Abstract
The temporomandibular joint (TMJ) is a unique synovial joint whose development differs from the formation of other synovial joints. Mutations have been associated with the developmental defects of the TMJ only in a few genes. In this study, we report the expression of the homeobox gene Shox2 in the cranial neural crest derived mesenchymal cells of the maxilla-mandibular junction and later in the progenitor cells and undifferentiated chondrocytes of the condyle as well as the glenoid fossa of the developing TMJ. A conditional inactivation of Shox2 in the cranial neural crest-derived cells causes developmental abnormalities in the TMJ, including dysplasia of the condyle and glenoid fossa. The articulating disc forms but fuses with the fibrous layers of the condyle and glenoid fossa, clinically known as TMJ ankylosis. Histological examination indicates a delay in development in the mutant TMJ, accompanied by a significantly reduced rate of cell proliferation. In situ hybridization further demonstrates an altered expression of several key osteogenic genes and a delayed expression of the osteogenic differentiation markers. Shox2 appears to regulate the expression of osteogenic genes and is essential for the development and function of the TMJ. The Shox2 conditional mutant thus provides a unique animal model of TMJ ankylosis.
Keywords: Shox2, temporomandibular joint, ankylosis, condyle, glenoid fossa, synovial disc, cartilage, bone, development
1. Introduction
The temporomandibular joint (TMJ) is a highly specialized synovial joint that is found only in mammals. It consists of the glenoid fossa of the temporal bone and the mandibular condyle, with an articulating disc separating these two bones. In contrast to other synovial joints, a fibrous cartilage rather than hyaline cartilage forms on the articular facets of the glenoid fossa and the condyle (Sperber, 2001). The TMJ is essential for the normal function of the jaw. TMJ disorders represent a collection of disorders related to the jaw joint, causing not only chronic myofacial pains but also food-taking. TMJ ankylosis, a major symptom of TMJ disorders, is clinically defined as limited mouth opening due to either a fibrous or bony union between the condyle and glenoid fossa. Most incidents of TMJ ankylosis occur after a trauma or an infection. Cases of congenital TMJ ankylosis are relatively rare, representing about 3% of TMJ ankylosis incidents (Converse, 1979; Tideman and Doddridge, 1987; Komorowska, 1997; Ajike et al., 2006; Mortazavi and Motamedi, 2007). However, little is known about genetic alterations that cause congenital TMJ ankylosis.
The embryonic development of the TMJ differs greatly from that of other synovial joints. Unlike the limb joints that form by the cleavage or segmentation within the single skeletal condensation, the TMJ develops from two distinct mesenchymal condensations: the glenoid fossa blastema, which arises from the otic capsule and undergoes intramembranous ossification, and the condylar blastema, which arises from the secondary condyle cartilage of the mandible and forms a bone through endochondral ossification (Sperber, 2001). The condylar blastema grows rapidly towards the glenoid fossa blastema. The intervening mesenchyme between the glenoid fossa and condylar blastemas condenses and differentiates into layers of fibrous tissues that form the articular disc separating the upper and lower synovial cavities.
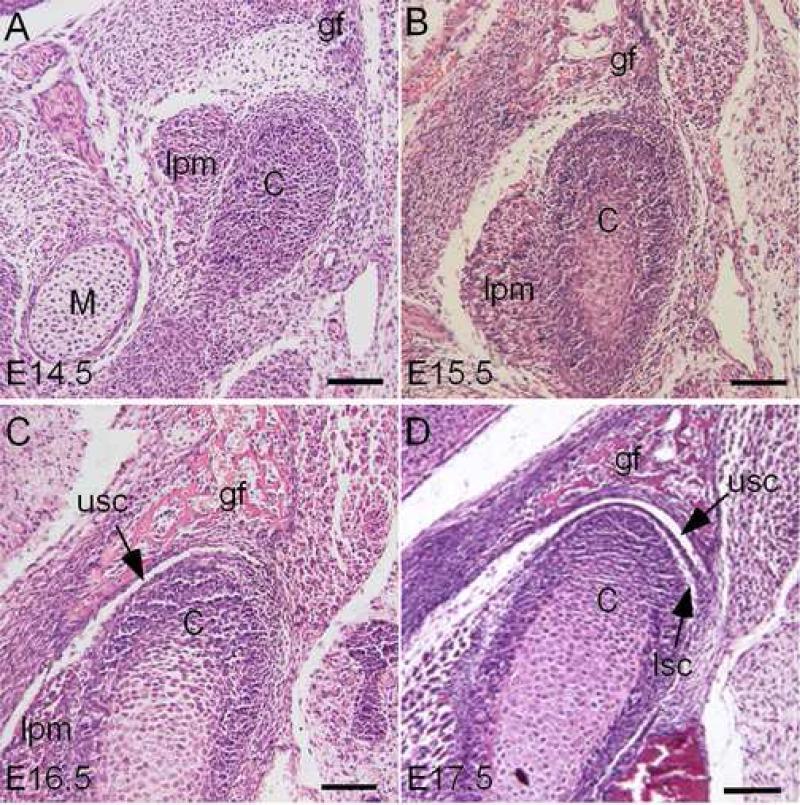
In the developing mouse, the mesenchymal condensation of the condyle, but not the glenoid fossa primordium, can be initially seen at embryonic day 13.5 (E13.5). At E14.5, the condensations of the condyle and glenoid fossa become clearly visible (Fig. 1A). At E15.5, the shape of the glenoid fossa and condyle is established, and the distance between them is narrowed due to the rapid growth of the condyle (Fig. 1B). The condylar chondrocytes that are distant from the condylar apex begin to differentiate. At E16.5, the glenoid fossa and condyle assume their position and complementary shape, with a joint disc beginning to form (Fig. 1C). The upper synovial cavity becomes discernible. Subsequently, all the major anatomical features of the TMJ are present at E17.5. A definite and compact synovial disc is clearly present, separating the upper and lower synovial cavities (Fig. 1D).
Figure 1.
Early development of the mouse TMJ. Coronal sections of E14.5 (A), E15.5 (B), E16.5 (C), and E17.5 (D) mouse embryonic heads show progression of TMJ formation. C, condyle; M, Meckel's cartilage; gf, glenoid fossa of the temporal bone; lpm, lateral pterygoid muscle; lsc, lower synovial cavity; usc, upper synovial cavity. Bar = 100 μm.
The mandibular condyle is actively involved in the endochondral ossification, and the condylar cartilage is an important growth site in the mandible, contributing to the elongation of the mandibular ramus (Silbermann and Frommer, 1972). The growth of the condylar cartilage goes through similar processes of chondrogenesis in the long bone formation, including proliferation, maturation, prehypertrophic and hypertrophic differentiation. The expression of many genes that are known to play critical role in cartilage growth and differentiation in the long bone formation has been documented in the developing condylar cartilage of rodents, including Bmp4, Fgf2, Ihh, Pthrp, Tgf-β2, Vegf, Cbfa1, Osterix, Sox9, Aggrecan, Col2 and Col10 (Fukada et al., 1999; Rabie and Hägg, 2002; Kuboki et al., 2003; Ogawa et al., 2003; Watahiki et al., 2003; Tang et al., 2004; Shibata et al., 2006, Shibukawa et al., 2007). However, the condylar cartilage is classified as a secondary cartilage (Beresford (1975), differing from primary cartilage in its rapid differentiation from progenitor cells to hypertrophic chondrocytes. Another unique feature of the condylar cartilage is that its chondrocytes are derived from alkaline phosphatase-positive progenitor cells that also express type I collagen (Silbermann, et al., 1987; Shibata et al., 1997, Miyake et al., 1997; Fukata et al., 1999; reviewed in Hall, 2005), suggesting that these progenitor cells have preosteoblast characteristics, capable of differentiating into either chondrocytes or osteoblasts. In addition, condyle elongation occurs mainly by appositional growth at its apical end, differing from that seen in other developing long bones in which chondrocyte proliferation makes major contribution (Kantomaa et al., 1994). Thus although the condylar cartilage shares many similarities with primary cartilage in development, they may utilize different molecular mechanisms that regulate osteogenesis.
The members of the short stature homeobox gene family, SHOX and SHOX2, are found only in vertebrates, implicating their unique role in the development of the internal skeleton and its related structures (Clement-Jones et al., 2000). Indeed, the mutations in the human SHOX have been associated with the Turner syndrome, Léri-Weill dyschondrosteosis, and Langer mesomelic dysplasia (Ellison et al., 1997; Rao et al., 1997; Belin et al., 1998; Shears et al., 1998; Zinn et al., 2002). All these syndromes exhibit abnormalities in the skeletal development. These skeletal defects are confined to the long bones, consistent with a restricted expression of SHOX in the limb mesenchyme and in the chondrocytes in the reserve, proliferating, and hypertrophic zones of the growth palate of long bones of the human fetus (Clement-Jones et al., 2000; Marchin et al., 2004; Munn et al., 2004). Similarly, SHOX2 expression was also found in the developing limbs of the human embryo in a complementary pattern to that of SHOX (Clement-Jones et al., 2000). Although no known human syndrome has been mapped or linked to the SHOX2 locus, the targeted inactivation of Shox2 in mice leads to the virtual elimination of the stylopod in the developing limbs (Cobb et al., 2006; Yu et al., 2007). A failure in growth, chondrogenesis, and endochondral ossification occurred in the mutant stylopodial cartilaginous element, accompanied by a down-regulation of several genes that are known to be essential for skeletogenesis, including Runx2, Runx3, and Ihh. Furthermore, over-expression of chick Shox in the developing limbs increased the length of the skeletal elements (Tiecke et al., 2006). These observations demonstrate a crucial role for the SHOX genes in the development of the long bone that undergoes the endochondral ossification.
