Abstract
Although parents and children are thought to influence one another's affect and behavior, few studies have examined the direction of effects from children to parents, particularly with respect to parental psychopathology. We tested the hypothesis that children's affective characteristics are associated with the course of mothers’ depressive symptoms. Children's affect expression was observed during a series of mother–child interaction tasks, and children's resting frontal electroencephalogram (EEG) asymmetry was assessed in a psychophysiology laboratory. Mothers’ depressive symptoms were assessed at two time points, approximately one year apart, at the mother–child interaction visits. Depressive symptoms increased over time for mothers with a history of childhood-onset depression whose children exhibited right frontal EEG asymmetry. Depressive symptoms were associated with high child negative affect at both time points for mothers whose children exhibited right frontal EEG asymmetry. Cross-lagged models with a subset of participants provided some evidence of both parent-to-child and child-to-parent directions of effects. Findings suggest that akin to other interpersonal stressors, children's affective characteristics may contribute to maternal depressive symptoms.
Keywords: Maternal depression, Child effects, Negative affect, Frontal EEG asymmetry
Investigations of families with a depressed parent have primarily focused on the effect of parental depression on child outcomes (Beardslee et al. 1998; Downey and Coyne 1990) and difficulties in interpersonal functioning (Cummings and Davies 1994). It is widely acknowledged that child characteristics can influence child development by eliciting specific parental behavior, which in turn influences subsequent child behavior (Bell and Harper 1977; Grusec and Lytton 1988; Scarr and McCartney 1983). In addition, because affect can be regulated by others as well as oneself, children can also influence parents’ behavior by serving as regulators of their parents’ affect (Cole et al. 2004; Eisenberg and Spinrad 2004). However, very few studies have examined whether child attributes are associated with the course of parental psychopathology. The current study sought to advance our understanding of child effects in relation to parental psychopathology by investigating the longitudinal effects of children's affective attributes in relation to maternal depressive symptomatology.
The transactional perspective emphasizes the reciprocal interplay of parent and child influences on outcomes over time (Bell and Harper 1977; Field 1994; Grusec and Lytton 1988; Sameroff and Chandler 1975). Bell's (1968) seminal paper argued that many “parent effects” findings in the socialization literature could be re-interpreted as supporting “child effects” on parents’ behavior. Over the past two decades, greater emphasis has been paid to reciprocal influences in outcomes such as early child psychopathology (Eisenberg et al. 2001; Sanson et al. 1991). Indeed, the field of affect regulation has addressed the importance of viewing affect as regulated by others (e.g., Cole et al. 2004).
Child effects on parental depression are hypothesized to occur because depression appears to be highly influenced by the interpersonal context (Hammen 2003). From an evolutionary perspective, the basis of depressed mood is interpersonal, with increases in depressed mood postulated to follow changes in social functioning (Allen and Badcock 2003). From a stressful life events perspective, interpersonal stressors contribute importantly to the onset and maintenance of depression in women in particular (Hammen 2003). Depressive episodes are likely to occur in conjunction with events such as family conflict and strained parent–child interactions (Hammen 2003), and interpersonal stress has been found to mediate changes in women's depressive symptoms over time (Davila et al. 1995). These findings suggest that the quality of the mother–child relationship is a potential influence on the course of maternal depressive symptoms.
One domain of children's behavior may be particularly salient in relation to the course of maternal depressive symptoms: Children's affective characteristics, including the expression and regulation of positive and negative affect. Symptoms of maternal depression may be particularly sensitive to individual differences in children's affect regulation because depression is considered to involve fundamental affect regulation difficulties (Gross and Muñoz 1995). Indeed, disruption of affect is thought to be a common and important feature in families of depressed individuals (Sheeber et al. 2001; Silk et al. 2006). While typical mothers might become affectively dysregulated in response to their children's negative affect, mothers with depression might be especially vulnerable, experiencing longer and more intense negative affect in response to the expression of child negative affect. This vulnerability to affective dysregulation in mothers with depression could occur as a result of tendencies toward high trait levels of negative affect (Kendler et al. 1993); genetic predisposition to affective reactivity (Caspi et al. 2003); physiological characteristics, including greater response to negative affective stimuli in brain regions such as the amygdala (Surguladze et al. 2005); or cognitive processes such as rumination about negative affect (Nolen-Hoeksema 2000) and beliefs of low parenting self-efficacy (Teti and Gelfand 1991). Furthermore, the impact of children's dysregulated affect expression on mothers’ mood may be cumulative, with mothers becoming increasingly sensitized to its presence over time.
