Abstract
MUC17 glycoprotein is a membrane-associated mucin that is mainly expressed in the digestive tract. It has been suggested that MUC17 expression is correlated with the malignancy potential of pancreatic ductal adenocarcinomas (PDACs). In the present study, we provided the first report of the MUC17 gene expression through epigenetic regulation such as promoter methylation, histone modification and microRNA (miRNA) expression. Near the transcriptional start site, the DNA methylation level of MUC17-negative cancer cell lines (e.g. PANC1) was high, whereas that of MUC17-positive cells (e.g. AsPC-1) was low. Histone H3-K9 (H3-K9) modification status was also closely related to MUC17 expression. Our results indicate that DNA methylation and histone H3-K9 modification in the 5′ flanking region play a critical role in MUC17 expression. Furthermore, the hypomethylation status was observed in patients with PDAC. This indicates that the hypomethylation status in the MUC17 promoter could be a novel epigenetic marker for the diagnosis of PDAC. In addition, the result of miRNA microarray analysis showed that five potential miRNA candidates existed. It is also possible that the MUC17 might be post-transcriptionally regulated by miRNA targeting to the 3′-untranslated region of its mRNA. These understandings of the epigenetic changes of MUC17 may be of importance for the diagnosis of carcinogenic risk and the prediction of outcomes for cancer patients.
Keywords: DNA methylation, epigenetics, MUC17, mucin, pancreatic cancer
Introduction
Pancreatic ductal adenocarcinomas (PDACs) are highly malignant neoplasms that are difficult to detect at an early stage (Bardeesy and DePinho 2002; Yonezawa et al. 2009). However, early detection of these neoplasms is important for the improvement of outcome and effective tools are needed for this purpose. Mucins are heavily O-glycosylated proteins found in the mucus layer and differentially expressed at the cell surface of many epithelia. They are responsible for the physical properties of mucus gels and are involved in epithelial cell protection and maintain the local molecular microenvironment (Bhaskar et al. 1992; Linden et al. 2004, 2008; Ho et al. 2006). There is also increasing evidence that mucin members are aberrantly expressed and contributes to the pathogenesis of cancer (Hollingsworth and Swanson 2004; Yonezawa et al. 2008). We have demonstrated that MUC1 and MUC4 expression is a poor prognostic factor, whereas MUC2 expression is related to a favorable outcome in various human neoplasms including PDACs and have stressed that they are indicators of potential for malignancy (Yonezawa and Sato 1997; Yonezawa et al. 2008) since our first report (Osako et al. 1993). In addition, we have reported that MUC5AC expression is also an effective tool for the early detection of the pancreatic neoplasms (Kim et al. 2002; Yonezawa et al. 2010).
MUC17 glycoprotein was identified and mapped to a mucin cluster at chromosome 7q22, along with MUC3A/B, MUC11 and MUC12 mucins, and it was categorized as a membrane-associated mucin (Williams et al. 1999; Gum et al. 2002). RNA blot analysis indicated that MUC17 is primarily expressed in the digestive tract, including the duodenum, ileum and the transverse (Gum et al. 2002; Moehle et al. 2006). Although the physiological function of MUC17 is still unclear, it may serve as a physical barrier molecule against microorganisms and cell-surface sensors. MUC17 may also conduct signals in response to external stimuli that lead to cellular responses, including proliferation, differentiation, apoptosis or secretion of cellular products such as other membrane-bound mucin members. Involvement of MUC17 expression in cancer pathogenesis, Moniaux et al. (2006) reported that aberrant MUC17 expression is observed in PDACs compared to no expression in the normal pancreas or pancreatitis. Recently, Hirono et al. (2010) revealed that MUC17 is an independent prognostic factor associated with lymph node metastasis in PDACs.
It has been reported that 5′ flanking region (1167 bp) of MUC17 possessing promoter activity harbors various transcriptional factor-binding elements, including GATA, VDR/RXR, Cdx-2, NFkB and Pdx-1 (Moniaux et al. 2006). However, the exact regulatory mechanism is still not fully understood. We have described the mechanisms of epigenetic regulation of MUC1, MUC2, MUC3A, MUC4 and MUC5AC expression (Yamada et al. 2006, 2008, 2009, 2010; Kitamoto et al. 2010), and thus we hypothesized that MUC17 may also be regulated epigenetically. In the present study, we mapped the CpG methylation status of MUC17 from −620 to +209 using MassARRAY analysis in various human cancer cell lines including pancreatic cancers.
Modification of histone tails also plays a critical role in epigenetic silencing (Nguyen et al. 2002). Therefore, to investigate the relationship between DNA methylation and histone modification, chromatin immunoprecipitation (ChIP) assays were performed in 10 cancer cell lines. Moreover, to examine whether MUC17 mRNA expression is regulated by the DNA methylation status and histone H3 modification, we also treated MUC17-negative cells or cells with low MUC17 expression (MUC17-negative/low cells) with a DNA methylation inhibitor, 5-aza-2′-deoxycytidine (5-AzadC) and a histone deacetylase inhibitor, trichostatin A (TSA).
In the present study, we describe that the tightly related combination of DNA methylation and histone H3 lysine 9 (H3-K9) modifications contribute to MUC17 gene expression. In addition, from the results of miRNA microarray analysis, we found five candidates of microRNAs (miRNAs) that might regulate MUC17 protein expression.
