Abstract
We have previously reported that CD8+ T cells significantly influence antibody production based on the observation that post-transplant alloantibody levels in CD8-deficient murine hepatocyte transplant recipients are markedly enhanced. However, the precise mechanism(s) contributing to enhanced alloantibody production in the absence of CD8+ T cells is not understood. We hypothesized that alloactivated CD8+ T cells inhibit antibody production by skewing towards a pro-inflammatory cytokine profile; whereas, when these cells are absent, an anti-inflammatory cytokine profile shifts the alloimmune response towards alloantibody production. To investigate this possibility, alloantibody isotype profiles were examined in CD8-deficient and wild-type hepatocyte recipients. We found that IgG1 (IL-4-dependent isotype) was the dominant alloantibody isotype in wild-type recipients as well as in CD8-deficient recipients, although the amount of alloantibody in the latter group was substantially higher. Utilizing real-time PCR we found that CD4+ T cells from wild-type recipients significantly upregulated IFN-γ but not IL-4 mRNA. In contrast, in the absence of CD8+ T cells, CD4+ T cells switched to significantly upregulate IL-4 mRNA, while IFN-γ was downregulated. IL-4 KO mice do not produce any post-transplant alloantibody. However, adoptive transfer of wild-type CD4+ T cells into CD8-depleted IL-4 KO mice restores high alloantibody levels observed in CD8-depleted wild-type recipients. This suggests that IL-4-producing CD4+ T cells are critical for post-transplant alloantibody production. Additionally, this CD8-mediated regulation of post-transplant alloantibody production is IFN-γ-dependent. Further elucidation of the mechanism(s) by which CD8+ T cells influence antibody production will significantly contribute to development of therapies to manipulate humoral responses to antigen.
Introduction
Transplantation has become the treatment of choice for end stage liver, renal, cardiac, and pulmonary disease. This modality of treatment can be life saving and in the cases of renal transplantation can vastly improve quality of life and prolong survival. The vast majority of current immunosuppressive treatments focus on inhibition of function and proliferation of alloreactive T cells central to the transplant rejection process. Despite the improvement in short-term graft survival, the half-life of transplants has remained the same due to chronic rejection, which represents the main cause of long-term graft failure (1, 2). Current experimental and clinical data implicate alloantibodies as important mediators of both acute and chronic rejection (3-6). Acute humoral graft rejection has emerged as an important cause of early graft dysfunction and is often more severe and resistant to immunotherapy than conventional T cell-mediated rejection responses (7, 8). Interestingly, transplant patients treated with immunosuppressants which inhibit T cell function still develop or are at risk for antibody-mediated rejection (9). Antibody-mediated allograft rejection and conditions which promote humoral immunity post-transplant are not well understood despite their critical impact on transplant outcomes.
In experimental models, post-transplant alloantibody, which is critical to acute humoral rejection, is MHC-directed (10, 11). While it is generally appreciated that CD4+ T cells and B cells collaborate for antibody production, we and others have noted a novel inhibitory function of CD8+ T cells manifested by the negative regulation of antibody production. Depletion of CD8+ T cells has been shown to significantly increase antigen specific antibody production in models of transplantation, allergy, bacterial infection, viral infection, and platelet transfusion (12-19). In our model, alloantibodies mediate in vivo allospecific cytotoxicity and acute hepatocellular allograft damage by a macrophage-dependent mechanism (20). We have also noted that IFN-γ critically inhibits alloantibody production, as alloantibody is significantly upregulated in IFN-γ KO recipient mice (12). It is well established that pro-inflammatory cytokines, such as IFN-γ, are produced by allo-activated CD8+ T cells (and other cells) that mediate inflammation and allograft rejection (21, 22). IFN-γ is also known to antagonize IL-4-induced B cell proliferation and IgG1 and/or IgE class switching (23, 24).
Since CD8+ T cells are known major producers of pro-inflammatory Th1-like cytokines, such as IFN-γ, CD8+ T cell depletion could result in a skewing towards a Th2-like cytokine dominant profile as has been suggested by Chan et al. (16). Therefore, we hypothesized that IFN-γ-producing CD8+ T cells may inhibit alloantibody production by skewing the immune response towards a pro-inflammatory dominant cytokine profile and, when they are absent, the anti-inflammatory dominant cytokine profile may drive alloantibody production of a given isotype. In order to explore this possibility, we evaluated alloantibody isotype and cytokine profiles in wild-type and CD8-deficient transplant recipients to identify a potential mechanism to explain the increased alloantibody produced when CD8+ T cells are absent.
Alloantibody isotype profiles in CD8 KO recipients showed that allospecific IgG1 (IL-4-dependent isotype) accounts for the majority of the increased alloantibody produced post-transplant. CD4+ T cells from wild-type recipients demonstrated an IFN-γ-dominant cytokine profile, while CD4+ T cells from CD8-deficient recipients switched to an IL-4-dominant cytokine profile. Unlike wild-type and CD8 KO transplant recipients that produce, primarily, an IgG1 alloantibody profile, IL-4 KO recipients revealed no alloantibody production post-transplant indicating that IL-4 is essential for IgG1 alloantibody production in this model. In contrast, IFN-γ KO recipients have an enhanced IgG1 alloantibody response. Following the adoptive transfer of wild-type CD8+ T cells into IFN-γ KO recipients, alloantibody is downregulated. Furthermore, adoptive transfer of IFN-γ KO CD8+ T cells into CD8 KO mice does not reduce alloantibody production, suggesting a critical role of IFN-γ-producing CD8+ T cells in the negative regulation of alloantibody production. Understanding the mechanisms of alloantibody production and regulation are necessary to develop novel diagnostic tools and targeted immunotherapeutic strategies to avoid alloantibody-mediated graft damage. Additionally, these results may be widely applicable to developing strategies to minimize immune responses to allergens or optimize responses to vaccination or infection.
Materials and Methods
Experimental animals
FVB/N (H-2q MHC haplotype, Taconic) and C57BL/6, CD8 KO, IL-4 KO, and IFN-γ KO (all H-2b, Jackson Labs) mouse strains (all 6-9 weeks of age) were used in this study. Transgenic FVB/N mice expressing human alpha-1 antitrypsin (hA1AT) were the source of “donor” hepatocytes, as previously described (25). All experiments were performed in compliance with the guidelines of the Institutional Laboratory Animal Care and Use Committee of The Ohio State University (Protocol 2008A0068).
Hepatocyte isolation, purification, and transplantation
Hepatocyte isolation and purification was completed, as previously described (25), by perfusing the liver with a 0.09% EGTA-containing calcium-free salt solution. The liver was then perfused with a 0.05% collagenase solution (type IV; Sigma Aldrich, St. Louis, MO) in 1% albumin. Liver tissue was minced, filtered, and washed with RPMI 1640 containing 10% fetal bovine serum. Hepatocytes were purified on a 50% Percoll gradient (Pharmacia Biotech, Uppsala, Sweden). Hepatocyte viability and purity has been determined to be consistently >95%. The hepatocytes were transplanted into recipient mice via intrasplenic injection with subsequent circulation of hepatocytes to the liver where they engraft and can be detected by immunohistochemical staining. Survival of transgenic donor hepatocytes was determined by serial measurement of recipient serum hA1AT levels. As previously reported, allogeneic hepatocytes are promptly rejected whereas syngeneic transgenic hepatocytes survive indefinitely. Similarly allogeneic hA1AT-hepatocytes survive indefinitely in immunodeficient SCID mice. Though in some cases the liver has been associated with immune tolerance, we have reported that allogeneic hepatocytes are highly immunogenic and elicit immune responses that are resistant to suppression by conventional immunosuppression (26). We have reported that hepatocyte rejection in this model occurs by both CD4-dependent and CD8-dependent pathways (12). The reporter protein hA1AT does not elicit an immune response which adversely affects hepatocyte survival since syngeneic, hA1AT-expressing hepatocytes survive long term (25).
CD8+ T cell depletion
Wild-type mice were given IP injections of 100 μg anti-CD8 antibody on day -1 and -2 prior to transplant (clone 53.6.72; Bioexpress Cell Culture Services, West Lebanon, New Hampshire).
Assay of total antibody
Host serum was collected on day 14 after transplant and assayed for specific IgG isotypes (IgG1, IgG2a, and IgG3) and IgE of antibody using ELISA. In brief, 96-well plates were coated with 2 μg/ml of goat anti-mouse IgG or goat anti-mouse IgE (eBioscience) in Phosphate Buffered Saline (PBS) with 0.02% azide overnight at 4°C. Plates were then blocked for non-specific binding with 20% FBS (PBS) at 37°C for 1 hr. To analyze IgG1, IgG2a, IgG2b and IgG3, serum from B6 and CD8 KO recipients was diluted from a range of 1 μg/ml to 0.5 ng/ml using 1:2 serial dilutions. The range of dilution for IgE was 50 μg/ml to 0.1 μg/ml. Specific binding was detected with goat anti-mouse IgG bound to alkaline phosphatase and developed with p-nitrophenyl phosphate substrate (PNPP, Sigma). Immunosorbance was detected at 460 nm. Quantitative measurement of IgG isotypes and IgE in serum of transplant recipients was determined by fold differences compared to amounts detected in control serum of naïve mice.
Assay of allospecific antibody
Antibody IgG isotypes from recipient serum was tested for allospecificity first by incubation with allogeneic FVB/N target splenocytes. The percent binding of alloantibody isotypes IgG1, IgG2a, IgG3, and total IgG to splenocyte targets was determined by a second incubation with FITC-conjugated goat anti-mouse IgG Fc (Organon Teknika, Durham, NC) or anti-mouse IgG isotypes (Invitrogen, Carlsbad, CA) and analysis by flow cytometry. Alloantibody level is represented as the percentage of target cells labeled by secondary fluorescent antibody as described previously (12). Positive controls for the assay were created by tagging allogeneic splenocytes with antigen specific (i.e., anti-CD8 mAb) antibodies of a known isotype (IgG1, IgG2a, and IgG3). All positive controls exhibited high percentile binding (data not shown).