Affect regulation in mothers and children may be especially compromised when mothers have a history of childhood-onset depression (COD). This subtype of depression tends to be chronic, severe, and familial (Harrington et al. 1990; Kovacs et al. 1997; Kovacs et al. 1984b; Newman et al. 1996; Weissman et al. 1999). Furthermore, COD is associated with impairments in the development of affect regulation and social relationship skills (Goodyer et al. 1997; Kovacs and Goldston 1991). These impairments may create vulnerability to interpersonal stressors in adulthood, which may then trigger future depressive episodes. Mothers with a history of COD are especially likely to develop parent–child interaction patterns characterized by high levels of negative affect, low levels of positive affect, and mutually dysregulated affect (Shaw et al. 2006).
The risk for depressive symptoms in the context of a challenging mother–child relationship may not be a static risk, as children's affective characteristics could predict change in mothers’ symptoms over time. Interactions with a troublesome child could exert a mounting pressure on mothers—just as enduring a high-conflict marriage, dangerous neighborhood, or interpersonally antagonistic work atmosphere could—and mothers’ symptoms could increase with exposure to this pressure. Mothers’ symptoms may be especially vulnerable during periods of their children's development in which affect regulation skills are typically developing. The preschool and early school-age periods are worthy of focus because they are periods at which cognitive and affective skills supporting affect regulation generally improve (Kopp 1982; Posner and Rothbart 1998). If children in this age range show only modest competence or little progress in regulating affect, their mothers’ depressive symptoms may be maintained or even exacerbated.
In considering the pathways by which children's affective characteristics may increase or contribute to mothers’ depressive symptoms, we focus on affective behavior and affective physiology. In the behavioral domain, there are several possible mechanisms by which children's affective characteristics can contribute to mothers’ depressive symptoms. Children's affective characteristics may elicit increases in mothers’ affective distress through reinforcement and punishment (Patterson 1982). A child who exhibits high rates of negative affect could serve as a negative reinforcer for her mother's sadness, social withdrawal, and helplessness (Shaw et al. in press; Shaw et al. 2006), and depressed mothers’ dysphoric affect can effectively suppress their children's aggressive behavior (Hops et al. 1987). Also, based on models of affect regulation that emphasize the role of others in regulating affect expression (Cole et al. 2004; Eisenberg and Spinrad 2004), children high in negative affect or low in positive affect may be less likely to contribute adaptively to mothers’ own affect regulation. Although little research has been conducted on the role of children in helping to regulate parents’ emotions, reciprocal effects have been found in research involving adult–adult (Gottman 1998; Levenson et al. 1994) and parent–child relationships (Cole et al. 2003). For example, when children experience and express intense or persistent negative affect, they may be less able to help their parent regulate affect through means such as distracting, offering comfort, and expressing positive affect.
Both behavioral and physiological components of child affectivity could be associated with mothers’ depressive symptoms. As noted above, behavioral components, especially children's behavior during mother–child interactions, provide a window into children's typical behavior in the family context. But physiological components offer another perspective that may provide additional, complementary information on affective tendencies. Although physiological components are valuable for investigating children's affective characteristics, they have rarely been examined in relation to maternal affective symptoms.
Resting frontal electroencephalogram (EEG) asymmetry is an especially promising index of physiologically based affective tendencies. Frontal EEG asymmetry reflects underlying disposition to approach or withdraw from appealing or aversive stimuli (Davidson et al. 2000a; Fox 1991). Left frontal EEG asymmetry is thought to index approach-related dispositional tendencies, while right frontal EEG asymmetry is thought to index withdrawal-related tendencies (Davidson 2004; Fox 1994). Right frontal EEG asymmetry is hypothesized to be a risk factor for depression (Davidson 1994; Tomarken and Keener 1998) and has been observed in children who are behaviorally inhibited or shy (e.g., Fox et al. 2001).
In addition to its association with depression, children’ right frontal EEG asymmetry at rest is thought to influence behaviors such as expression of fear and anxiety, active withdrawal from challenges, social withdrawal, and failure to pursue rewarding experiences (Fox 1994). Generally, right frontal EEG asymmetry is conceptualized as reflecting children's tendencies to pull themselves away from situations involving threat. These tendencies could contribute to mother–child interactions that mothers experience as aversive or unrewarding. If negative affect between mothers and children becomes mutually expressed, leading to reciprocal negative affect and interpersonal withdrawal, mothers might be more likely to maintain depressive symptoms such as persistent low mood, helplessness, worthlessness, and hopelessness. Right frontal EEG asymmetry in children could thus predict mothers’ depression over time via its behavioral manifestations. In addition, right frontal EEG asymmetry in children could capture aspects of children's affectivity that are independent of those measured in typical laboratory paradigms of affective behavior and, thus, could predict mothers’ symptoms in combination with other forms of child negative affectivity. Accordingly, we hypothesized that children's right frontal EEG asymmetry, possibly in combination with factors such as maternal history of depression and children's expressions of affect during mother–child interaction, would be associated with mothers’ depressive symptoms.
