reduce sleeping time produced by pentobarbital sodium. Various doses of TRH (3, 10, 30 and 100 mg/kg) were injected 1 to 5 minutes before various doses of pentobarbital (42, 50, 58, 66 and 74 mg/kg) in order to examine dose-response relationships of the TRH antagonism of pentobarbital sleeping time. The effect of TRH (5, 10, 30 and 100 mg/kg) on lethality of pentobarbital was examined by administering TRH simultaneously with higher doses of pentobarbital (50–130 mg/kg). TRH was also administered to rats, gerbils, hamsters and guinea pigs. Rectal temperature from animals kept at room temperature (25°C) was monitored in some experiments with a thermistor probe (Yellow Springs Instrument Company, Yellow Springs, Ohio). In other experiments, TRH, TRH derivatives somatostatin (somatotropin release-inhibiting factor; SRIF) and melanocyte-stimulating hormone release-inhibiting factor (MIF) were administered intracisternally 10 minutes after receiving pentobarbital sodium (55 mg/kg i.p.). Control mice received saline intracisternally. The effects of these compounds on pentobarbital-induced sleeping time and hypothermia were determined as described previously.
The ability of several centrally acting drugs to affect the actions of TRH was examined by pretreating animals with reserpine, chlorpromazine, diazepam or chloral hydrate. In the first series of experiments with these drugs, three mice were placed in circular activity cages obtained from Woodward Research Corporation (Herndon, Va.). Each cage consisted of a circular runway 9 cm wide with 20 cm high walls and an outside diameter of 31 cm. Six photocell sensors were mounted in the outer wall of the runway 1 cm above the wire mesh floor. Interruptions of the light beams were automatically recorded on electromechanical counters in 6-minute intervals for 1 hour. Before being placed in the cage, animals were treated with one of the following drugs: reserpine (2.5 mg/kg s.c., 24 hours prior to the experiment), diazepam (12.5 mg/kg i.p.), chlorpromazine (6.0 mg/kg i.p.) or chloral hydrate (360 mg/kg i.p.). Phenobarbital (95 mg/kg i.p.) was also included with this group of drugs and was administered 15 minutes before animals were placed in the activity chamber. Animals were allowed to habituate to the chamber for 24 minutes. At the end of the habituation period, each set of three mice received TRH (30 mg/kg i.p.) or saline vehicle. Motor activity was then recorded as described. Other groups of mice were treated with these drugs and rectal temperature was recorded prior to and 30 minutes after TRH administration (30 mg/kg i.p.).
Pentobarbital disposition and metabolism
The effect of TRH on the disposition of 3H-pentobarbital (New England Nuclear Corp., Boston, Mass.) with a specific activity of 1 μc/mg was determined at 10, 20, 30 and 40 minutes after administration of 5 mg/kg of TRH. At the time of killing, 50 μl of blood were collected in heparin-treated tubes to permit determination of total radioactivity in plasma. Analysis for unchanged drug and its metabolites followed the method outlined by Peters and Strother (1972) as modified by Randall and Lester (1974). Brain and liver were homogenized in 3.5 ml of 0.2 M sodium phosphate buffer (pH 4.5). Pentobarbital was isolated by extracting the homogenate (2 ml) with 30 ml of toluene scintillation solution containing 0.5% 2,5-diphenyloxazole (PPO) by shaking on a Vortex mixer for 15 seconds. After centrifugation, duplicate 12-ml aliquots of the supernatant toluene phase were transferred to glass scintillation vials for counting. This procedure was found to extract 99% of a 3H-pentobarbital standard. After two washings with 3 ml of toluene, the aqueous buffer was extracted with 6 ml of 95% ethanol by mixing for 15 seconds with a Vortex mixer to isolate the metabolites. After centrifugation, the alcohol supernatant was transferred to a counting vial containing 10 ml of 1:1 mixture of Triton X-100–scintillation solution. Samples were corrected for quenching by the use of an external standards ratio technique. This was verified by an internal standard of 3H-toluene.
Intracisternal administration of norepinephrine, phentolamine and atropine
Some mice were treated with pentobarbital and 10 minutes later given an intracisternal injection of either norepinephrine (15 or 30 μg), phentolamine (2.25 μg) or both phentolamine and norepinephrine. For comparison some mice received 2.25 μg of phentolamine intracisternally followed 30 seconds later by 10 mg/kg of TRH intraperitoneally. The effect of intracisternally administered atropine sulfate (20 μg) or atropine methyl nitrate (20 μg) on the actions of TRH against pentobarbital was also studied. Sleeping times were determined for all groups and rectal temperature was measured 30 minutes after pentobarbital injection.
Biogenic amine mechanisms
In these experiments, mice were treated with pargyline (100 mg/kg) 15 minutes before the injection of pentobarbital sodium. Half the animals received TRH and the other half saline 30 seconds later. Animals were killed 15 or 30 minutes after receiving the solution of pentobarbital sodium. Other animals received either TRH or TRH and α-methyltyrosine methyl ester (300 mg/Kg) 2 hours before being killed. Determinations of brain norepinephrine, dopamine and serotonin were carried out as described previously (Breese and Traylor, 1970). Appropriate controls were included where necessary.
In another series of experiments, mice were treated as above but 10 minutes later they received 10 μc of 3H-tyrosine (26 μg/mc) intracisternally. Mice were killed 30 minutes after the administration of the 3H-tyrosine. After the mice were killed, brains from three mice were pooled and homogenized in 10 ml of 0.4 N perchloric acid. Radioactive norepinephrine and dopamine were isolated according to the method described by Sedvall et al. (1968). Less than 0.5% dopamine was noted in the norepinephrine fraction and vice versa. Radioactive amine values are uncorrected for recovery which was 70% for dopamine and 80% for norepinephrine. An internal standard of 3H-toluene was used to correct for counting efficiency.
Statistics
Data was analyzed by the Dunnett's t test or Student's t test as appropriate. The LD50 determinations were calculated by the method of Bliss (1952) and displayed graphically on probit paper.
Results
Effect of TRH on pentobarbital-induced sleeping time and hypothermia
In accord with previous reports (Prange et al., 1974; Breese et al., 1974a), TRH significantly reduced pentobarbital-induced sleeping time and accompanying hypothermia (table 1). This effect was apparent in female mice as in male mice, indicating that sex of the animal is not a determinant in this action of TRH (table 1). However, when TRH was administered 45 minutes prior to pentobarbital, TRH was no longer effective in antagonizing the actions of this barbiturate (table 2).