Results
Influence of DNA methylation and histone deacetylation on the MUC17 expression induced by epigenetic-modifying agents
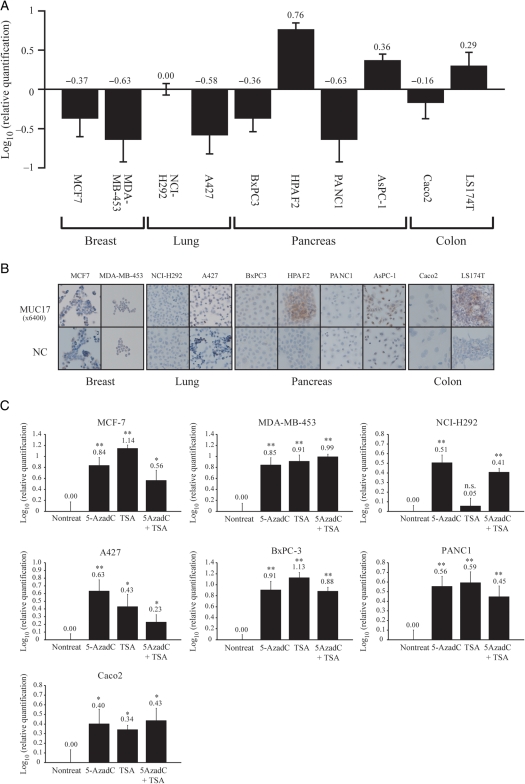
Experiments were performed using the 10 cancer cell lines derived from different tissues. Expression levels of MUC17 mRNA and MUC17 protein in the cell lines were examined by reverse transcription–PCR (RT–PCR) analysis and immunohistochemical staining (Figure 1A and B). HPAFII, AsPC-1 and LS174T cells expressed MUC17, but the other cells did not. To investigate the possible epigenetic regulation of MUC17 expression, quantitative RT–PCR analysis was performed in MUC17-negative/low cells treated with a DNA demethylating agent, 5-AzadC, a histone deacetylase inhibitor, TSA, or 5-AzadC/TSA in combination. The obvious tendency that the expression of MUC17 mRNA was restored by drug treatment was observed in MUC17-negative/low cells (Figure 1C). Consistent with the mRNA level, the MUC17 expression is also restored by the drug treatments (Supplementary data, Figure S1).
Fig. 1.
Expression of the MU17 gene examined by quantitative RT–PCR and immunohistochemistry. (A) Quantitative RT–PCR results in 10 cell lines. Bars, gene expression levels relative to those in NCI-H292 cells. HPAFII, AsPC1 and LS174T cells showed a high mRNA expression level in MUC17 gene, whereas MCF-7, MDA-MB-453, NCI-H292, A427, PANC1, BxPC-3 and Caco2 cells had no or low expression. (B) MUC17 immunoreactivity in 10 cancer cell lines. MUC17 expression was consistent with the results of quantitative RT–PCR. (C) Quantitative RT–PCR results before and after treatment with 5-AzadC, TSA and 5AzadC/TSA in combination in cells with little or no MUC17 expression. After drug treatments, these cells showed significant restoration of MUC17 expression. *P < 0.05, **P < 0.005, when compared with nontreated cells; Student's t-test; n.s., not significant.
Next, to rule out the contribution of single nucleotide polymorphisms (SNPs) in control of MUC17 expression, we sequenced a 1.2 kb putative promoter region of the human MUC17 gene from 10 cell lines. There are no differences from the sequence with GenBank accession number NT_007933 (Table I, Supplementary data, Figure S2).
Table I.
Synthetic oligonucleotides used in this study
| Name | Primer sequence | Position |
|---|---|---|
| MUC17 promoter sequencing primers | ||
| MUC17-S1F | CACTCTGGAATTTCCCTACCAA | −1190 to −1169 |
| MUC17-S1R | CCTGTAATCCCAGCTACTCAGG | −583 to −562 |
| MUC17-S2F | GGCACGATCTTGGCTCTC | −647 to −630 |
| MUC17-S2R | GACCTTTGGTCCCTGAGACA | −62 to −43 |
| MUC17-S3F | GTCATGAGGGAAGCTTCCAG | −117 to −98 |
| MUC17-S3R | TATGGGTTGAACACCAGTCTTG | +529 to +550 |
| 10mer-tagged or T7-tagged primers | ||
| MUC17-1F | AGGAAGAGAGTTTTAGATGGTTATGGGAGTTGTGT | −825 to −801 |
| MUC17-1R | CAGTAATACGACTCACTATAGGGAGAAGGCTTTTAACCCTAACCCCTAACAAAAAC | −372 to −348 |
| MUC17-2F | AGGAAGAGAGAGAGATAAAGGGGGTGTTTTTGTTA | −387 to −363 |
| MUC17-2R | CAGTAATACGACTCACTATAGGGAGAAGGCTCAAAAACCAACCTAAACCTAAATCA | −95 to −71 |
| MUC17-3F | AGGAAGAGAGGTTTTTGTTAGGGGTTAGGGTTAAA | −372 to −348 |
| MUC17-3R | CAGTAATACGACTCACTATAGGGAGAAGGCTAAAAACCAAAATCAACAAACACAAC | +77 to +101 |
| MUC17-4F | AGGAAGAGAGTTTGGTTTTTATAGGTTTTTTGGGT | −255 to −231 |
| MUC17-4R | CAGTAATACGACTCACTATAGGGAGAAGGCTAAACAAACAAAACAAACTAACCCCT | +179 to +203 |
| MUC17-5F | AGGAAGAGAGTGTTTTAGGGATTAAAGGTTTTTGG | −62 to −38 |
| MUC17-5R | CAGTAATACGACTCACTATAGGGAGAAGGCTCTAAACTCCAAACAAAAAACACACC | +241 to +265 |
| MSP primers | ||
| MUC17-UFa | GTTGATAATTTTTATGTTTATGGGTTGTT | −207 to −179 |
| MUC17-URa | CTAACCTTAACATCAAAACTCTAAACACA | +38 to +66 |
| MUC17-MFb | GTTGATAATTTTTATGTTTATGGGTTGTC | −207 to −179 |
| MUC17-MRb | CCTTAACATCGAAACTCTAAACACG | +38 to +62 |
| Semiquantitative PCR primers | ||
| MUC17-F1 | GCTGTGTCTGCTGACCTTGG | Exon 1 |
| MUC17-R1 | TGGCACTGACGGTTCAAGAC | Exon 2 |
| ChIP primers | ||
| MUC17-CF1 | CCAGTGTCTCAGGGACCAAAG | −66 to −46 |
| MUC17-CR1 | CCAAGGTCAGCAGACACAGC | +77 to +96 |
Synthetic oligonucleotides listed with the position number with respect to the transcriptional start site, respectively.
