Abstract
Background
There is controversy over whether exposure to stress precipitates relapse and/or increases alcohol (ethanol) intake. Our laboratory has demonstrated that repeated stress prior to withdrawal from a brief forced exposure to alcohol results in withdrawal-induced anxiety-like behavior. Because anxiety is often regarded as a precipitating factor in relapsing alcoholics, we decided to examine the consequences of stressing alcohol-preferring P rats on both voluntary alcohol drinking and withdrawal-induced anxiety.
Methods
P rats were subjected to 3 cycles of 5 days of voluntary alcohol drinking and 2 days of deprivation. Restraint stress (60 min) was applied to some animals during the first and second deprivations/withdrawals (at 4 h). Drugs (flumazenil, buspirone, SB242,084, CP154,526, CRA1000, naloxone, haloperidol, olanzapine, naloxone, and haloperidol) were given to some rats 30 min prior to restraint stress.
Results
Stressed, deprived P rats exhibited both a longer duration of elevated alcohol drinking and anxiety-like behavior in the social interaction test upon withdrawal after the third cycle of voluntary alcohol drinking. When given prior to each of the restraint stresses, the benzodiazepine receptor antagonist flumazenil (5 mg/kg), the corticotrophin releasing factor receptor antagonists CRA1000 (3 mg/kg) and CP154,526 (10 mg/kg), the serotonin 5-HT1A receptor partial agonist buspirone (0.6 mg/kg), and the mixed 5-HT2C/D2 receptor antagonist olanzapine were effective in reducing the increased duration of elevated alcohol drinking and the withdrawal-induced anxiety-like behavior. In contrast, while the opiate receptor antagonist naloxone (20 mg/kg), the 5-HT2C receptor antagonist SB242084 (3 mg/kg), and the dopamine receptor antagonist haloperidol (0.1 mg/kg) also reduced drinking, they did not significantly alter anxiety like behavior.
Conclusion
These results suggest that stress-induced facilitation of alcohol drinking and withdrawal-induced anxiety-like behavior in P rats may be closely but imperfectly linked.
Keywords: Alcohol-Preferring P Rats, Restraint Stress, Voluntary Alcohol Drinking, Alcohol Deprivation, Social Interaction Test, Anxiety-Like Behavior, Ethanol
Stress has been implicated in precipitating relapse in alcoholics (Breese et al., 2005; Sinha, 2001), but the relationship between stress and alcohol intake in both animals and humans has been and remains controversial (e.g., Champagne and Kirouac, 1987; Lynch et al., 1999; Pohorecky, 1990, 1991) despite the apparent simplicity of the tension reduction hypothesis (e.g., Kalodner et al., 1989; Young et al., 1990). Some of these discrepancies may relate to differences in animal strains, types of stressors, or other procedural variables. For example, Lynch et al. (1999) reported that restraint stress resulted in increased voluntary alcohol consumption in Wistar rats, while Chester et al., 2004) reported a decrease in voluntary alcohol drinking in the alcohol-preferring P rats or the high alcohol drinking (HAD) rats during the application of stress. Both groups reported increased drinking after termination of stress (Chester et al., 2004; Lynch et al., 1999). Thus, stress may have biphasic effects on alcohol intake depending upon the time at which measurements are made.
Footshock-induced stress resulted in a reduction in alcohol drinking in adolescent Sprague–Dawley (SD) rats (Brunell and Spear, 2005), but increased alcohol intake in a variety of alcohol-preferring rat lines, including the alcohol-preferring (P) and HAD rats (Vengeliene et al., 2003). On the other hand, repeated swim stress increased alcohol intake only in Wistar rats, not the alcohol-preferring rat lines (Vengeliene et al., 2003). Of particular relevance to the present study is the report by Funk et al. (2004). They applied repeated footshock or social defeat during 2-week deprivation periods and reported that the elevated alcohol drinking associated with the alcohol-deprivation effect (ADE) was enhanced in the stress groups. The present study shows that acute restraint stress during withdrawal from alcohol can have a similar effect in P rats.
Stress has also been implicated in the induction of anxiety-like behavior (Breese et al., 2004; Valdez et al., 2002). Indeed, it has been shown that exposure to restraint stress for 60 min is comparable to an episode of withdrawal from 5 days exposure to alcohol, with both procedures sensitizing rats to the anxiety-like behavior observed during withdrawal from a final episode of alcohol exposure (Breese et al., 2004; Overstreet et al., 2002). These studies have been carried out in SD rats on a forced alcohol diet. Because these rats do not drink substantial amounts of alcohol voluntarily, it was not possible to explore the relationship between stress, voluntary alcohol drinking, and subsequent withdrawal-induced anxiety-like behavior. By using a modified repeated withdrawal protocol (Overstreet et al., 2002, 2003, 2004) in the alcohol-preferring P rats, it was possible to explore the relationship between these variables, as reported in the current communication and described briefly in a previous report (Breese et al., 2004).
Another well-characterized phenomenon in the P rat is the ADE, where the rats exhibit increased drinking of and preference for alcohol following a period of deprivation (Holter et al., 1998; McKinzie et al., 1998; Rodd-Henricks et al., 2000a,b; Sinclair and Li, 1989; Spanagel and Holter, 1999, 2000). These studies often employ long-term exposure to water and alcohol solutions and have relatively long (2 weeks) deprivation periods. The present communication includes P rats that have been subjected to quite brief (5-day) exposures to water and alcohol and even briefer (2 days) deprivation (withdrawal) periods. The elevated alcohol intake and withdrawal-induced anxiety under these conditions are quite remarkable (see Breese et al., 2004). While others have reported both an increase in alcohol drinking and anxiety-like behavior in out-bred rats, the rats had access to alcohol for nearly 1 year (Holter et al., 1998; Spanagel and Holter, 1999), not just 15 days.