TABLE 1. Effect of TRH on sleep and hypothermia induced by pentobarbital in male and female micea.
| Treatment | N | Sleeping Time | Rectal Temperature |
|---|---|---|---|
| min ± S.E.M. | °C ± S.E.M. | ||
| Male mice | |||
| Control (saline) | 12 | 58.3 ± 7.3 | 33.2 ± 0.14 |
| TRH (5 mg/kg) | 12 | 28.8 ± 2.7b | 35.5 ± 0.18b |
| Female mice | |||
| Control (saline) | 28 | 57.0 ± 4.2 | 33.3 ± 0.12 |
| TRH (5 mg/kg) | 28 | 23.0 ± 1.9b | 35.7 ± 0.28b |
All animals were given 55 mg/kg of pentobarbital sodium intraperitoneally. TRH (5 mg/kg) was administered to mice just before the solution of pentobarbital sodium. Rectal temperature was measured 20 minutes after the mice were given the barbiturate.
P < .001 when compared with saline-treated control.
TABLE 2. Duration of pretreatment with TRH on antagonism of pentobarbital-induced sleep.
| Treatmenta | Time Before Pentobarbital | N | Sleeping Time | Rectal Temperatureb |
|---|---|---|---|---|
| min | min ± S.E.M. | °C ± S.E.M. | ||
| Control (saline) | 16 | 59.7 ± 2.7 | 33.0 ± 0.16 | |
| TRH (5 mg/kg) | 1 | 20 | 26.5 ± 2.2c | 34.9 ± 0.31c |
| TRH (5 mg/kg) | 15 | 20 | 29.3 ± 1.7c | 35.2 ± 0.17c |
| TRH (5 mg/kg) | 30 | 18 | 39.2 ± 3.5c | 35.0 ± 0.24c |
| TRH (5 mg/Kg) | 45 | 18 | 53.3 ± 5.0 | 33.6 ± 0.16 |
All mice were given pentobarbital sodium (55 mg/Kg). TRH was administered at 1, 15, 30 or 45 minutes before this treatment.
Rectal temperature was measured 20 minutes after the mice were given the barbiturate.
P < .001 when compared with control.
Furthermore, as shown in table 3, chronic administration of TRH (20 mg/kg) twice daily for 3 days with an additional dose of TRH injected 4 hours prior to the experiment did not influence the antagonism of sleeping time produced by the subsequent administration of 1 or 5 mg/kg of TRH.
TABLE 3. Effect of chronic treatment with TRH on acute antagonism of pentobarbital-induced sleepa.
| Pretreatmentb | Acute TRHb Dose | Sleeping Time | N |
|---|---|---|---|
| mg/kg | min ± S.E.M. | ||
| Control (saline) | 63.0 ± 4.32 | 10 | |
| Chronic TRH | 1 | 28.5 ± 3.2c | 13 |
| Chronic TRH | 5 | 21.2 ± 3.3c | 13 |
| Saline | 1 | 21.2 ± 5.5c | 10 |
| Saline | 5 | 24.0 ± 5.4c | 10 |
All mice were give pentobarbital sodium (55 mg/kg).
TRH (20 mg/kg) was administered to mice two times daily for 3 days with an additional dose of TRH (20 mg/kg) being given 4 hours prior to the acute administration of 1 or 5 mg/kg of TRH which was administered 1 minute before the pentobarbital sodium solution.
P < .001 when compared with saline-treated control.
Dose-response relationships of TRH and pentobarbital in mice
In preliminary experiments, a dose-response relationship of TRH antagonism of pentobarbital sleeping time was not apparent when doses from 1 to 10 mg/kg were administered (Prange et al., 1974). Since the interactions between TRH and pentobarbital are likely complex, the dose of pentobarbital was varied (42, 50, 58, 66 and 74 mg/kg) and various doses of TRH (3, 10, 30 or 100 mg/kg) were tested against each dose of pentobarbital to determine if a dose-response relationship could be obtained (fig. 1). From these data, an obvious dose-response relationship to the TRH antagonism of pentobarbital sleeping time could not be established in the TRH dose range explored. However, all doses of TRH administered 3 to 5 minutes prior to the pentobarbital effectively shifted the dose-response curve of pentobarbital to the right (fig. 1), indicating a consistent reduction of sleeping time.
Fig. 1.
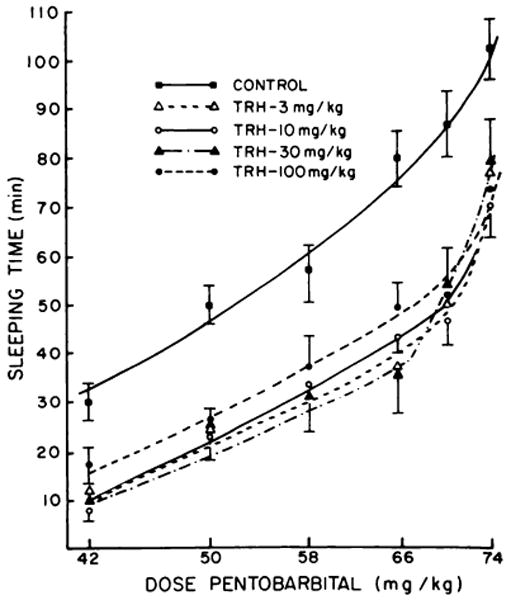
Effect of various doses of TRH on the dose–response relationships of pentobarbital sleeping time. The doses of TRH were administered 5 minutes before the injection of the doses of pentobarbital. Sleeping time was determined as described in “Methods.” Control values represent 20 to 40 determinations. Each point for the TRH curves represent the mean ± S.E.M. sleeping time of 10 to 30 mice. All mean values for TRH are significantly different from control (P < . 001).
In initial dose-response experiments it was observed that, when TRH and high doses of pentobarbital were administered together, the lethality of pentobarbital was increased rather than diminished as might have been expected. For that reason, a systematic study of the effect of TRH on pentobarbital lethality was performed (fig. 2). While 5 mg/kg of TRH caused a small reduction of pentobarbital lethality, doses of TRH of 30 mg/kg or greater administered with pentobarbital caused a significant increase in the lethality of pentobarbital (P < .01).