aThe U primer for unmethylated alleles in MSP analysis.
bThe M primer for methylated alleles in MSP analysis.
Quantification of DNA methylation status within the MUC17 gene promoter
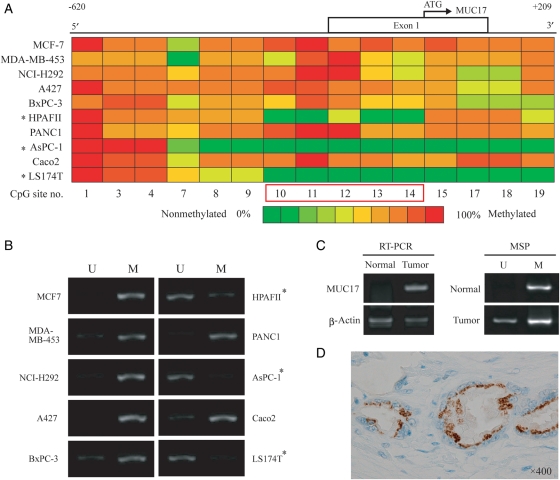
Quantification of MUC17 promoter CpG methylation levels in 10 cancer cell lines was examined by the MassARRAY compact system and mapped the efficacious data (Figure 2A). Near the transcriptional start site (−179 to +52), a high level of CpG methylation was observed in MUC17-negative/low cells (MCF-7, MDA-MB-453, NCI-H292, A427, BxPC-3, PANC1 and Caco2) compared with MUC17-positive cell lines (HPAFII, AsPC-1 and LS174T). These results indicate that hypomethylation near the transcriptional start site (corresponding to CpG site numbers 10–14) contributes to MUC17 expression. To confirm the MassARRAY analysis, methylation-specific-PCR (MSP) was done with primers targeting to five CpG sites on the 10 cell lines (Figure 2B, Table I). An unmethylated band (lanes indicated by U; Figure 2B) was clearly obtained in MUC17-positive HPAFII, AsPC-1 and LS174T cells, and a methylation band (lanes indicated by M; Figure 2B) was observed in MUC17-negative/low cells. These results were consistent with those from MassARRAY analysis.
Fig. 2.
Methylation pattern of MUC17 promoter and MSP analysis in cell lines and biological samples. (A) Quantitative methylation analysis of CpG sites located in the MUC17 promoter using a MassARRAY compact system. Different colors display relative methylation changes in 10% increments (green = 0%, red = 100% methylated). CpG sites that correlated well with MUC17 expression are boxed in red. *MUC17-positive cell lines. (B) MSP analysis of 10 cancer cell lines. The PCR products labeled M (methylated) were generated by methylation-specific primers and those labeled U (unmethylated) were generated by primers specific for unmethylated DNA. (C) Correlation between the mRNA expression level and methylation status in the 5′-flanking region in the normal pancreas (N) and tumoral pancreatic tissues (T) from patients with PDAC. Semi-quantitative RT–PCR and MSP analysis were carried out, respectively. (D) Immunohistochemical staining for MUC17 in a patient with PDAC. The carcinoma cells show granular staining in the supranuclear areas (×400, original magnification).
Moreover, we examined the methylation status of normal and tumoral pancreas tissues from patients with PDAC. First, we confirmed the mRNA and protein expression of MUC17 in tissues with PDAC. In tumor tissue, MUC17 was overexpressed compared with the normal pancreas (Figure 2C and D), which is in accordance with the previous study reported by Moniaux et al. (2006). Although islets of Langerhans were stained by rabbit anti-MUC17 polyclonal antibody, the unmethylated band was obtained in tumoral tissues with PDAC in six out of the eight cases we tested (Figure 2C and D, Table II).
Table II.
Methylation status in biological samples from patients with PDAC
| Unmethyl (U) | P-value | |
|---|---|---|
| Tissue/normal | 2/8 | 0.046 |
| Tissue/PDAC | 6/8 |
MSP analysis of eight normal and tumoral tissues from patients with PDAC.
