Abstract
We report a simple method to obtain stable asymmetric giant unilamellar vesicles (GUVs). Fluorescence correlation spectroscopy was used to quantitatively characterize vesicle properties. After brain sphingomyelin (bSM) was exchanged into dioleoylphosphatidylcholine (DOPC) GUVs, lateral diffusion in the bSM-containing outer leaflet decreased, whereas that in the DOPC-containing inner leaflet was largely unchanged, confirming asymmetry and a lack of coupling between the physical states of the inner and outer leaflets. In contrast, after bSM was exchanged into brain phosphatidylcholine vesicles, lateral diffusion decreased in both leaflets. Thus, asymmetric GUVs should be useful for investigating the molecular mechanisms behind interleaflet coupling.
Cell membranes exhibit an asymmetric distribution of lipids across the bilayer. The exoplasmic leaflet of the plasma membrane of eukaryotic cells often contains large amounts of phosphatidylcholine (PC) and sphingolipids such as sphingomyelin (SM), whereas the cytoplasmic leaflet is mainly composed of lipids such as phosphatidyl ethanolamine (PE) or phosphatidylserine (1).
The role of membrane asymmetry has only recently begun to be addressed in model systems (2). Asymmetric supported bilayers based on a leaflet-by-leaflet assembly have started to provide interesting information (3,4); however, such methods are limited in terms of what types of experiments can be carried out relative to those involving lipid vesicles, and are subject to the necessity of minimizing the effect of the support (4,5). Leaflet-by-leaflet assembly of free-standing asymmetric membranes has also been reported (5–7). However, it is a concern that these bilayers may incorporate the organic solvent used (e.g., mineral oil), which may perturb the membrane properties. A highly reproducible solvent-free method that is fast, has a high yield, employs conventional vesicle preparation techniques, and can be used with a wide variety of lipids is needed.
We recently developed a method to produce stable asymmetric small unilamellar vesicles (SUVs) with a wide variety of lipid compositions based on mβCD-mediated lipid exchange (8). Here, we show how a similar method can be used to produce asymmetric giant unilamellar vesicles (GUVs) with high yield. Such vesicles have dimensions comparable to those of cell membranes and, most importantly, can be characterized by optical methods. We investigated the asymmetry of the GUVs by using fluorescence correlation spectroscopy (FCS) to probe the lipid dynamics separately in the two leaflets. The results provide an example of how asymmetric GUV systems can be used to address questions of biological relevance.
We produced GUVs with an inner leaflet composed of dioleoylphosphatidylcholine (DOPC) and an outer leaflet composed of DOPC and brain SM (bSM) using an mβCD-mediated lipid exchange (see Supporting Material). Briefly, we first prepared symmetric GUVs made of DOPC using the electroformation method in observation chambers (9). This method results in the formation of a large amount of densely packed vesicles that can withstand subsequent washing steps. Lipid exchange was started by substituting the external medium of the GUVs with an mβCD-bSM donor solution at the desired concentration, with the final osmolarity matching that of the solution in which the GUVs were prepared. After 30–60 min, the now-asymmetric GUVs could be washed delicately to remove the mβCD.
To perform confocal imaging and FCS on the asymmetric GUVs, we labeled the outer and/or inner leaflets. To label the outer leaflet, we either introduced a lipid fluorophore together with bSM when preparing the mβCD donor solution or added a dye that does not translocate across membranes (NR12S, a Nile-red lipid analog (10)) at the end of the procedure. To label the inner leaflet, we included either 7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD)-PE or NR12S at 0.01 mol % of total lipids in the acceptor GUVs at the beginning of the procedure. After the exchange and washing steps were completed, we quenched the fluorescence of residual dye in the outer leaflet with sodium hydrosulfite (Fig. S1).
We next used FCS to measure the diffusion coefficient of the fluorescent probes (and thus indirectly the lipid packing) in each leaflet. Because we were interested exclusively in relative changes, all of the diffusion coefficients (D∗) were normalized assuming an approximate value of 10 μm2/s for symmetric DOPC vesicles, on the basis of what we measured with calibration-free scanning FCS (data not shown).