Humans have both SHOX and SHOX2. Rodents, however, do not have a SHOX orthologue, suggesting that during embryogenesis Shox2 in rodents may play a function which is broader than its human counterpart during embryogenesis. Indeed, besides the limb phenotype, Shox2-deficient mice exhibit severe defects in many other developing organs, including the heart and palate, which causes embryonic lethality during the midgestation stage (Yu et al., 2005; Blaschke et al., 2007; Gu et al., 2008). In the present study, we show the expression of Shox2 in the mesenchymal cells of the maxillamandibular junction and in the progenitor cells and chondrocytes of the developing condyle. To study the role of Shox2 in the TMJ development, we used a floxed Shox2 allele and the Wnt1-Cre transgenic mice to generate mice carrying a specific deletion of Shox2 in the neural crest-derived tissues, including the TMJ. Histological and molecular analyses indicate a delay in development and a down-regulation of the genes that are critical for skeletogenesis in the mutant developing TMJ, leading to a dysplasia of the condyle and glenoid fossa, and to an abnormal fusion of the synovial disc with the condyle and the glenoid fossa. Shox2 mutant mice thus provide an important animal model for studying the TMJ development and function.
2. Results
2.1. Shox2 expression in the developing TMJ
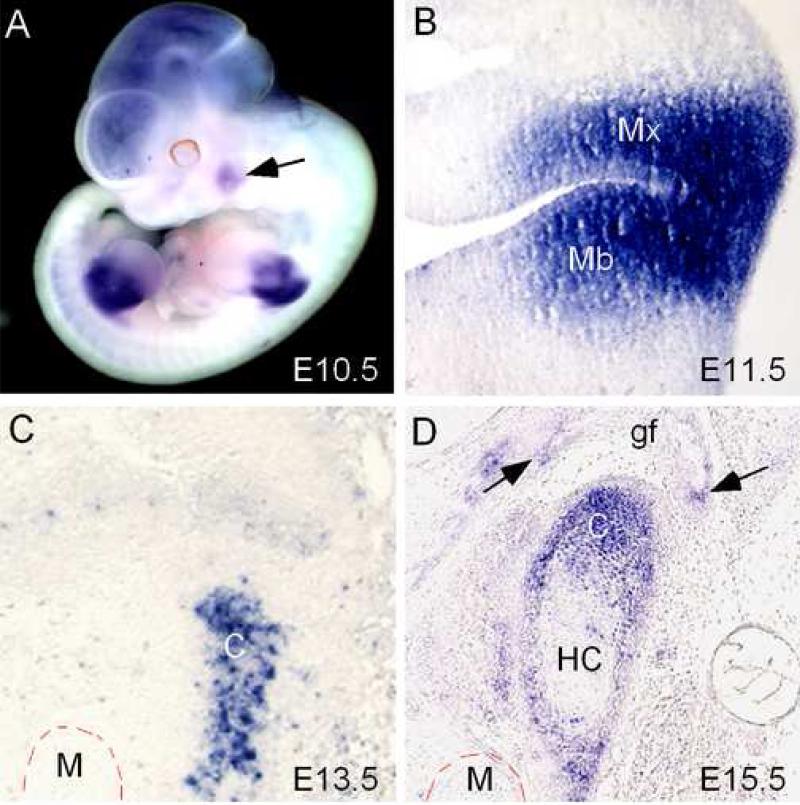
In a whole mount in situ hybridization survey for the expression pattern of Shox2 during early mouse embryonic development, a localized Shox2 expression domain was identified in the region of the maxilla-mandibular junction at embryonic day 10.5 (E10.5) (Fig. 2A). A similar expression domain was not found in the earlier stages of the embryos (data not shown). The expression intensity reached its highest level at E11.5, with Shox2 transcripts localized in the mesenchymal cells of the maxilla-mandibular junction as revealed by section in situ hybridization (Fig. 2B). However, the expression began to decrease at E12.5 (data not shown). At E13.5, when the condylar primordium condenses, Shox2 expression was restricted to the progenitor cells in the condensation (Fig. 2C). The expression then became localized to the progenitor cells and the undifferentiated chondrocytes of the developing chondyle at E15.5 and E16.5 (Fig. 2D, and data not shown). The expression was also detected in the perichondrium as well as in the growing glenoid fossa (Fig. 2D). Similarly to the developing long bone (Yu et al., 2007), the hypertrophic chondrocytes in the condyle also did not express Shox2. Thus, although the origins of the condylar and limb cells differ, they share many similarities in Shox2 expression pattern.
Figure 2.
Expression of Shox2 in the maxilla-mandibular junction and the developing TMJ. (A) Whole mount in situ hybridization of an E10.5 mouse embryo shows the presence of Shox2 transcripts in the brain, limb buds, and the maxilla-mandibular junction (arrow). (B) A coronal section of an E11.5 mouse head shows strong Shox2 expression in the mesenchymal tissue of the maxilla-mandibular junction. (C) A coronal section of an E13.5 mouse head shows a restricted Shox2 expression in the condylar condensation. (D) A coronal section through the developing TMJ of an E15.5 mouse head shows expression of Shox2 in the condylar chondrocytes. Note the absence of Shox2 expression in the hypertrophic chondrocyte zone (HC). Shox2 expression is also detected in the developing glenoid fossa (arrows). C, condyle; M, Meckel's cartilage; gf, glenoid fossa; HC, hypertrophic chondrocytes.
2.2 Wnt1-Cre;Shox2F/- mice exhibit a delay in TMJ initiation and abnormal TMJ architecture
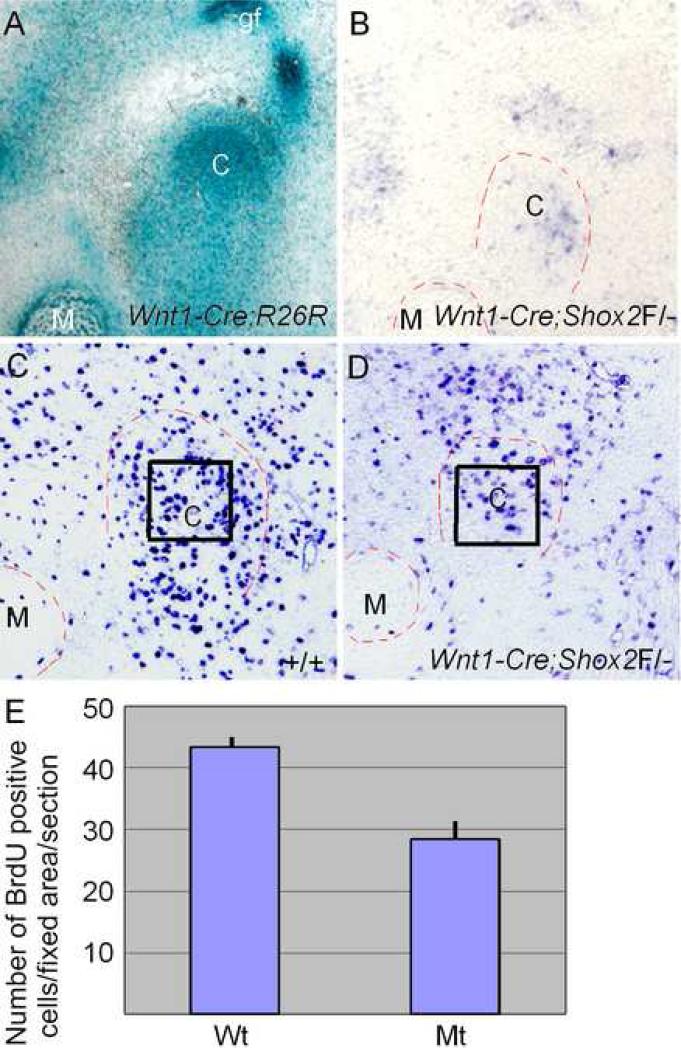
The Shox2 conventional knockout mice die during the mid-gestation stage, well before the formation of TMJ (Yu et al., 2005; Cobb et al., 2006, Blaschke et al., 2007). To study the role of Shox2 in the TMJ development, we took a conditional knockout approach to inactivate Shox2 in the craniofacial neural crest-derived cells by using a Wnt1-Cre transgenic line and a floxed Shox2 allele (Gu et al., 2008). We began with confirming the cranial neural crest (CNC) origin of the TMJ tissues by crossing the Wnt1-Cre mice with the R26R conditional reporter line. The LacZ staining of the Wnt1-Cre;R26R TMJ unambiguously demonstrated the CNC origin of both the condyle and glenoid fossa as well as of some surrounding cells (Fig. 3A). To determine the efficiency of Shox2 inactivation by Wnt1-Cre in the CNC-derived TMJ cells, we examined Shox2 expression in the developing TMJ area of Wnt1-Cre;Shox2F/- embryo by in situ hybridization. An extremely lower level of expression signals, if any, was detected. As shown in Fig. 3B, under the situation of an intense color-reaction, we detected a background level of in situ hybridization signals in the condylar condensation, as compared to the strong wild type expression shown in Fig. 2C. To further determine an efficient inactivation of Shox2 in the developing condyle, we examined the cell proliferation in the mutant. Consistent with a confirmed role of Shox2 in the cell proliferation regulation in the cartilaginous condensation of the developing limbs and in the palatal mesenchyme (Yu et al., 2005; 2007; Gu et al., 2008), the rate of cell proliferation was significantly decreased in the mutant condylar condensation (P < 0.01), as compared to that in the wild type controls (Fig. 3C-E). Based on these facts, we conclude an efficient ablation of Shox2 in the developing TMJ of Wnt1-Cre;Shox2F/-embryo.