Correlation between CpG methylation and histone H3-K9 modification in the MUC17 regulation
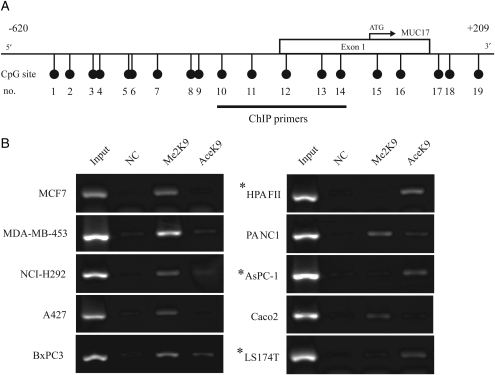
Furthermore, we investigated the possibility of another form of epigenetic regulation of MUC17 using a ChIP assay targeting to H3-K9 modification. To examine the relationship of MUC17 expression with DNA methylation and histone modification, ChIP primers were designed for the region containing CpG site numbers 10–14 (Figure 3A, Table I). The results showed that the dimethylation of histone H3-K9 was detected in cells with no or low MUC17 expression (MCF-7, MDA-MB-453, NCI-H292, A427, BxPC-3, PANC1 and Caco2). In contrast, histone H3-K9 acetylation was predominantly found in MUC17 high expression cells (HPAFII, AsPC-1 and LS174T). These results suggest that DNA methylation status and histone H3-K9 modification in its 5′ flanking region contribute to MUC17 expression (Figure 3B).
Fig. 3.
Histone modification status of MUC17 proximal promoter region in 10 cancer cell lines. (A) Schematic representation of the MUC17 gene promoter region. The relative positions of CpG sites and the ChIP primers used in this experiment are indicated. (B) ChIP analysis in the MUC17 promoter region was performed using antibodies against dimethylated H3-K9 (Me2-K9) and acetylated H3-K9 (Ace-K9). Input DNA was used as a positive control. NC indicates ChIP performed using rabbit IgGs as an isotype antibody control. Asterisks indicate MUC17-positive cell lines. Histone H3-K9 dimethylation was preferentially observed in cells with little or no MUC17 expression, whereas acetylation of histone H3-K9 was detected in all MUC17-positive cells. *MUC17-positive cell lines.
Identification of miRNA candidates in MUC17 expression in cancer cells
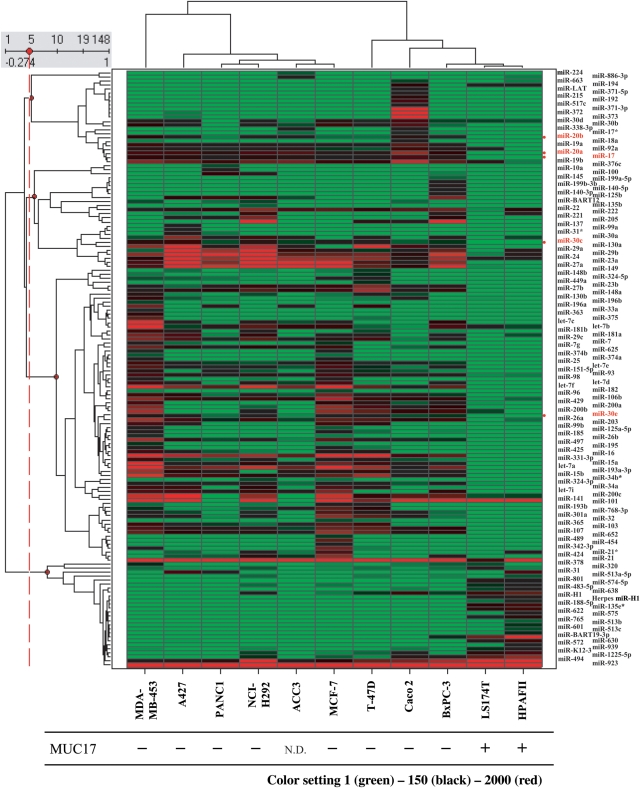
miRNAs are a class of noncoding RNA gene, product sizes are ∼22 nt sequences, that play important roles in the regulation of translation and degradation of mRNAs through imprecise base pairing in the untranslated regions of the message. The important roles of miRNA in cancer are intensively reported. Therefore, their regulatory impact has become more pronounced and impossible to overlook (Calin et al. 2002, 2005; Marcucci et al. 2008; Lujambio and Esteller 2009). We have previously performed miRNA microarray on 11 cancer cell lines (Yamada et al. 2010). The analysis has been carried out using an miRNA Complete Labeling and Hyb Kit (Human miRNA Microarray v.2, which contains probes for 723 human and 76 human viral miRNAs from the Sanger database v.10.1). In this analysis, 148 miRNAs with a maximum or minimum signal that varied by more than twice among the 11 cell lines were identified. A total of 18 of the 148 miRNAs had potential for MUC17 regulation based on target prediction using the miRBase. The comparison of these results with MUC17 expression revealed a significant relationship with five candidates to regulate MUC17 expression (miR-17, miR-20a, miR-20b, miR-30c, miR-30e; target prediction based on miRBase). Hence, these miRNAs might participate in the post-transcriptional regulation of MUC17 expression (Figure 4).
Fig. 4.
miRNA expression profiles of 11 human cancer cell lines. The heat map shows the 148 miRNAs for which the maximum or minimum signal differed by more than twice among the 11 cancer cells. The cluster shows correlated groups of miRNAs and cell lines. The five miRNAs with the potential for MUC17 regulation are highlighted in red. The MUC17 protein expression pattern is shown below. Reprinted, with permission and modification, from Yamada et al. (2010).
Discussion
It has been reported that the human MUC17 gene is predominantly expressed in PDACs and identified as an independent prognostic factor of the patients with PDAC carrying lymph node metastasis (Hirono et al. 2010). However, little is known about the functional role of MUC17 in cancer pathology. Understanding the expression mechanism of MUC17 can be a key step in developing new strategies for cancer diagnosis and treatment.