Fig. 1 shows the lateral diffusion coefficient of NR12S (∼0.01 mol %) localized in the whole bilayer, only in the inner leaflet, or only in the outer leaflet. Before lipid exchange occurs (i.e., in symmetric GUVs), there is of course no significant difference among the three values. For asymmetric GUVs, we would expect the outer leaflet, which contains bSM, to be more ordered (and thus have lower D∗) than the inner leaflet. Consistent with this prediction, in asymmetric GUVs (i.e., after exchange), diffusion occurs much more slowly in the outer leaflet than in the inner leaflet.
Figure 1.
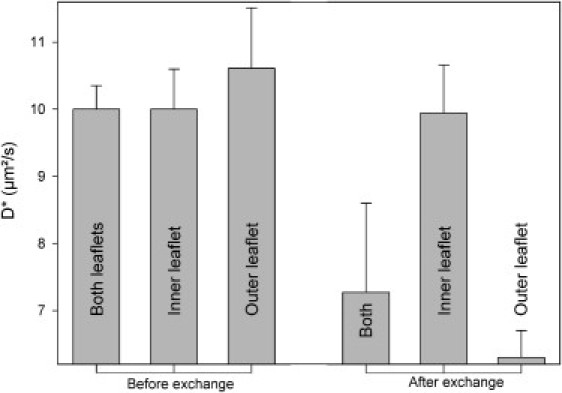
Lateral diffusion coefficients of NR12S in the outer leaflet of symmetric (before exchange) and asymmetric (after exchange) GUVs. The dye was located in the inner leaflet, outer leaflet, or both leaflets of the bilayer.
This could reflect a homogeneous increase in outer leaflet order, or the formation of ordered nanodomains that slow lipid dynamics by incorporating some of the dye and/or by acting as steric barriers to diffusion. As expected, when the dye is in both leaflets, D∗ has an intermediate value. Similar results were obtained with NBD-DOPE or NBD-DPPE in place of NR12S (data not shown). Of interest, the lipid packing in the inner leaflet seems comparable to that measured in symmetric DOPC GUVs, suggesting that its physical properties were barely affected by the bSM exchanged into the outer leaflet. This is also consistent with previous results obtained in asymmetric SUVs (8).
Of importance, the asymmetry of the obtained bilayer was stable for at least 4 h, with an approximate lower limit to the half-life time of decay of 18–22 h, as judged by the time necessary for inner and outer leaflet D∗ to equalize (Fig. S2).
Fig. 2 shows how the exchange is affected by the concentration of mβCD and bSM. Clearly, both higher mβCD and bSM concentrations enhance lipid delivery, as indicated by the decrease of D∗ in the outer leaflet. Controls involving exchange of DOPC into the outer leaflet with a DOPC-mβCD solution did not show an effect on D∗, meaning that the decrease in D∗ is not due to the mβCD itself.
Figure 2.
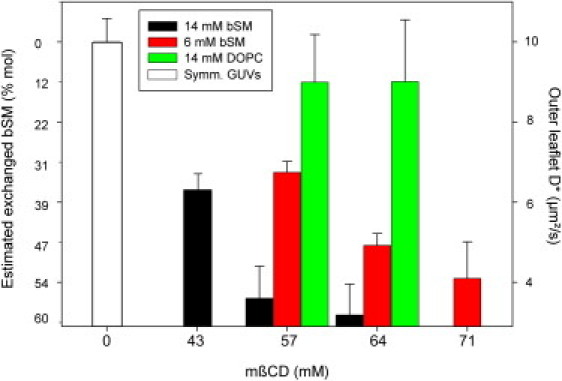
Estimation of the mol % of (exchanged) bSM in the outer leaflet of asymmetric GUVs prepared with different concentrations of mβCD and bSM in the donor solution. The mβCD concentrations are those used when the mβCD-bSM mixture is added to the acceptor GUVs at room temperature for 30 min. The bSM concentrations shown are those used when mβCD is first mixed with bSM. The amount of bSM in the GUV was estimated by measuring D∗ in the outer leaflet (see text).