CD8+ and CD4+ T cell isolation and purification
Isolation and purification of CD8+ T cells and CD4+ T cells was performed for each subset by negative selection columns as per the manufacturer's recommendations (R&D Systems, Minneapolis, MN). Briefly, a monoclonal antibody cocktail was added to isolated splenocytes to negatively select CD8+ T cells or CD4+ T cells. The cells were added to the T cell Subset Column, incubated, and eluted with column buffer. The purity of the recovered cells ranged from 90% to 95%.
Semi-quantitative Real-Time PCR of IFN-γ and IL-4 levels
Following Trizol extraction and RNeasy purification (Qiagen, Valencia, CA), 2 μg of total RNA were reverse transcribed. The resulting cDNA was used as a template to measure gene expression by real-time PCR and performed as described elsewhere (27). For real-time PCR, a common master mix (LightCycler-FastStart DNA SYBR Green I), MgCl2 (concentration adjusted to specific primer), 0.5 μM gene-specific primer, and 1.0 μl of cDNA were used at a final reaction volume of 10 μl (Roche, Basel, Switzerland). For each gene the following cycling protocol was used: 95°C for 10 min followed by 40 cycles of denaturing at 95°C for 15 s, a gene-specific annealing temperature for 2 s, and a 58°C extension for 20 s. IFN-γ (sense primer 5′-GCTCTGAGACAATGAACGCT-3′ and antisense 5′-AAAGAGATAATCTGGCTCTGC-3′) and IL-4 (sense primer 5′-TCGGCATTTTGAACGAGGTC-3′ and antisense 5′-GAAAAGCCCGAAAGAGTCTC-3′) were evaluated within wild-type and CD8 KO hepatocyte recipient mice (Integrated DNA Technologies, San Diego, CA). Mouse β-actin (sense primer 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and antisense 5′-AAAACGCAGCTCAGTAACAGTCCG-3′) was used as a normalization control. The gene-specific cDNA expression was evaluated by comparing cDNA from recipient mice to their respective naïve controls. Real-time PCR samples were performed in triplicate and analyzed using the Roto-Gene 2000 real-time cycler (Phoenix Research Products, Phoenix, AZ).
Statistical analysis
Statistical calculations were performed using a one-tailed Student's t test to analyze differences between experimental groups. P<0.05 was considered significant. To demonstrate the distribution of the data, results are listed as the mean plus or minus the standard deviation.
Results
CD8+ T cells downregulate the production of alloantibody
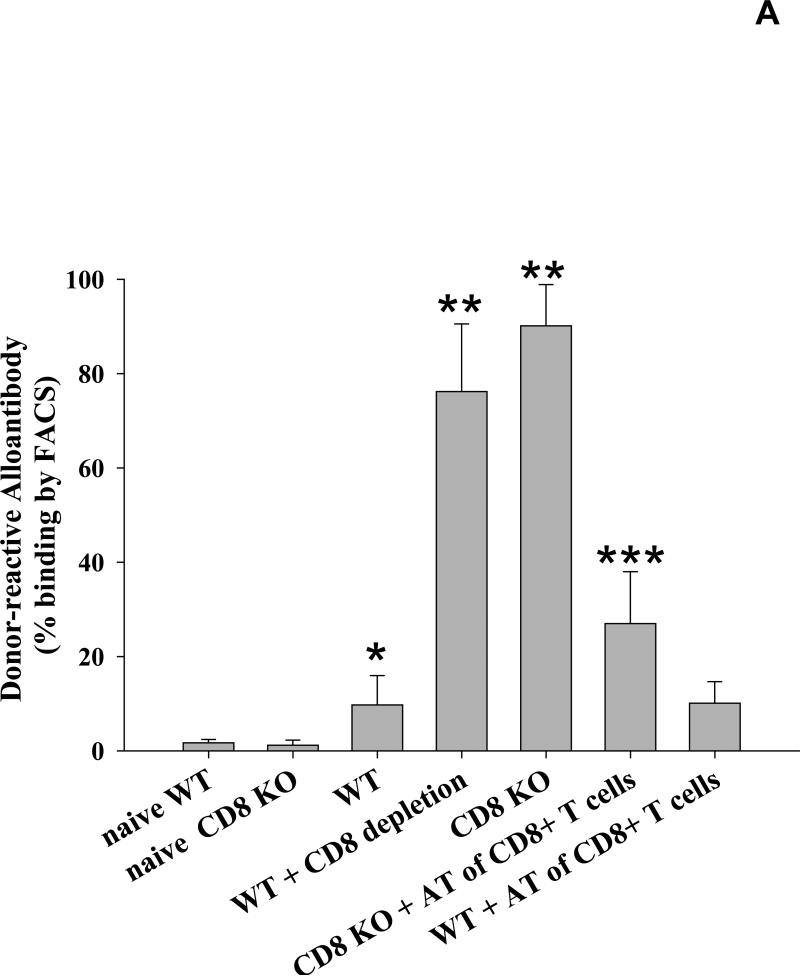
We have previously reported that following transplantation of allogeneic FVB/N (H-2q) hepatocytes, the production of alloantibodies detected in the serum of CD8-deficient (CD8 KO or CD8-depleted, H-2b) mice is significantly increased when compared to wild-type (H-2b) recipients and naïve (non-transplanted) controls (12, 20). To verify that CD8+ T cells are integral to this observation, CD8+ T cells were adoptively transferred (10×106 cells i.v.) to CD8 KO mice prior to hepatocyte transplant (day -1 or 0) and serial serum samples were collected for measurement of alloantibody levels. We found that the alloantibody response is significantly downregulated in CD8 KO recipients adoptively transferred with CD8+ T cells (27.0±11.1% vs. 90.1±8.7%; p<0.0001, Figure 1A). These results are consistent with the hypothesis that CD8+ T cells significantly regulate the level of alloantibody production following transplantation.
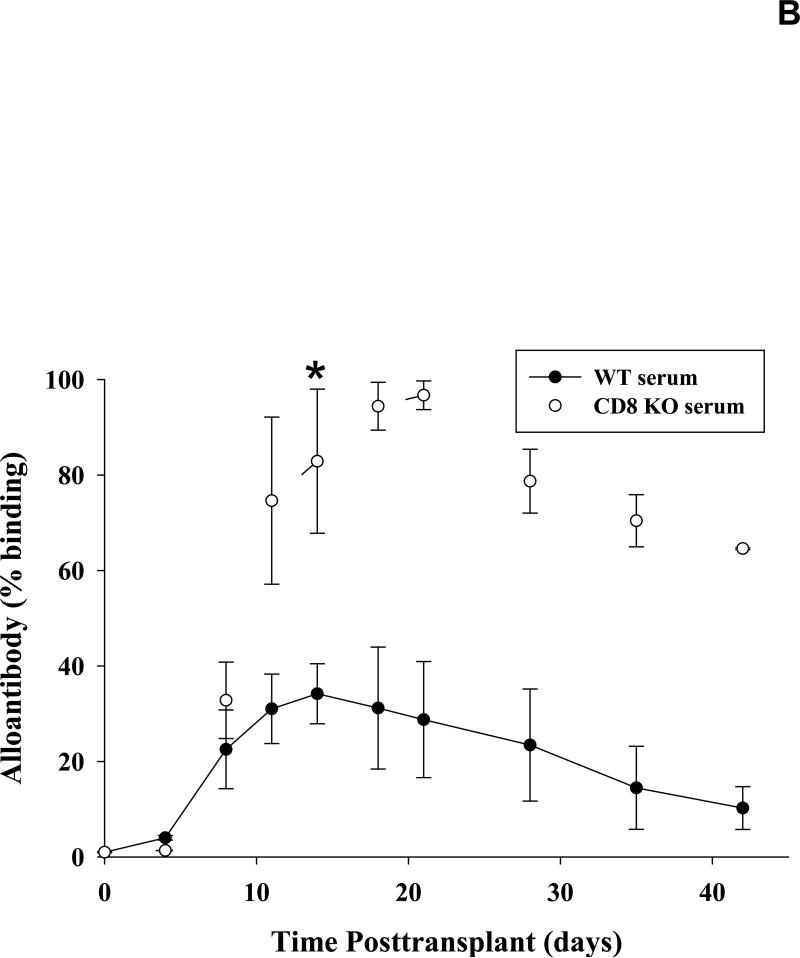
Figure 1. CD8+ T cells downregulate the production of alloantibody.
A) Wild-type and CD8 KO mice (H-2b) were transplanted with allogeneic FVB/N (H-2q) hepatocytes. Serum was tested for alloantibody on day 14 following transplantation by incubating sera with FVB/N splenocytes and staining with a secondary antibody specific to the Fc portion of mouse IgG. Transplanted wild-type mice showed a low but significant production of alloantibody (9.7±6.2%, n=25) as compared to naïve controls (non-transplanted; 1.7±0.7%, n=6; p=0.004, as denoted by “*”). The production of alloantibodies detected in the serum of CD8-deficient (CD8 KO, n=17 or CD8-depleted, n=8, H-2b) recipients is significantly increased when compared to wild-type (H-2b) recipients and naïve controls (90.1±8.7% and 76.2±14.3%, respectively; p<0.0001 for all comparisons, as denoted by “**”). Furthermore, when CD8+ T cells were adoptively transferred (AT; 10×106 cells i.v.) to CD8 KO mice prior to transplant, the alloantibody response is significantly downregulated (27.0±11.1%, n=5; p<0.0001, as denoted by “***”). B) Serum from wild-type and CD8 KO recipients collected over 42 days (n=4 for both). Alloantibody levels were maximal by day 14 in both CD8 KO (98.4±0.3%) and wild type recipients (28.8±12.1%; p=0.040, as denoted by “*”) and remained higher in CD8 KO recipients at all time points thereafter.