Goals of the Current Study
The current study applied a child effects perspective (Bell 1968) to the longitudinal course of mothers’ depressive symptoms. Specifically, we examined associations between behavioral and physiological markers of children's affect and the course of mothers’ depressive symptoms over one year. Some of the mothers in the sample had a history of COD, while others had no history of serious psychopathology. We predicted that both behavioral and physiological markers of children's affect would be associated with mothers’ depressive symptoms over time. Our hypothesis was that mothers of children who exhibit high negative affect, right frontal EEG asymmetry, or a combination of the two would exhibit higher overall depressive symptoms than mothers whose children exhibit lower levels of these markers. In addition, we examined whether mothers’ history of COD interacted with children's affective characteristics (i.e., high negative affect or right frontal EEG asymmetry) to predict mothers’ depressive symptoms overall or change in symptoms over time. Finally, we conducted exploratory cross-lagged models to consider whether child effects were a plausible explanation of observed relations between children's affective characteristics and mothers’ depressive symptoms.
Method
Participants
Participants were 74 children and their mothers from 61 families who were participating in a longitudinal program project on affect regulation in families with a parent history of COD. Of the 74 children, 44 had a maternal history of COD. All offspring in the COD group in the target age range were assessed (31 families total, with a range of 1–4 children per family); as a result, nine families contributed more than one child. One child per control family was assessed. The effect of including multiple siblings from some families in the COD group was accounted for statistically by using random effects modeling to control for shared variance among siblings. A total of 40 dyads had child frontal EEG asymmetry data from both time points; 60 had child affective behavior data from both time points; and 34 families had both types of data from both time points.
Families were recruited either through prior research studies or community advertisements. A subgroup of the COD mothers (n=17) had participated in a longitudinal, naturalistic follow-up study of COD during childhood (Kovacs et al. 1984a). The remaining mothers in the COD group (n=14) were recruited from the community during adulthood. Mothers in both COD subgroups had documented histories of childhood-onset mood disorders. Mothers in the two COD subgroups did not differ in depressive symptoms at either time point or in lifetime comorbid disorders. In the control group, families (n=30) were recruited by accessing individuals who had participated in studies during their childhood or adolescence as “normal controls,” soliciting participation using the Cole directory of households in neighborhoods comparable in socioeconomic status to the COD group, and advertising in the general community or through special community programs. For adults in the control group, criteria for participation in the program project included a lifetime history free of major psychiatric disorder. All adult participants were required to be free of major systemic medical disorders.
Families in the current study were selected from the larger program project sample if five criteria were met: (1) the adult participant was a woman with children; (2) children were in the age range of 3–9 years; (3) children had been assessed in both the psychophysiology study and the mother–child interaction study; (4) children had complete resting EEG data from the psychophysiology assessment; and (5) mothers had completed symptom questionnaires in the mother–child interaction study at the initial assessment and again one year after the child's participation in the psychophysiology and mother–child interaction studies. The age range of 3–9 years was selected because of the increasing acquisition of affect regulation skills during this period and the inclusion of this age range in similar studies (e.g., Cole 1986). For program project families with children age 3–9 years who completed the mother–child interaction study at time 1, 63% of children returned for another assessment one year later. Of those, 76% also completed the psychophysiology study at time 1.
As shown in Table 1, children of mothers in the COD and control groups did not differ in age, ethnicity, or handedness, although the control group contained a greater proportion of European–American children than did the COD group (X2=4.01, p<0.05). Mothers in the COD and control groups did not differ in education level, with 80% in the control group and 92% in the COD group having at least a high school diploma. Mothers in the control group were older than mothers in the COD group (M=30.57, SD=5.67 and M=26.71, SD=3.54, respectively), but because mother's age was unrelated to the variables of interest, it was not included in analyses.
Table 1.