The increase in withdrawal-induced anxiety-like behavior in SD rats has been blocked or reduced by treatments with specific pharmacological actions during the early withdrawal periods (Breese et al., 2004; Knapp et al., 2005; Overstreet et al., 2003, 2004) despite the fact that the drug treatments were given nearly a week before the behavioral test. Among the compounds that counteracted the withdrawal-induced anxiety were flumazenil, a benzodiazepine (BZD) receptor antagonist, buspirone, a 5-HT1A receptor partial agonist, and CP154,526, a corticotropin-releasing factor type 1 (CRF1) receptor antagonist. These compounds were, therefore, examined in the P rats subjected to multiple cycles of alcohol exposure and withdrawal/deprivation and stress. In addition, the following drugs known to affect alcohol intake or the ADE were tested: naloxone, an opiate receptor antagonist, reduces alcohol intake (Overstreet et al., 1999), while haloperidol, a dopamine receptor antagonist, reduces the ADE (Salimov et al., 2000) and olanzapine, an atypical antipsychotic that blocks both dopamine and serotonin receptors, reduces alcohol consumption and craving in humans (Hutchison et al., 2006). The findings of this study, which greatly extend the results previously reported (Breese et al., 2004), suggest a close but imperfect link between the elevated voluntary drinking induced by stress and the withdrawal-induced anxiety-like behavior.
METHODS
Animals
The alcohol-preferring P rats were selected from the breeding colonies in the Bowles Center for Alcohol Studies at an initial weight of about 180 g (50 days of age). They were initially housed in groups of 4 to adapt them to the experimental treatment room maintained at 50% humidity, 22°C and a reversed light:dark cycle (lights off from 1000 to 2200). After the 1-week adaptation period, the rats were transferred to special plastic cages fitted with 2 holes for the placement of calibrated drinking tubes (Dyets, Inc., Bethlehem, PA). Rats had free access to food throughout the study, but their access to fluids was restricted according to the research design, as described below. Rats assigned to the various groups gained weight at comparable rates.
Research Design
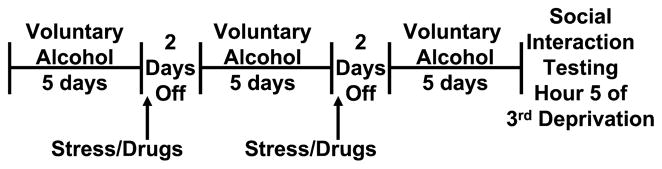
The experiment was divided into 4 primary treatment groups: Group 1 had access to water only throughout the study and served as the control group. Group 2 was adapted to drink alcohol voluntarily and was then provided with continuous, simultaneous access to water and 10% alcohol solution. This group served as a control for the length of exposure to alcohol in the groups that were cycled. Group 3 was adapted to drink alcohol voluntarily and then subjected to 3 cycles of 5 days’ exposure to a choice between tap water and 10% alcohol. Two 2-day periods of deprivation/withdrawal were interposed after the first and second cycles. Group 4 was adapted to drink alcohol voluntarily and then was subjected to the same conditions as Group 3, with an additional variable. At 4 h into the first and second withdrawals, these rats were subjected to restraint stress for 1 h. Group 4 rats were also divided into multiple subgroups based upon drug treatments that they received 30 min prior to the application of restraint stress during the first and second deprivation/withdrawal periods. Figure 1 illustrates the important aspects of this design.
Fig. 1.
Research design for drug treatment and stress application in P rats repeatedly withdrawn from alcohol.
Several (2 to 4) rats from Groups 1 to 3 were tested simultaneously with each group of drug-treated rats in Group 4, so there were larger sample sizes in these groups at the end of the study: 18 in Group 1, 24 in Group 2, and 20 in Group 3, while the individual subgroups of drug-treated rats contained 7 to 10 rats.
Two-Bottle Choice
The two-bottle choice design for access to alcohol has been routinely used in our laboratory (e.g., Overstreet et al., 1997; Kampov-Polevoy et al., 2000). The rats are adapted to the procedure by giving them 1 day of access to water only and 3 days of access to alcohol (10%, v/v) only. Then the choice between water and alcohol began and was present for 15 days. Some rats were on the choice procedure for 15 consecutive days while others received 3 cycles of 5 days’ exposure. During the deprivation/withdrawal periods these cycled rats had access to water only.
Drug Treatments
The following drug treatments were given:
Flumazenil (5 mg/kg), a BZD antagonist, was given because it prevents anxiety-like behavior in rats subjected to repeated withdrawals (Knapp et al., 2005) and stress (Breese et al., 2004).
CRA1000 (3 mg/kg) and CP154,526 (10 mg/kg), CRF1 receptor antagonists, were given because they also have prophylactic effects against withdrawal- and stress-induced anxiety-like behavior (Breese et al., 2004; Overstreet et al., 2004).
Buspirone (0.6 mg/kg), a 5-HT1A receptor partial agonist, was given because it has prophylactic effects against withdrawal- and stress-induced anxiety-like behavior (Breese et al., 2004; Overstreet et al., 2003).
SB242,084, a 5-HT2C receptor antagonist, was used because it prevented withdrawal-induced anxiety-like behavior (Overstreet et al., 2003).
Naloxone (20 mg/kg) was used because the opiate antagonists are widely used to prevent alcohol relapse (e.g, Anton et al., 1999) and reduce alcohol drinking in rodents (e.g., Overstreet et al., 1999).
Haloperidol (0.1 mg/kg) was given because Salimov et al. (2000) reported that it prevented the ADE in mice.
Olanzapine (5 mg/kg) was given because this atypical antipsychotic is an antagonist at both dopamine D2 and 5-HT2C receptors and has been reported to reduce alcohol craving and consumption in heavy drinkers (Hutchison et al., 2001, 2003, 2006).
Flumazenil, the CRF antagonists and SB242,084 were suspended in carboxymethylcellulose (0.5%), naloxone and buspirone were dissolved in isotonic saline, and haloperidol and olanzapine were dissolved in a small amount of 1 N HCl and the pH adjusted to 5. All injections were i.p.
Social Interaction Test
To obtain an index of anxiety-like behavior during withdrawal from the third cycle of choice between water and 10% alcohol (or 15th day of exposure), the rats were placed in a social interaction arena 5 h after removal of alcohol. The arena was 60 × 60 cm, with sixteen 10 × 10 cm squares marked out on the floor. Rats with similar weights and treatments were placed in the arena and time spent in social interaction (grooming, sniffing, crawling over or under) and line crosses was recorded during the 5-min session (File and Seth, 2003). The scores for individual animals were taken, as previous reports indicated that analyses of individual scores were similar to those for pairs (Overstreet et al., 2002, 2003).
Statistical Analysis
The voluntary drinking data was averaged over days for the basic 3 cycles and the data subjected to mixed two-way ANOVAs, with cycle as the repeated factor and treatment as the independent factor. If there were significant main or interaction effects, follow-up tests were carried out with Tukey’s protected t-tests. For analysis of drug effects on drinking, the cumulative alcohol intake over the last 5 days (Days 11 to 15) was compared to the amount over the first 5 days. These cumulative alcohol intake data were analyzed by one-way ANOVA and Tukey’s protected t-tests. Measures from the social interaction test (seconds spent in social interaction and line crosses) were also analyzed by one-way ANOVA and Tukey’s tests. Significance was set at p < 0.05.