Although alloantibody production is higher in CD8-deficient mice on day 14 as compared to CD8-sufficient mice, it was unclear if this difference was due to differential kinetics of alloantibody production post-transplant between the two groups. To analyze this possibility, we quantified serum alloantibody levels over the course of 42 days in both wild-type and CD8 KO recipient mice. Serum was collected and analyzed for alloantibody production as previously described (12). Alloantibody was detectable within CD8 KO mice on day 7 following transplantation and was maximal by day 14 (98.4±0.4%; Figure 1B). Serum from wild-type recipients had significantly less alloantibody 14 days post-transplant (28.8±12.1%; p=0.040); maximal alloantibody production also occurred on day 14 following transplant. Alloantibody levels in wild-type and CD8 KO recipients begin to progressively decline after days 14 and 21, respectively. Thus it appears that the difference in quantity of post-transplant alloantibody production in CD8-deficient and CD8-sufficient recipients is not attributable to differences in the kinetics of alloantibody production.
It is also unclear if the presence of CD8+ T cells rapidly kill the hepatocytes, thereby eliminating the source of alloantigen required for generation of the alloantibody response. Upon analyzing recipient serum hA1AT levels to measure allogeneic hepatocellular graft survival, we found that there is no statistically significant difference in hA1AT levels between our CD8 KO and wild-type recipients on day 7 following transplantation (Supplemental Figure 1A). However, to address this further, we have performed a second sequential hepatocyte transplant into wild-type recipients and measured subsequent alloantibody production compared to CD8-depleted WT recipients with only one hepatocyte transplant. Wild-type recipients that underwent a successive transplant on day 5 had superimposable serial hA1AT levels as a CD8-depleted wild-type mouse following a single transplant (Supplemental Figure 1B). Nevertheless, the same marked differences in serum alloantibody were noted in WT versus CD8-depleted WT recipients (Supplemental Figure 1C). These data support the contention that the differences in amounts or persistence of alloantigen does not contribute to the enhanced alloantibody response in CD8-deficient recipients. We next addressed the possibility that CD8+ T cells, through influence on the pro-inflammatory/inflammatory cytokine balance, skew the immune response away from humoral immunity.
Post-transplant alloantibody isotype analysis reveals an IgG1-dominant profile
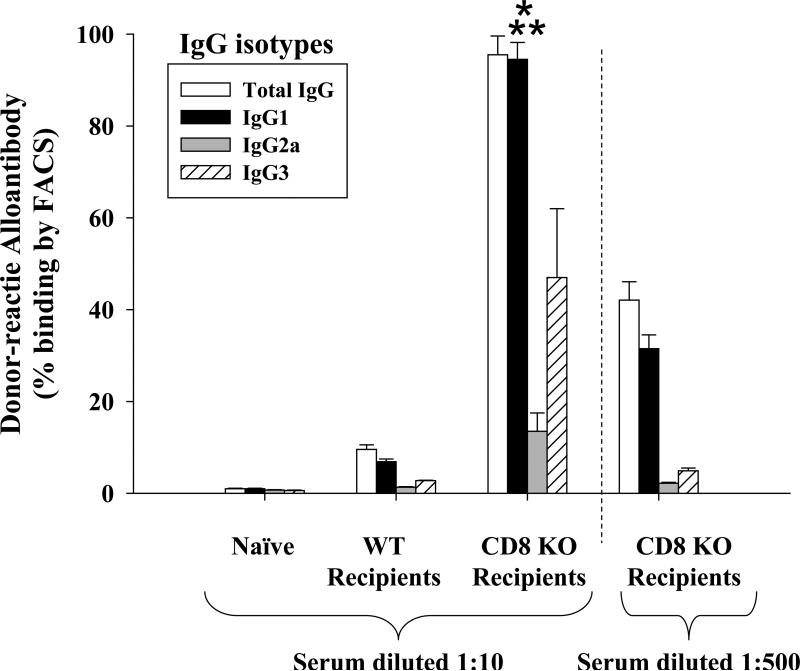
If CD8+ T cells influenced the amount of alloantibody produced by skewing towards a pro-inflammatory cytokine balance, then, in their absence, we would predict that a relatively anti-inflammatory Th2-type cytokine milieu would develop, driving the production of Th2-isotype dominant alloantibody responses. We next determined the isotypes of post-transplant alloantibodies in the presence or absence of CD8+ T cells. Serum total antibody was tested for alloantibody isotypes IgG1, IgG2a, IgG3 and IgE by ELISA. Serum from CD8 KO transplant recipients was found to have significant enhancement of IgG1 (5.07±0.71 fold; p<0.002) isotype, but not IgG2a, IgG3, or IgE as compared to naïve control mice. Wild-type transplant recipients also exhibited elevated IgG1 levels (1.8±0.5 fold; p=0.020), but to a significantly lesser degree than CD8 KO recipients (Supplemental Figure 2; p<0.0001). To determine the post-transplant antibody allospecificity, we performed a flow cytometric analysis using allogeneic target splenocytes. Serum from wild-type and CD8 KO transplant recipient mice was diluted (1:10 or 1:500) and incubated with splenocytes harvested from allogeneic FVB/N mice. Naïve serum from wild-type and CD8 KO mice served as negative controls. The binding percentage of total IgG and IgG isotypes including IgG1, IgG2a, and IgG3 to allogeneic splenocytes was measured using FITC labeled secondary antibodies. As previously noted, there was an increase in total alloantibody in the serum from CD8 KO transplant recipients. As predicted, there was a dominant antibody isotype which was allospecifc (IgG1 subtype) and significantly enhanced in amount in CD8-deficient recipients (94.5±3.7%) compared to serum from both naïve (1.0±0.1%) and wild-type recipient mice (6.9±0.6%) at the 1:10 dilution; (p<0.0001 for both comparisons; Figure 2). Wild-type recipient serum also showed subtle, but significant levels of IgG1 as compared to naïve mice (6.9±0.6%; p=0.0001). IgG2a and IgG3 subtypes in serum from either wild-type or CD8 KO recipients did not show significant binding to allogeneic targets. This preference for an IgG1-dominant alloantibody profile, following CD8-depletion, has been verified in other transplant strain combinations (i.e., FVB/N recipients of C57BL/6 hepatocytes; data not shown). Although IgG1 antibody isotype is the dominant alloantibody produced in both CD8-deficient and wild-type recipients, the overall amount of IgG1 alloantibody is significantly enhanced in CD8-deficient mice following transplantation. IgG1 production is predominantly regulated by IL-4 cytokine production which is the hallmark of a Th2 immune response (28). Therefore, it is possible that CD8+ T cells regulate IgG1 production by influencing the relative magnitude of Th2 cytokine production in recipient mice. We next investigated cytokine production by CD4+ T cells in CD8-sufficient and CD8-deficient recipients.
Figure 2. Post-transplant alloantibody isotype analysis reveals an IgG1-dominant profile.
Serum from naïve and recipient mice was collected on post-transplant day 14 and allospecific antibody levels were measured by flow cytometry, as previously described. Total IgG levels are significantly higher in CD8 KO recipients as compared to wild-type recipients and naïve control (p<0.05). Wild-type recipient serum (diluted 1:10) showed elevated levels of IgG1 (6.9±0.6%) which account for a majority of the allospecific IgG. CD8 KO recipient serum (diluted 1:10) also showed enhanced allospecific IgG1 antibody isotype (94.5±3.7%) compared to serum from both naïve (1.0±0.1%) and wild-type recipient mice (6.9±0.6%; p<0.0001 for both comparisons, as denoted by “*” and “**”, respectively). Diluted serum (1:500) from CD8 KO recipients also showed that elevated levels of IgG1 (31.5±3.2%) accounts for the majority of the allospecific IgG. IgG2a and IgG3 subtypes did not show significant allospecificity from either wild-type or CD8 KO recipients. The graph depicts representative data of 3 experiments. Standard deviations were calculated by 3 samples per experiment.
CD4+ T cells switch from an IFN-γ-dominant to an IL-4-dominant cytokine expression in the absence of CD8+ T cells
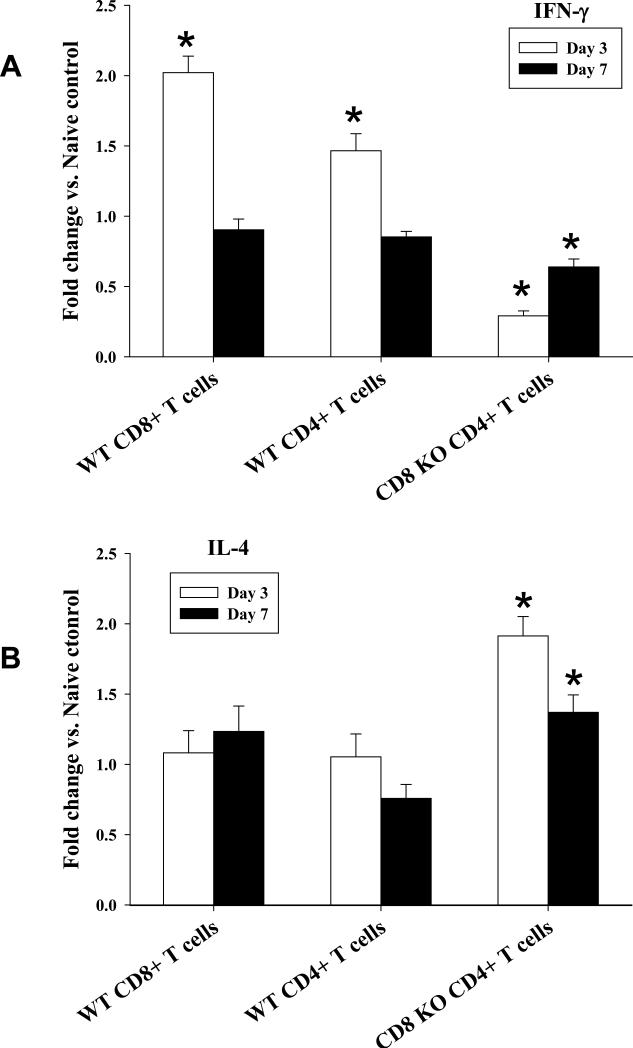
To examine the expression of IFN-γ and IL-4 within T cells in transplant recipient mice, splenocytes were isolated from CD8 KO and wild-type recipients on day 3 and 7 following transplant. CD4+ and CD8+ T cells were isolated from recipients splenocytes using negative selection columns and purity was confirmed to be >90% by FACS analysis. Phenol/chloroform extraction was utilized to harvest mRNA followed by reverse transcription to cDNA. IFN-γ and IL-4 mRNA was then quantified using real-time PCR and data is given as fold increase as compared to naïve control. On day 3 following transplantation, IFN-γ mRNA derived from wild-type recipients was shown to be significantly upregulated in CD8+ T cells (2.0±0.1 fold, p<0.0001) and to a lesser degree in CD4+ T cells (1.5±0.1 fold, p=0.0020; Figure 3A). Conversely, CD4+ T cells from CD8 KO recipients demonstrated downregulation of IFN-γ (0.3±0.01 fold, p=0.0004). IL-4 expression in CD4+ T cells isolated from wild-type recipients did not significantly change as compared to naïve controls. However, in the absence of CD8+ T cells, CD4+ T cells from CD8 KO recipients exhibited significantly enhanced levels of IL-4 mRNA as compared to naïve control (1.9±0.1 fold, p=0.0005; Figure 3B). This demonstrates that the cytokine profile of CD4+ T cells shifts towards IL-4 expression in the absence of CD8+ T cells. On day 7 posttransplant, there is evidence of a reversion to baseline for both IFN-γ and IL-4. Similar results were demonstrated when evaluating whole splenocytes from wild-type and CD8 KO recipient mice (Supplemental Figure 3).