Figure 3.
Efficient inactivation of Shox2 in the cranial neural crest derived TMJ cells. (A) LacZ staining shows the cranial neural crest origin of the condyle and the glenoid fossa in an E16.5 Wnt1-Cre;R26R embryo. (B) In situ hybridization assay detects Shox2 expression signals at the background level in a coronal section through the developing TMJ of an E13.5 Wnt1-Cre;Shox2F/- embryo. (C) BrdU labeling of cell proliferation in the condylar condensation of an E13.5 wild type embryo. (D) Reduced level of cell proliferation, as indicated by BrdU labeling, is seen in the condylar condensation of an E13.5 Wnt1-Cre;Shox2F/- embryo. (E) Comparison of numbers of BrdU-positive cells in the fixed area of the condylar primorida in wild type (Wt) and mutant (Mt) embryos shown in (C) and (D). Standard deviation values were presented as error bars. C, condyle; M, Meckel's cartilage; gf, glenoid fossa.
The Wnt1-Cre;Shox2F/- mice indeed survived embryonic lethality, but develop a wasting syndrome from postnatal day 4 (P4) on, and died around P15 (Gu et al., 2008). Gross and histological examinations of the mutant mice revealed no other obvious craniofacial abnormalities, except a minor anterior clefting of the palate (Gu et al., 2008), and the TMJ defects described below.
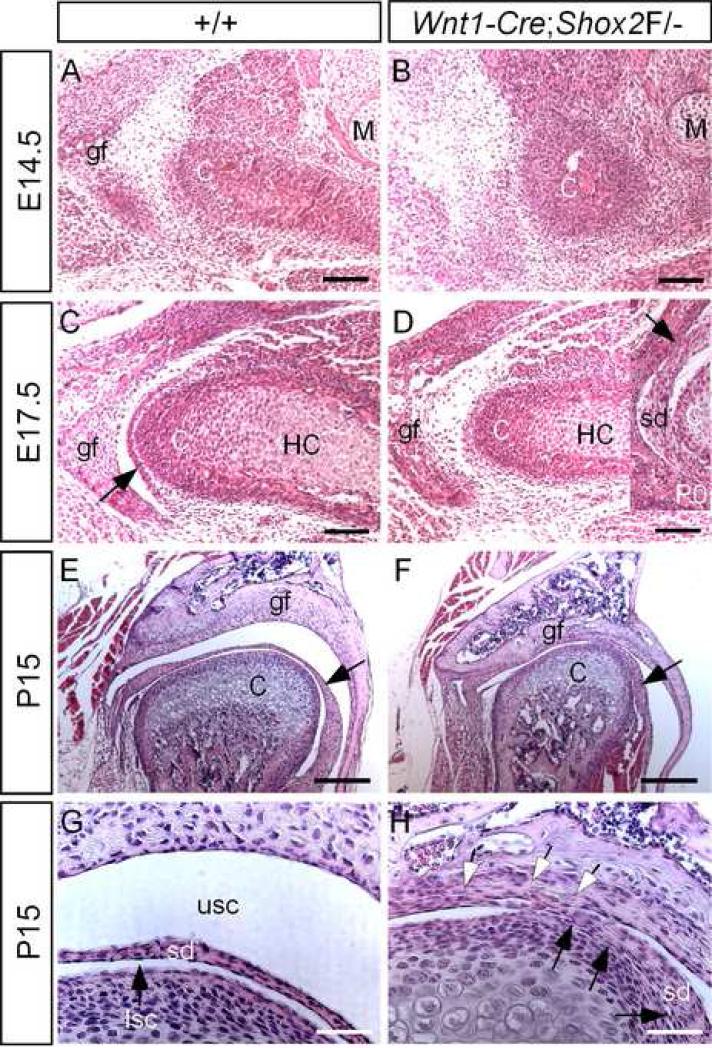
Histological analyses of the developing mutant embryos demonstrate a delay in the TMJ development. At E14.5, when the condensations of the condyle and glenoid fossa become discernible in the wild type control, a small condylar condensation just began to form in the mutant (Fig. 4A, 4B). The glenoid fossa condensation in the mutant was invisible at this stage (Fig. 4B), but became clear at E15.5 (data not shown; Fig. 6J). At E17.5, when a definite synovial disc forms and separates the joint cavities in the wild type, a disc primordium was indiscernible in the mutant (Fig. 4C, 4D). A synovial disc in the mutant TMJ did not form until the newborn stage, but appeared to adhere to both the glenoid fossa and the condyle (insert in Fig. 4D). More severe architectural abnormalities were observed in the mutant TMJ at P15 (Fig. 4E-H). The mutant condyle exhibited a reduced size, about a quarter shorter in the diameter, as compared to the wild type control (Fig. 4E, 4F). Most notably, the synovial disc fused with the fibrous layers of the condyle and the glenoid fossa in the mutant, resulting in the formation of incomplete upper and lower synovial cavities (Fig. 4G, 4H). A definite and completely separated disc was never observed. This phenotype was consistently observed in all five mutant mice that were subjected to histological analysis. This fibrous adhesion of the joint components, clinically defined as TMJ ankylosis, could restrain the jaw movement, at least to some extent, and lead to the difficulty or even inability to eat and drink.
Figure 4.
Histological analysis of the TMJ. (A) Coronal section through the developing TMJ of an E14.5 wild type embryo shows discernible condensations of the condyle and the glenoid fossa. (B) A section through an E14.5 Wnt1-Cre;Shox2F/- TMJ region shows a delay in the development of the TMJ. The condylar condensation appears smaller, and the glenoid fossa primordium is invisible. (C) A section through an E17.5 wild type TMJ show well defined structures of the condyle and the glenoid fossa, as well as the definite synovial disc (arrow) which has separated the upper synovial cavity from the lower joint cavity. (D) A section from an E17.5 Wnt1-Cre;Shox2F/- TMJ shows definite structures of the condyle and the glenoid fossa. However, a synovial disc has not yet formed. Insert: at P0, a synovial disc forms but does not completely separated from the condyle and the glenoid fossa in the mutant TMJ. Arrow points to the fused site of the disc and the glenoid fossa. (E, F) Sections through the TMJ of P15 wild type (E) and Wnt1-Cre;Shox2F/- mice (F) display the joint dysplasia in the mutant. The condyle exhibits a reduction in size, and the synovial disc does not separate the joint cavities completely. Arrows point to the disc. (G) A higher magnification of the image in (E) shows a distinct synovial disc and the clearly separated upper synovial cavity and lower synovial cavity. (H) A higher magnification of image in (F) shows abnormal fusions of the disc with condyle (black arrows) and the glenoid fossa (open arrows). C, condyle; M, Meckel's cartilage; gf, glenoid fossa; sd, synovial disc; lsc, lower synovial cavity; usc, upper synovial disc. Bars = 100 μm in A-D, 200 μm in E, F, 800 μm in G, H.
Figure 6.