Fig. 2.
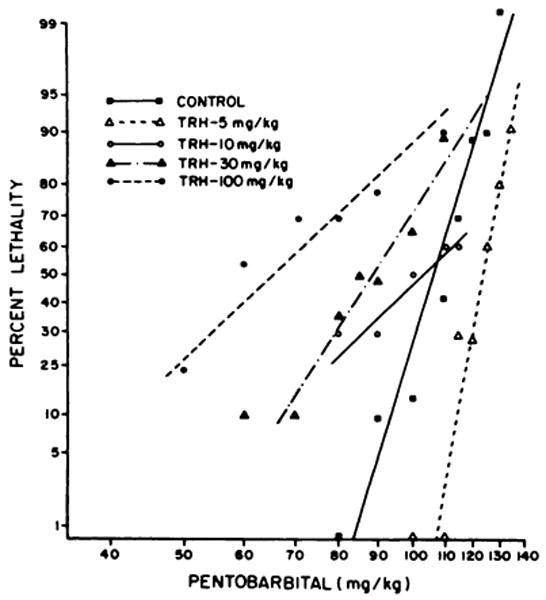
Effect of TRH on the lethality of pentobarbital. In contrast to the procedure used in Figure 1, TRH was administered simultaneously with pentobarbital sodium in these experiments. The LD50 values for 30 and 100 mg/kg of TRH are significantly different from control (P < .01). Each point represents not less than 10 nor more than 40 mice.
Relationship of TRH antagonism of pentobarbital-induced sleeping time to pituitary function
In order to test the possibility that the effect of TRH on pentobarbital sleeping time might be due to release of thyrotropin, mice treated with pentobarbital were given either thyrotropin (10 mg/kg) or l-triiodothyronine (T3; 0.1 and 1 mg/kg). Neither agent significantly altered sleeping time or the hypothermia produced by pentobarbital (P > .1). It was also established that hypophysectomy did not affect the ability of TRH (5 mg/kg) to reverse the actions of pentobarbital (45 mg/kg). Pentobarbital sleeping time in control hypophysectomized mice was 68.3 ± 81 minutes vs. 35.7 ± 2.7 minutes for hypophysectomized mice treated with TRH. TRH antagonism of pentobarbital hypothermia was also unaffected by hypophysectomy (TRH-treated mice = 34.8 ± 0.26°C vs. saline treatment = 32.3 ± 0.42°C). These latter findings indicate that the pituitary is not essential for TRH antagonism of pentobarbital.
Effect of TRH on pentobarbital metabolism
Since TRH could reduce sleeping time by increasing the metabolism or excretion of pentobarbital, this possibility was examined by administering TRH (5 mg/kg) 5 minutes before the injection of 50 mg/kg of 3H-pentobarbital The level of 3H-pentobarbital in these TRH-treated mice was determined 10, 20, 30 and 40 minutes after administration and compared with the amount of radioactivity found in mice that received only 3H-pentobarbital. As shown in figure 3, the content of 3H-pentobarbital found in brain was not altered by treatment with TRH. Furthermore, it was also found that neither total plasma radioactivity nor 3H-pentobarbital isolated from liver differed between groups at any of the time periods (P > .1).
Fig. 3.
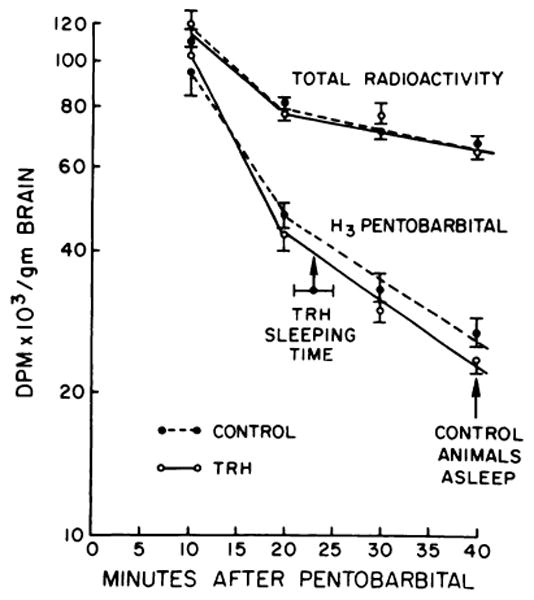
Effect of TRH on the content of 3H-pentobarbital in brain. Mice were treated with 3H-pentobarbital (50 mg/kg i.p.) and 30 seconds later received 5 mg/kg of TRH i.p. Animals were killed 10, 20, 30 and 40 minutes after receiving the pentobarbital. Sleeping time for a group of mice treated in a similar fashion was 22.5 ± 3.5 minutes. This is indicated on the graph as “TRH sleeping time.” The arrow at 40 minutes indicates that all control animals remained asleep at this time.
Effect of TRH on biogenic amine mechanisms
Since alterations in monoamine content can alter pentobarbital-induced sleep (Boissier and Simon, 1968), the possibility that TRH might in some way be affecting monoamine systems was examined. In confirmation of previous findings (Plotnikoff et al., 1974a; Breese et al., 1974a; Reigle et al., 1974), TRH alone did not alter the content of norepinephrine and dopamine in brain (fig. 4). However, when given in combination with α-methyltyrosine, TRH was found to facilitate the reduction of brain norepinephrine produced 2 hours after α-methyltyrosine. In contrast to this finding, TRH was not found to enhance the incorporation of 3H-tyrosine into 3H-norepinephrine (NE) in pentobarbital-treated mice 30 minutes after the 3H-tyrosine (3018 ± 136 cpm of NE per g for control mice and 3110 ± 150 cpm of NE per g for TRH-treated mice).
Fig. 4.