RESULTS
Effects of Deprivation and Stress
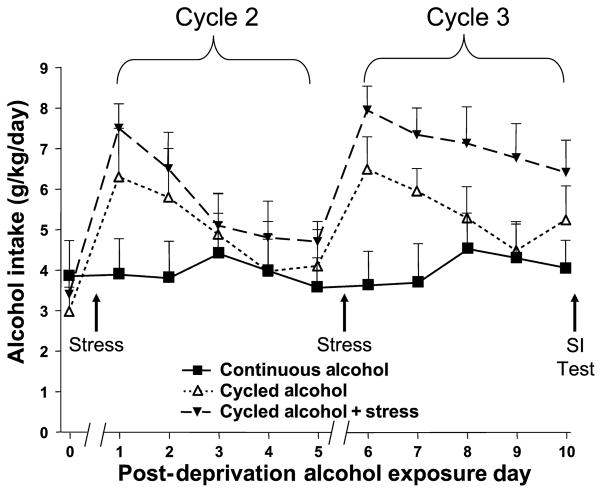
The effects of 2 periods of 2 days’ alcohol deprivation with and without restraint stress on subsequent alcohol intake in the alcohol-preferring P rats are illustrated in Fig. 2. As expected, the rats given continuous access to a choice between alcohol and water do not increase their alcohol intake, while the rats subjected to deprivation or deprivation and restraint stress exhibited short-term increases in alcohol intake [F(2,21) = 4.50, p = 0.02 for treatment]. The two-way ANOVA also confirmed that the amount of alcohol intake was different over days [F(5,105) = 7.45, p < 0.0001]. However, an important aspect of Fig. 2 was that the alcohol intake of the group that received restraint stress during deprivation had a prolonged increase in alcohol intake during the third cycle of exposure, a result supported by the significant group × day interaction effect [F(10,105) = 2.31, p < 0.02].
Fig. 2.
Daily alcohol intake (g/kg/d) in P rats continuously exposed to alcohol, deprived of alcohol, or deprived and stressed. Average intake for the first 5 days is presented as Treatment Day 0. The data represent the means ± SEM for n = 8 animals in each of the 3 groups. There was a 2-day break between Days 0 and 1 and between Days 5 and 6 of postdeprivation alcohol exposure during which time the rats had water only. SI, social interaction.
To illustrate this effect more clearly, cumulative alcohol intake during first cycle was subtracted from that for the third cycle to obtain increase in cumulative alcohol intake. There were dramatic differences, as described initially and briefly in Breese et al. (2004). The rats that had continuous access to alcohol exhibited almost no change in drinking, while the deprived rats exhibited a modest increase and the deprived, restrained rats exhibited a significantly larger increase. Alcohol was withdrawn from these groups of rats after their 15th session of access to alcohol and water and the social interaction test was carried out to assess anxiety-like behavior.
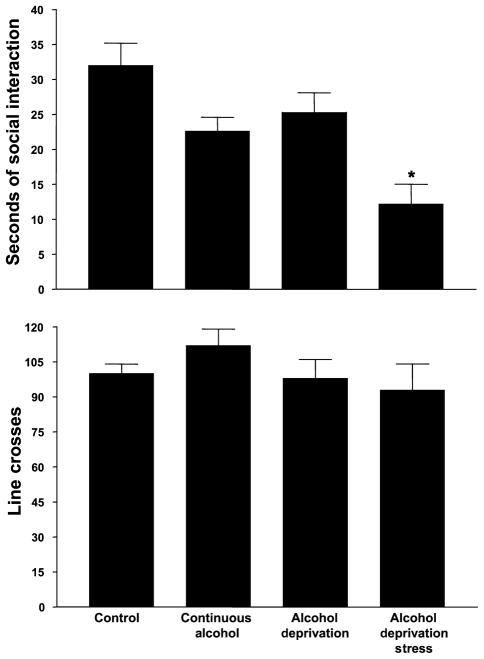
A group that was maintained on water throughout was used as a reference control group. As illustrated in the upper panel of Fig. 3, those rats that were subjected to stress during the 2 deprivation periods exhibited the least amount of time spent in social interaction, while the rats that had access to water only exhibited the most. A one-way ANOVA confirmed that there were significant group differences [F(3,32) = 25.25, p < 0.0001] and Tukey’s protected t-tests established that deprived and stressed group was significantly different from all of the others. Importantly, the rats that were deprived only were not different from the control group (Fig. 3, upper panel. In contrast, there were no differences in line crosses among the 4 groups [F(3,32) = 1.07, NS], as illustrated in the lower panel of Fig. 3. These findings replicate those of Breese et al., 2004).
Fig. 3.
Social interaction behavior in alcohol-withdrawn P rats continuously exposed to alcohol, deprived twice from alcohol, or deprived and stressed twice. The test was carried out 5 h after the last alcohol session. Data represent the mean s ± SEM time spent in social interaction for 16 to 24 rats in the upper panel and line crosses in the lower panel. *Significantly different from the Continuous Alcohol Group.
Thus, restraint stress applied during deprivation from alcohol both increased the amount of alcohol intake during the third cycle (Fig. 2) and induced anxiety-like behavior upon withdrawal from alcohol (Fig. 3). Deprivation alone also increased alcohol drinking somewhat, but did not induce anxiety-like behavior.