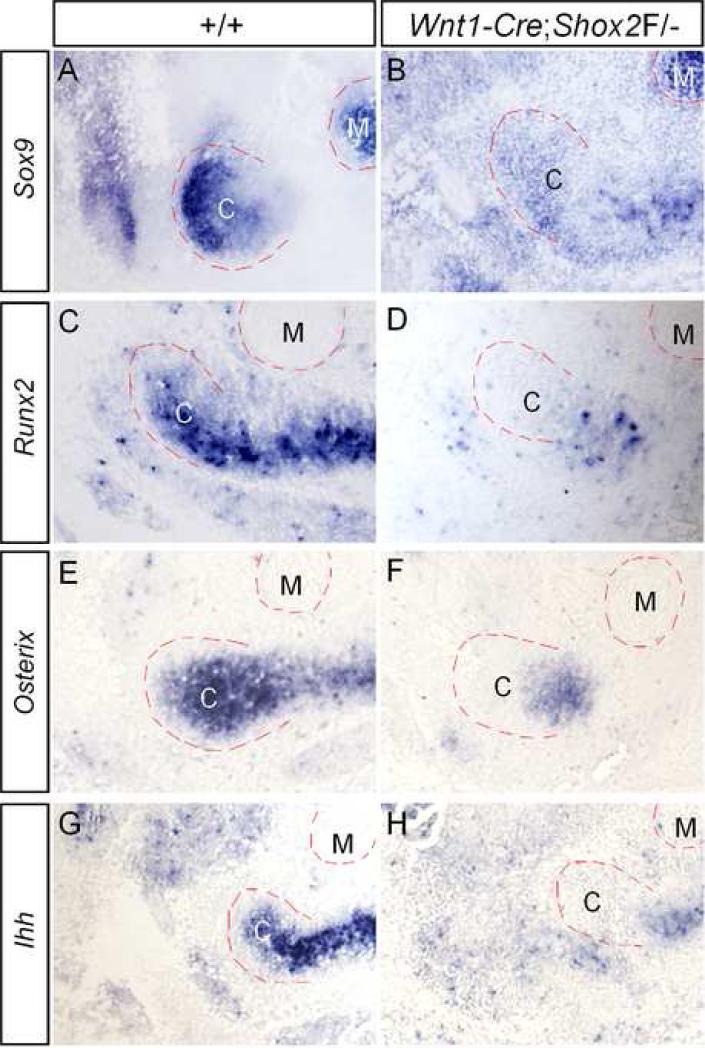
Down-regulated expression of osteogenic genes in the developing condyle of Wnt1-Cre;Shox2F/- mice. (A, C, E, G) The expression of Sox9 (A), Runx2 (C), Osterix (E) and Ihh (G) is detected in the condylar condensation of E13.5 wild type embryo. (B, D, F, H) Significantly down-regulated expression of Sox9 (B), Runx2 (D), Osterix (F) and Ihh (H) is observed in the condylar condensation of E13.5 Wnt1-Cre;Shox2F/- embryo. Note that only Sox9 expression (A, B) is seen in Meckel's cartilage. C, condyle; M, Meckel's cartilage.
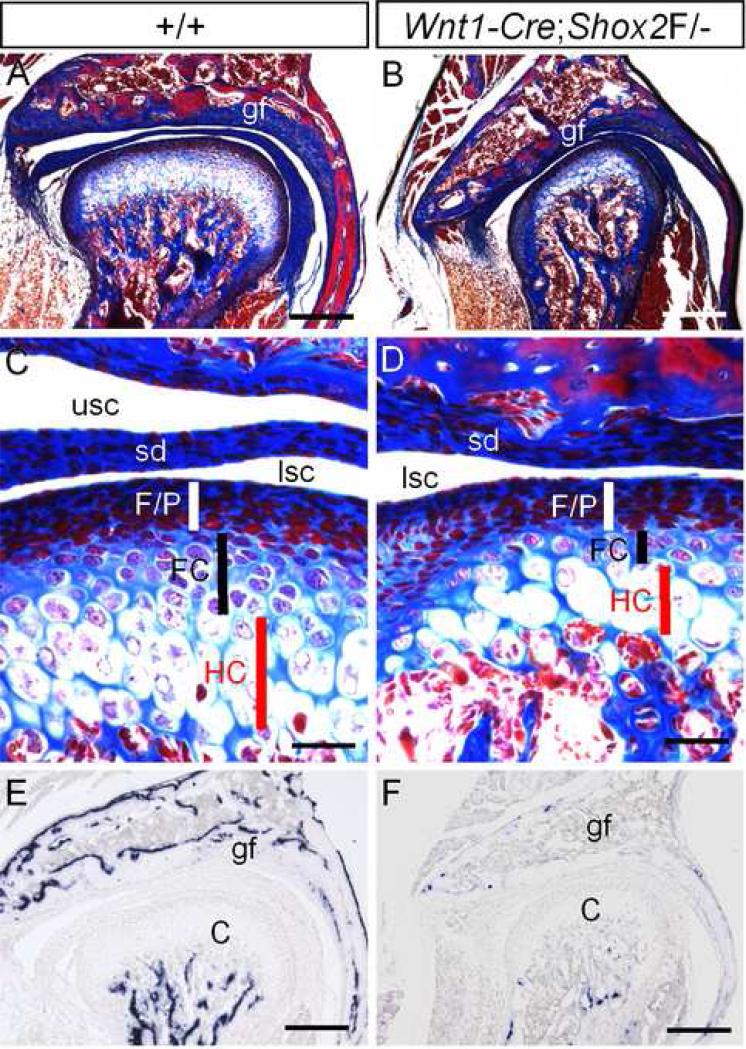
Since the SHOX genes have been implicated in skeletogenesis, we next examined in the TMJ the osteogenic consequence in the absence of Shox2. By using the Azon red/Anilin blue staining method, which stains bone matrix in red, we readily detected a large amount of ossified bone matrix in the condyle and glenoid fossa of the P15 wild type TMJ (Fig. 5A). In contrast, both the condyle and glenoid fossa in the age-matched mutant mice showed a significantly reduced amount of bone tissues (Fig. 5B). A close examination of the condyle revealed a severe dysplasia of the growth palate-like structure. Although the fibrous/polymorphic zone in the mutant appears similar to the wild type control, a significant reduction in thickness of both flattered chondrocyte zone and hypertrophic chondrocyte zone was identified (Fig. 5C, 5D). Osteocalcin is an osteoblast-specific gene and a direct downstream target of Runx2 (Ducy, 2000). It encodes a noncollagenous bone matrix protein that binds to calcium and regulates bone formation and mineralization, In situ hybridization assays for further revealed a greatly reduced level of Osteocalcin expression in both the condyle and glenoid fossa of the mutant TMJ (Fig. 5E, 5F). Shox2 is thus essential not only for chondrogenesis but also for osteoblast differentiation in both the endochondral and intramembranous bones.
Figure 5.
Wnt1-Cre;Shox2F/- mice exhibit TMJ dysplasia. (A, B) Sections through the TMJ of a P15 wild type mouse (A) and an age-matched mutant (B) show significantly reduced bone formation in the mutant TMJ, as determined by the Azon red/Anilin blue staining method, which stains bone matrix in red. (C, D) Sections through the condyles of a P15 wild type mouse (C) and a P15 mutant (D) show condylar dysplasia of the mutant. The mutant exhibits three distinct zones in the growth plate-like structure, but the flattered chondrocyte zone and the hypertrophic chondrocyte zone appear much thinner, as compared to the wild type control. (E, F) In situ hybridization assay shows a high level of Osteocalcin expression in the condyle and the glenoid fossa of a P15 wild type mouse (E), and an extremely reduced expression of Osteocalcin in the TMJ of an age-matched mutant mouse. C, condyle; FC, flattered chondrocyte zone; F/P. fibrous/polymorphic layers; gf, glenoid fossa; HC, hypertrophic chondrocyte zone; sd, synovial disc; lsc, lower synovial cavity; usc, upper synovial cavity. Bars = 200 μm in A, B, E, F, and 800 μm in C and D.
2.3. Down-regulated expression of genes critical for skeletogenesis in the developing TMJ of Wnt1-Cre;Shox2F/- mice
Previous studies have demonstrated a role for Shox2 in skeletogenesis by controlling the expression of Runx2 and Ihh in the developing limbs (Cobb et al., 2006; Yu et al., 2007). Since the developmental defect in the mutant TMJ becomes morphologically recognizable at E14.5 (Fig. 4), we initially surveyed the expression of several osteogenic genes, including Sox9, Runx2, Osterix, and Ihh, in the mutant condyle primordium at E13.5. The expression of these genes has been documented in the developing condyle at late stages (Shibata et al., 2006; Shibukawa et al., 2007). In our in situ hybridization experiments, we were able to readily detect the expression of these genes in the condylar condensation of the wild type controls at E13.5 (Fig. 6A, 6C, 6E, and 6G). Interestingly, among these genes, only Sox9 expression was detected in Meckel's cartilage. In contrast, in the mutant condylar primordium, Runx2 and its downstream gene Osterix, and Ihh, exhibited a marked down-regulation (Fig. 6D, 6F, 6H), indicating a requirement of Shox2 for the expression of these genes. This observation is consistent with the expression of Runx2 and Ihh in the limbs lacking Shox2. Surprisingly, we also observed a significantly reduced Sox9 expression (Fig. 6B), a result that was not seen in the Shox2-/- limbs (Yu et al., 2007). In the Shox2-/- developing limbs, an up-regulated Bmp4 expression was found, which is thought to be responsible for the repression of Runx2 expression (Yu et al., 2007). We set to determine if this is the case in the developing condyle. However, we did not detect obviously elevated Bmp4 expression in or around the condylar condensation of Wnt1-Cre;Shox2F/- embryos at E13.5 and E14.5 (data not shown). These observations indicate that Shox2 appears to exert its role through different mechanisms in the development of the limbs and the condyle.
