Abstract
OBJECTIVE
To develop and validate an algorithm to identify and classify noninvasive infections due to Staphylococcus aureus by using positive clinical culture results and administrative data.
DESIGN
Retrospective cohort study.
SETTING
Veterans Affairs Maryland Health Care System.
METHODS
Data were collected retrospectively on all S. aureus clinical culture results from samples obtained from nonsterile body sites during October 1998 through September 2008 and associated administrative claims records. An algorithm was developed to identify noninvasive infections on the basis of a unique S. aureus–positive culture result from a nonsterile site sample with a matching International Classification of Diseases, Ninth Revision (ICD-9-CM), code for infection at time of sampling. Medical records of a subset of cases were reviewed to find the proportion of true noninvasive infections (cases that met the Centers for Disease Control and Prevention National Healthcare Safety Network [NHSN] definition of infection). Positive predictive value (PPV) and negative predictive value (NPV) were calculated for all infections and according to body site of infection.
RESULTS
We identified 4,621 unique S. aureus–positive culture results, of which 2,816 (60.9%) results met our algorithm definition of noninvasive S. aureus infection and 1,805 (39.1%) results lacked a matching ICD-9-CM code. Among 96 cases that met our algorithm criteria for noninvasive S. aureus infection, 76 also met the NHSN criteria (PPV, 79.2% [95% confidence interval, 70.0%–86.1%]). Among 98 cases that failed to meet the algorithm criteria, 80 did not meet the NHSN criteria (NPV, 81.6% [95% confidence interval, 72.8%–88.0%]). The PPV of all culture results was 55.4%. The algorithm was most predictive for skin and soft-tissue infections and bone and joint infections.
CONCLUSION
When culture-based surveillance methods are used, the addition of administrative ICD-9-CM codes for infection can increase the PPV of true noninvasive S. aureus infection over the use of positive culture results alone.
During the past decade, there has been a dramatic increase in the incidence of infections due to community-associated methicillin-resistant Staphylococcus aureus (MRSA), many of which are skin and soft-tissue infections.1–3 Although the incidence of S. aureus infection in specific populations, and according to certain infection types, has been reported, the overall burden of S. aureus infection remains unclear. To improve the public health response to the nationwide epidemic of community-associated MRSA infections, a better estimate of the overall burden and distribution of all S. aureus infections is necessary. This requires a validated approach to identifying and classifying S. aureus infections according to type and severity, which is relatively straightforward for invasive S. aureus infections, because the presence of S. aureus in a specimen obtained from a normally sterile body site is highly predictive of infection. However, invasive S. aureus infections comprise only a small proportion of S. aureus infections. The greater challenge is to identify and classify noninvasive S. aureus infections, largely because in a clinical culture, S. aureus that is isolated from a sample obtained from a nonsterile body site can represent either infection or colonization. Although data on culture results are often available from large clinical databases, many of these results represent colonization rather than infection, because S. aureus is an opportunistic pathogen.
Thus, a more predictive approach to identifying noninvasive S. aureus infections is needed, particularly because a substantial proportion of infections due to community-associated MRSA—specifically, skin and soft-tissue infections—are noninvasive.1,4 In addition, there is a need for an automated approach to identifying S. aureus infections for use in large epidemiological research studies for which medical record review is impractical or when medical records are unavailable. To address these needs, we performed a retrospective study in a large population of patients who receive healthcare services through the Veterans Affairs Maryland Health Care System (VAMHCS). Our research objective was to develop and estimate the accuracy of a new algorithm to identify and classify noninvasive S. aureus infections.
METHODS
Design and Setting
This retrospective cohort study was conducted in the VAMHCS, which operates approximately 730 inpatient (mostly nonacute care) beds and provides comprehensive care to approximately 49,000 veterans annually. VAMHCS electronic medical records, administrative data (including International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM], codes), and results of microbiological cultures served as the primary data sources for this study. The study took place during the period from October 1, 1998, through September 30, 2008. The VAMHCS Research and Development Committee and the institutional review board of the University of Maryland, Baltimore, approved this study.