Figure 3. CD4+ T cells switch from an IFN-γ-dominant to an IL-4-dominant cytokine expression in the absence of CD8+ T cells.
Splenocytes were isolated from CD8 KO and wild-type recipient mice on day 3 and 7 post-transplant. Total RNA was then purified and cDNA for IFN-γ and IL-4 was amplified utilizing real-time PCR. CD4+ and CD8+ T cells were isolated and purified from splenocytes using negative selection columns A) IFN-γ mRNA derived from wild-type recipients was significantly upregulated in CD8+ T cells (2.0±0.1 fold, p<0.0001) and in CD4+ T cells (1.5±0.1 fold, p=0.0020). Conversely, CD4+ T cells from CD8 KO recipients demonstrated downregulation of IFN-γ (0.3±0.01 fold, p=0.0004). B) IL-4 mRNA was significantly upregulated in CD4+ T cells isolated from CD8 KO (1.9±0.1 fold; p=0.0005) but not in wild-type recipients. Data were expressed as the mean fold increase relative to cells collected form naïve control mice. All real-time PCR data were normalized to the level of mouse β-actin mRNA. Error bars denote the standard deviation of duplicate experiments (triplicate wells/sample). Significant upregulation or downregulation is denoted by “*”.
IL-4 producing CD4+ T cells critically regulate production of allospecific antibody post-transplant
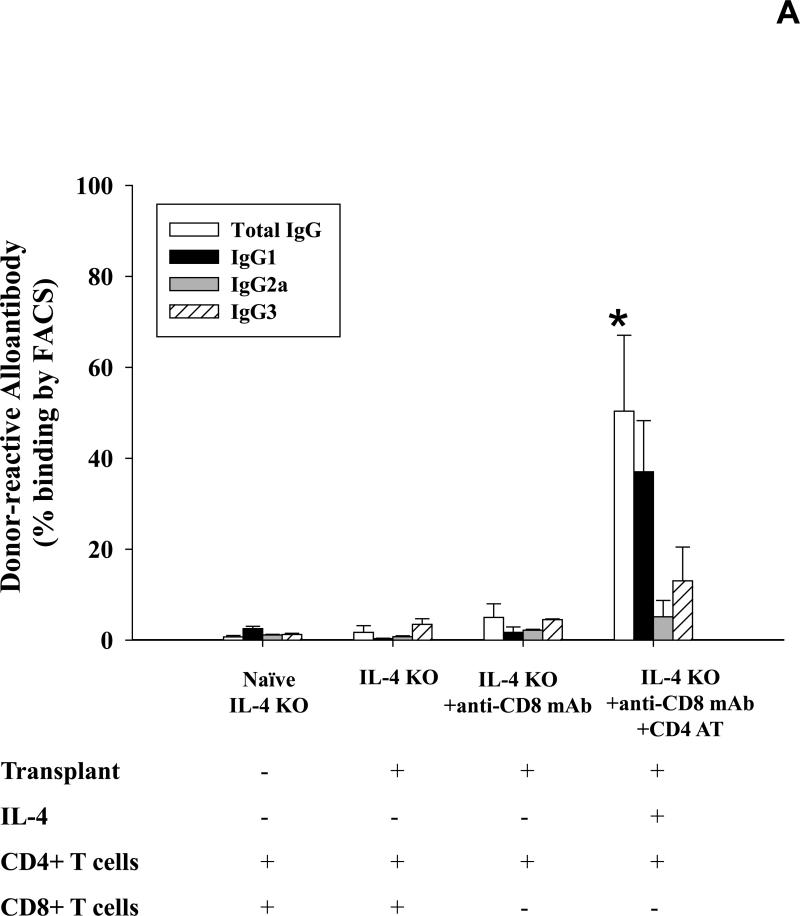
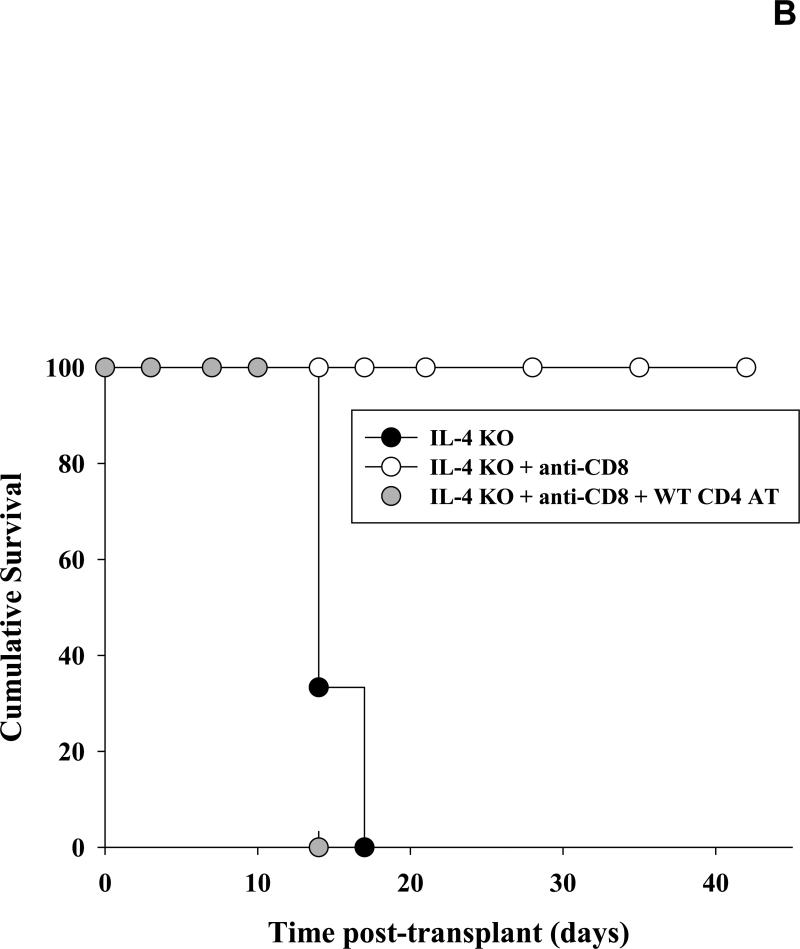
To further determine the role of IL-4 cytokine in alloantibody production we analyzed serum alloantibody levels in IL-4 KO recipients on day 14 following transplantation. Allospecific binding by total IgG and isotypes IgG1, IgG2a and IgG3 was again measured using flow cytometry. Post-transplant alloantibody (total IgG) production was abrogated in IL-4 KO recipients (1.7±1.5%; Figure 4A) when compared to serum alloantibody levels detected in wild-type recipient mice (9.7±6.2%; p=0.0025; Figure 1). CD8+ T cell depletion (100 μg, days -1, -2) enhanced alloantibody production in IL-4 KO recipients (5.0±3.0%; p=0.013), but not to the extent that it did in CD8-depleted wild-type recipients (76.2±14.3%; Figure 1) suggesting that IL-4 is a critical cytokine which drives alloantibody production following transplantation. To verify that IL-4 KO mice have allo-responsive B cells, IL-4 KO mice were adoptively transferred with wild-type (IL-4 sufficient) CD4+ T cells prior to transplantation. Adoptive transfer of CD4+ T cells significantly upregulated alloantibody production in CD8-depleted IL-4 KO mice (50.4±16.7% vs. 5.0±3.0; p<0.0001, Figure 4A). Without CD8-depletion, the adoptive transfer of CD4+ T cells stimulates marginal levels of alloantibody production (8.1±1.7%; data not shown) demonstrating that alloantibody production in IL-4 KO mice is also regulated by CD8+ T cells. As expected, IgG1 remained the dominant post-transplant alloantibody isotype following the adoptive transfer of CD4+ T cells. This data suggests that IL-4-producing CD4+ T cells are critical for the development of alloantibody production post-transplant in this model. Interestingly, IL-4 KO mice that are CD8-depleted have markedly enhanced hepatocyte survival (all survive beyond 42 days) when compared to IL-4 KO mice or IL-4 KO mice adoptively transferred with wild-type CD4+ T cells (Figure 4B). This is likely due to the elimination of both CD8+ T cell-mediated and CD4-dependent antibody-mediated rejection mechanisms. This transplant outcome is notable since in all other experimental conditions performed in this study (i.e., wild type, CD8 KO, and IFN-γ KO recipients), transplanted hepatocytes have a median survival time of 10 to 14 days post-transplant. The results also imply that in the absence of CD8+ T cells, CD4-dependent hepatocyte rejection is mediated entirely by alloantibody and/or IL-4.
Figure 4. IL-4 producing CD4+ T cells critically regulate production of allospecific antibody post-transplant.