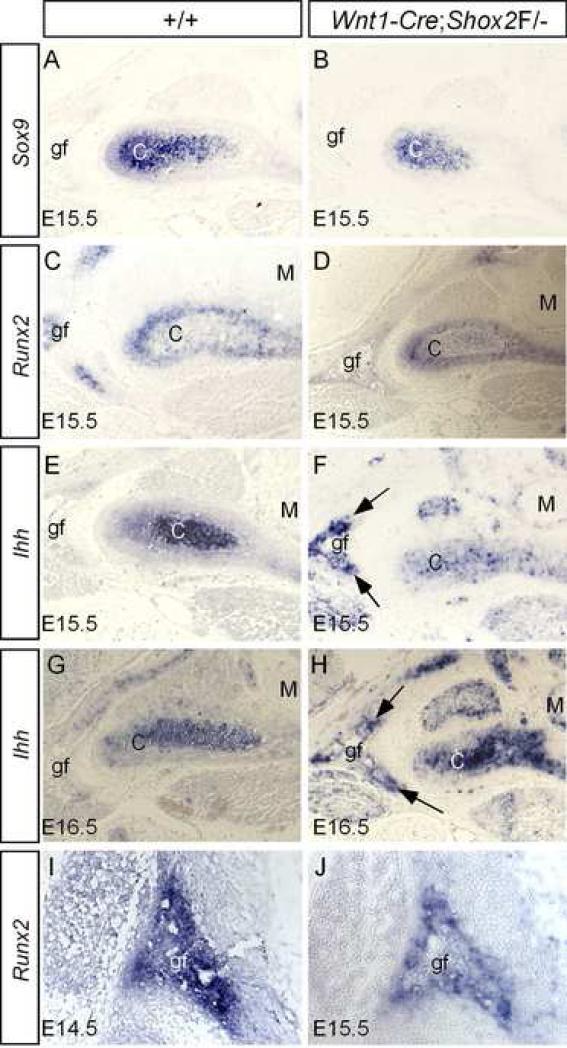
We further examined the expression of Sox9, Runx2 and Ihh at later stages. As shown in Fig. 7, at E15.5, the expression of Sox9 and Runx2 remained a slightly down-regulation in the mutant condyle, as compared to their wild type controls (Fig. 7A-D). Surprisingly, at E15.5, the mutant exhibited a consistent down-regulation of Ihh in the condyle, but an ectopic Ihh expression in the glenoid fossa (Fig. 7E, 7F). At E16.5 and E17.5, Ihh remained ectopically activated in the glenoid fossa of the mutant TMJ (Fig. 7G, 7H; and data not shown). We never detect an Ihh expression in the developing glenoid fossa of the wild type mice (from E13.5 to E17.5), which is consistent with the fact that the intramembranous ossification is unaffected in Ihh-null embryos (St-Jacques et al., 1999). The ectopic Ihh expression in mice lacking Shox2 indicates a repressive effect of Shox2 on Ihh expression in the glenoid fossa. Shox2 thus appears to exert different regulatory effects on Ihh expression in the developing condyle and glenoid fossa.
Figure 7.
Altered expression of osteogenic genes in the developing TMJ of Wnt1-Cre;Shox2F/- mice. (A) Strong Sox9 expression is detected in the chondrocytes of an E15.5 wild type condyle. No expression is seen in the glenoid fossa. (B) Sox9 expression, although at relatively reduced level, is detected in the chondrocytes of the condyle of an E15.5 Wnt1-Cre;Shox2F/- embryo. (C) The developing TMJ of an E15.5 wild type embryo shows Runx2 expression in the condyle and glenoid fossa. Note that the expression in the condyle at this stage is mainly restricted to the perichondrial region. (D) Reduced Runx2 expression is detected in the condyle and glenoid fossa of an E15.5 Wnt1-Cre;Shox2F/- embryo. (E) Ihh expression is seen in the condylar chondrocytes of an E15.5 wild type embryo. No Ihh expression is detected in the glenoid fossa. (F) In an E15.5 Wnt1-Cre;Shox2F/- embryo, although Ihh expression remains at a reduced level in the condyle, ectopic Ihh expression is detected in the glenoid fossa (arrows). (G) An E16.5 wild type condyle shows a reduced Ihh expression in the condyle. (H) In an E16.5 mutant, an increased level of Ihh expression is seen in the condyle. Ectopic Ihh expression remains in the glenoid fossa (arrows). (I) Strong Runx2 expression is detected in the glenoid fossa primordium of an E14.5 wild type embryo. (J) A reduced level of Runx2 expression is detected in an E15.5 Wnt1-Cre;Shox2F/- embryo.
Since Runx2 is expressed in all developing skeletal components and is essential for the osteoblast differentiation, we wondered if a down-regulation of Runx2 could also occur in the developing glenoid fossa of Wnt1-Cre;Shox2F/- embryo, which would account for the reduced bone formation. A strong Runx2 expression can be detected in the developing glenoid fossa of E14.5 wild type embryo (Fig. 7I). In contrast, Runx2 expression was barely detected in the glenoid fossa of an age-matched mutant (data not shown). At E15.5, when the mutant glenoid fossa assumed a shape which was morphologically similar to that of E14.5 wild type controls, we indeed detected Runx2 expression, at a reduced level, in the mutant glenoid fossa (Fig. 7H). Shox2 thus positively controls Runx2 expression in the skeletal primordia undergoing either an endochondral or an intramembranous ossification process.
2.4. Wnt1-Cre;Shox2F/- mice display a delayed expression of molecular markers for chondrogenic differentiation in the developing condyle
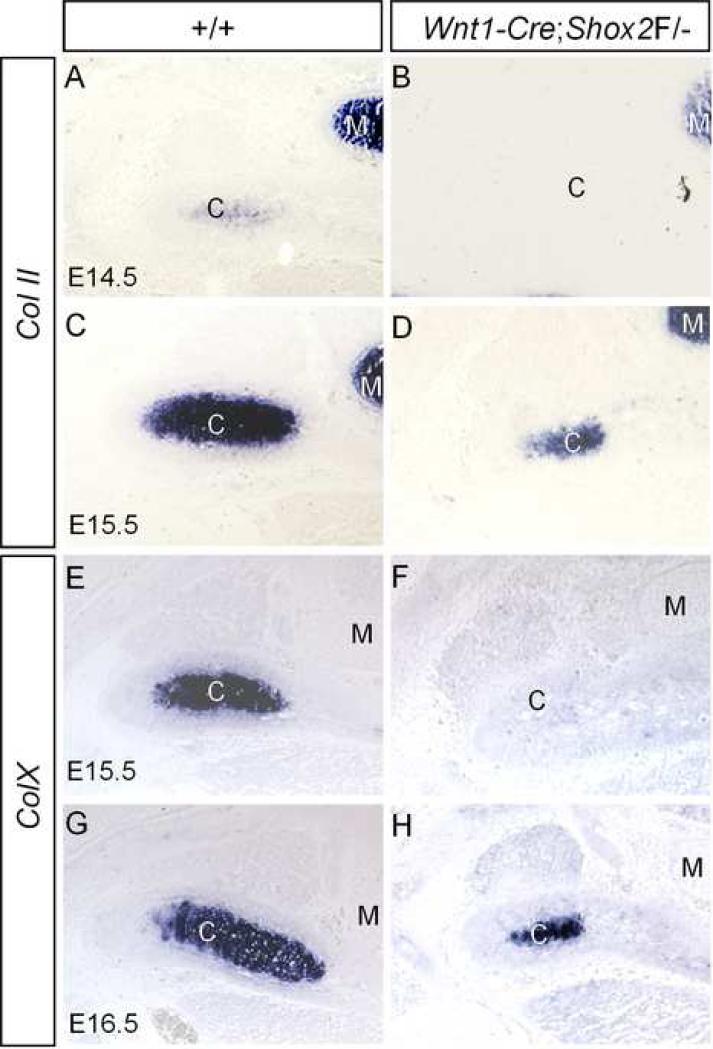
Shox2 has been shown to regulate chondrocyte maturation in the developing limbs (Cobb et al., 2006; Yu et al., 2007). To determine if Shox2 plays a similar role in the developing condyle, we examined the expression of chondrogenic differentiation markers: the collagen type II (Col II), a marker for immature chondrocytes, and the collagen X (Col X), a marker for hypertrophic chondrocytes. In the wild type controls, Col II expression initially becomes detectable in the condylar condensation at E14.5 (Fig. 8A). Strong Col II expression can be seen in both the immature and the hypertrophic chondrocytes at E15.5 (Fig. 8C). Col X expression appears later than Col II, undetectable at E14.5 (data not shown). From E15.5 on, Col X transcripts can be detected with a restricted localization in the hypertrophic chondrocytes of the condyle (Fig. 8E, 8G). This overlapped expression of Col II and Col X in the hypertrophic chondrocytes is different from their expression in the long bones, in which they exhibit complementary expression patterns. In the developing condyle of Wnt1-Cre;Shox2F/- mice, we have found that the expression of both Col II and Col X was delayed for one day in development. Col II transcripts were undetectable at E14.5, but were detected at E15.5 (Fig. 8B, 8D). Col X expression was not detectable until E16.5 (Fig. 8F, 8H). These results indicate that the differentiation of chondrocytes into hypertrophic chondrocytes, although delayed, indeed occurs in the developing condyle lacking Shox2. This observation is in contrast to that concerning the developing limbs of mice lacking Shox2, where the stylopodial cartilaginous element does not have hypertrophic chondrocytes and never expresses Col X, which leads to the virtual absence of the humerus and femur (Cobb et al., 2006; Yu et al., 2007).