Drug Effects on Alcohol Drinking
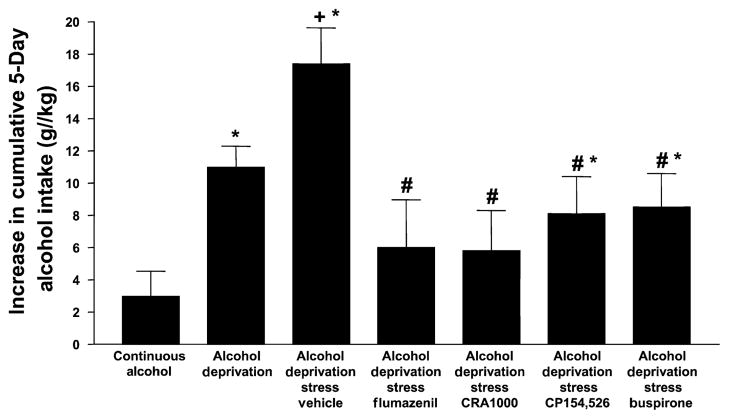
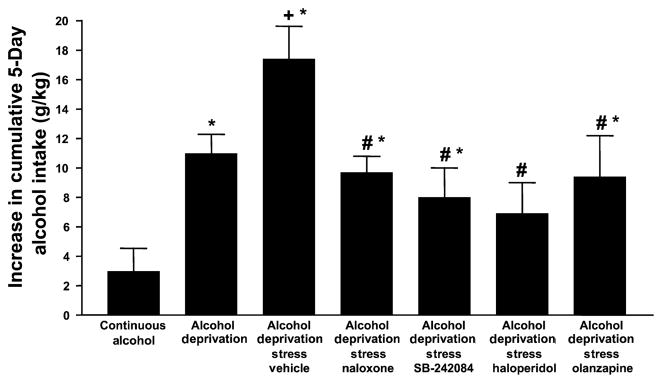
The one-way ANOVA indicated that there were significant differences among the drug-treated rats for increase in cumulative alcohol intake [F(11,145) = 9.45, p < 0.001] and Tukey’s protected t-tests established which groups differed from each other. Because of the large number of drugs tested, it was decided to present the data in 2 figures, with the key control and experimental groups being repeated to permit easy comparison. As shown in Fig. 4, flumazenil, the BZD receptor antagonist, CRA1000 and CP154,526, CRF1 receptor antagonists, and buspirone, 5-HT1A receptor partial agonist, all reduced the elevated drinking induced by restraint stress but only flumazenil and CRA1000 also appeared to reduce the drinking stimulated by deprivation only. The groups cannot be directly compared statistically because of multiple differences in treatment, but these 2 groups were not significantly different from the rats drinking alcohol and water continuously. A similar pattern of effects was seen (Fig. 5) for naloxone, the opiate receptor antagonist, SB242,084, the 5-HT2C receptor antagonist, and 5-HT2C and dopamine D2 receptor antagonist olanzapine (the atypical antipsychotic). However, haloperidol, the selective dopamine receptor antagonist, reduced alcohol drinking to a greater extent, so that the value was not significantly different from that of the rats exposed continuously to alcohol and water.
Fig. 4.
Cumulative alcohol intake of P rats after treatment with flumazenil, CRA1000, CP154,526, and buspirone. Drug-treated rats were injected 30 min before the application of 1 h of restraint stress during the first and second withdrawal/deprivation periods. Values represent the mean increase in cumulative alcohol intake between the first and third cycles for 7 to 10 rats. *Significantly different from Continuous Alcohol; +significantly different from Deprivation alone; #significantly different from Vehicle.
Fig. 5.
Cumulative alcohol intake of P rats after treatment with naloxone, SB242084, haloperidol, and olanzapine. Drug-treated rats were injected 30 min before the application of 1 h of restraint stress during the first and second withdrawal/deprivation periods. Values represent the mean increase in cumulative alcohol intake between the first and third cycles for 7 to 10 rats. The bars for Continuous Alcohol, Deprivation only and Vehicle groups are repeated from Fig. 4. *Significantly different from Continuous Alcohol, +significantly different from Deprivation alone; #significantly different from Vehicle.
Drug Effects on Anxiety-Like Behavior
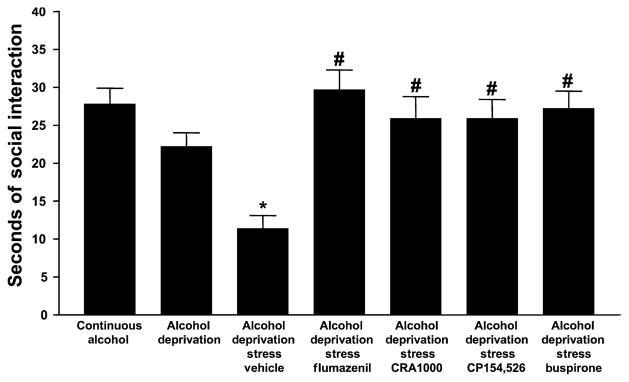
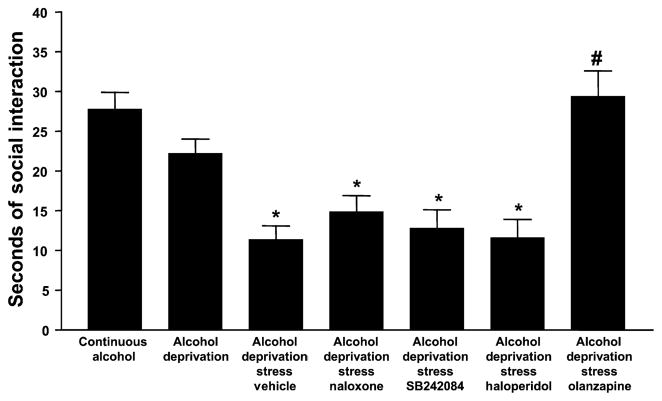
A one-way ANOVA comparing all 13 experimental groups revealed significant differences for time spent in social interaction, an index of anxiety-like behavior [F(12,155) = 11.08, p < 0.0001]. As was found above (Fig. 3), the rats subjected to both deprivation and restraint stress exhibited the lowest amount of time spent in social interaction and the rats exposed to water only exhibited the greatest. The groups that were continuously exposed to alcohol and water or repeatedly deprived of alcohol were not significantly different from the control group exposed to water only, as illustrated in Fig. 6. Flumazenil, CP154,526, CRA1000 and buspirone all completely counteracted the anxiety-like behavior induced by restraint stress and deprivation, such that their times spent in social interaction were not significantly different from the control rats exposed only to water. In contrast, as illustrated in Fig. 7, naloxone, SB242,084, and haloperidol failed to counteract the anxiety-like behavior at all, while olanzapine was effective.
Fig. 6.
Social interaction behavior in alcohol-withdrawn P rats after prior treatment with flumazenil, CRA1000, CP154,516, and buspirone. Drug-treated rats were injected 30 min before the application of 1 h of restraint stress during the first and second withdrawal/deprivation periods. Social interaction behavior was tested approximately 5 h after the removal of the alcohol tube at the end of the third cycle. Data represent the s + SEM for 7 to 10 rats. *Significantly different from Continuous Alcohol; #significantly different from Vehicle.
Fig. 7.