Characteristics of the children in the sample, by maternal history of depression
| Control (n = 30) | COD (n = 44) | |
|---|---|---|
| Child age (years) | 5.09 (1.55) | 5.06 (1.77) |
| Child gender (female, %) | 43 | 43 |
| Child ethnicity | ||
| European–American (%) | 60 | 36 |
| African–American (%) | 27 | 32 |
| Latino (%) | 0 | 5 |
| Asian–American (%) | 3 | 0 |
| Mixeda (%) | 10 | 27 |
| Child affect expression | 0.17 (0.65) | –0.12 (1.18) |
| Child frontal EEG asymmetry | –0.0034 (0.1307) | –0.0122 (0.1128) |
| Mothers’ depressive symptoms | ||
| Time 1 | 3.34 (3.36) | 14.63 (8.49) |
| Time 2 | 3.07 (4.08) | 15.05 (10.37) |
| Mothers’ anxiety symptoms | 23.47 (3.17) | 32.36 (8.96) |
Values are mean (SD) or percentages. Data for affect expression are factor scores from principal components analysis. Negative frontal asymmetry scores reflect right frontal asymmetry, and positive scores reflect left frontal asymmetry. Anxiety symptoms were measured at time 1.
COD Maternal history of childhood-onset depression.
Primarily mixed European–American and African–American.
Diagnosis
Mothers’ history of depression was determined through the administration of structured clinical interviews and a review of childhood psychiatric records. All mothers in the COD group had extensive clinical or research records supporting the presence of operational DSM-III, DSM-III-R, or DSM-IV diagnoses (American Psychiatric Association 1980, 1987, 1994) of either depression (major depressive and/or dysthymic disorder) by age 14 (n=22), or bipolar spectrum disorder (bipolar I, bipolar II, or cyclothymic disorder) by age 17 (n=9). In addition, all COD participants’ diagnoses were confirmed through current administration of the Structured Clinical Interview for DSM-IV (SCID; First et al. 1995). The results reported below did not differ when families with early-onset bipolar spectrum disorders were excluded. The subgroup of mothers who had participated in a longitudinal, naturalistic follow-up study of COD was evaluated during childhood using the Interview Schedule for Children and Adolescents (Sherrill and Kovacs 2000). During adulthood, this subgroup was evaluated using the SCID. The subgroup containing the remaining mothers in the COD group was diagnosed with COD based on psychiatric and/or research documentation dating from childhood. In addition, this subgroup was administered the SCID at the time of entry into the study to confirm the presence of childhood mood disorder and to assess current disorders.
Design, Procedure, and Data Quantification
Laboratory Visits
At both time points, participants visited two laboratories: a psychophysiology laboratory, in which child resting EEG was recorded; and a parent–child interaction laboratory, in which mother–child interaction was observed. Participants completed affect-eliciting tasks in both laboratories. The visits to the two laboratories occurred within 2 months of each other for 88% of the participants, with 6 months as the greatest lag between the visits. After the physiology assessment, children were administered an 11-item behavioral version of the Edinburgh Handedness Inventory (Oldfield 1971) adapted for children. During the mother–child interaction assessments, mothers completed questionnaires about their current depressive and anxiety symptoms. At time 2, approximately one year later, the psychophysiology and mother–child behavior assessments were repeated.
Children's Affective Behavior
Mothers and children engaged in a series of 4–5 tasks lasting 25 min designed to elicit (1) both positive and negative affect in children and (2) comparable levels of affect for children varying in developmental status. For example, at age 3, tasks involved a dinosaur puzzle; Etch-a-Sketch (fine-motor drawing board), stack-n-pop (motor and balance), and toss-a-cross (gross motor and hand–eye coordination) games; and exposure to a wiggle ball (a ball with flashing lights that emits shrill sounds). At age 5, tasks included “Hungry Hungry Hippos” (a competitive game involving fine motor skills and speed), Marble Works (a building task using marbles), a naming game (e.g., name things that fly), a shape sorter task, and a tractor treader (a large wheel that turns when a child crawls while inside it). At least one task within each group was selected to provide a provocative or frightening element (e.g., wiggle ball at age 3). Because the tasks were designed to elicit similar amounts of positive and negative affect across the age range of children in the study, we did not expect to find age effects on behavior.
Children's affective behavior was subsequently coded from videotapes. The following behaviors were coded within 10-s intervals: positive affect, negative affect, disruptiveness, task involvement, and task uninvolvement. Additionally, coders rated the following behaviors on four-point global scales after viewing all tasks: positivity toward mother, negative affect toward mother, involvement with tasks, involvement with mother, appropriate affect, inhibition, and sociability. All coders were blind to the group status of participants. Kappas for coder reliability ranged between 0.59–0.76, with a mean of 0.65. These levels are consistent with established reliability expectations for observational codes (Mitchell 1979).