To examine the possible epigenetic regulation of MUC17 expression, we treated MUC17-negative/low cells with 5-AzadC and/or TSA. In MCF-7, A427, BxPC3 and PANC1 cells the restoration level of MUC17 mRNA decreased in 5-AzadC/TSA treatments rather than in treatment with 5-AzadC or TSA alone. Yet, it is unclear whether 5-AzadC and TSA caused an antagonistic effect in three cells. We showed that treatment with TSA did not restore the MUC17 mRNA in NCI-H292 cells. Kondo et al. (2003) showed that 5-AzadC or a combination of 5-AzadC and TSA, but not TSA alone, reactivates tumor suppressor gene expression at the silenced loci (e.g. p16). Our results in NCI-H292 cells are in agreement with their observation. Although there were differences in the restoration level of MUC17 mRNA among treated cell lines, our overall results suggested the possibility that MUC17 expression is regulated by epigenetic mechanisms.
Before beginning the epigenetic analysis, we performed a sequence analysis of the MUC17 promoter in 10 cancer cells to examine the possible regulatory SNPs (rSNPs). rSNPs are usually promoter region mutations that cause variation in gene expression levels (Knight 2005). Our results were congruent in the 10 cell lines and matched the data for GenBank accession number NT_007933. Therefore, we examined the DNA methylation status of the MUC17 promoter in 10 cancer cell lines.
In this study, we used MassARRAY analysis to examine the methylation status of 19 CpG sites in the promoter of the MUC17 gene and identified five CpG sites (site numbers 10–14) involved in MUC17 expression. We also examined the methylation status of normal and tumoral pancreatic tissues from patients with PDAC. As a result of the MSP analysis, the methylation status in the MUC17 promoter was consistent with the expression of MUC17 in both mRNA and protein levels (Figure 2C and D, Table II). These results raise the possibility that human pancreatic tissue might be regulated with the epigenetic mechanism similar to cell lines. Although further studies are needed to clarify the relationship, these findings indicate that the methylation status in the MUC17 promoter could be a novel epigenetic marker for the diagnosis of PDAC.
We also examine the relationship between DNA methylation and histone modification in MUC17 expression by ChIP assay, resulting in the presence of dimethyl-H3-K9 in cells with no or low MUC17 confirmed, whereas histone H3-K9 was more highly acetylated in MUC17-positive cells than in cells with no or low MUC17 (Figure 3B). These results suggest that the combination of DNA methylation and histone H3-K9 modification contribute to MUC17 expression.
Epigenetic regulation of small non-coding RNAs plays a critical role in the modulation of mammalian gene expression (Baek et al. 2008; Siomi H and Siomi MC 2009). In addition, miRNAs have been reported to regulate both tumor suppressor genes and oncogenes. We have previously examined the expression profiles of miRNAs in 11 cancer cell lines (Yamada et al. 2010). The result of miRNA microarray analysis showed 148 miRNAs with a maximum or a minimum signal that varied by more than twice among the 11 cells lines that were identified. Furthermore, the comparison of these results with MUC17 expression revealed a significant relationship with five candidates to regulate MUC17 expression (miR-17, miR-20a, miR-20b, miR-30c, miR-30e; target prediction based on miRBase). Of all the potential candidates of a given mRNA of MUC17, it is still unclear which ones are authentic miRNA in vivo. Our data suggest that the MUC17 gene may be regulated post-transcriptionally by miRNAs (Figure 4).
In this study, our data suggested that the epigenetic regulation of the MUC17 gene in cancer as follows: (1) open chromatin can be characterized by unmethylated status in the proximal MUC17 promoter region and histones modification with acetylated H3-K9. This allows the assembly of transcription factors and transcription by RNA polymerase. DNA methylation and histone deacetylation result in the condensation of chromatin into a compact state that is inaccessible by transcription factors. (2) The methylation of the proximal MUC17 promoter region directly blocks binding of transcription factors and prevents transcription. It may also recruit methyl-CpG-binding domain proteins such as MeCP2 and MBD1 that have associated histone deacetylases. (3) Post-transcriptional regulation by miRNA might also participate in MUC17 expression.
Although the functional roles of MUC17 in cancer development are still unknown, there are some common structural features in other mucins. First, MUC17 is similar to MUC1 in the aspect of possessing SEA (sperm protein, enterokinase and agrin) domain, hypothesized to be the proteolytic cleaveage site. Mahanta et al. (2008) showed that MUC1 cleavage by an SEA domain leads to activation of the MAP kinase signaling and stimulates tumor cell growth. MUC17 also has two EGF-like domains separated by the SEA domain. Other experimental data have shown that the extracellular EGF-like motif of MUC4 can interact with ERBB2 (Jepson et al. 2002; Hollingsworth and Swanson 2004). Additionally, the soluble EGF-ligand on a mucin might serve as a mediator of inflammatory or immune responses (Agrawal et al. 1998; Correa et al. 2003). These findings indicate that MUC17 might have multiple functions, including cell growth, differentiation or immunoregulatory mediation. Our results indicate that MUC17 is regulated tightly through epigenetic manners including DNA methylation, histone modification and miRNA regulation. These findings suggest the possible functional importance of MUC17 in both physiological and pathological conditions. The understanding of these intimately correlated epigenetic changes for the MUC17 gene expression may also be of importance for the diagnosis of carcinogenic risk and the prediction of outcomes of patients with PDAC.