Social interaction in alcohol-withdrawn P rats after prior treatment with naloxone, SB242084, haloperidol, or olanzapine. Drug-treated rats were injected 30 min before the application of 1 h of restraint stress during the first and second withdrawal/deprivation periods. Social interaction behavior was tested approximately 5 h after the removal of the alcohol tube at the end of the third cycle. Data represent the s + SEM for 7 to 8 rats. The first 3 bars are repeated from Fig. 6. *Significantly different from Continuous Alcohol; #significantly different from Vehicle.
The effects of the drug treatments on locomotor activity were quite different from their effects on time spent in social interaction. Although there were significant group differences [F(12,155) = 3.96, p < 0.001], almost all treatments were not significantly different from the control group or from each other. Virtually all of the significance could be attributed to the fact that rats given CP154,526 or olanzapine were about 40% more active (148 ± 6 and 141 ± 8 lines crossed, respectively) than the other groups (about 110 ± 7).
DISCUSSION
Neither the rats continuously exposed to alcohol and water nor those repeatedly deprived of alcohol spent less time in social interaction than the rats exposed to water only. Thus, P rats that are exposed to alcohol voluntarily for 15 days do not exhibit anxiety-like behavior upon withdrawal. This finding is consistent with a previous report indicating that anxiety-like behavior and seizure susceptibility can be observed in P rats after 6 weeks of voluntary drinking but not after 2 or 4 weeks (Kampov-Polevoy et al., 2000). The likely reason for the rats in this study to exhibit normal behavior is that their alcohol intakes (4 to 6 g/kg) are much lower than those of rats exposed to alcohol (8 to 13 g/kg) in liquid diets (Breese et al., 2004; Overstreet et al., 2002, 2003, 2005).
The data for time spent in social interaction in the untreated P rats provide support for a key role of stress as a risk factor for anxiety-like behavior during alcohol withdrawal. It must be emphasized that the restraint stress was employed twice, 7 and 14 days prior to the conduct of the social interaction test. The fact that the deprived rats that were stressed exhibited significantly lower time spent in social interaction confirms our previous finding in SD rats that were forced to drink alcohol in a liquid diet (Breese et al., 2004). However, these P rats were drinking the alcohol voluntarily and their alcohol intakes (4 to 6 g/kg) were substantially lower than those of the SD rats maintained on forced 4.5 (8 to 10 g/kg) or 7% (11 to 13 g/kg) alcohol diets. Even the alcohol intake obtained on the first day of the third cycle (Fig. 2) is less than the level normally obtained by rats forced to drink alcohol in liquid diets or in P rats maintained on 2 bottle choice for long periods (Kampov-Polevoy et al., 2000). Therefore, the increased anxiety-like behavior of the stressed and deprived rats cannot be due exclusively to the fact that they drink more alcohol than the rats that were continuously exposed to alcohol and water or were repeatedly deprived of alcohol.
These experiments also confirm the existence of an ADE in the alcohol-preferring P rats. Although the increase in alcohol intake after a period of deprivation has been observed on numerous occasions by others (Holter et al., 1998; McKinzie et al., 1998; Rodd-Henricks et al., 2000a,b; Sinclair and Li, 1989; Vengeliene et al., 2003), they normally use much longer alcohol exposures (e.g., 2 to 4 weeks) and deprivation (e.g., 1 to 2 weeks) periods. These P rats exhibited small short-term increases in alcohol intake after only 5 days of exposure to alcohol and with only a 2-day deprivation period (Fig. 2). When restraint stress was applied to the deprived rats 4 h after removal of the alcohol during the first and second cycles of deprivation/withdrawal, there was also a short-term increase in alcohol intake. This increase was similar in magnitude to that observed for the deprived only group, but the elevated drinking persisted for a longer time (Fig. 2). Indeed, the stressed P rats exhibited a pattern of drinking during the third cycle that was reminiscent of P rats subjected to multiple deprivations (Rodd-Henricks et al., 2000a,b). To the extent that this elevated drinking during the ADE represents an index of craving (e.g., McKinzie et al., 1998; Spanagel and Holter, 1999, 2000) or positive rewarding effects of alcohol (Funk et al., 2004), it may be suggested that restraint stress applied during alcohol deprivation periods increases craving (See Funk et al., 2004; Sinha, 2001).
The present findings on the facilitated alcohol drinking in the P rats subjected to restraint stress are consistent with much of the previous literature on stress and alcohol drinking. Although Chester et al. (2004) and Lynch et al. (1999) reported opposite changes in drinking during the application of stress, both reported increases in drinking after termination of stress. Access to alcohol in this study began approximately 48 h after termination of the stress. Funk et al. (2004) also reported an increase in the ADE when stressors were applied during the deprivation period. In addition, they reported that the aversive effects of alcohol, as assessed by place conditioning, was reduced by the application of stress. However, few investigators examined the possibility of withdrawal-induced anxiety in their stress-treated rats. The one study that looked for withdrawal effects did not see them in adolescent rats whose alcohol drinking was increased by stress (Brunell and Spear, 2005).
Thus, restraint stress in alcohol-deprived P rats both increased the duration of the elevated drinking and induced anxiety-like behavior upon the final withdrawal from alcohol at the end. Whether these 2 effects represent separate phenomena controlled by independent mechanisms or are mutually controlled by the same mechanisms cannot be determined on the basis of these data alone. However, the present experiments included a variety of pharmacological treatments that will permit a more definitive conclusion. Before these individual treatments are discussed, it is important to emphasize the treatment protocol used. The drugs were given 30 min before the application of restraint stress or 3.5 h after the alcohol was removed at the first and second cycles. There was no drug in the rats when the alcohol intakes or social interaction behavior were being measured. Therefore, any effects of the drugs on these measures must be due to a modification of an adaptive process that contributes to the elevated drinking and/or anxiety-like behavior.
Flumazenil, a BZD receptor antagonist, the CRF1 receptor antagonists CRA1000 and CP154,526, and buspirone, a 5-HT1A partial agonist, have been reported to counteract alcohol withdrawal-induced anxiety-like behavior when given shortly before the behavior task (File et al., 1989; Knapp et al., 1998, 2004; Moy et al., 1997, 2000), during repeated withdrawals from alcohol (Knapp et al., 2005; Overstreet et al., 2003, 2004, 2006), or prior to repeated stresses (Breese et al., 2004). Thus, the fact that the anxiety-like behavior of the stressed, alcohol-deprived rats is also blocked by these compounds was expected. However, they also counteracted the elevation in drinking induced by stress exposure, suggesting that these 2 key behavioral changes may both be mediated, in part, by BZD, CRF1, and 5-HT1A receptor mechanisms. There have been previous reports of these compounds suppressing voluntary alcohol intake (June et al., 1998; Lodge and Lawrence, 2003; Schmitt et al., 2002), but the designs were quite different from that used in this study. The fact that all 3 systems (BZD, CRF, and 5-HT1A) counteracted both behaviors supports the contention that the rats may be drinking alcohol to relieve the anxiety-like behavior and not because it is intrinsically more rewarding.