Behavioral data were reduced using principal components analysis with varimax rotation, which resulted in a two-factor solution. The two factors that emerged explained 53% of the variance and corresponded to task engagement and affect expression.1 The affect expression factor explained 16.5% of the variance (eigenvalue=1.97, internal consistency alpha=0.60) and involved low negative affect (loading=−0.60), low negative affect toward mother (−0.47), high positive affect toward mother (0.67), high sociability (0.79), and high appropriate affect (0.73). Based on our hypotheses and the literature on children at risk for depression, we included the affect expression factor in hypothesis tests. At low levels, this factor represents frequent expression of negative affect; at high levels, the factor represents frequent expression of positive affect. Because the factor includes sociability and appropriate affect, high levels are likely to reflect the suitability of affect expression for the social context. The inclusion of both negative affect and positive affect in this empirically derived factor is consistent with research indicating that the balance of positive and negative affect is related to human flourishing, which represents optimal mental health (Fredrickson and Losada 2005), and that positive and negative affect are somewhat related (e.g., Watson 2000).
Frontal EEG Asymmetry
Children's EEG was recorded during six 30-s resting segments, during which children sat quietly and alternately looked at a small model spaceship and closed their eyes. EEG was recorded with an electrode cap (Electro-Cap International) placed according to standard landmarks. The following sites were included for children: mid-frontal (F3, F4), lateral frontal (F7, F8), central (C3, C4), anterior temporal (T7, T8), mid-parietal (P3, P4), and occipital (O1, O2).
On-line recordings were referenced to the vertex (Cz), then re-referenced to a whole-head average. The signal was amplified with a gain of 5,000 and bandpass filtered at 1–100 Hz. Data were digitized on-line at a sampling rate of 512 Hz per channel. Electrode impedances were below 5 kΩ, and impedances for homologous sites were within 0.5 kΩ. Vertical and horizontal electrooculogram (EOG) data were used to identify and manually remove eye movement artifact. Artifact rejection was conducted by two trained coders who were blind to group status. Coders visually inspected data from EOG and EEG channels for the entire resting period and manually removed data from epochs that included blinks, eye movements, or motor activity. EOG was recorded using tin cup electrodes; vertical EOG electrodes were placed on the suborbital and supraorbital areas around the right eye, and horizontal EOG electrodes were placed on the left and right outer canthi.
The EEG signal was quantified with discrete Fourier transformation (DFT) using a Hanning window 1-s wide and with 50% overlap. Prior to DFT computation, the mean voltage was subtracted from each data point to eliminate any influence of DC offset. Power (in units of picowatt–Ohms or squared microvolts) was computed for 1-Hz frequency bins for frequencies between 1 and 30 Hz. The frequency range of interest was the alpha band, which is putatively inversely related to brain activation (Pfurtscheller et al. 1996). Based on an examination of each participant's EEG activity in single-Hz bins, as well as previous developmental findings (Marshall et al. 2002), the alpha range was defined as 7–10 Hz for 3- to 5-year-olds and 8–11 Hz for 6-to 9-year-olds. Alpha power values (in picowatt–Ohms or squared microvolts) for each electrode site were weighted by the number of artifact-free epochs in each segment and averaged across segments. Average values were subjected to a natural-logarithm transformation to normalize distributions (Gasser et al. 1982). Following a widely used approach (Davidson et al. 2000b), asymmetry scores were computed as the difference of transformed power scores for midfrontal leads (lnF4–lnF3). Positive scores reflect greater relative left frontal activity, and negative scores reflect greater relative right frontal activity.
Mothers’ Symptoms
Mothers’ current depressive and anxiety symptoms were measured using the Beck Depression Inventory (BDI; Beck et al. 1988a) and the Beck Anxiety Inventory (BAI; Beck et al. 1988b), respectively. These questionnaires are reliable and valid self-report measures of depressive and anxiety symptoms. Each questionnaire contains 21 items, with four possible responses per item. Internal consistency for the entire set of items was high for both the BDI and the BAI (Cronbach's α=0.92 for each).
Data Analysis
Hypotheses were tested using repeated measures random effects models, which allow modeling of within-family similarity (across siblings) and within-mother similarity (across time) and which have important advantages over traditional models that assume independence of cases. Random effects models adjust the degrees of freedom for each factor to accommodate the presence of both fixed and random factors in the model.