Case Definition
Data on all S. aureus–positive results from nonsurveillance specimens obtained during the period from October 1, 1998, through September 30, 2008, were identified. Only S. aureus–positive clinical culture results from samples that were obtained from a nonsterile body site were included in the study. Clinical culture results from samples obtained from a normally sterile body site (blood, cerebrospinal fluid, pleural fluid, pericardial fluid, peritoneal fluid, joint or synovial fluid, bone, internal body site, muscle, or other normally sterile site, including deep tissue that is sampled during surgery), as defined by the US Centers for Disease Control and Prevention (CDC) Active Bacterial Core surveillance systems criteria, were omitted.5 A unique culture was defined as the first S. aureus–positive clinical culture result from a sample of a nonsterile site obtained from the same patient during a 6-month period without a concurrent S. aureus–positive culture result from a sterile site specimen.
We collected data on all ICD-9-CM administrative claim codes and corresponding ICD-9-CM terms associated with either the outpatient visit or the inpatient assessment during which each unique S. aureus–positive culture result from a nonsterile site specimen was obtained. For a S. aureus–positive culture result from a nonsterile site specimen obtained during an outpatient visit for which the patient was not subsequently hospitalized within a 72-hour period after culture sampling, we obtained all ICD-9-CM codes associated with all of the patient’s outpatient visits on the day of culture sampling. For a S. aureus–positive culture result from a non-sterile site specimen obtained during an outpatient visit for which the patient was subsequently hospitalized within a 72-hour period after culture sampling, we obtained the outpatient visit ICD-9-CM codes along with the hospital discharge ICD-9-CM codes. Last, we collected the ICD-9-CM hospital discharge codes for all S. aureus–positive culture results from nonsterile site specimens obtained during a hospitalization, rehabilitation, or long-term care stay.
We developed an algorithm (Figure 1) to identify noninvasive S. aureus infections on the basis of a confirmed S. aureus–positive culture result from a nonsterile site specimen without a concurrent S. aureus–positive culture result from a sterile site during the same 6-month period, and at least 1 ICD-9-CM code for S. aureus–related infection from the visit or hospitalization associated with the positive culture result. A comprehensive list of ICD-9-CM codes for S. aureus–related infections was developed on the basis of ICD-9-CM codes used in previous studies that described S. aureus infections (refer to Appendix, Table A1, for the complete list of codes). ICD-9-CM codes were categorized according to the site of infection most consistent with the associated code (eg, septic arthritis was classified as a bone and joint infection). Once cultures were linked with respective ICD-9-CM codes, each code was categorized according to type of infection. Bone and joint infections were ranked as the most important and most likely associated with S. aureus, followed in order by infections of skin and soft tissue, endovascular site, respiratory tract, intra-abdominal or pelvic site, central nervous system, urinary tract, nonspecific site, and other or site not specified. For culture results with more than 1 matching ICD-9-CM code, the primary code was defined as the code that corresponded to the most likely infection type.
FIGURE 1.
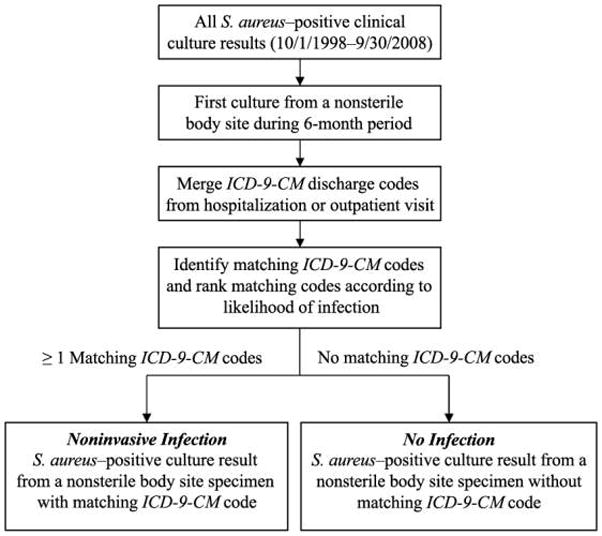
Algorithm to identify noninvasive infections due to Staphylococcus aureus on the basis of a confirmed S. aureus–positive culture result from a specimen obtained from a nonsterile body site (all cultures not classified as coming from a normally sterile body site according to the US Centers for Disease Control and Prevention Active Bacterial Core surveillance systems criteria) and at least 1 International Classification of Diseases, Ninth Revision (ICD-9-CM), code for S. aureus–related infection (ICD-9-CM code from outpatient visit or hospitalization during which culture sample was obtained or hospitalization within 72 hours of culture sampling).