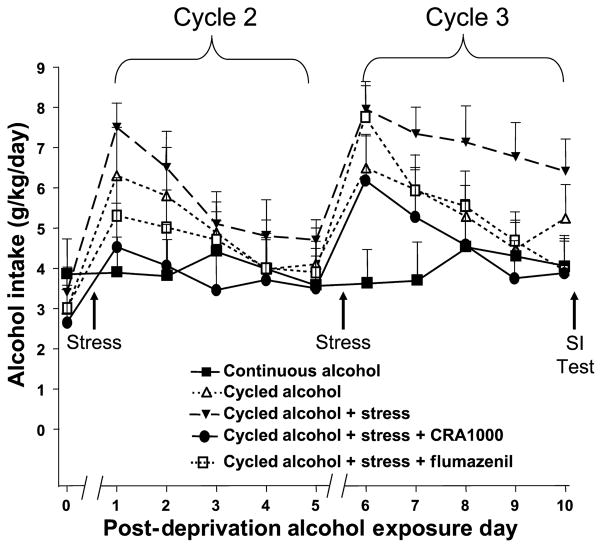
Before discussing other pharmacological agents, a more detailed consideration of the effects of flumazenil and the CRF1 receptor antagonist, CRA1000, on alcohol drinking would be informative. As shown in Fig. 2 and in other studies, the ADE is characterized by an initial elevation of drinking followed by a gradual decline. As shown in Fig. 8, neither flumazenil nor CRA1000 have any discernible effect on the alcohol intake during the first day (neither does stress). However, while the stressed, deprived group continues to exhibit elevated drinking, the rats treated with flumazenil or CRA1000 show a decrease. Thus, the drugs counteract the effects of stress on the duration of drinking, not the effects of deprivation on the peak drinking.
Fig. 8.
Effects of Flumazenil and CRA1000 on the daily pattern of drinking in stressed, alcohol deprived P rats. Rats were subjected to the protocol illustrated in Fig. 1, with flumazenil, and CRA1000 being injected 30 min prior to the application of stress. Animals with continuous access to alcohol and water were compared to: (1) those with cycled exposure to alcohol with two 2-day deprivation periods interspersed between three 5-day cycles of exposure to water and alcohol and (2) those with cycled exposure to alcohol with stress added during the deprivation periods with and without flumazenil or CRA1000.
A consideration of other drug effects suggests that these 2 behaviors may indeed be at least partially mediated by separate neurochemical mechanisms. Naloxone, the opiate receptor antagonist, counteracted the stress-induced increase in alcohol drinking, but did not affect withdrawal-induced anxiety. Thus, the 2 behaviors can be distinctly modulated. SB242,084, the 5-HT2C antagonist, produced a pattern similar to that for naloxone. The failure to block the anxiety-like behavior in the stressed, deprived P rats is consistent with its lack of effect in preventing the sensitizing effects of stress on SD rats exposed to forced alcohol diets (Breese et al., 2004). Haloperidol, the dopamine D2 receptor antagonist, was even more effective in reducing alcohol drinking in the P rats, a result that supports the findings of Salimov et al. (2000) in mice. However, haloperidol-treated rats exhibited as much anxiety-like behavior in the social interaction test as did rats treated with Vehicle. Thus, it appears that opioid, 5-HT2C, and D2 mechanisms are not involved in the anxiety-like behavior resulting from multiple stresses and deprivations, but they may contribute to the elevated drinking.
The atypical antispsychotic olanzapine, which blocks both D2 and 5-HT2C receptors, counteracted the stress-induced elevation in alcohol drinking and anxiety-like behavior even though the more select compounds, haloperidol and SB242,084, did not alter the anxiety-like behavior. The reduction in alcohol drinking is consistent with clinical reports that found that olanzapine reduces craving for alcohol (Hutchison et al., 2001). A recent report suggested that this effect may be related to the dopamine D4 receptor because it varied with polymorphic differences in the D4 receptor and because cyproheptadine, a 5-HT2C antagonist, did not mimic the effects of olanzapine (Hutchison et al., 2003). More recently it was shown that olanzapine may also reduce alcohol consumption (Hutchison et al., 2006).
In general, the drugs had little effect on locomotor activity in the social interaction test, a finding consistent with the results from rats repeatedly exposed to alcohol diets and/or stresses (Breese et al., 2004; Knapp et al., 2005; Overstreet et al., 2002, 2003, 2004). However, both olanzapine and CP154,526 increased locomotor activity. It must be emphasized that the drugs were administered 1 week prior to the behavioral test, so their effects on activity must be the consequence of an interaction with the adaptive processes underlying alcohol withdrawal. What these processes are cannot be specified at this time, particularly since haloperidol, SB242,084, and CRA1000, which share some of the properties of olanzapine and CP154,526, did not affect activity.
An assessment of the utility and validity of this paradigm can be attempted by comparing the effects of the drugs used in these experiments with clinical and preclinical reports on these agents under other conditions. For example, the BZD antagonist flumazenil inhibited both the increased alcohol drinking and anxiety-like behaviors in the present study and there are reports that it has both a suppressing effect on alcohol drinking (June et al., 1998; Schmitt et al., 2002) as well as anxiolytic effects (File et al., 1989; Knapp et al., 1998; Moy et al., 1997, 2000). The anxiolytic properties of CRF1 antagonists are also well known (Okuyama et al., 1999; Overstreet et al., 2004; Seymour et al., 2003) and recent studies suggest that these antagonists may also reduce alcohol drinking in anxious or alcohol-dependent rats (Lodge and Lawrence, 2003; Valdez et al., 2002). However, neither of this class of compound has been used in the treatment of alcoholics. In contrast, buspirone, a 5-HT1A receptor partial agonist, has well-known anxiolytic properties in humans but a mixed history in treating alcoholics (Fawcett et al., 1999; George et al., 1999; Kranzler and Meyer, 1992; Malcolm et al., 1992; Malec et al., 1996; Tollefson et al., 1992). There is stronger evidence that 5-HT1A receptor agonists and 5-HT2C receptor antagonists reduce alcohol drinking in animals (Hedlund and Wahlstrom, 1996; Knapp et al., 1993; McKenzie-Quirk and Miczek, 2003; Tomkins et al., 2002). Although olanzapine has been reported to decrease craving and alcohol consumption (Hutchison et al., 2001, 2003, 2006), the only clinical trial published to date has been negative (Guardia et al., 2004). Based upon these observations, it would be unwise to predict that drugs which reduced both alcohol drinking and anxiety-like behavior in stressed, deprived P rats will necessarily be useful treatments for alcoholism.