By calibrating from the dependence of D∗ on the SM content in symmetric DOPC/bSM vesicles (Fig. S3), we can estimate the extent of lipid exchange, which reaches ∼60 mol % bSM in the outer leaflet. As a practical note, we observed that increasing the concentration of mβCD and decreasing that of bSM in the donor solution lowered the asymmetric GUV yield, with 14 mM bSM and <60 mM mβCD being the most convenient conditions we tried.
As an example of a problem that can be investigated through the use of FCS applied to asymmetric GUVs, we show how coupling of the physical state between the inner and outer leaflets (interleaflet coupling) can be studied. Interleaflet coupling has been invoked to explain domain induction and registration in opposite leaflets, but its dependence on lipid structure is poorly understood (2). Because an exhaustive study would exceed the scope of this work, we simply demonstrate here how FCS on asymmetric GUVs can address this question. We prepared asymmetric GUVs with DOPC in the inner leaflet and different ratios of DOPC/bSM in the outer leaflet. The black triangles in Fig. 3 show D∗ values in the inner and outer leaflets for four samples containing different amounts of bSM in the outer leaflet. Although the outer leaflet D∗ can be reduced to ∼30% of the value in pure DOPC vesicles, as shown by the x-axis value for the leftmost black point in Fig. 3, the inner leaflet seems almost unaware of the presence of bSM, and its D∗ is comparable to that of symmetric DOPC GUVs. The weak coupling can be quantified by the small slope of the line going through the experimental points.
Figure 3.
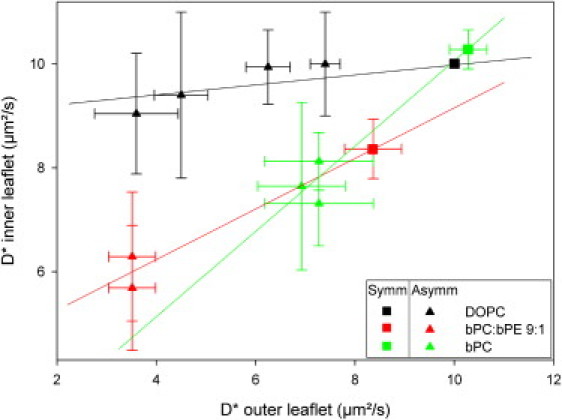
Correlation between D∗ in the outer and inner leaflets for different asymmetric GUV preparations. The colors indicate the lipid in the inner leaflet, and that mixed in the outer leaflet with the bSM. Solid lines serve as a guide for the eye. Each point represents the average D∗ measured in ∼10 vesicles of a single GUV preparation. Symm: Upper-right squares, which refer to the GUVs before lipid exchange. Asymm: GUVs after exchange.
In contrast, for GUVs composed of brain PC (bPC) or brain PC/brain PE (bPE) mixtures, exchange of bSM into the outer leaflet results in a large decrease in the inner leaflet D∗ (green and red points). The slope of the line passing through the points measured for these GUVs seems significantly higher than that for the DOPC mixtures. Of interest, in planar bilayers, phase separation in one leaflet has been shown to induce domain formation in an opposing bPC (or bPC/bPE) but not in a DOPC leaflet (3). Thus, the GUV results most likely reflect a physical coupling between the two leaflets when brain lipids are used. An alternative explanation—that bPC bilayers are characterized by faster flip-flop rates and thus form symmetric bilayers after bSM is introduced—is unlikely, on the basis of supported bilayer results (3) and control experiments (Fig. S4).