Statistical Analysis
Among all unique S. aureus–positive culture results obtained from samples of nonsterile body sites, we calculated the proportions with and without a matching ICD-9-CM code for infection and described the site of infection for the ones with a matching code. To validate this approach, we randomly selected a sample of cases that met the definition of noninvasive infection (ie, positive culture result with matching ICD-9-CM code) and a sample of cases that did not meet the definition (ie, positive culture result without matching ICD-9-CM code). Focusing on the clinical and laboratory information pertaining to the date(s) of hospitalization or outpatient visit associated with the date of the positive S. aureus culture result, the first author (L.A.T.) performed a detailed review of the medical records of all cases to identify and confirm the presence or absence of infection. A “true infection” was determined by using the CDC National Healthcare Safety Network (NHSN) definitions (reference standard), which were adapted for our study by sorting according to body site of infection (defined as bone and joints, skin and soft tissue, endovascular site, respiratory tract, intra-abdominal or pelvic site, central nervous system, or urinary tract) and removing the requirement that the infection be healthcare associated.6 The positive predictive value (PPV) was calculated as the probability that an infection identified with use of our algorithm was confirmed as an infection according to the reference standard criteria. Analogously, the negative predictive value (NPV) was calculated as the probability that a case that did not meet the definition of noninvasive S. aureus infection (positive culture result that lacked a matching ICD-9-CM code for infection) on the basis of our algorithm also did not meet the reference standard criteria. A 20% subset of the medical records was randomly selected and independently reviewed by the senior author (M.-C.R.). The Cohen κ was calculated to assess the level of interreviewer agreement.7
The validation study sample size was based on the minimum number of cases required to achieve 95% confidence that the true (population) PPV was within 10% of the observed (sample) PPV value.8,9 Similar calculations were performed to determine the appropriate sample size required to estimate the NPV in the validation study.
RESULTS
We identified 4,621 unique S. aureus–positive clinical culture results from samples obtained during the period October 1, 1998, through September 30, 2008, of which 2,816 positive results (60.9%; 2,511 unique patients) met the definition of noninvasive S. aureus infection and 1,805 positive results (39.1%; 1,666 unique patients) did not meet the definition because they lacked a matching ICD-9-CM infection code. Among all noninvasive S. aureus infections, 1,807 (64%) of 2,816 were methicillin resistant. Skin and soft tissue were the most common sites of infection, accounting for 1,704 (60.5%) of the 2,816 noninvasive S. aureus infections, according to the highest priority (most likely noninvasive infection) matching ICD-9-CM code. The second most common site was the respiratory tract, accounting for 345 (12.3%) infections, followed by the urinary tract (322 infections [11.4%]), non-specified site of infection (184 infections [6.5%]), bone and joints (173 infections [6.1%]), endovascular site (40 infections [1.4%]), intra-abdominal or pelvic site (23 infections [0.8%]), other or infection not specified (21 infections [0.7%]), and central nervous system (4 infections [0.1%]). The site from which the culture sample was obtained matched the site of infection, as determined according to ICD-9-CM code, for 163 (94%) of 173 bone and joint infections, for 1,620 (95%) of 1,704 skin and soft-tissue infections, for 289 (84%) of 345 respiratory tract infections, and for 180 (56%) of 322 urinary tract infections.
Validation of Algorithm to Identify and Classify Noninvasive S. aureus Infections
Among a sample of 96 cases that met our algorithm criteria for noninvasive S. aureus infection, 76 (79.2% [95% confidence interval {CI}, 70.0%–86.1%]) cases also met the criteria for a clinical infection on the basis of the NHSN surveillance definitions (Table 1). On the basis of CIs, these results suggest that the probability that an infection identified with use of our case definition is also an infection on the basis of accepted clinical definitions is in the range 70.0%–86.1%. Among the 2,816 unique S. aureus–positive clinical culture results with matching ICD-9-CM codes, we could expect that approximately 2,225 were true noninvasive S. aureus infections on the basis of estimated PPV. There was substantial agreement (κ = 0.60–0.70) between reviewer findings in the mutually reviewed subset of records. The estimated PPV varied widely according to infection site: 95% for skin and soft tissue, 78% for bone and joints, 67% for endovascular sites, 55% for respiratory tract, 71% for nonspecific sites, and 22% for urinary tract (Table 2).