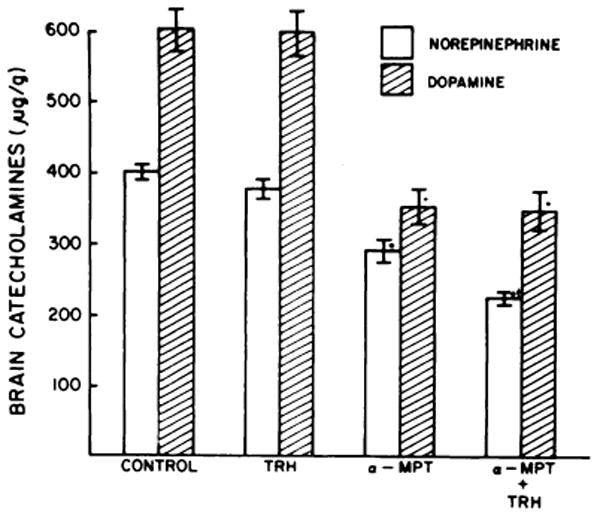
Effect of TRH on α-methyltyrosine reduction of brain catecholamine content. Mice received 10 mg/kg of TRH or TRH plus α-methyl-tyrosine methyl ester (300 mg/kg) 2 hours before sacrifice. Each column represents the mean ± S.E.M. of at least 17 determinations. *P < .001 when compared with control; P < .01 when compared with α-methyltyrosine.
The possibility was also examined that amine alterations might be observed if degradation of the monoamines was reduced with pargyline. In mice that received pentobarbital 15 minutes after pargyline, TRH produced no significant changes in brain norepinephrine, dopamine or serotonin content when these animals were killed 45 minutes after pargyline (P > 0.1).
Effect of phentolamine and atropine on TRH antagonism of pentobarbital-induced sleep and hypothermia
The possibility that TRH might be acting through a noradrenergic mechanism was explored further by examining the actions of TRH after phentolamine, a compound which was found to block the ability of intracisternally administered norepinephrine (30 μg) to reduce pentobarbital sleep and hypothermia (table 4). While the absolute sleeping time in phentolamine-treated mice given TRH was significantly longer than that for mice that received TRH alone, the percent reduction in sleeping time caused by TRH in phentolamine-treated mice (66% of phentolamine-treated control) was comparable to that observed in TRH-treated mice without the phentolamine pretreatment (63% of control) (table 4). Furthermore, phentolamine did not alter significantly the increased temperature response caused by TRH in pentobarbital-treated mice. These latter findings suggest that noradrenergic mechanisms are not responsible for the actions of TRH.
TABLE 4. Effects of phentolamine and atropine on the actions of TRH to reduce pentobarbital-induced sleep and hypothermia.
| Treatmenta | N | Sleeping Time | Rectal Temperature |
|---|---|---|---|
| min ± S.E.M. | °C ± S.E.M. | ||
| Experiment I | |||
| Saline | 37 | 65.4 ± 3.00 | 33.6 ± 0.23 |
| TRH (10 mg/kg) | 24 | 41.5 ± 3.15b | 36.6 ± 0.08b |
| NE (30 μg) | 20 | 54.3 ± 2.90c | 39.5 ± 0.26b |
| Phentolamine (2.25 μg) | 20 | 80.4 ± 4.88c | 34.3 ± 0.19 |
| Phentolamine (2.25 μg) + NE (30 μg) | 10 | 78.2 ± 7.92 | 37.2 ± 0.33b,d |
| Phentolamine (2.25 μg) + TRH (10 mg/kg) | 17 | 53.7 ± 2.81b,d | 36.4 ± 0.25b,d |
| Experiment II | |||
| Saline | 20 | 44.6 ± 5.31 | 33.0 ± 0.50 |
| TRH | 20 | 16.9 ± 2.23b | 37.3 ± 0.31b |
| Atropine sulfate (20 μg) | 10 | 43.3 ± 4.35 | 34.0 ± 0.66 |
| Atropine CH3–nitrate (20 μg) | 10 | 44.7 ± 8.84 | 34.4 ± 0.82 |
| Atropine sulfate (20 μg) + TRH (10 mg/kg) | 20 | 40.0 ± 4.81 | 36.9 ± 0.53b |
| Atropine CH3-nitrate (20 μg) + TRH (10 mg/kg) | 20 | 44.8 ± 6.37 | 36.0 ± 0.35b |
In experiment I mice were given 55 mg/kg of pentobarbital sodium 10 minutes before TRH, norepinephrine or phentolamine. Phentolamine (2.25 μg) and norepinephrine (15 or 30 μg) were administered intracisternally. NE and TRH were administered 30 seconds after phentolamine. In experiment II, mice were given 50 mg/kg of pentobarbital sodium 10 minutes before TRH, atropine sulfate or atropine methyl nitrate. The atropine SO4 and atropine CH3–nitrate were injected intracisternalry. TRH (10 mg/kg) was injected intraperitoneally. Rectal temperacture measured 30 minutes after TRH.
P < .001 when compared with saline-treated control.
P < .05 when compared with saline-treated control.
P < .001 when compared with group that received phentolamine.
Intracisternally administered atropine sulfate and atropine methyl nitrate were used to examine possible cholinergic involvement of TRH in its antagonism of pentobarbital. Support for activation of cholinergic systems by TRH was provided by the finding that atropine sulfate as well as atropine methyl nitrate antagonized the effect of TRH on pentobarbital sleep (table 4). Neither agent affected the ability of TRH to reverse pentobarbital-induced hypothermia Peripherally administered atropine methyl nitrate did not significantly alter TRH antagonism of pentobarbital (P > .1).
Effect of intracisternally administered TRH derivatives and other hypothalamic releasing factors on pentobarbital-induced sleep and hypothermia
After the observation that TRH would alter pentobarbital-induced sleep and hypothermia, several TRH derivatives and other hypothalamic releasing factors were administered intracisternally to determine whether they might also reduce the actions of this barbiturate. As shown in table 5, structural analogs of TRH that were found to possess activity comparable to TRH included pyrazolyl TRH, β-Ala-TRH and 3-methyl-His-TRH. A lysine-substituted analog (lysine TRH) significantly reduced sleeping time but less so than TRH. Diiodo substituted TRH (diiodo TRH) was inactive in this paradigm as was the deamidated derivative which had previously been shown to be inactive after intraperitoneal administration (Breese et al., 1974b). Furthermore, melanocyte-stimulating hormone release-inhibiting factor (MIF) and somatostatin at the doses chosen did not significantly alter pentobarbital-induced sleep or hypothermia (table 5). However, since somatostatin has been shown to enhance pentobarbital sleeping time after peripheral administration (A. J. Prange, unpublished data) a higher dose of somatostatin (30 μg) was injected intracisternally into mice treated with pentobarbital (50 mg/kg). This dose of somatostatin killed 5 of 15 of the pentobarbital-treated mice and significantly prolonged sleep of surviving mice to 166 ± 17% of control (P < .01).