A) Serum from IL-4 KO recipients was collected 14 days following transplantation and analyzed for allospecific IgG alloantibody isotypes IgG1, IgG2a, and IgG3 using flow cytometry. Alloantibody production in IL-4 KO recipients was abrogated (1.7±1.5%, n=6) as compared to wild-type recipient mice (9.7±6.2%; p=0.0025; Figure 1). CD8+ T cell depletion enhanced alloantibody production in IL-4 KO recipients (5.0±3.0%, n=6; p=0.013) but not to the extent that it did in CD8-depleted wild-type recipients (76.2±14.3%; Figure 1). Adoptive transfer of wild type CD4+ T cells (n=5) resulted in significant upregulation of alloantibody production in CD8-depleted IL-4 KO recipient mice (50.4±16.7% vs. 5.0±3.0; p<0.0001, as denoted by “*”). B) To analyze hepatocyte survival, cohorts of IL-4 KO recipient mice were untreated (n=6), CD8-depleted (n=6), or both CD8-depleted and adoptively transferred with 10×106 wild-type CD4+ T cells (n=5). Hepatocyte grafts were monitored for survival by serum hA1AT levels. IL-4 KO recipients that were untreated or were adoptively transferred with wild-type CD4+ T cells (and CD8-depleted) rejected their transplants with median survival time of 14 days. However, IL-4 KO recipients that were only CD8-depleted exhibited significantly enhanced graft survival, with 100% cumulative survival 42 days post-transplant (p<0.0001).
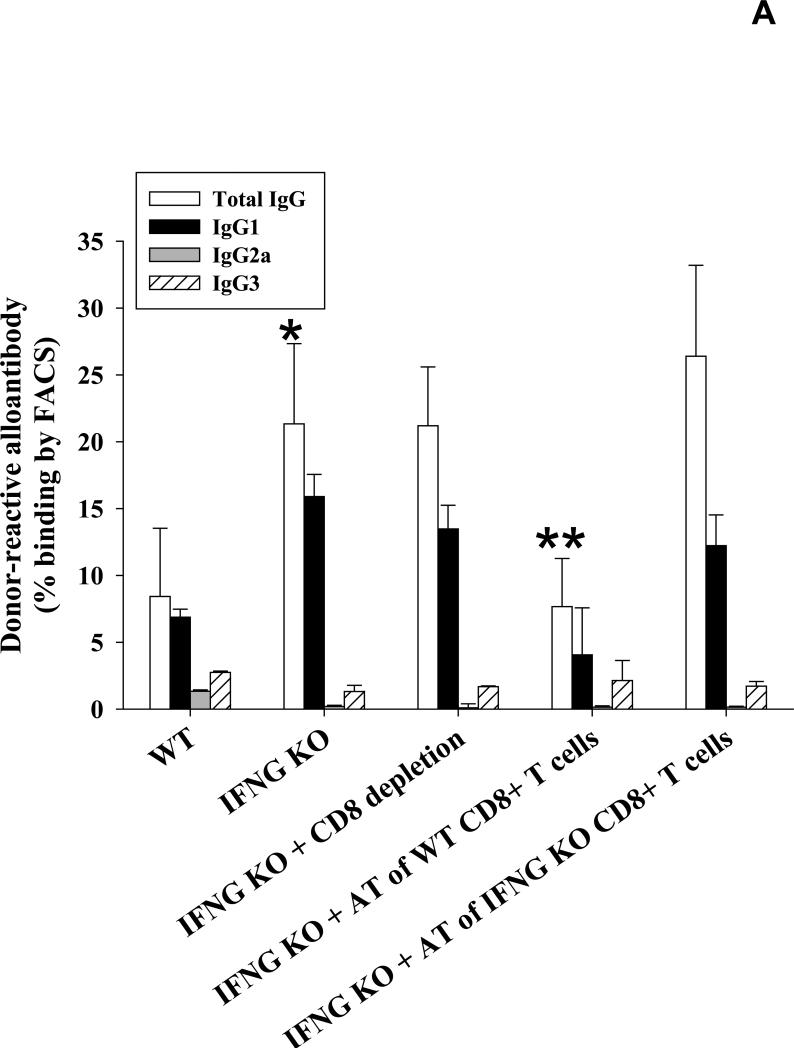
IFN-γ positive CD8+ T cells suppress post-transplant IgG1-dominant alloantibody production in IFN-γ KO recipients
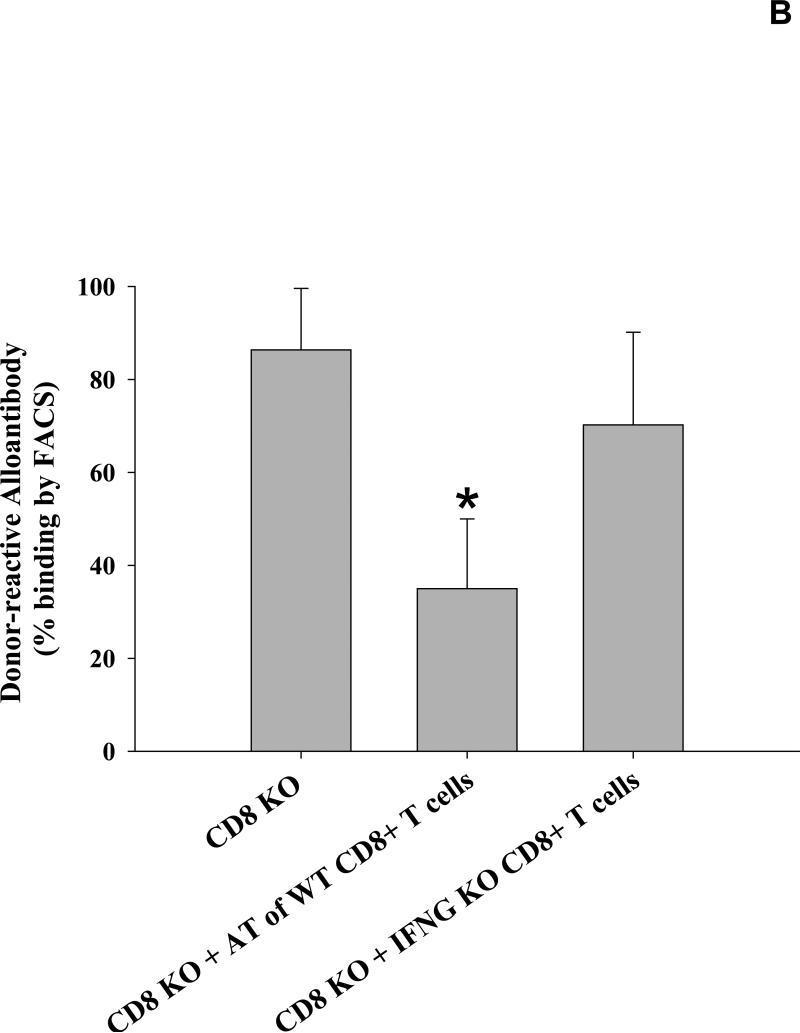
We have previously published that post-transplant alloantibody levels are increased in IFN-γ KO recipients (21.3±6.0%; p=0.01) (12), though the reason for these results were not clear. Upon further investigation, IgG isotypes were largely skewed towards IgG1 with negligible levels of IgG2a in these IFN-γ KO recipients (Figure 5A). In light of the current findings, we postulated that the relatively suppressed pro-inflammatory cytokine milieu in these recipients led to conditions favoring humoral immunity. CD8-depletion in IFN-γ KO recipients did not further enhance alloantibody production as compared to untreated IFN-γ KO recipients (21.2±4.4% vs. 21.3±6.0%, respectively; p=ns). We concluded that this was likely due to the absence of IFN-γ producing CD8+ T cells. To investigate this hypothesis, we adoptively transferred wild-type (IFN-γ-sufficient) CD8+ T cells into IFN-γ KO recipients to restore a (CD8-mediated) pro-inflammatory cytokine dominance which we suspected was necessary to suppress post-transplant alloantibody production. As predicted, the adoptive transfer of wild-type CD8+ T cells (10×106 cells, day 0 with respect to transplantation) resulted in significant inhibition of post-transplant alloantibody production as compared to that of IFN-γ KO recipients (7.7±3.6% vs. 21.3±6.0%; p=0.0001). In contrast, the adoptive transfer of IFN-γ KO CD8+ T cells into IFN-γ KO recipients did not significantly affect alloantibody production (26.4±6.8%; p=ns). Furthermore, the adoptive transfer of IFN-γ KO CD8+ T cells into CD8 KO mice did not reduce alloantibody production when compared to alloantibody levels in CD8 KO mice (70.2±19.9% vs. 86.4±13.2%, respectively; p=ns, Figure 5B) unlike results we observed when wild type CD8+ T cells were transferred (27.0±11.1%, Figure 1A). These data suggest that IFN-γ is essential for CD8+ T cell-mediated regulation of post-transplant alloantibody production.
Figure 5. IFN-γ positive CD8+ T cells suppress post-transplant IgG1-dominant alloantibody production in IFN-γ KO recipients.
A) To determine whether recipient IFN-γ critically influences alloantibody production post-transplant, wild-type (H-2b) and IFN-γ KO mice (H-2b) were tested for the production of allo-specific antibodies 14 days following the transplantation of allogeneic FVB/N (H-2q) hepatocytes. Allospecific binding of total IgG as well as isotypes IgG1, IgG2a and IgG3 was measured by flow cytometry. IFN-γ KO recipients had increased alloantibody levels compared to wild-type recipient mice (21.3±6.0%, n=8; p=0.01, as denoted by “*”). CD8-depletion of IFN-γ KO recipients did not further enhance the level of alloantibody production (21.2±4.4%, n=5; p=ns). The adoptive transfer of 10×106 wild-type CD8+ T cells into IFN-γ KO mice on day 0 (day of transplant, n=6) significantly suppressed post-transplant alloantibody production (7.7±3.6% vs. 21.3±6.0%; p=0.0001, as denoted by “**”). Similar to wild-type recipients, IgG1 is the dominant alloantibody produced in IFN-γ KO recipients. The adoptive transfer of IFN-γ KO CD8+ T cells into IFN-γ KO recipients did not significantly affect alloantibody production (26.4±6.8%, n=5; p=ns). B) Additionally, the adoptive transfer of IFN-γ KO CD8+ T cells into CD8 KO recipient mice (n=5) did not reduce alloantibody production as compared to untreated CD8 KO recipient mice (70.2±19.9% vs. 86.4±13.2%, respectively, p=ns).