Mothers’ level of depressive symptoms was the dependent variable. COD group, child frontal EEG asymmetry, and child affect expression were fixed effects. Family and mother were random effects. The family effect could be modeled only for the COD group, since siblings were present in this group but not the control group. Time was a within-subjects factor. Rather than consider time 1 depressive symptoms an independent variable and time 2 depressive symptoms the dependent variable, we included time as an independent variable in a repeated-measures analysis. We could then test whether depressive symptoms changed with time and whether the relation between child characteristics and maternal depression varied with time (i.e., time interacted with other fixed effects). The compound symmetry covariance structure was used.
We computed a random effects model with only main effects, then a full model with both main and interaction effects. As the emphasis of the full-model analyses was on factors related to change in mothers’ symptoms, hypothesis testing was focused on two- and three-way interactions that included time (e.g., time X COD X frontal EEG asymmetry).
Level of anxiety symptoms at time 1 was included as a covariate, allowing us to examine an index of maternal depression that is somewhat “pure” and independent of anxiety. Because depressive symptoms and anxious symptoms tend to be strongly correlated (r=0.78, p<0.001 in this sample), this represents a conservative approach. However, results were generally similar when anxiety symptoms were left out of the model.
Significant interaction effects were examined two ways. In our primary approach, we graphed means and conducted post hoc tests using traditional methods. For graphs, groups for continuous variables were created by selecting cases that were at least 1 SD below and above the mean (e.g., high and low affect expression) and plotting the mean level of maternal depressive symptoms, with a separate line for each group, at each level of the interacting variable. The resulting groups were small, often with fewer than 10 cases each. Groups were then included in random effects regressions to elucidate the nature of interaction effects. Secondarily, to further specify the contributions to interaction effects, we used an adaptation of the Johnson–Neyman technique as described by Preacher, Bauer, and Curran (Bauer and Curran 2005; Preacher et al. 2006) to plot regions of significance and confidence bands for interaction effects. In this more precise approach, regions of significance indicate the high and low values of the moderator variable at which the relation of the focal predictor and the dependent variable (displayed as a simple slope) becomes significant. Confidence bands are continuously plotted confidence intervals that correspond to conditional values of the moderator.
Finally, in an attempt to examine whether findings might reflect a direction of effects from children to mothers (in addition to effects from mothers to children), we conducted exploratory cross-lagged path analysis models using data from both time points. Because only a subset of cases had child data from both time points (e.g., 40 for frontal EEG asymmetry), limiting our power to detect effects with large models, we conducted separate models for child frontal EEG asymmetry and child affect expression, with the child variable and maternal depressive symptoms included at both time points. Within each model, child and mother effects were estimated simultaneously, accounting for the autoregressive relations between child and mother measures at two time points and the relations with COD group.
Preliminary Findings
Preliminary random effects regression models examined the effects of child age, gender, ethnicity, or family SES on child physiology, child affect expression, and mothers’ symptoms of depression. None of these characteristics was related to the variables of interest. In addition, results of the full model for depressive symptoms did not differ when we included ethnicity (defined as European–American or non-European–American), when left-handed participants were removed from EEG-related analyses, or when child age was included as a covariate. Thus, these variables were subsequently excluded from the main analyses. Furthermore, the pattern of findings did not differ when models were computed with child age as a factor or when siblings were excluded from the analyses. Models with significant frontal EEG asymmetry effects were re-computed with parietal rather than frontal asymmetry, confirming that effects were specific to the frontal region.
Bivariate correlation analyses indicated that age, maternal history of COD, child affect expression, and child frontal EEG asymmetry were uncorrelated (rs=−0.01 to 0.14, ps>0.14; Spearman's rho=0.01 to 0.07, ps>0.10 for correlations with COD group). Maternal history of COD was moderately correlated with depressive symptoms and anxiety symptoms (Spearman's rho=0.60 p<0.001, Spearman's rho=0.55, p<0.001, respectively), and depressive symptoms and anxiety symptoms were strongly correlated with each other (r=0.78, p<0.001). In addition, the correlation between continuous indices of affect expression and frontal EEG asymmetry indicated that they were fairly independent of one another (r=0.12, p=0.17). Finally, comparisons of children of mothers with a history of COD and children of control mothers revealed no difference in frontal EEG asymmetry or affective behavior.