TABLE 5. Effect of intracisternally administered TRH, TRH analogs or other releasing factors on pentobarbital sleep and hypothermiaa.
| Treatment | Dose | N | Sleeping Time | Rectal Temperature | Thyroid Potency (%TRH)b |
|---|---|---|---|---|---|
| μg | min ± S.E.M. | °C± S.E.M. | |||
| Control (Saline) | 34 | 55.8 ± 3.95 | 34.3 ± 0.16 | ||
| TRH | 10 | 34 | 27.3 ± 2.18c | 37.8 ± 0.23c | 100 |
| Pyrazolyl TRHd | 10 | 20 | 27.7 ± 2.86c | 37.1 ± 0.35c | 5 |
| β-Ala-TRHd | 10 | 20 | 26.9 ± 2.72c | 37.8 ± 0.17c | 10 |
| Lysine TRHd | 10 | 20 | 35.4 ± 2.05c | 36.7 ± 0.46c | 0.02 |
| 3-Methyl-His-TRHd | 10 | 20 | 25.4 ± 1.77c | 36.9 ± 0.30c | 800 |
| Diiodo TRHd | 10 | 10 | 57.8 ± 5.70 | 36.4 ± 0.34c | |
| Deamidated TRHd | 10 | 10 | 59.3 ± 2.73 | 35.1 ± 0.22e | 0.02 |
| MIFd | 10 | 12 | 52.7 ± 3.21 | 34.2 ± 0.27 | |
| Somatostatin | 15 | 10 | 68.4 ± 6.33 | 34.5 ± 0.23 | |
Mice were given 55 mg/kg of pentobarbital sodium 10 minutes before the various compounds intracisternally. Rectal temperature was measured 30 minutes after mice were given the pentobarbital.
Values taken from Vale et al. (1973) except for β-Ala-TRH (W. Lotz, personal communication).
P < .001 when compared with control.
TRH, pGlu-His-Pro-NH2; pyrazoly 1 TRH,pGlu-(pyrazolyl-3) Ala-Pro-NH2; lysine TRH, pGlu-Lys-Pro-NH2; β-Ala-TRH pGlu-His-Pro-β-Ala-NH3; 3-Methyl-His-TRH, pGlu-3-methyl-His-Pro-NH2; diiodo TRH, pGlu-2,4-Diiodo-His-Pro-NH2; MIF, Pro-Leu-Gly-NH2.
P < .01 when compared with control.
In view of reports that somatostatin can antagonize the release of thyrotropin by TRH (Vale et al., 1973), mice were treated intracisternally with somatostatin (15 μg) 30 seconds before they were given TRH (10 mg/kg) intraperitoneally. TRH produced about the same percent time reduction in sleeping time in somatostatin-treated mice (66.4 ± 7.4 minutes) as was observed in saline-treated mice (60.4 ± 3.2 minutes). Temperature increase to TRH was similar 30 minutes after TRH in somatostatin and control mice (36.3 ± 0.30°C for TRH in saline-treated mice vs. 36.4 ± 0.33°C in somatostatin-TRH-treated mice). These results suggest that a comparable antagonism of TRH by somatostatin reported for pituitary does not occur in brain, at least with regard to the antagonism of pentobarbital-sleeping time and hypothermia.
Effect of TRH on pentobarbital anesthesia in other mammalian species
The purpose of this portion of the study was to determine whether TRH would alter pentobarbital sleep in mammals other than mice (table 6). In accord with previous studies (Prange et al., 1974), the reaction reported in rats was evident in this study. However, in this former work which used rats weighing approximately 300 g, TRH produced apparent awakening and reduced hypothermia without always reinstating righting reflex (Prange et al., 1974). In the present study which employed smaller rats (140 g), TRH not only produced the apparent awakening effect and reduced hypothermia but also reinstated righting reflex. TRH also produced a significant reduction of pentobarbital-induced sleeping time and hypothermia in hamsters, gerbils and guinea pigs (table 6).
TABLE 6. Effects of TRH on pentobarbital-induced sleep and hypothermia in various speciesa.
| Animal | Pentobarbital Dose | N | Sleeping Time | Rectal Temperature | ||
|---|---|---|---|---|---|---|
| Control | TRH | Control | TRH | |||
| mg/kg | min ± S.E.M | °C ± S.E.M. | ||||
| Rat | 32 | 8 | 73 ± 6.8 | 29 ± 4.1b | 35.9 ± 0.10 | 38.4 ± 0.10b |
| Guinea pig | 26 | 4 | 190 ± 12.5 | 130 ± 12.7c | 35.5 ± 0.17 | 37.9 ± 0.16b |
| Hamster | 50 | 10 | 35 ± 3.6 | 24 ± 2.0c | 36.2 ± 0.22 | 37.9 ± 0.16b |
| Gerbil | 50 | 13 | 130 ± 8.4 | 88 ± 2.6b | 32.0 ± 0.13 | 36.7 ± 0.59b |
Doses of pentobarbital were administered intraperitoneally. All animals were given 10 mg/kg of TRH intraperitoneally 1 minute after pentobarbital. Sleeping time was considered the time from loss of righting reflex until righting reflex was regained. [Rectal temperature was measured 30 to 60 minutes after TRH.]
P < .001 when compared with control.
P < .025 when compared with control.
Effect of TRH on sleeping time and hypothermia produced by other barbiturates
The effect of TRH after treatment with several types of barbiturates was examined to assure that the actions of TRH on pentobarbital were not peculiar to this drug. As shown in table 7, TRH shortened the sleeping time produced by thiopental sodium, secobarbital sodium, amobarbital sodium and phenobarbital sodium. In the case of phenobarbital sodium, TRH was administered after the animals lost their righting reflex. It also should be pointed out that TRH antagonized the actions of phenobarbital sodium (100 mg/kg) in hypophysectomized animals (354 ± 44 minutes for hypophysectomized control vs. a sleeping time of 9.3 ± 2.9 minutes for hypophysectomized mice treated with TRH; P < .001), providing additional evidence that an intact hypophyseal-thyroid system was not required for this action of TRH.