Discussion
Both clinical and experimental studies highlight the barrier that alloantibody-mediated acute and chronic rejection pose to successful allograft survival (3-6, 29, 30). Acute antibody-mediated rejection occurs despite the use of maintenance immunosuppression to prevent rejection and is associated with worse graft outcome than T cell-mediated rejection (9). Many current immunosuppressive treatments effectively inhibit alloreactive T cell responses. Therefore, it is somewhat surprising that de novo acute antibody-mediated rejection occurs in the setting of effective regulation of CD4+ T cells known to be critical to antibody production. We and others have previously reported that post-transplant production of alloantibody is markedly enhanced in the absence of CD8+ T cells (12, 16, 17, 31). Thus it is possible that immunosuppressive agents or other conditions which impair or deplete CD8+ T cell function might promote alloantibody production post-transplant. The current studies investigate the novel hypothesis that CD8+ T cells regulate the amount and isotype of alloantibody produced after transplant by modulating the cytokine phenotype of CD4+ T cells.
We have previously shown that in wild-type recipients, CD8+ T cell-mediated rejection is dominant and humoral immunity is negligible after hepatocyte transplant (32). Activated CD8+ T cells can produce high levels of pro-inflammatory cytokines including IFN-γ which has been shown to be essential in CD8+ T cell-mediated rejection (32, 33). Cytokines are also important to humoral immunity; it is well established that IFN-γ and IL-4 cytokines mediate antibody class switching, with IFN-γ promoting IgG2a and IL-4 promoting IgG1/IgE production (34). Yet, we do not detect significant levels of alloantibody in the setting of hepatocyte rejection in wild-type recipients across multiple strain combinations (unpublished results). Interestingly, in the absence of IFN-γ, we have noted that IgG1 alloantibody levels are significantly enhanced (32). Based on our observations that deficiency of CD8+ T cells or the cytokine IFN-γ are independently associated with enhanced alloantibody, we postulated that CD8+ T cell-mediated regulation of post-transplant alloantibody production might be IFN-γ-dependent. If so, adoptive transfer of wild-type, but not IFN-γ KO, CD8+ T cells into IFN-γ KO and CD8 KO recipients should suppress alloantibody production in these recipients. Indeed, this is what we found (Figure 5A and 5B). We are aware that CD8+ T cells can be induced to produce IL-4 under conditions that promote Th2 cytokine responses (35, 36). However, if this was a significant contribution to alloantibody production in our model, we would have expected a significant increase in alloantibody production when IFN-γ-/- CD8+ T cells were transferred into IFN-γ-/- recipients. This did not occur indicating that CD8+ T cell-mediated IL-4 production does not appear to be a major path under these conditions (Figure 5A).
Furthermore, we found that CD8+ (and to a lesser degree CD4+) T cells have an IFN-γ-dominant cytokine profile in wild-type recipients which is consistent with our hypothesis that IFN-γ producing CD8+ T cells downregulate alloantibody production. Although IFN-γ is known to drive IgG2a antibody responses in mice, we only detected low levels of IgG2a in wild-type recipient serum. Since C57BL/6 mice have a predominant Th1 response, it is surprising that IgG2a alloantibody is not produced in these mice following transplantation. However, the absence of IgG2a, a complement fixing antibody isotype, is consistent with our previous results demonstrating that complement is not required for antibody-mediated hepatocyte rejection or in vivo allocytotoxicity (20) and that complement deposition is not observed histologically (data not shown). In contrast, in CD8-deficient recipients, high levels of alloantibody are produced and we found that IgG1 is the predominant allospecific antibody isotype. Since it is known that IL-4 is the signature cytokine responsible for IgG1 production as well as the hallmark cytokine necessary to drive a Th2 immune response (37, 38), we investigated the cytokine profile of CD4+ T cells. We revealed the novel finding that the cytokine profile of CD4+ T cells switches, from an IFN-γ to an IL-4-dominant profile in the absence of CD8+ T cells. This suggests that the production of IFN-γ by CD8+ T cells drives the post-transplant immune response towards an IFN-γ pro-inflammatory cytokine profile and suppression of an IL-4 anti-inflammatory cytokine profile which influences the amount and type of alloantibody produced post-transplant. This scenario is congruent with the known antagonizing role of IFN-γ in the regulation of IL-4. This IFN-γ-mediated control exists at several levels of regulation including suppression of Th2 CD4+ T cells differentiation and/or inhibition of IL-4 signal transduction (39, 40). In our transplant model, IFN-γ producing CD8+ T cells might similarly suppress CD4+ T cell differentiation, IL-4 production, and/or IL-4 signaling resulting in the downregulation of IgG1 alloantibody levels post-transplant. In the absence of IFN-γ producing CD8+ T cells, CD4+ T cells express an anti-inflammatory, IL-4-dominant cytokine profile which drives IgG1 alloantibody production. In the absence of IL-4, no alloantibody is detected post-transplant. Interestingly, these CD8-depleted IL-4 KO recipients have markedly enhanced graft survival, despite the presence of CD4+ T cells. These results are consistent with previous reports in our laboratory by Horne et al. showing enhanced graft survival in CD8-depleted B cell KO mice suggesting that CD4-mediated graft rejection in this model is primarily antibody-mediated (12). However, high levels of alloantibody production in IL-4 KO hepatocyte recipients can be triggered by recipient depletion of CD8+ T cells and adoptive transfer of wild-type CD4+ T cells and is correlated with prompt hepatocellular graft rejection.
Specific cytokine producing CD8+ T cells are also reported to critically influence the development of other antibody responses in vivo. For example, Sanchez et al. reported enhanced levels of IgE antibody, which is IL-4-mediated, in ovalbumin immunized CD8-deficient mice (41). Further studies determined that the regulation of antigen specific IgE production by CD8+ T cells was in part due to IFN-γ and IL-12 (18). However, this group concluded that CD8-derived IFN-γ is not essential for regulation of alloantibody because wild-type CD8+ T cells adoptively transferred into IFN-γ KO OVA immunized mice failed to suppress IgE antibody levels. Furthermore, OVA specific IgG1 levels in wild-type mice were unaffected by the adoptive transfer of antigen specific CD8+ T cells. Thomas et al. proposed that CD8+ T cell regulation of antibody was isotype specific to IgE production (18). These results differ from our studies which show that the adoptive transfer of wild-type CD8+ T cells into IFN-γ KO recipients significantly suppresses IgG1 alloantibody levels to the low levels observed in wild-type recipients. This difference might be explained by differences in immune responses to nominal peptide antigen versus cellular alloantigen. Although Thomas et al. have demonstrated that substantial levels of allospecific IgE antibody exist on day 14 following OVA/alum immunization, in our studies at comparable time points IgE was not detectable by ELISA in wild-type or CD8-deficient mice following hepatocyte transplantation (18). Thus, although both of these antibody isotypes appear to be regulated by CD8+ T cells and are dependent on IL-4 cytokine signals, there are many other factors (number of B cell divisions, concentration of IL-4, etc.) which determine commitment towards the production of IgG1 or IgE isotypes (42, 43). Compartmentalization of transplanted hepatocytes to the liver likely also impacts the immune responses observed as we have previously published that the site of hepatocyte engraftment influences the cellular versus humoral dominance of alloimmune responses (44 and unpublished data).
The precise phenotype of IFN-γ+ allo-activated CD8+ T cells and effector mechanism(s) by which they regulate alloreactive CD4+ T cell cytokine phenotype and subsequent alloantibody quantity and isotype remain to be determined. CD8+ T cells may act directly on CD4+ T cells and/or B cells to inhibit IL-4 production and IgG1 antibody production, respectively. Alternatively, it is possible that CD8+ T cells could indirectly produce these effects through interaction with other cell subsets. It has been reported that subsets of activated CD8+ T cells upregulate IL-10 and TGF-β, which are able to induce CD4+ T regulatory cells and/or Th17 cells which could influence B cell production of antibody (45-52). In other models there is evidence that CD8+ T cells can induce dendritic cells (DCs) to secrete IL-12 which regulates IgE levels in vivo (18) by promoting the generation of IFN-γ-producing cells that inhibit the generation of Th2 cells and IgE class switching (18, 53). Yoshimoto et al. also suggested that B cells, in response to stimulation from IL-12/IL-18, might produce IFN-γ as a potential autocrine cytokine to inhibit IgG1/IgE production (54).
Perhaps CD8+ T cells directly regulate antibody production by impacting alloprimed B cell survival. Precedence for (CD25+) T cell-mediated apoptosis of autoreactive B cells exists in models of peripheral tolerance (55). If a similar mechanism were operative in our model we would expect to see more alloprimed B cells in CD8 KO recipients. Results to date showing similar numbers of IgG1+ B cells in WT and CD8 KO recipients on days 5 and 7 but markedly increased numbers in CD8 KO recipients by day 11 demonstrate that B cells appear to have a greater propensity for proliferation and/or survival in CD8-deficient recipient mice as compared to wild-type recipient mice (unpublished results). Further studies are necessary to determine if CD8+ T cells regulate post-transplant alloantibody production through direct or indirect effects on CD4+ T cells, dendritic cells, and/or B cells.
In summary, our in vivo studies provide first evidence in that in the absence of CD8+ T cells, a distinct cytokine milieu emerges which is IL-4-dominant; whereas, in wild-type recipients the cytokine milieu is IFN-γ-dominant. CD8+ T cells influence the proinflammatory/anti-inflammatory cytokine phenotype of CD4+ T cells that correlates with the level of the subsequent IgG1-dominant alloantibody isotype and quantity of alloantibody produced. Further elucidation of the mechanisms by which CD8+ T cells regulate the amount and isotype of post-transplant alloantibody will be important to the development of immunotherapeutic strategies to suppress alloantibody production and humoral-mediated allograft damage post-transplant. Our findings may also have relevance to in vivo regulation of antibody production in response to autoimmune or infectious antigenic stimuli.