Figure 8.
Delayed expression of chondrogenic differentiation genes in the developing condyle of Wnt1-Cre;Shox2F/- mice. (A, C) Col II expression is initially detected in the condylar of E14.5 wild type embryo (A) but becomes stronger at E15.5 (C). (B, D) In the mutant condyle, Col II expression is undetectable at E14.5 (B) but becomes visible at E15.5 (D). (E, G) Strong Col X expression is seen in the wild type condyle at E15.5 (E) and E16.5 (G). (F, H) In the mutant condyle, Col X expression is not detected at E15.5 (F) but becomes detectable at E16.5 (H).
3. Discussion
Mice lacking Shox2 die during mid-gestation and exhibit severe developmental defects in several organs (Yu et al., 2005; 2007; Cobb et al., 2006; Blaschke et al., 2007). Shox2-/- mice display chondrodysplasia in the developing limbs, supporting a role proposed for the SHOX genes in skeletogenesis (Clement-Jones et al., 2000; Cobb et al., 2006; Tiecke et al., 2006; Yu et al., 2007). In this study, we report an initial expression of Shox2 in the cranial neural crest-derived mesenchymal cells of the maxilla-mandibular junction and a subsequently restricted Shox2 expression in the developing TMJ. A specific ablation of Shox2 from the neural crest cells in the mouse by the Wnt1-Cre transgenic allele circumvents embryonic lethality in the Shox2 conventional knockouts, providing an opportunity to study the role of Shox2 in the development of the TMJ. The defects found in the Wnt1-Cre;Shox2F/- TMJ demonstrate an essential role for Shox2 in the TMJ development and function.
The developmental processes of the TMJ appear distinct from those of other synovial joints. The TMJ develops from two distinct mesenchymal condensations, the condylar and glenoid fossa blastemas, each of which undergoes a different ossification process. The development of the condyle shares many similarities to that of the long bones, including the endochondral ossification process and gene expression profile. However, because of its neural crest origin, the condylar cartilage displays some distinct characteristics and behaviors as compared to the cartilaginous elements that form long bones, and is classified as secondary cartilage (Beresford, 1975). The condylar cartilage exhibits a rapid differentiation from the progenitor cells to the hypertrophic chondrocytes, expressing Col I, Col II and Col X simultaneously (Shibata et al., 1997; Fukada et al., 1999). In addition, the alkaline phosphatase-positive, Col I-expressing progenitor cell-derived condylar cartilage appears to possess preosteoblast characteristics (Silbermann et al., 1987). Some osteogenic genes, such as Runx2, Osterix, and Bsp, exhibit slightly different expression patterns in the developing condylar cartilage as compared to that in primary cartilage (Shibata et al., 2002; 2006). Furthermore, same genes may exert different function in the development of primary and secondary cartilage. For example, in Runx2-deficient mice, primary cartilage forms, but the condylar cartilage is completely eliminated (Ducy et al., 1997, Komori et al., 1997; Otto et al., 1997; Shibata et al., 2004).
Shox2 appears to have similar and distinct effects in the development of the condyle and the limbs. For example, Shox2 regulates the cell proliferation rate and the expression of Runx2 and Ihh in both the developing condyle and limbs (Cobb et al., 2006; Yu et al., 2007). On the other hand, unlike the stylopod of limbs, the development of the mutant condyle is not completely arrested but is only delayed. The condylar chondrocytes differentiate into the hypertrophic chondrocytes eventually. Although a down-regulated but not completely diminished Runx2 expression may explain the delayed differentiation of the hypertrophic chondrocytes in the mutant condyle, the rapid differentiation feature of the condylar chondrocytes could at least partially account for the formation of hypertrophic chondrocytes. In the developing condyle lacking Shox2, Sox9 expression is down-regulated and the mesenchymal condensation is delayed, which was not observed in the limbs lacking Shox2. Since Sox9 expression is essential for mesenchymal condensation (Akiyama et al., 2002), a reduced level of Sox9 expression may contribute to the formation of the smaller condyle in the mutants. These different consequences observed in the condyle and limbs lacking Shox2 could be attributed to their distinct origins. Furthermore, a similar but slightly different expression pattern of Shox2 in the developing condyle may also contribute to the different outcomes. In the developing limbs, Shox2 is expressed in the surrounding mesenchymal cells but is absent from the stylopodial cartilage condensation. In contrast, Shox2 is exclusively expressed in the condensing mesenchyme of the condylar primordium.
Although Shox2 regulates Runx2 expression in several developing organs, including the limbs, TMJ, and palate, different mechanisms must be utilized (Yu et al., 2007; Gu et al., 2008; and this study). This is concluded based on the observations that in the developing limbs, Shox2 appears to control Runx2 expression through an intermediate means, by regulating Bmp4 expression (Yu et al., 2007). However, in the developing TMJ, Shox2 expression overlaps with that of Runx2 in the condyle and glenoid fossa. In addition, Bmp4 expression was not altered in the mutant TMJ region. It is thus possible that in the developing TMJ, Shox2 regulates Runx2 directly. This hypothesis is supported by the fact that the mouse Runx2 upstream region contains two consensus SHOX binding sites, and Shox2 can activate the Runx2 promoter in a reporter assay in cell culture (Liu, H. and Chen, Y.P, unpublished observations). The down-regulated Runx2 expression most likely account for the reduced expression level of Ihh and Osterix in the mutant condylar primordia, as the latter genes are known down-stream targets of Runx2 (Nakashima et al., 2002; Yoshida et al., 2004). The ectopic Ihh expression in the developing glenoid fossa lacking Shox2 indicates a repressive effect of Shox2 on Ihh expression. However, whether or not Shox2 represses Ihh expression is not unknown. The fact that Shox2 can perform opposite roles on gene expression, functioning either as a transcription activator or a repressor, implicates Shox2 in a direct repression of Ihh expression (Yu et al., 2007).
A dysplasia of the glenoid fossa found in the mutant TMJ identifies a new role for Shox2 in the development of skeletons that undergo intramembranous ossification. This conclusion is consistent with a significantly reduced bone formation in the hard palate lacking Shox2 (Gu et al., 2008). A down-regulation of Runx2 in the developing glenoid fossa could account for the reduced bone formation in the mutant. The extremely reduced expression of Osteocalcin indicates the presence of an extremely reduced number of osteoblasts in the glenoid fossa and condyle of the mutant. Since Shox2 is expressed in the developing glenoid fossa and in the perichondrium of the condyle, where the osteoblasts differentiate, we can not rule out the possibility that Shox2 may control the osteoblast differentiation directly.
One important component of the TMJ is the synovial disc, which separates the joint cavities and is essential for the TMJ function: the jaw movement. The disc is believed to form from an independent mesenchymal condensation between the developing condyle and glenoid fossa (Shibukawa et al., 2007). Since Shox2 expression is not detected in the disc prospective areas between the condylar and glenoid fossa primordia, a delay in the disc development and subsequent adhesion phenotype seen in the TMJ of Wnt1-Cre;Shox2F/- mice suggest an indirect effect of Shox2 in disc formation. Mice lacking Ihh failed to form a disc primordium, leading to a complete absence of the normal disc and joint cavities (Shibukawa et al., 2007). Ihh produced from the condyle is believed to exert a direct effect on the disc development, particularly on the disc initiation. In Wnt1-Cre;Shox2F/- embryo, the development of the disc is delayed, and a complete disc never forms. It is likely that the initial down-regulation of Ihh expression in the condylar primordium and the ectopic Ihh expression in the glenoid fossa at late stages attribute the disc abnormalities found in the mutants.