To mimic natural membranes more closely, we incorporated cholesterol into asymmetric GUVs. We found that simple inclusion of ∼30 mol % cholesterol in symmetric DOPC GUVs caused a ∼40% decrease of D∗. Similarly, when symmetric DOPC GUVs were incubated with ∼10–12 mM cholesterol-mβCD complexes (see Supporting Material), there was a ∼40% decrease of D∗ relative to its initial value. Incubation of asymmetric GUVs that had a bSM-DOPC mixture outside and DOPC inside, with ∼7–10 mM cholesterol-mβCD complexes, resulted in a >50% drop of D∗ (measured in the inner leaflet). Thus, it is possible to incorporate a significant amount of cholesterol into asymmetric GUVs. Our previous studies in asymmetric SUVs (8) showed that cholesterol incorporation does not destroy asymmetry.
In conclusion, we have presented a method to produce asymmetric GUVs. As we previously showed for SUVs (8), this method can be finely tuned to create bilayers of different compositions. Also, we have shown that FCS can monitor the asymmetry and physical properties of the two leaflets independently. Finally, to illustrate an application of this method, we described an approach to systematically study the molecular basis of interleaflet coupling. The issue of whether the apparent coupling between bSM outer leaflets and brain lipid inner leaflets is due to a high tendency of brain lipids to form ordered states relative to DOPC, or is the result of asymmetric (interdigitating) acyl chains, is worthy of further study. Future work may also address cholesterol-induced phase separation and the lateral organization of integral membrane proteins in asymmetric GUVs.
Acknowledgments
We thank N. Kahya and U. Golebiewska for useful discussions and S. McLaughlin for the use of the Zeiss ConfoCor2.
This work was supported by the National Institutes of Health (grant GM 48596). S.C. is a Howard Hughes Medical Institute Fellow of the Life Sciences Research Foundation.
Supporting Material
References
- 1.Kiessling V., Wan C., Tamm L.K. Domain coupling in asymmetric lipid bilayers. Biochim. Biophys. Acta. 2009;1788:64–71. doi: 10.1016/j.bbamem.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida P.F.F. Thermodynamics of lipid interactions in complex bilayers. Biochim. Biophys. Acta. 2009;1788:72–85. doi: 10.1016/j.bbamem.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Wan C., Kiessling V., Tamm L.K. Coupling of cholesterol-rich lipid phases in asymmetric bilayers. Biochemistry. 2008;47:2190–2198. doi: 10.1021/bi7021552. [DOI] [PubMed] [Google Scholar]
- 4.Garg S., Rühe J., Naumann C.A. Domain registration in raft-mimicking lipid mixtures studied using polymer-tethered lipid bilayers. Biophys. J. 2007;92:1263–1270. doi: 10.1529/biophysj.106.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins M.D., Keller S.L. Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc. Natl. Acad. Sci. USA. 2008;105:124–128. doi: 10.1073/pnas.0702970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pautot S., Frisken B.J., Weitz D.A. Engineering asymmetric vesicles. Proc. Natl. Acad. Sci. USA. 2003;100:10718–10721. doi: 10.1073/pnas.1931005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamada T., Miura Y., Takagi M. Construction of asymmetric cell-sized lipid vesicles from lipid-coated water-in-oil microdroplets. J. Phys. Chem. B. 2008;112:14678–14681. doi: 10.1021/jp807784j. [DOI] [PubMed] [Google Scholar]
- 8.Cheng H.T., Megha, and E., London Preparation and properties of asymmetric vesicles that mimic cell membranes: effect upon lipid raft formation and transmembrane helix orientation. J. Biol. Chem. 2009;284:6079–6092. doi: 10.1074/jbc.M806077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahya N., Scherfeld D., Schwille P. Probing lipid mobility of raft-exhibiting model membranes by fluorescence correlation spectroscopy. J. Biol. Chem. 2003;278:28109–28115. doi: 10.1074/jbc.M302969200. [DOI] [PubMed] [Google Scholar]
- 10.Kucherak O.A., Oncul S., Klymchenko A.S. Switchable Nile red-based probe for cholesterol and lipid order at the outer leaflet of biomembranes. J. Am. Chem. Soc. 2010;132:4907–4916. doi: 10.1021/ja100351w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