Supplementary Material
Supplemental Figure 1. Comparable antigen load in wild-type and CD8-deficient recipients. Wild-type and CD8 KO mice were transplanted with FVB/N hepatocytes. Serum hA1AT levels were serially analyzed to measure allogeneic hepatocellular graft survival. A) We found that there is no significant difference in hA1AT levels between our CD8 KO and wild-type recipients on day 7 following transplantation. B) Wild-type recipients that underwent a successive transplant on day 5 had similar hA1AT levels as a CD8-depleted wild-type mouse following a single transplant. C) However, this increased antigen exposure did not stimulate significantly enhanced alloantibody production in wild-type recipients.
Supplemental Figure 2. CD8-deficient mice exhibit an IgG1 alloantibody response to transplantation. The serum from wild-type transplant recipient and CD8 KO transplant recipient mice was collected 14 days after hepatocyte transplantation and tested for total IgG antibody isotypes IgG1, IgG2a, IgG3, and IgE using ELISA. Fold measurements of the total IgG isotypes were quantified and compared to the serum from naïve control mice. Serum from wild-type transplant recipient mice contained significant levels of IgG1 alloantibody isotype as compared to naïve control serum (1.8±0.5 fold; p=0.020, as denoted by “*”). Serum from CD8 KO transplant recipient mice had a significant increase of the IgG1 alloantibody isotype (5.0±0.7 fold) compared to both serum from naïve and transplanted wild-type mice (p<0.002 for both comparisons, as denoted by “*” and “**”, respectively). Serum from wild-type and CD8 KO recipients did not show significant levels of IgG2a, IgG3, or IgE. The graph depicts representative data of duplicate experiments. Standard deviations were calculated by 2 samples per experiments (triplicate wells/sample).
Supplemental Figure 3. IL-4 mRNA is upregulated in CD8 KO mice following transplantation. To examine the mRNA expression of IFN-γ and IL-4 in wild-type and CD8-deficient mice following hepatocyte transplantation, we performed semi-quantitative real-time PCR of recipient splenocytes. Splenocytes were harvested from recipient mice on day 5 and 7 post-transplant. A) IFN-γ mRNA was significantly upregulated in wild-type C57BL/6 recipients on day 5 and 7 (1.3±0.1 and 1.7±0.2 fold, respectively; p<0.05 for both days). CD8 KO recipients did not have a significant change in IFN-γ expression as compared to naïve control. B) IL-4 mRNA was significantly upregulated in CD8 KO recipients as compared to naïve control on day 5 and 7 (2.0±0.1 and 1.9±0.4 fold, respectively; p<0.03 for both days). These results were compared to wild-type recipient mice which have significantly decreased levels of IL-4 on post transplant days 5 and 7 (0.7±0.1 and 0.3±0.1 fold, respectively; p<0.05 for both). Significant upregulation or downregulation is denoted by “*”. Data were expressed as the mean fold increase relative to cells collected form naïve control mice. All real-time PCR data were normalized to the level of mouse β-actin mRNA. Error bars denote the standard deviation of duplicate experiments (triplicate wells/sample).
Acknowledgements
We would like to thank Scot S. Erbe (Ohio State University) for informative discussions and assistance with the antibody isotype ELISA experimental protocol and analysis. We would also like to thank Leslie E. King (Ohio State University; Samuel J. Roessler Memorial Medical Scholar) for assistance with performance of the ELISAs.
Support: This work was supported in part by grants from the Roche Organ Transplantation Research Foundation (to G.L.B.), the ASTS-NKF (National Kidney Foundation) Folkert Belzer, MD, Research Award (to T.A.P.), and National Institutes of Health grants DK072262 (to G.L.B.), and F32 DK082148 (NIDDK; to J.M.Z.).. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Nonstandard Abbreviations
- AT
Adoptive transfer
- hA1AT
human alpha-1 antitrypsin
Footnotes
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
References
- 1.Ponticelli C. Progression of renal damage in chronic rejection. Kidney Int Suppl. 2000;75:S62–70. [PubMed] [Google Scholar]
- 2.Tilney NL, Paul LC. Antigen-independent events leading to chronic graft dysfunction. In: Tilney NL, Strom TB, Paul LC, editors. Transplantation biology: cellular and molecular aspects. Lippincott-Raven; Philadelphia: 1996. pp. 629–637. [Google Scholar]
- 3.Terasaki PI, Cai J. Humoral theory of transplantation: further evidence. Curr Opin Immunol. 2005;17:541–545. doi: 10.1016/j.coi.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Moll S, Pascual M. Humoral rejection of organ allografts. Am J Transplant. 2005;5:2611–2618. doi: 10.1111/j.1600-6143.2005.01086.x. [DOI] [PubMed] [Google Scholar]
- 5.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5:807–817. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu A, Colvin RB. Pathological features of antibody-mediated rejection. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:199–214. doi: 10.2174/1568006054064744. [DOI] [PubMed] [Google Scholar]
- 7.Trpkov K, Campbell P, Pazderka F, Cockfield S, Solez K, Halloran PF. Pathologic features of acute renal allograft rejection associated with donor-specific antibody, Analysis using the Banff grading schema. Transplantation. 1996;61:1586–1592. doi: 10.1097/00007890-199606150-00007. [DOI] [PubMed] [Google Scholar]
- 8.McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000;69:319–326. doi: 10.1097/00007890-200002150-00001. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz M, Regele H, Schillinger M, Exner M, Rasoul-Rockenschaub S, Wahrmann M, Kletzmayr J, Silberhumer G, Horl WH, Bohmig GA. Risk factors for capillary C4d deposition in kidney allografts: evaluation of a large study cohort. Transplantation. 2004;78:447–452. doi: 10.1097/01.tp.0000128344.94808.03. [DOI] [PubMed] [Google Scholar]
- 10.Terasaki P, Lachmann N, Cai J. Summary of the effect of de novo HLA antibodies on chronic kidney graft failure. Clin Transpl. 2006:455–462. [PubMed] [Google Scholar]
- 11.Terasaki PI, Cai J. Human leukocyte antigen antibodies and chronic rejection: from association to causation. Transplantation. 2008;86:377–383. doi: 10.1097/TP.0b013e31817c4cb8. [DOI] [PubMed] [Google Scholar]
- 12.Horne PH, Lunsford KE, Eiring AM, Wang Y, Gao D, Bumgardner GL. CD4+ T-Cell-Dependent Immune Damage of Liver Parenchymal Cells Is Mediated by Alloantibody. Transplantation. 2005;80:514–521. doi: 10.1097/01.tp.0000168342.57948.68. [DOI] [PubMed] [Google Scholar]
- 13.Coutelier JP. Enhancement of IgG production elicited in mice by treatment with anti-CD8 antibody. Eur J Immunol. 1991;21:2617–2620. doi: 10.1002/eji.1830211046. [DOI] [PubMed] [Google Scholar]
- 14.Byrom B, Barbet AF, Obwolo M, Mahan SM. CD8(+) T cell knockout mice are less susceptible to Cowdria ruminantium infection than athymic, CD4(+) T cell knockout, and normal C57BL/6 mice. Veterinary parasitology. 2000;93:159–172. doi: 10.1016/s0304-4017(00)00336-8. [DOI] [PubMed] [Google Scholar]
- 15.Sayeh E, Sterling K, Speck E, Freedman J, Semple JW. IgG antiplatelet immunity is dependent on an early innate natural killer cell-derived interferon-gamma response that is regulated by CD8+ T cells. Blood. 2004;103:2705–2709. doi: 10.1182/blood-2003-10-3552. Epub 2003 Dec 2704. [DOI] [PubMed] [Google Scholar]
- 16.Chan SY, DeBruyne LA, Goodman RE, Eichwald EJ, Bishop DK. In vivo depletion of CD8+ T cells results in Th2 cytokine production and alternate mechanisms of allograft rejection. Transplantation. 1995;59:1155–1161. [PubMed] [Google Scholar]
- 17.Ensminger SM, Spriewald BM, Sorensen HV, Witzke O, Flashman EG, Bushell A, Morris PJ, Rose ML, Rahemtulla A, Wood KJ. Critical role for IL-4 in the development of transplant arteriosclerosis in the absence of CD40-CD154 costimulation. J Immunol. 2001;167:532–541. doi: 10.4049/jimmunol.167.1.532. [DOI] [PubMed] [Google Scholar]
- 18.Thomas MJ, Noble A, Sawicka E, Askenase PW, Kemeny DM. CD8 T cells inhibit IgE via dendritic cell IL-12 induction that promotes Th1 T cell counter-regulation. J Immunol. 2002;168:216–223. doi: 10.4049/jimmunol.168.1.216. [DOI] [PubMed] [Google Scholar]
- 19.Salagianni M, Wong KL, Thomas MJ, Noble A, Kemeny DM. An essential role for IL-18 in CD8 T cell-mediated suppression of IgE responses. J Immunol. 2007;178:4771–4778. doi: 10.4049/jimmunol.178.8.4771. [DOI] [PubMed] [Google Scholar]
- 20.Horne PH, Zimmerer JM, Fisher MG, Lunsford KE, Nadasdy G, Nadasdy T, van Rooijen N, Bumgardner GL. Critical role of effector macrophages in mediating CD4-dependent alloimmune injury of transplanted liver parenchymal cells. J Immunol. 2008;181:1224–1231. doi: 10.4049/jimmunol.181.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 22.Diamond AS, Gill RG. An essential contribution by IFN-gamma to CD8+ T cell-mediated rejection of pancreatic islet allografts. The Journal of Immunology. 2000;165:247–255. doi: 10.4049/jimmunol.165.1.247. [DOI] [PubMed] [Google Scholar]
- 23.Hitoshi Y, Mita S, Tominaga A, Kikuchi Y, Sonoda E, Takatsu K, Watanabe Y. Interferon-gamma inhibits the proliferation but not the differentiation of murine B cells in response to IL-5. Int Immunol. 1989;1:185–190. doi: 10.1093/intimm/1.2.185. [DOI] [PubMed] [Google Scholar]
- 24.Young HA, Hardy KJ. Role of interferon-gamma in immune cell regulation. J Leukoc Biol. 1995;58:373–381. [PubMed] [Google Scholar]
- 25.Bumgardner GL, Heininger M, Li J, Xia D, Parker-Thornburg J, Ferguson RM, Orosz CG. A Functional Model of Hepatocyte Transplantation for in Vivo Immunologic Studies. Transplantation. 1998;65:53–61. doi: 10.1097/00007890-199801150-00011. [DOI] [PubMed] [Google Scholar]