In conclusion, adding restraint stress to deprivation periods in P rats having voluntary access to alcohol both increases the duration of postdeprivation alcohol drinking and decreases time spent in social interaction behavior. Flumazenil, CRF1 receptor antagonists, buspirone, and olanzapine reduce the effects of stress on both alcohol drinking and social interaction. However, naloxone, haloperidol, and SB242,084 affected only the increase in alcohol drinking. These results suggest that the 2 behaviors are modulated by multiple systems, some of which are involved in both behaviors.
Acknowledgments
We thank, Lara Marr, Qi Yu, and Mili Senapati for technical assistance, Taisho Company for supply of CRA1000, Pfizer, Inc. for supply of CP154,526, and GlaxoSmithKline for supply of SB242,084.
References
- Anton RE, Moak DH, Ward LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156:1658–1764. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a “kindling”/stress hypothesis. Psychopharmacology (Berl) 2005;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Champagne F, Kirouac G. Effects of unavoidable electric shocks on voluntary alcohol consumption in the rat. Percept Mot Skills. 1987;64:335–338. doi: 10.2466/pms.1987.64.1.335. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Zweifel M, Froehlich JM. Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcohol Clin Exp Res. 2004;28:385–393. doi: 10.1097/01.alc.0000117830.54371.7a. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Kravitz HM, McGuire M, Easton M, Ross J, Pisani V, Fogg LF, Clark D, Whitney M, Kravitz G, Javaid J, Teas G. Pharmacological treatments for alcoholism: revisiting lithium and considering buspirone. Alcohol Clin Exp Res. 1999;24:666–674. [PubMed] [Google Scholar]
- File SE, Baldwin HA, Hitchcot PK. Flumazenil but not nitrendipine reverses the increased anxiety during ethanol withdrawal in the rat. Psychopharmacology (Berl) 1989;98:262–264. doi: 10.1007/BF00444702. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Funk D, Vohra S, Le AD. Influence of stressors on the rewarding effects of alcohol in Wistar rats: studies with alcohol deprivation and place conditioning. Psychopharmacology (Berl) 2004;176:82–87. doi: 10.1007/s00213-004-1859-x. [DOI] [PubMed] [Google Scholar]
- George DT, Rawlings R, Eckhardt MJ, Phillips MJ, Shoaf SE, Linnoila M. Buspirone treatment of alcoholism: age of onset, and cerebrospinal fluid 5-hydroxyindolacetic acid and homovanillic acid concentrations, but not medication treatment, predict return to drinking. Alcohol Clin Exp Res. 1999;23:272–278. doi: 10.1111/j.1530-0277.1999.tb04110.x. [DOI] [PubMed] [Google Scholar]
- Guardia J, Segura L, Gonzalvo B, Iglesias L, Roncero L, Cardus M, Casas M. A double-blind, placebo-controlled study of olanzapine in the treatment of alcohol-dependence disorder. Alcohol Clin Exp Res. 2004;28:736–745. doi: 10.1097/01.alc.0000125352.06688.f7. [DOI] [PubMed] [Google Scholar]
- Hedlund L, Wahlstrom G. Buspirone as an inhibitor of voluntary ethanol intake in male rats. Alcohol Alcohol. 1996;31:149–156. doi: 10.1093/oxfordjournals.alcalc.a008126. [DOI] [PubMed] [Google Scholar]
- Holter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long-term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety-related behaviour during ethanol deprivation in rats. Behav Pharmacol. 1998;9:41–48. [PubMed] [Google Scholar]
- Hutchison KE, Ray L, Sandman E, Rutter MC, Peters A, Davidson D, Swift R. The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology. 2006;31:1310–1317. doi: 10.1038/sj.npp.1300917. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Swift R, Rohsenow DJ, Monti PM, Davidson D, Almeida A. Olanzapine reduces urge to drink after drinking cues and a priming dose of alcohol. Psychopharmacology (Berl) 2001;155:27–34. doi: 10.1007/s002130000629. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Wooden A, Swift RM, Smolen A, McGeary J, Adler L, Paris L. Olanzapine reduces craving for alcohol: a DRD4VNTR polymorphism by pharmacotherapy interaction. Neuropsychopharmacology. 2003;28:1882–1888. doi: 10.1038/sj.npp.1300264. [DOI] [PubMed] [Google Scholar]
- June HL, Devaraju Sl, Eggers MW, Williams JA, Cason CR, Green TL, Leveige T, Braun MR, Torres L, Murphy JM. Benzodiazepine receptor antagonists modulate the actions of ethanol in alcohol-preferring and -nonpreferring rats. Eur J Pharmacol. 1998;342:139–151. doi: 10.1016/s0014-2999(97)01489-1. [DOI] [PubMed] [Google Scholar]
- Kalodner CR, Delucia JL, Ursprung AW. An examination of the tension reduction hypothesis: the relationship between anxiety and alcohol in college students. Addict Behav. 1989;14:649–654. doi: 10.1016/0306-4603(89)90007-5. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res. 2000;24:278–284. [PubMed] [Google Scholar]
- Knapp DJ, Benjamin D, Pohorecky LA. Effects of gepirone on ethanol consumption, exploratory behavior, and motor performance in rats. Drug Dev Res. 1993;26:319–341. [Google Scholar]
- Knapp DJ, Duncan GE, Crews FT, Breese GR. Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res. 1998;22:481–493. [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Modulation of ethanol withdrawal-induced anxiety-like behavior during later withdrawals by treatment of early withdrawals with benzodiazepine/gamma-aminobutyric acid ligands. Alcohol Clin Exp Res. 2005;29:553–563. doi: 10.1097/01.alc.0000158840.07475.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Meyer RF. An open trial of buspirone in alcoholics. J Clin Psychopharmacol. 1992;9:379–380. [PubMed] [Google Scholar]
- Lodge DJ, Lawrence AJ. The CRF1 receptor antagonist antalarmin reduces volitional ethanol consumption in isolation-reared fawn-hooded rats. Neuroscience. 2003;117:243–247. doi: 10.1016/s0306-4522(02)00793-5. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kushner MG, Rawleigh JM, Fiszdon J, Carroll ME. The effects of restraint stress on voluntary ethanol consumption in rats. Exp Clin Psychopharmacol. 1999;7:318–323. doi: 10.1037//1064-1297.7.4.318. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Anton RF, Randall CL, Johnston A, Brady K, Thevos A. A placebo-controlled trial of buspirone in anxious inpatient alcoholics. Alcohol Clin Exp Res. 1992;16:1007–1013. doi: 10.1111/j.1530-0277.1992.tb00691.x. [DOI] [PubMed] [Google Scholar]