TABLE 7. Effects of TRH on sleep and hypothermia produced by secobarbital, amobarbital, phenobarbital and thiopentala.
| Barbiturate | Dose | Sleeping Time | Rectal Temperatureb | ||
|---|---|---|---|---|---|
| Control | TRH | Control | TRH | ||
| mg/kg | min ± S.E.M. | °C ± S.E.M. | |||
| Secobarbital Na | 50 | 91.1 ± 9.54 | 39.9 ± 4.56c | 32.5 ± 0.24 | 36.0 ± 0.28c |
| Amobarbital Na | 110 | 73.8 ± 5.55 | 48.7 ± 5.23c | 33.8 ± 0.02 | 35.9 ± 0.20c |
| Phenobarbital Na | 150 | 96.8 ± 5.34 | 8.4 ± 4.96c | 34.4 ± 0.22 | 35.6 ± 0.38d |
| Thiopental Na | 55 | 70.1 ± 16.6 | 33.9 ± 3.93c | 34.7 ± 0.65 | 37.1 ± 0.40d |
| Pentobarbital Na | 50 | 59.3 ± 4 10 | 22.4 ± 2.93c | 33.8 ± 1.22 | 36.7 ± 0.40e |
Mice were treated with TRH (5 mg/kg i.p.) 1 minute before the various barbiturate derivatives (i.p.) except for the phenobarbital group which received the TRH 30 minutes after the phenobarbital. Values represent the mean ± S.E.M. of 12 animals for all drugs.
Rectal temperature was measured 20 minutes after the barbiturate
P < .001 when compared with control.
P < .025 when compared with control
P < .05 when compared with control
Effect of TRH on the actions of various centrally acting depressants
Since TRH was found to antagonize the actions of pentobarbital and other barbiturates, experiments were designed to determine whether TRH would have a similar action against other centrally acting drugs (table 8). In this case, the ability of TRH to antagonize hypothermia and to increase motor activity was used to assess the reversal of such compounds as chloral hydrate, reserpine, chlorpromazine, diazepam and phenobarbital. As is apparent, TRH significantly antagonized the hypothermia and sedation produced by all compounds. In the case of reserpine, TRH not only antagonized the hypothermia but also produced a mild hyperthermia.
TABLE 8. Effect of TRH on locomotor activity and temperature after treatment with reserpine, chloral hydrate, chlorpromazine, diazepam, and phenobarbital.
| Treatmenta | Dose | Motor Activity | Rectal Temperature |
|---|---|---|---|
| mg/kg | Counts/hr ± S.E.M | °C ± S.E.M. | |
| Reserpine | 2.5 | 48 ± 27 | 33.3 ± 0.26 |
| Reserpine + TRH | 2.5 + 30 | 1610 ± 290b | 38.3 ± 0.56b |
| Chloral hydrate | 360.0 | 33 ± 8 | 31.4 ± 0.85 |
| Chloral hydrate + TRH | 360.0 + 30 | 608 ± 74b | 33.1 ± 0.33 |
| Chlorpromazine | 6.0 | 91 ± 29 | 34.0 ± 0.56 |
| Chlorpromazine + TRH | 6.0 + 30 | 882 ± 96b | 38.4 ± 0.26b |
| Diazepam | 12.5 | 80 ± 16 | 35.2 ± 0.37 |
| Diazepam + TRH | 12.5 + 30 | 1131 ±479b | 37.3 ± 0.42c |
| Phenobarbital | 95.0 | 294 ± 143 | 35.9 ± 0.25 |
| Phenobarbital + TRH | 95.0 + 30 | 2634 ± 284b | 38.0 ± 0.22b |
All animals received 30 mg/kg of TRH after the various drugs. Details are described in “Methods.” Values in animals that did not receive TRH indicate base-line data after animals were injected with saline rather than TRH. Temperature was recorded in a separate group of mice 30 minutes after animals received TRH. Temperature in untreated control mice was 37.8 ± 0.19°C and with TRH was 38.5 ± 0.25°C (P < .01). Each value represents the mean ± S.E.M. of at least 10 mice.
P < .001 when compared with control.
P < .005 when compared with control.
Discussion
In addition to the recognized action of TRH to release thyrotropin and prolactin, the findings in the present work indicate that TRH also has effects on brain. This effect would appear to be independent of its pituitary actions, since TRH antagonism of pentobarbital was not imitated by l-triiodothyronine or thyrotropin. Furthermore, hypophysectomy did not alter the effects of TRH against pentobarbital or phenobarbital sleeping time and hypothermia. The observation that 10 μg of TRH administered intracisternally reduced pentobarbital sleeping time provides further support for a central action of this tripeptide.
In addition to providing evidence that the central action of TRH did not involve the pituitary-thyroid axis, the present results also delineated other aspects of TRH antagonism of pentobarbital. It was found that the duration of the effects of exogenously administered TRH against pentobarbital appeared to be less than an hour. This result would not be surprising in view of its short half-life in plasma (May and Donabedian, 1973). However, in hypophysectomized rats, TRH has been found to potentiate dopa after pargyline pretreatment when administered longer than 1 hour before the test (Plotnikoff et al., 1972, 1974a; Breese et al., 1974a). Since this latter observation reduces possible involvement of thyroid hormones which also potentiate l-dopa after pargyline pretreatment (Breese et al., 1972, 1974c), the reason for the difference in the duration of the effectiveness of TRH in these procedures is unclear.
Although a wide range of TRH doses reduced the effects of pentobarbital, at none of the doses of pentobarbital did the TRH antagonism show a satisfactory dose-response relationship. Furthermore, there does not presently seem to be a satisfactory explanation for the increased lethality of pentobarbital when administered simultaneously with increasing doses of TRH. However, it should be mentioned that the dose of TRH which enhanced pentobarbital lethality is well in excess of the smallest dose of TRH that will reduce pentobarbital sleeping time and hypothermia (Prange et al., 1974). Thus, there would appear to be considerable margin of safety in giving small doses of TRH to reverse the effects of pentobarbital, even when the dose of pentobarbital is in the potentially lethal range.
Another important consideration in this work was the structure specificity of TRH to antagonize pentobarbital anesthesia. Results obtained from the study of MIF and somatostatin indicate that the antagonism of pentobarbital by TRH is not generally shared by other hypothalamic polypeptides. A methyl group addition (3-methyl-His-TRH) or a pyrazolyl group substitution (pyrazolyl TRH) on TRH produced compounds capable of reversing pentobarbital, whereas iodination of histidine (diiodo TRH) eliminated this action of TRH against pentobarbital. Furthermore, by contrasting the effects of 3-methyl-His-TRH and pyrazolyl TRH on thyrotropin release and reversal of pentobarbital sleep, it can be concluded that there is no relationship between potency of these compounds to release thyroid-stimulating hormone from the pituitary (Vale et al., 1973) and their ability to antagonize pentobarbital sleep. Such separation of these actions of TRH may be particularly relevant to studies in which the actions on brain are desired without the complication of an action on pituitary.