Materials and methods
Cells and treatment
Human pancreatic carcinoma cell lines HPAFII, BxPC-3, PANC1 and AsPC-1, human breast cancer cell lines MCF-7 and MDA-MB-453, human lung cancer cell lines NCI-H292 and A427 and human colon adenocarcinoma cell lines Caco2 and LS174T were obtained from the American Type Culture Collection. MCF-7, A427, HPAFII, Caco2 and LS174T cells were cultured in Eagle's minimum essential medium (Sigma, St. Louis, MO); PANC1 cells were cultured in D-MEM (Sigma); NCI-H292, BxPC3, AsPC-1 and ACC3 cells were cultured in RPMI-1640 medium (Sigma) and MDA-MB-453 cells were cultured in Leibovitz's L-15 medium (Invitrogen, Carlsbad, CA). All media were supplemented with 10% fetal bovine serum (Invitrogen) and 100 U/mL of penicillin/100 μg/mL of streptomycin (Sigma). MUC17-negative/low cells were split 24 h before treatment. BxPC-3, NCI-H292 cells were incubated with 100 μM 5-AzadC (Sigma) and/or 100 nM TSA (Sigma) for 5 days; MCF7, PANC1 and Caco2 cells were incubated with 100 μM 5-AzadC and/or 50 nM TSA for 5 days; MDA-MB-453 cells were incubated with 100 μM 5-AzadC and/or 10 nM TSA for 5 days and A427 cells were incubated with 1 μM 5-AzadC and/or 50 nM TSA for 5 days. Media were changed every 24 h.
Patients and biological samples
This study was approved by the ethical committee of Kagoshima University Hospital. Fresh pancreatic tumor tissues were obtained from the surgically resected pancreatic specimens from eight patients with PDAC. The normal pancreatic tissues from the same resected specimens were obtained from the areas apart from the tumor in each patient. To conduct the following study, all tissue samples were stored at –80°C. The adjacent areas of cancerous and normal tissues were fixed in buffered formalin (pH 7.4) and were examined histologically and immunohistochemically.
Quantitative RT–PCR analysis
Total RNA from cells that did or did not undergo 5-AzadC, TSA or 5-AzadC + TSA treatment was purified with an RNeasy Mini kit (Qiagen, Valencia, CA). Total RNA (20 μL) was reverse-transcribed with a High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA) as described previously (Yamada et al. 2008). The primers and probes were designed and synthesized by Applied Biosystems. The product number of the Target Assay Mix used for MUC17 was Hs00959753_s1. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH; product number 4310884E) was used to calibrate the original concentration of mRNA; i.e. the concentration of mRNA in the cell was defined as the ratio of target mRNA copies versus GAPDH mRNA copies. In this analysis, data from three separate experiments were averaged.
Immunohistochemical staining
MUC17 protein expression levels were assessed by immunohistochemistry. For cultured cells, MUC17 was detected by rabbit anti-MUC17 polyclonal antibody (generated by one of us, by S.K.B., University of Nebraska Medical Center, Omaha; dilution rate at 1:6400 for overnight at 4°C). Immunohistochemical staining was performed by an immunoperoxidase method using a Vectastain Elite ABC kit (Vector Laboratories), as described previously (Yonezawa et al. 1997; 2008). For tissue staining, antigen retrieval was performed using CC1 antigen retrieval buffer (Ventana Medical Systems, Tucson, AZ) for all sections. Following incubation with the primary antibody (dilution rate at 1:20,000), sections were stained on the Ventana automated slide stainer (Benchmark XT) using the Ventana diaminobenzidine detection kit (Ventana Medical Systems).
MUC17 gene promoter sequencing
Genomic DNA was extracted from the 10 cell lines using a DNeasy Tissue System (Qiagen) according to the manufacturer's instructions. Sequencing was carried out using three pairs of forward and reverse primers (Table I) in MUC17 promoter.
Quantitative methylation analysis
Quantitative methylation analysis of the MUC17 promoter (GenBank accession number: NT_007933) was performed using the MassARRAY Compact system (Sequenom), as described previously (Ehrich et al. 2005). The target regions were amplified using the primer pairs shown in Table I. Each forward primer is tagged with a 10mer (5′-AGG AAG AGA G-3′) to balance the PCR and a reverse primer (5′-CAG TAA TAC GAC TCA CTA TAG GGA GAA GGC T-3′) with a T7-promoter tag for in vitro transcription. The resultant methylation cells were analyzed with EpiTyper software v1.0 (Sequenom) to generate quantitative results for each CpG site or an aggregate of multiple CpG sites.
DNA extraction and MSP analysis
DNA from cell lines was extracted using a DNeasy Tissue System (Qiagen), according to the manufacturer's instructions. Bisulfite modification of the genomic DNA was carried out using an Epitect Bisulfite kit (Qiagen), and the modified DNA was amplified by PCR using AmpliTaq Gold (Applied Biosystems). The target regions were amplified using the primer pairs shown in Table I. The PCR conditions were 95°C for 10 min, 40 cycles at 96°C for 5 s, 59°C for 5 s and 68°C for 5 s, with a final extension reaction at 72°C for 1 min. The amplified products were subjected to 1.0% agarose gel electrophoresis.
ChIP assay
ChIP assay was performed using a Shearing-ChIP kit and a 1 day ChIP kit (Diagenode, Philadelphia, PA), according to the manufacturer's instructions. Briefly, the nucleoprotein complexes were sonicated to reduce the sizes of DNA fragments from 300 to 500 bp using a Bioruptor (Cosmo Bio). One microgram of normal mouse immunoglobulin was used as the negative control (NC), and anti-dimethyl histone H3-K9 antibody (Abcam) and anti-acetyl histone H3-K9 antibody (Abcam) were used for each immunoprecipitation. Immunoprecipitated DNA was amplified by PCR using AmpliTaq Gold (Applied Biosystems). The ChIP primers were designed to target a region similar to the target region of the MSP primers (Table I). The PCR conditions were 95°C for 5 min, 34 cycles at 96°C for 5 s, 59°C for 5 s and 68°C for 7 s, with a final extension reaction at 72°C for 1 min. The amplified products were subjected to 1.0% agarose gel electrophoresis.