Results
As indicated in Table 2, the main effects model indicated an effect for COD group (η2=0.19), with higher depressive symptoms in mothers with a history of COD than in mothers with no history of psychopathology (M=14.83, SD=9.36 for COD; M=3.21, SD=3.69 for control). The main effect for time was not significant, indicating that for the sample as a whole, mothers’ depressive symptoms did not tend to increase or decrease from time 1 to time 2. The main effects for frontal EEG asymmetry and affect expression were also nonsignificant in the main effects model.
Table 2.
Results of random effects regression models for mothers’ depressive symptoms
| Variable | df | F |
|---|---|---|
| Main effects model | ||
| Time | 1, 70.00 | 0.53 |
| COD group | 1, 61.06 | 13.33** |
| Affect expression | 1, 108.38 | 0.58 |
| Frontal asymmetry | 1, 106.91 | 0.75 |
| Anxiety symptoms (covariate) | 1, 88.53 | 15.83** |
| Full model | ||
| Time | 1, 63.70 | 0.67 |
| COD group | 1, 59.96 | 9.88** |
| Affect expression | 1, 59.85 | 0.14 |
| Frontal asymmetry | 1, 93.44 | 0.01 |
| Anxiety symptoms (covariate) | 1, 81.58 | 25.81** |
| Time × COD group | 1, 63.79 | 0.01 |
| Time × Affect expression | 1, 62.48 | 0.22 |
| Time × Frontal asymmetry | 1, 64.52 | 6.02* |
| Time × COD group × Affect expression | 2, 61.90 | 0.57 |
| Time × COD group × Frontal asymmetry | 2, 77.02 | 3.85* |
| Time × Affect expression × Frontal asymmetry | 2, 80.09 | 6.07** |
COD group Presence or absence of childhood-onset depression in the mother, frontal asymmetry balance of child EEG alpha power at left and right midfrontal sites, affect expression child behavior during mother–child interaction. Degrees of freedom were adjusted for each predictor variable as part of the random effects regression procedure.
p<0.05
p<0.005.
As indicated in Table 2, the full model (including interaction effects) for maternal depressive symptoms indicated a significant COD group main effect (η2=0.14) qualified by a time X COD group X frontal EEG asymmetry interaction (η2=0.09). The time X frontal EEG asymmetry interaction was significant (η2=0.08), as was the time X frontal EEG asymmetry X affect expression interaction (η2=0.13).
Time X COD Group X Frontal EEG Asymmetry Interaction
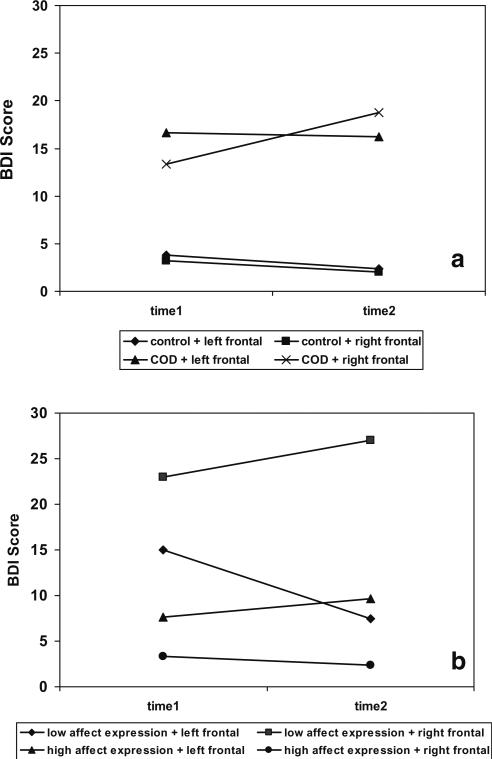
The pattern of the three-way interaction involving time X COD group X frontal EEG asymmetry for maternal depressive symptoms is depicted in Fig. 1a. To examine this interaction, the sample was split by COD group, and random effects regression models were conducted for each group. The time X frontal EEG asymmetry interaction was significant within the COD group, F(1, 41.92)=9.21, p<0.005, but not within the control group. Random effects regression models within the COD group at each time point indicated that at time 1 only, child frontal EEG asymmetry was associated with maternal depressive symptoms, F(1, 39)=5.78, p<0.05. In other words, depressive symptoms at time 1 were higher for mothers with a history of COD whose children had left frontal EEG asymmetry.
Fig. 1.