In addition to its action against pentobarbital, TRH was found to reduce hypothermia and sleep induced by a variety of barbiturates and to antagonize the sedation produced by other centrally acting drugs. While TRH reduced chloral hydrate sedation, it had less effect on the hypothermia produced by this centrally acting depressant, providing evidence that reversal of hypothermia is not essential for the effects of TRH. Similar to preliminary findings of Kruse (1975), TRH antagonized the sedation and hypothermia caused by reserpine, chlorpromazine and diazepam, providing additional evidence for the actions of TRH on the central nervous system. In view of reports that TRH has transient antidepressant properties (Prange et al., 1972; Kastin et al., 1972), the action of TRH in reserpine-treated rats seems a particularly important observation because reversal of reserpine hypothermia has been a common pharmacological screen for antidepressant activity (Garattini et al., 1962).
Since norepinephrine-containing fibers appear to influence activity of neurons which contain TRH (Grimm and Reichlin, 1973), it seemed plausible that TRH might influence the activity of this monoamine system. Observations that TRH enhances the reduction of brain norepinephrine by α-methyltyrosine and increases the content of 3-methoxy-4-hydroxyphenylglycol in brain (Keller et al., 1974) are in accord with this view and suggest that norepinephrine synthesis is increased by this tripeptide. Furthermore, a high dose of norepinephrine administered intracisternally reduced pentobarbital sleeping time and hypothermia, an effect which was antagonized by phentolamine. In contrast to these findings suggesting a possible role for noradrenergic fibers in the action of TRH, phentolamine did not reduce the effect of TRH to diminish pentobarbital anesthesia nor did TRH alter the synthesis of norepinephrine from tyrosine in pentobarbital-treated mice. Since phentolamine has been found to block the action of dibutyryl cyclic adenosine monophosphate to shorten amobarbital-induced narcosis (Cohn and Cohn, 1974), the lack of effect of phentolamine on TRH reduces the likelihood that a direct cyclic adenosine monophosphate mechanism is involved in the effects of TRH. Therefore, regardless of the actions of TRH on noradrenergic fibers in unanesthetized animals, our experiments do not provide support for an involvement of noradrenergic fibers in the antagonism of pentobarbital by TRH. However the observation that atropine will block the action of TRH against pentobarbital suggests that cholinergic mechanisms may be involved in the effects of TRH and provides a new avenue of research to define the mechanism (s) by which this tripeptide acts.
The continued study of the actions of TRH on brain could provide clues for the development of new classes of therapeutic agents. Based on present observations, TRH might be an important treatment for the overdose of certain depressant drugs. Furthermore, that TRH is widely localized in areas of the central nervous system other than the hypothalamus may have even broader implications (Jackson and Reichlin, 1974; Winokur and Utiger, 1974). If one considers the potent central action (s) of TRH along with its anatomical localization, an intriguing question is raised as to whether TRH might not play an important role in the function of brain (Breese et al., 1974a). Such important possibilities for TRH will likely be resolved as future studies delineate the biological and pharmacological activities of this endogenous substance in the central nervous system.
Acknowledgments
Dr. Jean Rivier of the Salk Institute provided our laboratory with linear somatostatin. Pyrazolyl TRH, β-Ala-TRH, diiodo-TRH, deamidated TRH and lysine TRH were furnished by Hoffmann La Roche (Nutley, N.J.) and the 3-methyl-His-TRH by Wyeth Laboratories (Philadelphia, Pa.). The authors acknowledge the excellent assistance of Joseph Farmer, Marcine Kinkead, Susan Hollister and Edna Edwards.
Footnotes
This work was supported by U.S. Public Health Service Grants MH-16522, HD-03110 and MH-15631.
References
- Bliss CI. The Statistics of Bioassay. Academic Press; New York: 1952. [Google Scholar]
- Boissier JR, Simin P. Factors influencing the interaction between hypnotics and a variety of other drugs. In: Tedeschi DH, Tedeschi RE, editors. Importance of Fundamental Principles in Drug Evaluation. 1974b. pp. 1053–1063. [Google Scholar]
- Bowers CY, Friesen HG, Hwang P, Guyda HJ, Folkers K. Prolactin and thyrotropin release in man by synthetic pyroglutamyl-histidyl-prolinamide. Biochem Biophys Res Commun. 1971;45:1003–1041. doi: 10.1016/0006-291x(71)90441-4. [DOI] [PubMed] [Google Scholar]
- Breese GR, Cooper BR, Prange AJ, Cott JM, Lipton MA. Interactions of thyrotropin-releasing hormone with centrally acting drugs. In: Prange AJ, editor. The Thyroid Axis, Drugs, and Behavior. Raven Press; New York: 1974a. pp. 115–127. [Google Scholar]
- Breese GR, Cott JM, Cooper BR, Prange AJ, Lipton MA. Antagonism of ethanol narcosis by thyrotropin releasing hormone. Life Sci. 1974b;14:1053–1063. doi: 10.1016/0024-3205(74)90230-6. [DOI] [PubMed] [Google Scholar]
- Breese GR, Prange AJ, Lipton MA. Parmacological studies of thyroid-imipramine interactions in animals. In: Prange AJ Jr, editor. The Thyroid Axis, Drugs, and Behavior. Raven Press; New York: 1974c. pp. 29–48. [Google Scholar]