Statistical analysis
Statistical analyses were conducted using Pearson's χ2 test. The statistical analysis software, SPSS (version 18.0), was used and P < 0.05 was considered significant.
Supplementary data
Supplementary data for this article is available online at http://glycob.oxfordjournals.org/.
Funding
This study was supported by Grant-in-Aid for Scientific Research on Priority Areas 20014022 and Scientific Research (C) 20590345 to S.Y. and Scientific Research (C) 21590399 from the Ministry of Education, Science, Sports, Culture and Technology, Japan, the Kodama Memorial Foundation to M.H., Scientific Research on Priority Areas 219447 to N.Y. from the Grant-in-Aid for JSPS Fellow and International educational research support project for islands, environment and medicine (S.Y.) from Kagoshima University, and National Institutes of Health USA (RO1 CA78590 and EDRN UO1CA111294) to S.K.B.
Acknowledgements
The authors thank Ms. Yukari Nishimura and Ms. Sayuri Yoshimura for their excellent technical assistance.
Conflict of interest
None declared.
Abbreviations
ChIP, chromatin immunoprecipitation; 5-AzadC, 5-aza-2′-deoxycytidine; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MSP, methylation-specific-PCR; NC, negative control; rSNPs, regulatory SNPs; RT, reverse transcription; SEA, sperm protein, enterokinase and agrin; SNPs, single nucleotide polymorphisms; TSA, trichostatin A
References
- Agrawal B, Krantz MJ, Parker J, Longenecker BM. Expression of MUC1 mucin on activated human T cells: Implications for a role of MUC1 in normal immune regulation. Cancer Res. 1998;58:4079–4081. [PubMed] [Google Scholar]
- Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- Bhaskar KR, Garik P, Turner BS, Bradley JD, Bansil R, Stanley HE, LaMont JT. Viscous fingering of HCl through gastric mucin. Nature. 1992;360:458–461. doi: 10.1038/360458a0. [DOI] [PubMed] [Google Scholar]
- Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, et al. A microRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- Correa I, Plunkett T, Vlad A, Mungul A, Candelora-Kettel J, Burchell JM, Taylor-Papadimitriou J, Finn OJ. Form and pattern of MUC1 expression on T cells activated in vivo or in vitro suggests a function in T-cell migration. Immunology. 2003;108:32–41. doi: 10.1046/j.1365-2567.2003.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich M, Nelson MR, Stanssens P, Zabeau M, Liloglou T, Xinarianos G, Cantor CR, Field JK, van den Boom D. Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proc Natl Acad Sci USA. 2005;102:15785–15790. doi: 10.1073/pnas.0507816102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gum JR, Jr., Crawley SC, Hicks JW, Szymkowski DE, Kim YS. MUC17, a novel membrane-tethered mucin. Biochem Biophys Res Commun. 2002;291:466–475. doi: 10.1006/bbrc.2002.6475. [DOI] [PubMed] [Google Scholar]
- Hirono S, Yamaue H, Hoshikawa Y, Ina S, Tani M, Kawai M, Ushijima M, Matsuura M, Saiki Y, Saiura A, et al. Molecular markers associated with lymph node metastasis in pancreatic ductal adenocarcinoma by genome-wide expression profiling. Cancer Sci. 2010;101:259–266. doi: 10.1111/j.1349-7006.2009.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SB, Dvorak LA, Moor RE, Jacobson AC, Frey MR, Corredor J, Polk DB, Shekels LL. Cysteine-rich domains of muc3 intestinal mucin promote cell migration, inhibit apoptosis, and accelerate wound healing. Gastroenterology. 2006;131:1501–1517. doi: 10.1053/j.gastro.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: Protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Jepson S, Komatsu M, Haq B, Arango ME, Huang D, Carraway CA, Carraway KL. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27(kip), but does not activate mitogen-activated kinase or protein kinaseB/Akt pathways. Oncogene. 2002;21:7524–7532. doi: 10.1038/sj.onc.1205970. [DOI] [PubMed] [Google Scholar]
- Kim GE, Bae HI, Park HU, Kuan SF, Crawley SC, Ho JJ, Kim YS. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 2002;123:1052–1060. doi: 10.1053/gast.2002.36018. [DOI] [PubMed] [Google Scholar]
- Kitamoto S, Yamada N, Yokoyama S, Houjou I, Higashi M, Yonezawa S. Promoter hypomethylation contributes to the expression of MUC3A in cancer cells. Biochem Biophys Res Commun. 2010;397:333–339. doi: 10.1016/j.bbrc.2010.05.124. [DOI] [PubMed] [Google Scholar]