- Malec TS, Malec EA, Dongier M. Efficacy of buspirone in alcohol dependence: a review. Alcohol Clin Exp Res. 1996;20:853–858. doi: 10.1111/j.1530-0277.1996.tb05263.x. [DOI] [PubMed] [Google Scholar]
- McKenzie-Quirk SB, Miczek KA. 5-HT1A agonists. Alcohol drinking in rats and squirrel monkeys. Psychopharmacology (Berl) 2003;167:145–152. doi: 10.1007/s00213-003-1395-0. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, Li T-K. The alcohol deprivation effects in the alcohol-preferring P rat under free-drinking and operant access conditions. Alcohol Clin Exp Res. 1998;22:1170–1176. [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Criswell HE, Breese GR. Flumazenil blockade of anxiety following ethanol withdrawal in rats. Psychopharmacology (Berl) 1997;131:354–360. doi: 10.1007/s002130050303. [DOI] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Duncan GE, Breese GR. Enhanced ultrasonic vocalization and Fos protein expression following ethanol withdrawal: effects of flumazenil. Psychopharmacology (Berl) 2000;152:208–215. doi: 10.1007/s002130000507. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Chaki S, Kawashima N, Suzuki Y, Ogawa S, Nakazato A, Kumagai T, Okubo T, Tomisawa K. Receptor binding, behavioral, and electrophysiological profiles of nonpeptide corticotropin-releasing factor subtype 1 receptor antagonists CRA1000 and CRA1001. J Pharmacol Exp Ther. 1999;289:926–935. [PubMed] [Google Scholar]
- Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Braun C, Bartus RB, Crews FT. Suppression of alcohol intake in P rats: tolerance development and elevation of opiate receptor binding. Alcohol Clin Exp Res. 1999;23:1761–1771. [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Angel RA, Navarro M, Breese GR. Reduction in repeated ethanol-withdrawal-induced anxiety-like behavior by site-selective injections of 5-HT(1A) and 5-HT (2C) ligands. Psychopharmacology (Berl) 2006;187:1–12. doi: 10.1007/s00213-006-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decreases in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1269. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Pharmacological modulation of repeated ethanol withdrawal-induced anxiety-like behavior differs in alcohol-preferring P and Sprague–Dawley rats. Pharmacol Biochem Behav. 2005;81:122–130. doi: 10.1016/j.pbb.2005.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2C antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharmacology (Berl) 2003;167:344–352. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, McArthur RA, Rezvani AH, Post C. Selective inhibition of alcohol intake in diverse alcohol-preferring rat strains by the 5-HT2A antagonists amperozide and FG5974. Alcohol Clin Exp Res. 1997;21:1448–1454. [PubMed] [Google Scholar]
- Pohorecky LA. Interaction of ethanol and stress: research with experimental animals—an update. Alcohol Alcohol. 1990;25:263–276. doi: 10.1093/oxfordjournals.alcalc.a045000. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Stress and alcohol interaction: an update of human research. Alcohol Clin Exp Res. 1991;15:438–459. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li TK. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcohol Clin Exp Res. 2000a;24:747–753. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, Li T-K. Alcohol deprivation effect is prolonged in the alcohol-preferring (P) rat after repeated deprivations. Alcohol Clin Exp Res. 2000b;24:8–16. [PubMed] [Google Scholar]
- Salimov RM, Salimova NB, Shvets LN, Maisty AI. Haloperidol administered subchronically reduces the alcohol deprivation effect in mice. Alcohol. 2000;20:61–68. doi: 10.1016/s0741-8329(99)00057-9. [DOI] [PubMed] [Google Scholar]
- Schmitt U, Waldhofer S, Weigelt T, Hiemke L. Free-choice ethanol consumption under the influence of GABAergic drugs in rats. Alcohol Clin Exp Res. 2002;26:457–462. [PubMed] [Google Scholar]
- Seymour PA, Schmidt AW, Schultz DW. The pharmacology of CP154,526, a non-peptide antagonist of the CRH1 receptor. A review. CNS Drug Rev. 2003;9:57–96. doi: 10.1111/j.1527-3458.2003.tb00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, Li TK. Long and short alcohol deprivation effects on AA and alcohol-preferring rats. Alcohol. 1989;6:505–509. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Holter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Holter SM. Pharmacological validation of a new animal model of alcoholism. J Neural Transm. 2000;107:669–680. doi: 10.1007/s007020070068. [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Montagu-Clouse J, Tollefson SF. Treatment of comorbid generalized anxiety in a recently detoxified alcoholic population with a selective serotonergic drug (buspirone) J Clin Psychopharmacol. 1992;12:19–25. doi: 10.1097/00001573-199202000-00004. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Joharchi N, Tampakeras M, Martin JR, Wichmann J, Higgins GA. An investigation of the role of 5-HT(2C) receptors in modifying ethanol self-administration behaviour. Pharmacol Biochem Behav. 2002;71:735–744. doi: 10.1016/s0091-3057(01)00710-9. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Cham L, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R. A comparative study on alcohol-preferring rat lines: effects of deprivation and stress phases on voluntary alcohol intake. Alcohol Clin Exp Res. 2003;27:1048–1054. doi: 10.1097/01.ALC.0000075829.81211.0C. [DOI] [PubMed] [Google Scholar]
- Young RM, Oei TP, Knight RG. The tension reduction hypothesis revisited: an alcohol expectancy perspective. Br J Addict. 1990;85:31–40. doi: 10.1111/j.1360-0443.1990.tb00621.x. [DOI] [PubMed] [Google Scholar]