TABLE 1.
Positive Predictive Value (PPV) and Negative Predictive Value (NPV) of Case Definition of Noninvasive Infection due to Staphylococcus aureus from a Random Sample of Patients with S. aureus–Positive Culture Results from a Sample Obtained from a Nonsterile Body Site
| Variable | Noninvasive infection due to S. aureus present according to reference standard |
Predictive value (% [95% CI]) | |
|---|---|---|---|
| Yes | No | ||
| Positive culture result and matching ICD-9-CM code | 76 | 20 | PPV, 76/96 (79.2 [70.0–86.1]) |
| Positive culture result alone | 18 | 80 | NPV, 80/98 (81.6 [72.8–88.0]) |
NOTE. Reference standard as determined by means of medical chart review and application of the CDC/NHSN surveillance definitions for specific type of infection. Cases of noninvasive infection due to S. aureus that meet the criteria are those with a positive clinical culture result and a matching International Classification of Diseases, Ninth Revision (ICD-9-CM), code for infection (refer to Appendix 1 for list of codes) from a same-day outpatient encounter or associated hospitalization. Cases that do not meet the criteria are those with positive clinical culture result without ICD-9-CM code for infection. CI, confidence interval.
TABLE 2.
Positive Predictive Value (PPV) According to Site of Infection for Noninvasive Infections due to Staphylococcus aureus
| Site of infection | True infectionsa (n = 76) | False-positive resultsb (n = 20) | PPV, % (95% CI)c |
|---|---|---|---|
| Skin and soft tissue | 54 | 3 | 95 (86–98) |
| Bone and joints | 7 | 2 | 78 (45–94) |
| Endovascular site | 2 | 1 | 67 (21–94) |
| Respiratory tract | 6 | 5 | 55 (28–79) |
| Nonspecific site | 5 | 2 | 71 (36–92) |
| Urinary tract | 2 | 7 | 22 (8–64) |
NOTE. Site of infection based on matching ICD-9-CM code for infection. CI, confidence interval.
Cases that were identified with use of algorithm and also meet reference standard criteria.
Cases that were identified with use of algorithm but do not meet reference standard criteria.
According to site of infection based on random sample of validation cohort.
Among 98 randomly selected cases that failed to meet the algorithm criteria for noninvasive S. aureus infection, 80 (81.6% [95% CI, 72.8%–88.0%]) cases did not meet the NHSN clinical criteria for infection on the basis of medical record review (Table 1). These results suggest a case definition NPV (the absence of an infection determined on the basis of the clinical definition although a positive culture result was present) of approximately 72.8%–88.0%. Among the 1,805 unique S. aureus–positive clinical culture results that lacked a matching ICD-9-CM code for infection, approximately 332 could represent a clinical infection on the basis of estimated NPV.
Increase in PPV with Matching ICD-9-CM Code
To estimate the increase in PPV with the additional requirement of a matching ICD-9-CM code for infection, we also estimated the PPV of any S. aureus–positive clinical culture result. On the basis of 79.2% PPV, we calculated that among the noninvasive S. aureus infections (n = 2,816), 2,230 (2,816 × 0.792) cases would represent clinical infection on the basis of the minimum requirement of S. aureus–positive clinical culture; and on the basis of 81.6% NPV, we calculated that among the cases that did not meet the definition for infection (n = 1,805 ), 332 ([1,805 × 0.184], where 0.184 is 1 – NPV) would represent clinical infection on the basis of the minimum requirement of S. aureus–positive clinical culture, for a total of 2,562 (55.4%) of 4,621 cases (Table 3). Thus, the additional requirement of a matching ICD-9-CM code for infection with S. aureus–positive clinical culture result increases the PPV for a true noninvasive S. aureus infection over that of a positive clinical culture result from 55.4% to 79.2%, a 23.8% increase.
TABLE 3.