- Knight JC. Regulatory polymorphisms underlying complex disease traits. J Mol Med. 2005;83:97–109. doi: 10.1007/s00109-004-0603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Shen L, Issa JP. Critical role of histone methylation in tumor suppressor gene silencing in colorectal cancer. Mol Cell Biol. 2003;23:206–215. doi: 10.1128/MCB.23.1.206-215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden S, Mahdavi J, Hedenbro J, Boren T, Carlstedt I. Effects of pH on Helicobacter pylori binding to human gastric mucins: Identification of binding to non-MUC5AC mucins. Biochem J. 2004;384:263–270. doi: 10.1042/BJ20040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujambio A, Esteller M. How epigenetics can explain human metastasis: A new role for microRNAs. Cell Cycle. 2009;8:377–382. doi: 10.4161/cc.8.3.7526. [DOI] [PubMed] [Google Scholar]
- Mahanta S, Fessler SP, Park J, Bamdad C. A minimal fragment of MUC1 mediates growth of cancer cells. PLoS ONE. 2008;3:e2054. doi: 10.1371/journal.pone.0002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucci G, Radmacher MD, Maharry K, Mrozek K, Ruppert AS, Paschka P, Vukosavljevic T, Whitman SP, Baldus CD, Langer C. MicroRNA expression in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1919–1928. doi: 10.1056/NEJMoa074256. [DOI] [PubMed] [Google Scholar]
- Moehle C, Ackermann N, Langmann T, Aslanidis C, Kel A, Kel-Margoulis O, Schmitz-Madry A, Zahn A, Stremmel W, Schmitz G. Aberrant intestinal expression and allelic variants of mucin genes associated with inflammatory bowel disease. J Mol Med. 2006;84:1055–1066. doi: 10.1007/s00109-006-0100-2. [DOI] [PubMed] [Google Scholar]
- Moniaux N, Junker WM, Singh AP, Jones AM, Batra SK. Characterization of human mucin MUC17. Complete coding sequence and organization. J Biol Chem. 2006;281:23676–23685. doi: 10.1074/jbc.M600302200. [DOI] [PubMed] [Google Scholar]
- Nguyen CT, Weisenberger DJ, Velicescu M, Gonzales FA, Lin JC, Liang G, Jones PA. Histone H3-lysine 9 methylation is associated with aberrant gene silencing in cancer cells and is rapidly reversed by 5-aza-2′-deoxycytidine. Cancer Res. 2002;62:6456–6461. [PubMed] [Google Scholar]
- Osako M, Yonezawa S, Siddiki B, Huang J, Ho JJ, Kim YS, Sato E. Immunohistochemical study of mucin carbohydrates and core proteins in human pancreatic tumors. Cancer. 1993;71:2191–2199. doi: 10.1002/1097-0142(19930401)71:7<2191::aid-cncr2820710705>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Siomi H, Siomi MC. On the road to reading the RNA-interference code. Nature. 2009;457:396–404. doi: 10.1038/nature07754. [DOI] [PubMed] [Google Scholar]
- Williams SJ, McGuckin MA, Gotley DC, Eyre HJ, Sutherland GR, Antalis TM. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res. 1999;59:4083–4089. [PubMed] [Google Scholar]
- Yamada N, Hamada T, Goto M, Tsutsumida H, Higashi M, Nomoto M, Yonezawa S. MUC2 expression is regulated by histone H3 modification and DNA methylation in pancreatic cancer. Int J Cancer. 2006;119:1850–1857. doi: 10.1002/ijc.22047. [DOI] [PubMed] [Google Scholar]
- Yamada N, Nishida Y, Tsutsumida H, Hamada T, Goto M, Higashi M, Nomoto M, Yonezawa S. MUC1 expression is regulated by DNA methylation and histone H3 lysine 9 modification in cancer cells. Cancer Res. 2008;68:2708–2716. doi: 10.1158/0008-5472.CAN-07-6844. [DOI] [PubMed] [Google Scholar]
- Yamada N, Nishida Y, Tsutsumida H, Goto M, Higashi M, Nomoto M, Yonezawa S. Promoter CpG methylation in cancer cells contributes to the regulation of MUC4. Br J Cancer. 2009;100:344–351. doi: 10.1038/sj.bjc.6604845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Nishida Y, Yokoyama S, Tsutsumida H, Houjou I, Kitamoto S, Goto M, Higashi M, Yonezawa S. Expression of MUC5AC, an early marker of pancreatobiliary cancer, is regulated by DNA methylation in the distal promoter region in cancer cells. J Hepatobiliary Pancreat Sci. 2010;17:844–854. doi: 10.1007/s00534-010-0278-0. [DOI] [PubMed] [Google Scholar]
- Yonezawa S, Goto M, Yamada N, Higashi M, Nomoto M. Expression profiles of MUC1, MUC2, and MUC4 mucins in human neoplasms and their relationship with biological behavior. Proteomics. 2008;8:3329–3341. doi: 10.1002/pmic.200800040. [DOI] [PubMed] [Google Scholar]
- Yonezawa S, Higashi M, Yamada N, Yokoyama S, Goto M. Significance of mucin expression in pancreatobiliary neoplasms. J Hepatobiliary Pancreat Surg. 2010;17:108–124. doi: 10.1007/s00534-009-0174-7. [DOI] [PubMed] [Google Scholar]
- Yonezawa S, Sato E. Expression of mucin antigens in human cancers and its relationship with malignancy potential. Pathol Int. 1997;47:813–830. doi: 10.1111/j.1440-1827.1997.tb03713.x. [DOI] [PubMed] [Google Scholar]
- Yonezawa S, Sueyoshi K, Nomoto M, Kitamura H, Nagata K, Arimura Y, Tanaka S, Hollingsworth MA, Siddiki B, Kim YS, et al. MUC2 gene expression is found in noninvasive tumors but not in invasive tumors of the pancreas and liver: Its close relationship with prognosis of the patients. Hum Pathol. 1997;28:344–352. doi: 10.1016/s0046-8177(97)90134-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.