One significant anomaly we found in the TMJ of Wnt1-Cre;Shox2F/- mice is the adhesion of the disc to the fibrous layers of the condyle and glenoid fossa. Although the disc does not fuse completely with the condyle and the glenoid fossa, it mediates in fact the fusion between these two contiguous elements, representing a typical feature of TMJ ankylosis. We do not know to what extent that this fibrous adhesion of the TMJ could restrain the jaw movement of the mutant mice. However, the fact that all the Wnt1-Cre;Shox2F/- mice inevitably developed a wasting syndrome and died around P15 demonstrates a difficulty or even an inability to drink and eat (Gu et al., 2008). This notion is further supported by the fact that manned feeding before the death of the mice is able to partially relieve the wasting syndrome and extend the mouse life extensively (unpublished data). However, since Wnt1-Cre;Shox2F/- mice also develop an anterior clefting phenotype, it is possible that the clefting defect may also cause an inefficient sucking action, contributing to the development of the wasting syndrome (Gu et al., 2008).
Most incidents of TMJ ankylosis in humans occur after a trauma or an infection, but congenital cases, although rare, have been reported (Converse, 1979; Tideman and Doddridge, 1987; Komorowska, 1997; Mortazavi and Motamedi, 2007; Ajike et al., 2007). However, little is known about genetic alterations that cause congenital TMJ ankylosis at present. Our studies demonstrate an essential role for Shox2 in the TMJ formation and function, providing direct evidence for a genetic association with the congenital TMJ ankylosis. Even though patients with Turner syndrome do not exhibit significant alteration in the TMJ function (Szilagyi et al., 2000), and no information is available relating a TMJ phenotype to patients with Langer mesomelic dysplasia syndrome, SHOX2 could represent a potential candidate gene for congenital TMJ ankylosis. The fact that the congenital TMJ ankylosis has been reported to a patient who also exhibited a short stature phenotype supports this idea (Komorowska, 1997). Nevertheless, the Wnt1-Cre;Shox2F/- mice provide an unique model for studying the TMJ development and ankylosis.
4. Experimental procedures
4.1. Mice and mouse embryo collection
The generation of Shox2 conventional knockout mice and mice carrying a floxed Shox2 allele have been described previously (Yu et al., 2005; Cobb et al., 2006). The Wnt1-Cre transgenic mice (Danielian et al., 1998) and the R26R conditional reporter line (Soriano, 1999) were obtained from the Jackson Laboratories. Wnt1-Cre;Shox2F/- mice were obtained by mating Wnt1-Cre;Shox2+/- with Shox2F/F mice, and the genotype of the mice were determined by PCR-based methods using tail DNA, as described previously (Chai et al., 2000; Yu et al., 2005; Cobb et al., 2006). The age of the embryo was defined as the embryonic day 0.5 (E0.5) in the morning of the day when the vaginal plug was discovered.
4.2 Histological analyses and LacZ staining
For histological analysis of the developing TMJ, mouse heads were collected in ice-cold phosphate-buffered saline (PBS), and fixed in 4% paraformaldehyde (PFA)/PBS at 4°C overnight. The heads from E17.5 and the older mice were decalcified in 10% EDTA in fixative for various days depending on the age of the mouse. Samples were then dehydrated through a graded ethanol series, and thenprocessed for paraffin sectioning. Serial sections were made at 6-μm and subjected to standard Hematoxylin/Eosin staining or Azon red/Anilin blue staining, as described (Presnell and Schreibman, 1997). To detect the β-galactosidase activity in the TMJ region of Wnt1-Cre;R26R embryo, E16.5 embryos were collected from the cross of Wnt1-Cre with R26R mice The heads were removed and embedded in OCT. After the genotyping, the heads carrying both Wnt1-Cre and R26R alleles were processed for frozen sectioning at 20-μm and subjected for LacZ staining according to the standard protocol (Chai et al., 2000).
4.3. In situ hybridization and cell proliferation assay
Samples for in situ hybridization were fixed in 4% PFA/PBS at 4°C. For whole mount in situ, samples were dehydrated gradually with methanol. For section in situ, the samples were dehydrated through graded ethanol, embedded in paraffin, and sectioned at 10 μm. The non-radioactive riboprobes were synthesized by an in vitro transcription labeling with Digoxigenin-UTP according to the manufacturer's instructions. Whole mount and section in situ hybridizations were performed as described previously (St. Amand et al., 2000). Cell proliferation rate was monitored by the intraperitoneal BrdU injection into timed pregnant mice at a dose of 1.5 ml of labeling reagent/100 g body weight using the BrdU labeling and Detection Kit II from Roche. Two hour after the BrdU injection, the mice were sacrificed, and the samples were fixed in Carnoy's fixative, ethanol-dehydrated, paraffin-embedded, and sectioned at 5-μm. The sections were subjected to immunodetection according to the manufacturer's protocol. Two samples of both wild type and mutant were subjected for cell proliferation assay, and three adjacent sections from each sample were counted for BrdU labeling. BrdU-labeled cells within an arbitrarily defined area of the condylar primordia in wild type and mutant embryos were counted.
Acknowledgements
We are grateful to Drs. Dennis Duboule and John Cobb for providing Shox2 floxed mice. We are also grateful to the members of the Chen lab for their suggestion and discussion. We thank Mr. Catalin Anghelina for his excellent editing work on the manuscript. This work was supported by the NIH grants R01DE12329 and R01DE14044 to Y.P.C., and by National Natural Science Foundation of China (30671022) to S.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajike SO, Chom ND, Amanyeiwe UE, Ononiwu CN, Anyiam JO, Ogala WN. Non-syndromal, true congenital ankylosys of temporomandibular joint: a case report. West Indian Med. J. 2007;55:444–446. doi: 10.1590/s0043-31442006000600015. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Chaboissier M-C, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin V, Cusin V, Viot G, Girlich D, Toutain A, Moncla A, Vekemans M, Le Merrer M, Munnich A, Cormier-Daire V. SHOX mutations in dyschondrosteosis (Lèri-Weill syndrome). Nat. Genet. 1998;19:67–69. doi: 10.1038/ng0198-67. [DOI] [PubMed] [Google Scholar]
- Beresford WA. Schemes of zonation in the mandibular condyle. Am. J. Orthod. 1975;68:189–195. doi: 10.1016/0002-9416(75)90207-9. [DOI] [PubMed] [Google Scholar]
- Blaschke RJ, Hahurij ND, Kuijper S, Just S, Wisse LJ, Deissler K, Maxelon T, Anastassiadis K, Spitzer J, Hardt SE, Scholer H, Feitsma H, Rottbauer W, Blum M, Meijlink F, Rappold G, Gittenberger-de Groot AC. Targeted mutation reveals essential functions of the homeodomain transcription factor Shox2 in sinoatrial and pacemaking development. Circulation. 2007;115:1830–1838. doi: 10.1161/CIRCULATIONAHA.106.637819. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch D, Soriano P, McMahon A, Sucov H. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Clement-Jones M, Schiller S, Rao E, Blaschke RJ, Zuniga A, Zeller R, Robson SC, Binder G, Glass I, Strachan T, Lindsay S, Rappold GA. The short stature homeobox gene SHOX is involved in skeletal abnormalities in Turner syndrome. Hum. Mol. Genet. 2000;9:695–702. doi: 10.1093/hmg/9.5.695. [DOI] [PubMed] [Google Scholar]
- Cobb J, Dierich A, Huss-Garcia Y, Duboule D. A mouse model for human short-stature syndrome identifies Shox2 as a upstream regulator of Runx2 during long-bone development. Proc. Natl. Acad. Sci. USA. 2006;103:4511–4515. doi: 10.1073/pnas.0510544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Converse JM. Surgical release of bilateral, intractable, temporomandibular joint. Clin. Otolaryng. 1979;3:127–132. doi: 10.1097/00006534-197909000-00035. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in uterus by a tamoxifeninducible form of Cre recombinase. Curr. Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Ducy P. Cbfa1: a molecular switch in osteoblast biology. Dev. Dyn. 2000;219:461–471. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1074>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall A, Karsenty G. Osf2/Cbfa1: a transcription activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Ellison JW, Wardak Z, Young MF, Robey PG, Laig-Webster M, Chiong W. PHOG, a candidate gene for involvement in the short stature of Turner syndrome. Hum. Molec. Genet. 1997;6:1341–1247. doi: 10.1093/hmg/6.8.1341. [DOI] [PubMed] [Google Scholar]