Estimated Positive Predictive Value (PPV) of Any Staphylococcus aureus–Positive Culture Result from a Sample Obtained from a Nonsterile Body Site
| Variable | Estimated no. |
Total infections, no. | Estimated PPV, % | |
|---|---|---|---|---|
| Infection present | Infection absent | |||
| Positive culture result with matching ICD-9-CM code | 2,230 | 586 | 2,816 | 79.2 |
| Positive culture result without matching ICD-9-CM code | 332 | 1,473 | 1,805 | 18.4 |
| All positive culture results | 2,562 | 2,059 | 4,621 | 55.4 |
NOTE. ICD-9-CM, International Classification of Diseases, Ninth Revision.
DISCUSSION
In this study, we measured the validity of an algorithm consisting of clinical culture plus ICD-9-CM coding for identification of noninvasive S. aureus infection. On the basis of medical record documentation of clinical infection for a representative sample of cases that meet the algorithm criteria and cases that do not meet the criteria, we calculated a PPV of 79.2% (95% CI, 70.0%–86.1%) and an NPV of 81.6% (95% CI, 72.8%–88.0%). Furthermore, we estimated that coding in addition to culture may increase the probability of identifying true infection by approximately 23.8% over positive clinical culture result alone. The sites of infection with the highest PPVs were skin and soft tissue and bone and joints. We found some discordance between the body site from which the specimen was collected and the ICD-9-CM code for infection, specifically for cultures of urine and sputum samples, which coincides with findings that the algorithm was less predictive of true infection for respiratory tract and urinary tract sites. Although our calculated PPV for pneumonia was only 54.5% (95% CI, 28.0%–78.7%), it is consistent with other reported estimates in the literature.10,11 We attribute this low PPV to the difficulty in the clinical diagnosis of these infections, particularly of pneumonia, as a result of ambiguity in clinical symptoms and radiographic results as determined during the review of medical records. Requiring a match between the culture site and the ICD-9-CM–based site of infection would have been technically difficult, because the culture sampling site is not always consistent with the diagnosis. In addition, multiple culture samples are often obtained from a patient during a single infection period, which would complicate any matching approach.
A study by Sherman et al12 assessed the sensitivity, PPVs, and NPVs of identifying healthcare-associated infections on the basis of infection-specific ICD-9-CM codes assigned at hospital discharge. True infections were those that met the definitions of the CDC National Nosocomial Infections Surveillance system (the predecessor to the CDC NHSN) on the basis of medical record review. They reported a sensitivity of 61% and a PPV of only 20% for identification of healthcare-associated infections on the basis of ICD-9-CM coding. They concluded that review of administrative records did not provide accurate data and often led to misclassification of the 4 most common types of healthcare-associated infection (central line–associated bloodstream infection, surgical-site infection, catheter-associated urinary tract infection, and ventilator-associated pneumonia). Such infections are commonly caused by S. aureus; therefore, these findings are relevant to concerns about the limitations of ICD-9-CM coding alone to identify S. aureus infection. Although a few studies have examined the validity of ICD-9-CM coding and other healthcare administrative data, such as antimicrobial use data in predicting infections, they assessed only surgical-site infections or did not focus specifically on S. aureus infections.13,14 A few studies have attempted to estimate the national burden of S. aureus infection but used administrative coding only and not clinical culture results and coding.15–17
There are some limitations to our study. First, our use of clinical culture results to define infection leads to an underestimation of the true number of infections, which leads to a decrease in sensitivity, because for some S. aureus infections it is not often feasible to culture (eg, cellulitis) and others are not always cultured (eg, furuncles). However, because many of these infections can be due to bacteria other than S. aureus, the requirement of a positive culture result increases the specificity of our definition. Second, we cannot exclude the possibility that there was a change in microbiologic culturing practices during the 10-year study period. However, the annual number of S. aureus–positive culture results was similar from year to year and there was no significant change in the size of our veteran population (data not shown). Third, the coding practices at the VAMHCS may differ from those used at other centers; for example, inpatient hospitalizations may have fewer codes assigned than in other types of hospitals. However, with the requirement of a positive culture result and ICD-9-CM code, we would expect differences in coding practices to have a small effect on our results.