- 26.Bumgardner GL, Orosz CG. Unusual patterns of alloimmunity evoked by allogeneic liver parenchymal cells. Immunological reviews. 2000;174:260–279. doi: 10.1034/j.1600-0528.2002.017409.x. [DOI] [PubMed] [Google Scholar]
- 27.Pongratz G, McAlees JW, Conrad DH, Erbe RS, Haas KM, Sanders VM. The level of IgE produced by a B cell is regulated by norepinephrine in a p38 MAPK- and CD23-dependent manner. J Immunol. 2006;177:2926–2938. doi: 10.4049/jimmunol.177.5.2926. [DOI] [PubMed] [Google Scholar]
- 28.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Chilton PM, Tanner MK, Huang Y, Schanie CL, Dy-Liacco M, Yan J, Ildstad ST. Humoral immunity is the dominant barrier for allogeneic bone marrow engraftment in sensitized recipients. Blood. 2006;108:3611–3619. doi: 10.1182/blood-2006-04-017467. Epub 2006 Aug 3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vongwiwatana A, Tasanarong A, Hidalgo LG, Halloran PF. The role of B cells and alloantibody in the host response to human organ allografts. Immunol Rev. 2003;196:197–218. doi: 10.1046/j.1600-065x.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 31.Ensminger SM, Spriewald BM, Witzke O, Morrison K, van Maurik A, Morris PJ, Rose ML, Wood KJ. Intragraft interleukin-4 mRNA expression after short-term CD154 blockade may trigger delayed development of transplant arteriosclerosis in the absence of CD8+ T cells. Transplantation. 2000;70:955–963. doi: 10.1097/00007890-200009270-00013. [DOI] [PubMed] [Google Scholar]
- 32.Horne PH, Koester MA, Jayashankar K, Lunsford KE, Dziema HL, Bumgardner GL. Disparate Primary and Secondary Allospecific CD8+ T Cell Cytolytic Effector Function in the Presence or Absence of Host CD4+ T Cells. J Immunol. 2007;179:80–88. doi: 10.4049/jimmunol.179.1.80. [DOI] [PubMed] [Google Scholar]
- 33.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 34.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science (New York, N.Y. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 35.Rodolfo M, Zilocchi C, Accornero P, Cappetti B, Arioli I, Colombo MP. IL-4-transduced tumor cell vaccine induces immunoregulatory type 2 CD8 T lymphocytes that cure lung metastases upon adoptive transfer. J Immunol. 1999;163:1923–1928. [PubMed] [Google Scholar]
- 36.Apte SH, Groves P, Olver S, Baz A, Doolan DL, Kelso A, Kienzle N. IFN-gamma inhibits IL-4-induced type 2 cytokine expression by CD8 T cells in vivo and modulates the anti-tumor response. J Immunol. 185:998–1004. doi: 10.4049/jimmunol.0903372. [DOI] [PubMed] [Google Scholar]
- 37.Cher DJ, Mosmann TR. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by Th1 clones. J Immunol. 1987;138:3688. [PubMed] [Google Scholar]
- 38.Nakamura T, Kamogawa Y, Bottomly K, Flavell RA. Polarization of IL-4- and IFN-gamma-producing CD4+ T cells following activation of naive CD4+ T cells. J Immunol. 1997;158:1085–1094. [PubMed] [Google Scholar]
- 39.Paludan SR. Interleukin-4 and interferon-gamma: the quintessence of a mutual antagonistic relationship. Scandinavian journal of immunology. 1998;48:459–468. doi: 10.1046/j.1365-3083.1998.00435.x. [DOI] [PubMed] [Google Scholar]
- 40.Venkataraman C, Leung S, Salvekar A, Mano H, Schindler U. Repression of IL-4-induced gene expression by IFN-gamma requires Stat1 activation. J Immunol. 1999;162:4053–4061. [PubMed] [Google Scholar]
- 41.Diaz-Sanchez D, Noble A, Staynov DZ, Lee TH, Kemeny DM. Elimination of IgE regulatory rat CD8+ T cells in vivo differentially modulates interleukin-4 and interferon-gamma but not interleukin-2 production by splenic T cells. Immunology. 1993;78:513–519. [PMC free article] [PubMed] [Google Scholar]
- 42.Hasbold J, Lyons AB, Kehry MR, Hodgkin PD. Cell division number regulates IgG1 and IgE switching of B cells following stimulation by CD40 ligand and IL-4. European journal of immunology. 1998;28:1040–1051. doi: 10.1002/(SICI)1521-4141(199803)28:03<1040::AID-IMMU1040>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 43.Snapper CM, Finkelman FD, Paul WE. Differential regulation of IgG1 and IgE synthesis by interleukin 4. The Journal of experimental medicine. 1988;167:183–196. doi: 10.1084/jem.167.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horne PH, Lunsford KE, Walker JP, Koester MA, Bumgardner GL. Recipient immune repertoire and engraftment site influence the immune pathway effecting acute hepatocellular allograft rejection. Cell Transplant. 2008;17:829–844. doi: 10.3727/096368908786516792. [DOI] [PubMed] [Google Scholar]
- 45.Endharti AT, Rifa IM, Shi Z, Fukuoka Y, Nakahara Y, Kawamoto Y, Takeda K, Isobe K, Suzuki H. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175:7093–7097. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 46.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. Journal of Experimental Medicine. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunological reviews. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Myers L, Croft M, Kwon BS, Mittler RS, Vella AT. Peptide-specific CD8 T regulatory cells use IFN-gamma to elaborate TGF-beta-based suppression. J Immunol. 2005;174:7625–7632. doi: 10.4049/jimmunol.174.12.7625. [DOI] [PubMed] [Google Scholar]
- 49.Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Pomie C, Menager-Marcq I, van Meerwijk JP. Murine CD8+ regulatory T lymphocytes: the new era. Human immunology. 2008;69:708–714. doi: 10.1016/j.humimm.2008.08.288. [DOI] [PubMed] [Google Scholar]
- 51.Hirota K, Martin B, Veldhoen M. Development, regulation and functional capacities of Th17 cells. Seminars in immunopathology. 32:3–16. doi: 10.1007/s00281-009-0187-y. [DOI] [PubMed] [Google Scholar]
- 52.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. The Journal of experimental medicine. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schreiber RD, Darnell JE, Jr., Murphy KM. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. The Journal of experimental medicine. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshimoto T, Okamura H, Tagawa YI, Iwakura Y, Nakanishi K. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-gamma production from activated B cells. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3948–3953. doi: 10.1073/pnas.94.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ludwig-Portugall I, Hamilton-Williams EE, Gottschalk C, Kurts C. Cutting Edge: CD25+ regulatory T cells prevent expansion and induce apoptosis of B cells specific for tissue autoantigens. J Immunol. 2008;181:4447–4451. doi: 10.4049/jimmunol.181.7.4447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Comparable antigen load in wild-type and CD8-deficient recipients. Wild-type and CD8 KO mice were transplanted with FVB/N hepatocytes. Serum hA1AT levels were serially analyzed to measure allogeneic hepatocellular graft survival. A) We found that there is no significant difference in hA1AT levels between our CD8 KO and wild-type recipients on day 7 following transplantation. B) Wild-type recipients that underwent a successive transplant on day 5 had similar hA1AT levels as a CD8-depleted wild-type mouse following a single transplant. C) However, this increased antigen exposure did not stimulate significantly enhanced alloantibody production in wild-type recipients.
Supplemental Figure 2. CD8-deficient mice exhibit an IgG1 alloantibody response to transplantation. The serum from wild-type transplant recipient and CD8 KO transplant recipient mice was collected 14 days after hepatocyte transplantation and tested for total IgG antibody isotypes IgG1, IgG2a, IgG3, and IgE using ELISA. Fold measurements of the total IgG isotypes were quantified and compared to the serum from naïve control mice. Serum from wild-type transplant recipient mice contained significant levels of IgG1 alloantibody isotype as compared to naïve control serum (1.8±0.5 fold; p=0.020, as denoted by “*”). Serum from CD8 KO transplant recipient mice had a significant increase of the IgG1 alloantibody isotype (5.0±0.7 fold) compared to both serum from naïve and transplanted wild-type mice (p<0.002 for both comparisons, as denoted by “*” and “**”, respectively). Serum from wild-type and CD8 KO recipients did not show significant levels of IgG2a, IgG3, or IgE. The graph depicts representative data of duplicate experiments. Standard deviations were calculated by 2 samples per experiments (triplicate wells/sample).
Supplemental Figure 3. IL-4 mRNA is upregulated in CD8 KO mice following transplantation. To examine the mRNA expression of IFN-γ and IL-4 in wild-type and CD8-deficient mice following hepatocyte transplantation, we performed semi-quantitative real-time PCR of recipient splenocytes. Splenocytes were harvested from recipient mice on day 5 and 7 post-transplant. A) IFN-γ mRNA was significantly upregulated in wild-type C57BL/6 recipients on day 5 and 7 (1.3±0.1 and 1.7±0.2 fold, respectively; p<0.05 for both days). CD8 KO recipients did not have a significant change in IFN-γ expression as compared to naïve control. B) IL-4 mRNA was significantly upregulated in CD8 KO recipients as compared to naïve control on day 5 and 7 (2.0±0.1 and 1.9±0.4 fold, respectively; p<0.03 for both days). These results were compared to wild-type recipient mice which have significantly decreased levels of IL-4 on post transplant days 5 and 7 (0.7±0.1 and 0.3±0.1 fold, respectively; p<0.05 for both). Significant upregulation or downregulation is denoted by “*”. Data were expressed as the mean fold increase relative to cells collected form naïve control mice. All real-time PCR data were normalized to the level of mouse β-actin mRNA. Error bars denote the standard deviation of duplicate experiments (triplicate wells/sample).