- Breese GR, Traylor TD. Effect of 6-hydroxydopamine on brain norepinephrine and dopamine: Evidence for selective degeneration of catecholamine neurons. J Pharmacol Exp Ther. 1970;174:413–420. [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Traylor TD, Prange AJ. The effect of triiodothyronine on the disposition and actions of imipramine. Psychopharmacologia. 1972;25:101–111. doi: 10.1007/BF00423187. [DOI] [PubMed] [Google Scholar]
- Cohn ML, Cohn M. Norepinephrine—An antagonist of dibutyryl cyclic AMP in the regulation of narcosis in the rat. Fed Proc. 1974;33:494. [PubMed] [Google Scholar]
- Garattini S, Giachetti A, Jori A, Pieri L, Valzelli L. Effect of imipramine, amitriptyline, and their monomethyl derivatives on reserpine activity. J Pharm Pharmacol. 1962;14:509–514. doi: 10.1111/j.2042-7158.1962.tb11130.x. [DOI] [PubMed] [Google Scholar]
- Grimm Y, Reichlin S. Thyrotropin-releasing hormone (TRH): Neurotransmitter regulation of secretion by mouse hypothalamic tissue in vitro. Endocrinology. 1973;93:626–631. doi: 10.1210/endo-93-3-626. [DOI] [PubMed] [Google Scholar]
- Hollander CS, Mitsuma T, Shenkman L, Woolf P, Gershengorn MC. Thyrotropin-releasing hormone: Evidence for thyroid response to intravenous injection in man. Science (Washington) 1972;175:209–210. doi: 10.1126/science.175.4018.209. [DOI] [PubMed] [Google Scholar]
- Jackson IMD, Reichlin S. Thyrotropin releasing hormone (TRH): Distribution in the brain, blood and urine of the rat. Life Sci. 1974;14:2259–2266. doi: 10.1016/0024-3205(74)90107-6. [DOI] [PubMed] [Google Scholar]
- Jacobs LS, Snyder PJ, Utiger RD, Wilber JF, Daughaday WH. Increased serum prolactin after administration of synthetic thyrotropin releasing hormone (TRH) in man. J Clin Endocrinol Metab. 1971;33:996–998. doi: 10.1210/jcem-33-6-996. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Ehrensing RH, Schalch DS, Anderson MS. Improvement in mental depression with decreased thyrotropin response after administration of thyrotropin-releasing hormone. Lancet. 1972;2:740–742. doi: 10.1016/s0140-6736(72)92028-4. [DOI] [PubMed] [Google Scholar]
- Keller HH, Bartholini G, Pletscher A. Enhancement of cerebral noradrenaline turnover by thyrotropin-releasing hormone. Nature (London) 1974;248:528–529. doi: 10.1038/248528a0. [DOI] [PubMed] [Google Scholar]
- Kruse H. Neurotropic effects of thyrotropin releasing hormone. Naunyn-Schmeideberg's Arch Pharmacol. 1975;282 in press. [PubMed] [Google Scholar]
- May P, Donabedian RK. Factors in blood influencing the determination of thyrotropin releasing hormone. Clin Chim Acta. 1973;46:377–382. doi: 10.1016/0009-8981(73)90250-7. [DOI] [PubMed] [Google Scholar]
- Peters MA, Strother A. A study of some possible mechanisms by which glucose inhibits drug metabolism in vivo and in vitro. J Pharmacol Exp Ther. 1972;180:151–157. [PubMed] [Google Scholar]
- Plotnikoff NP, Prange AJ, Jr, Breese GR, Anderson MS, Wilson IC. Thyrotropin releasing hormone: Enhancement of DOPA activity by a hypothalamic hormone. Science (Washington) 1972;178:417–418. doi: 10.1126/science.178.4059.417. [DOI] [PubMed] [Google Scholar]
- Plotnikoff NP, Prange AJ, Breese GR, Anderson MS, Wilson IC. The effects of thyrotropin-releasing hormone on DOPA response in normal, hypophysectomized, and thyroidectomized animals. In: Prange AJ, editor. The Thyroid Axis, Drugs, and Behavior. Raven Press; New York: 1974a. pp. 103–113. [Google Scholar]
- Plotnikoff NP, Prange AJ, Breese GR, Wilson IC. Thyrotropin releasing hormone: Enhancement of DOPA activity in thyroidectomized rats. Life Sci. 1974b;14:1271–1278. doi: 10.1016/0024-3205(74)90435-4. [DOI] [PubMed] [Google Scholar]
- Prange AJ, Jr, Breese GR, Cott JM, Martin BR, Cooper BR, Wilson IC, Plotnikoff NP. Thyrotropin releasing hormone: Antagonism of pentobarbital in rodents. Life Sci. 1974;14:447–455. doi: 10.1016/0024-3205(74)90359-2. [DOI] [PubMed] [Google Scholar]
- Prange AJ, Wilson IC, Lara PP, Alltop LB, Breese GR. Effects of thyrotropin-releasing hormone in depression. Lancet. 1972;2:999–1002. doi: 10.1016/s0140-6736(72)92407-5. [DOI] [PubMed] [Google Scholar]
- Prange AJ, Wilson IC, Breese GR, Plotnikoff NP, Lara PP, Lipton MA. Frontiers in Catecholamine Research. Pergamon Press; New York: 1973. Hypothalamic releasing hormones and catecholamines: A new interface; pp. 1149–1155. [Google Scholar]
- Randall CL, Lester D. Differential effects of ethanol and pentobarbital on sleeping time in C57B and BALB mice. J Pharmacol Exp Ther. 1974;188:27–33. [PubMed] [Google Scholar]
- Reigle TG, Avni J, Platz PA, Schildkraut JJ, Plotnikoff NP. Norepinephrine metabolism in the rat brain following acute and chronic administration of thyrotropin releasing hormone. Psychopharmacologia. 1974;37:1–6. doi: 10.1007/BF00426676. [DOI] [PubMed] [Google Scholar]
- Sedvall GC, Weise VK, Kopin IJ. The rate of norepinephrine synthesis measured in vivo during short intervals: Influence of adrenergic nerve impulse activity. J Pharmacol Exp Ther. 1968;159:274–282. [PubMed] [Google Scholar]
- Tiwary CM, Rosenbloom AL, Robertson MF, Parker JC. Improved behavior of hyperkinetic children given TRH. Abstracts Fourth International Congress of the International Society of Psychoneuroendocrinology; Berkeley. September 7-11, 1973. [Google Scholar]
- Vale W, Grant G, Guillemin R. Chemistry of the hypothalamic releasing factors—Studies on structure-function relationships. In: Ganong WF, Martini L, editors. Frontiers in Neuroendocrinology. Oxford University Press; New York: 1973. pp. 375–413. [PubMed] [Google Scholar]
- Winokur A, Utiger RD. Regional distribution of thyrotropin releasing hormone in rat brain. Science (Washington) 1974;185:265–267. doi: 10.1126/science.185.4147.265. [DOI] [PubMed] [Google Scholar]