Our study has several important implications. It is the first study to assess the validity of the use of clinical culture results plus ICD-9-CM codes for identifying noninvasive S. aureus infections. In addition, this study revealed that surveillance with use of positive culture results alone can lead to low predictability of true infection. Our algorithm was highly predictive of noninvasive S. aureus infections, specifically for skin and soft-tissue infections. However, it is also important to recognize that a single automated algorithm is unlikely to accurately identify all infections, particularly those with complex clinical presentations. These results offer a starting point from which future epidemiological studies, using large clinical databases, can be focused to identify and quantify noninvasive S. aureus infections, which will provide a better estimate of the public health burden of S. aureus infections.
Acknowledgments
We acknowledge the contributions of Jingkun Zhu in abstracting the culture and administrative data.
Financial support. This work was supported by a VA Clinical Science Research and Development Merit Award to M.C.R. J.P.F. was supported by a NIH NIAID K01 award (5K01AI071015–02) and A.D.H. by a NIH NIAID K24 award (1K24AI079040–01A1) while this work was performed.
APPENDIX
TABLE A1.
International Classification of Diseases, Ninth Revision, Codes for Staphylococcus aureus–Related Infections
| Site of infection | ICD-9-CM code |
|---|---|
| Bone and joints | |
| Septic arthritis18 | 711.00–711.09, 996.66, 996.67 |
| Joint effusions and/or pain | 719.06–.08 (.42, .49) |
| Osteomyelitis18 | 730.00–730.09, 730.10–730.19 (.9, .20–.29, .81, .88, .96–.97) |
| Skin and soft tissue | |
| Oral soft-tissue disease, not elsewhere classified | 528.9 (.3) |
| Anal rectal abscess | 566 |
| Inflammatory disease of breast19 | 611.0 |
| Infective mastitis or nonpurulent mastitis19 | 675.2, 771.50 |
| Breast abscess | 675.1 |
| Carbuncle or furuncle2,19 | 680 (.0–.9) |
| Felon2,19 | 681.01 |
| Cellulitis and abscess of finger and toe2,19 | 681.00, 681.9, 681.10 |
| Other cellulitis and abscess2,19 | 682 (.0–.9) |
| Acute lymphadenitis | 683 |
| Impetigo2,19 | 684 |
| Pilonidal cyst with abscess | 685.0 |
| Pyoderma | 686.0 |
| Unspecified local infection19 | 686.9 |
| Other specified diseases of hair and hair follicles2,19 | 704.8 |
| Hydradenitis2,19 | 705.83 |
| Myositis19 | 728.0 |
| Gangrene | 785.4 |
| Posttraumatic wound infection, not elsewhere classified | 958.3 |
| Amputation stump infection, chronic | 997.62 |
| Surgical-site infection18 | 998.3 (.31–.32), 998.5 (.51, .59) |
| Endovascular system | |
| Tricuspid valve disease | 397.0 |
| Endocarditis18 | 421.0 (.9), 996.61 |
| Aortic valve disorder | 424.1 |
| Endocarditis, not otherwise specified | 424.90–.91, .99 |
| Phlebitis and thrombophlebitis of superficial veins of upper extremities | 451.82 |
| Thrombophlebitis, not otherwise specified | 451.9 |
| Due to vascular device, implant, and graft | 996.62 |
| Respiratory tract | |
| Bacterial pneumonia, not otherwise specified | 482.9 |
| Pneumonia, organism, not otherwise specified | 486 |
| Bacterial pleural effusion, not tuberculosis | 511.1 |
| S. aureus pneumonia15,16,18 | 482.41 |
| Pneumonia due to Staphylococcus | 482.4 |
| Empyema without fistula | 510.9 |
| Intra-abdominal or pelvic | |
| Staphylococcal enterocolitis8 | 008.41 |
| Suppurative peritonitis, not elsewhere classified | 567.2 |
| Peritonitis, not otherwise specified | 567.9 |
| Acute pancreatitis | 577.0 |
| Cystitis, not otherwise specified | 595.9 |
| Acute cholecystitis | 575.0 |
| Central nervous system | |
| Meningitis, not otherwise specified | 322.9 |
| Central nervous system abscess, not otherwise specified | 324.9 |
| Intraspinal abscess | 324.1 |
| Urinary tract | |
| Chronic pyelonephritis, not otherwise specified | 590 |
| Acute pyelonephritis, not otherwise specified | 590.10 |
| Urinary tract infection, not otherwise specified | 599.0 |
| Bacteremia without focus | |
| Bacteremia18 | 038.1, 790.7 |
| Bacterial diseases, not elsewhere classified | 040.89 |
| Bacterial infection, not otherwise specified | 041.9 |
| S. aureus, nonspecifica | |
| S. aureus septicemia15,16,18 | 038.11 (.1, .8, .9) |
| S. aureus infection in conditions classified elsewhere or of unspecified site15,16,18 | 041.11 |
| Methicillin-resistant S. aureus18 | V09.0 |
| Vancomycin-resistant S. aureus18 | V09.8 |
| Other or not specifieda | |
| Infection with microbial resistance, cephalosporin and other | V09.1 |
| Infection with microbial resistance, other specified antimicrobial | V09.70 |
| Infection with microbial resistance, other specified drugs | V09.80, .81 |
| Infection complicating medical care, not elsewhere classified | 999.3 |
NOTE. Values in parentheses are additional subcodes added subsequent to review of study ICD-9-CM codes.