- Fukada K, Shibata S, Suzuki S, Ohya K, Kuroda T. In situ hybridization study of type I, II, X collagens and aggrecan mRNAs in the developing condylar cartilage of fetal mouse mandible. J. Anat. 1999;195:321–329. doi: 10.1046/j.1469-7580.1999.19530321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Wei N, Jiang Y, Fei J, Chen YP. Mice with an anterior cleft of the palate survive neonatal lethality. Dev. Dyn. 2008 doi: 10.1002/dvdy.21534. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. Elsevier Academic Press; San Diego, CA: 2005. [Google Scholar]
- Kantomaa T, Tuminen M, Pirttiniemi P. Effect of mechanical forces on chondrocyte maturation and differentiation in the mandibular condyle of the rat. J. Dent. Res. 1994;73:1150–1156. doi: 10.1177/00220345940730060401. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao Y-H, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Komorowska A. Congenital temporomandibular joint ankylosis: a case report. Eur. J. Orthod. 1997;19:243–248. doi: 10.1093/ejo/19.3.243. [DOI] [PubMed] [Google Scholar]
- Kuboki T, Kanyama M, Nakanishi T, Akiyama K, Nawachi K, Yatani H, Yamashita K, Takano-Yamamoto T, Takigawa M. Cbfa1/Runx2 gene expression in articular chondrocytes of the mice temporomandibular and knee joints in vivo. Arch. Oral Biol. 2003;48:519–525. doi: 10.1016/s0003-9969(03)00088-8. [DOI] [PubMed] [Google Scholar]
- Marchin A, Marttila T, Winter A, Caldeira S, Malanchi I, Balschke RJ, Hacker B, Rao E, Karperien M, Wit JM, Richter W, Tommasino M, Rappold GA. The short stature homeodomain protein SHOX induces cellular growth arrest and apoptosis and is expressed in human growth plate chondrocytes. J. Biol. Chem. 2004;279:37103–37114. doi: 10.1074/jbc.M307006200. [DOI] [PubMed] [Google Scholar]
- Mortazavi SH, Motamedi MH. Congenital fusion of the jaws. Indian J. Pediatr. 2007;74:416–418. doi: 10.1007/s12098-007-0071-5. [DOI] [PubMed] [Google Scholar]
- Munns CF, Haase HR, Crowther LM, Hyes MT, Blaschke R, Rappold G, Glass IA, Batch JA. Expression of SHOX in human fetal and childhood growth plate. J. Clin. Endocrinol. & Metabol. 2004;89:4130–4135. doi: 10.1210/jc.2003-032230. [DOI] [PubMed] [Google Scholar]
- Miyake T, Cameron AM, Ha. BK. Stage-specific expression patterns of alkaline phosphatase during development of the first arch skeleton in inbred C57BL/6 mouse embryos. J. Anat. 1997;190:239–260. doi: 10.1046/j.1469-7580.1997.19020239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Shimokawa H, Fukada K, Suzuki S, Shibata S, Ohya K, Kuroda T. Localization and inhibitory effect of basic fibroblast growth factor on chondrogenesis in cultured mouse mandibular condyle. J. Bone Miner. Metab. 2003;21:145–153. doi: 10.1007/s007740300023. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GWH, Beddington RSP, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Presnell JK, Schreibman MP. Humason's Animal Tissue Techniques. Fifth Edition The Johns Hopkins University Press; Baltimore, MD: 1997. [Google Scholar]
- Rabie AB, Hägg U. Factors regulating mandibular condylar growth. Am. J. Orthod. Dentofacial. Orthop. 2002;122:401–109. doi: 10.1067/mod.2002.125713. [DOI] [PubMed] [Google Scholar]
- Rao E, Weiss B, Fukami M, Rump A, Niesler B, Mertz A, Muroya K, Binder G, Kirsch S, Winkelmann M, Nordsiek G, Heinrich U, Breuning MH, Ranke MB, Rosenthal A, Ogata T, Rappold GA. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat. Genet. 1997;16:54–63. doi: 10.1038/ng0597-54. [DOI] [PubMed] [Google Scholar]
- Shears DJ, Vassal HJ, Goodman FR, Palmer RW, Reardon W, Superti-Furga A, Scamber PJ, Winter RM. Mutation and deletion of the pseudoautosomal gene SHOX cause Lèri-Weill dyschondrosteosis. Nat. Genet. 1998;19:70–73. doi: 10.1038/ng0198-70. [DOI] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Suzuki S, Yamashita Y. Immunohistochemistry of collagen types II and X, and enzyme-histochemistry of alkaline phosphatase in the developing condylar cartilage of the fetal mouse mandible. J. Anat. 1997;191:561–570. doi: 10.1046/j.1469-7580.1997.19140561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Suzuki S, Ogawa T, Yamashita Y. In situ hybridization and immunohistochemistry of bone sialoprotein and secreted phosphoprotein 1 (osteopontin) in the developing mouse mandibular condyle cartilate compared with limb bud cartilage. J. Anat. 2002;200:309–320. doi: 10.1046/j.1469-7580.2002.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Suda N, Yoda S, Fukuoka H, Ohyama K, Yamashita Y, Komori T. Runx2-deficient mice lack mandibular condyle cartilage and have deformed Meckel's cartilage. Anat. Embryol. 2004;208:273–280. doi: 10.1007/s00429-004-0393-2. [DOI] [PubMed] [Google Scholar]
- Shibata S, Suda N, Suzuki S, Fukuoka H, Yamashita Y. An in situ hybridization study of Runx2, Osterix, and Sox9 at the onset of condylar cartilage formation in fetal mouse mandible. J. Anat. 2006;208:169–177. doi: 10.1111/j.1469-7580.2006.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibukawa Y, Young B, Wu C, Yamada S, Long F, Pacifici M, Koyama E. Temporomandibular koint formation and condyle growth require Indian hedgehog signaling. Dev. Dyn. 2007;236:426–434. doi: 10.1002/dvdy.21036. [DOI] [PubMed] [Google Scholar]
- Silbermann M, Frommer J. The nature of endochondrial ossification in the mandibular condyle of the mouse. Anat. Rec. 1972;172:659–668. doi: 10.1002/ar.1091720406. [DOI] [PubMed] [Google Scholar]
- Shibermann M, Reddi AH, Hand A>R, Leapman RD, von der mark K, Franzen A. Further characterization of the extracellular matrix in the mandibular condyle in neonatal mice. J. Anat. 1987;151:169–188. [PMC free article] [PubMed] [Google Scholar]
- Sperber GH. Craniofacial development. BC Decker Inc.; Hamilton, Otario: 2001. [Google Scholar]
- Soriano P. Generalized LacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- St. Amand TR, Zhang YD, Semina E, Zhao X, Hu YP, Nguyen L, Murray JC, Chen YP. Antagonistisc signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth forming anlage. Dev. Biol. 2000;217:323–332. doi: 10.1006/dbio.1999.9547. [DOI] [PubMed] [Google Scholar]
- St. Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilagyi A, Kesztav G, Nagy G, Madlena M. Oral manifestation of patients with Turner syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000;89:577–584. doi: 10.1067/moe.2000.104475. [DOI] [PubMed] [Google Scholar]
- Tang GH, Rabie ABM, Hägg U. Indian hedgehog: A mechanotransduction mediator in condylar cartilage. J. Dent. Res. 2004;83:434–438. doi: 10.1177/154405910408300516. [DOI] [PubMed] [Google Scholar]
- Tideman H, Doddridge M. Temporomandibular joint ankylosis. Austral. Dent. J. 1987;3:171–177. doi: 10.1111/j.1834-7819.1987.tb01850.x. [DOI] [PubMed] [Google Scholar]
- Tiecke E, Bangs F, Blaschke R, Farrell ER, Rappod G, Tickle C. Expression of the short stature homeobox gene Shox is restricted by proximal and distal signals in chick limb buds and affects the length of skeletal elements. Dev. Biol. 2006;298:585–596. doi: 10.1016/j.ydbio.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Watahiki J, Yamaguchi T, Irie T, Nakano H, Marki K, Tachikawa T. Gene expression profiling of mouse condylar cartilage during mastication by means of laser microdissection and cDNA array. J. Dent. Res. 2003;83:245–249. doi: 10.1177/154405910408300312. [DOI] [PubMed] [Google Scholar]
- Yoshida CA, Fujita T, Furuichi T, Ito K, Inoue K, Yamana K, Zanma A, Takada K, Ito Y, Komori T. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian Hedgehog. Genes Dev. 2004;18:952–963. doi: 10.1101/gad.1174704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Gu S, Alappat S, Song Y, Yan M, Zhang X, Zhang G, Jiang Y, Zhang ZY, Zhang YD, Chen YP. Shox2-deficient mice exhibit a rare type of imcomplete clefting of the secondary palate. Development. 2005;132:4397–4406. doi: 10.1242/dev.02013. [DOI] [PubMed] [Google Scholar]
- Yu L, Liu H, Yan M, Yang J, Long F, Muneoka K, Chen YP. Shox2 is required for chondrocyte proliferation and maturation in proximal limb skeleton. Dev. Biol. 2007;306:549–559. doi: 10.1016/j.ydbio.2007.03.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn AR, Wei F, Zhang L, Elder FF, Scott CI, Jr, Marttila P, Ross JL. Complete SHOX deficiency causes Langer mesomelic dysplasia. Am. J. Med. Genet. 2002;110:158–163. doi: 10.1002/ajmg.10422. [DOI] [PubMed] [Google Scholar]