These ICD-9-CM codes are generally used with an infection code; V codes are designed to modify other codes.
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.
The views expressed by M.S. are not necessarily those of the US Food and Drug Administration.
References
- 1.Johnson JK, Khoie T, Shurland S, Kreisel K, Stine OC, Roghmann MC. Skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus USA300 clone. Emerg Infect Dis. 2007;13(8):1195–1200. doi: 10.3201/eid1308.061575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA., Jr Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2008;51(3):291–298. doi: 10.1016/j.annemergmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 4.King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144(5):309–317. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 5.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 6.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70(4):213–220. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 8.Arkin CF, Wachtel MS. How many patients are necessary to assess test performance? JAMA. 1990;263(2):275–278. [PubMed] [Google Scholar]
- 9.Li J, Fine J. On sample size for sensitivity and specificity in prospective diagnostic accuracy studies. Stat Med. 2004;23(16):2537–2550. doi: 10.1002/sim.1836. [DOI] [PubMed] [Google Scholar]
- 10.Skull SA, Andrews RM, Byrnes GB, et al. ICD-10 codes are a valid tool for identification of pneumonia in hospitalized patients aged ≤65 years. Epidemiol Infect. 2008;136(2):232–240. doi: 10.1017/S0950268807008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronsky D, Haug PJ, Lagor C, Dean NC. Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328. doi: 10.1177/1062860605280358. [DOI] [PubMed] [Google Scholar]
- 12.Sherman ER, Heydon KH, St John KH, et al. Administrative data fail to accurately identify cases of healthcare-associated infection. Infect Control Hosp Epidemiol. 2006;27(4):332–337. doi: 10.1086/502684. [DOI] [PubMed] [Google Scholar]
- 13.Platt R, Yokoe DS, Sands KE. Automated methods for surveillance of surgical site infections. Emerg Infect Dis. 2001;7(2):212–216. doi: 10.3201/eid0702.010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoe DS, Classen D. Improving patient safety through infection control: a new healthcare imperative. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S3–S11. doi: 10.1086/591063. [DOI] [PubMed] [Google Scholar]
- 15.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13(12):1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuehnert MJ, Hill HA, Kupronis BA, Tokars JI, Solomon SL, Jernigan DB. Methicillin-resistant-Staphylococcus aureus hospitalizations, United States. Emerg Infect Dis. 2005;11(6):868–872. doi: 10.3201/eid1106.040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noskin GA, Rubin RJ, Schentag JJ, et al. National trends in Staphylococcus aureus infection rates: impact on economic burden and mortality over a 6-year period (1998–2003) Clin Infect Dis. 2007;45(9):1132–1140. doi: 10.1086/522186. [DOI] [PubMed] [Google Scholar]
- 18.Noskin GA, Rubin RJ, Schentag JJ, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med. 2005;165(15):1756–1761. doi: 10.1001/archinte.165.15.1756. [DOI] [PubMed] [Google Scholar]
- 19.McCaig LF, McDonald LC, Mandal S, Jernigan DB. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg Infect Dis. 2006;12(11):1715–1723. doi: 10.3201/eid1211.060190. [DOI] [PMC free article] [PubMed] [Google Scholar]