Illustrations of three-way interactions for the influence of child affective characteristics on mothers’ depressive symptoms. The time X group X frontal asymmetry (a) and time X affect expression X frontal asymmetry (b) interactions are depicted. Groups for affect expression and frontal asymmetry were created by selecting cases that were at least 1 SD below or above the sample mean. At high levels, the affect expression variable reflects high positive affect, high sociability, high appropriate affect, low negative affect, and low negative affect toward mother. COD Maternal history of childhood-onset depression. Left frontal Greater relative left frontal brain activity in child; right frontal Greater relative right frontal brain activity in child
To examine whether, as suggested in Fig. 1a, depressive symptoms increased with time in the group of mothers with a history of COD and a child with right frontal EEG asymmetry, random effects regression models were computed to examine the main effect of time within groups that were created with combinations of maternal history of COD and child frontal EEG asymmetry. For frontal EEG asymmetry, the sample was split into right and left frontal groups based on cases at least 1 SD below or above the mean, respectively. The time effect was significant for the group with a maternal history of COD and child right frontal EEG asymmetry, F(1, 21.71)=10.44, p<0.005, and marginally significant for the group with a maternal history of COD and child left frontal EEG asymmetry, F(1, 17.78)=3.67, p=0.07. That is, in mothers with a history of COD, depressive symptoms tended to increase in those whose children had right frontal asymmetry and tended to decrease in those whose children had left frontal asymmetry.
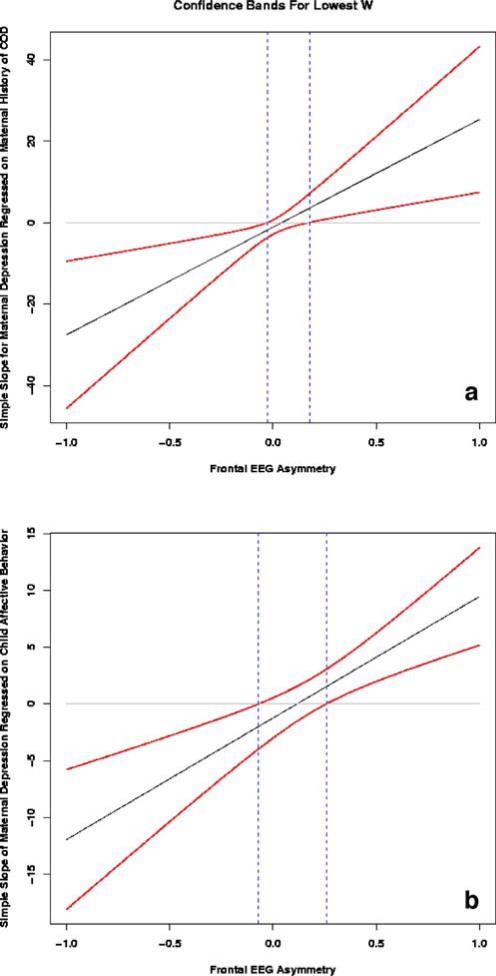
Secondary probing of interactions using the technique proposed by Preacher et al. (2006) yielded generally similar findings to traditional methods. COD group was considered the focal predictor variable and frontal EEG asymmetry the moderator variable. At time 1, the slope of maternal depressive symptoms regressed on COD group was significant for frontal EEG asymmetry values <−0.028 and >0.177 (see Fig. 2a). As both of these values fall within the range of frontal EEG asymmetry scores, the relation between mothers’ history of COD and depressive symptoms was significant for women whose children had extreme right frontal EEG asymmetry (i.e., higher depressive symptoms) or extreme left frontal EEG asymmetry (i.e., lower depressive symptoms). As would be expected, the confidence band around this regression line was wider at more extreme asymmetry values (Bauer and Curran 2005). There was no region of significance for this interaction at time 2.
Fig. 2.
Illustration of regions of significance and confidence bands for the time X COD group X frontal asymmetry (a) and time X affect expression X frontal asymmetry (b) interactions. In both graphs, frontal EEG asymmetry is considered the moderator variable (depicted on x-axis), and the regression of maternal depressive symptoms on a maternal COD group or b child affect expression is plotted as a simple slope. Dotted lines indicate regions of significance: Values above the upper limit and below the lower limit are values of frontal EEG asymmetry for which the relation between maternal depressive symptoms and the focal predictor (either COD group or child affect expression) is considered significant. Confidence bands are continuously plotted confidence intervals for the relation of maternal depressive symptoms and the focal predictor at values of frontal EEG asymmetry. COD Maternal history of childhood-onset depression
Time X Affect Expression X Frontal EEG Asymmetry Interaction
The three-way interaction involving time X affect expression X frontal EEG asymmetry for maternal depressive symptoms is depicted in Fig. 1b. To examine this interaction, the sample was first split into right and left frontal EEG asymmetry groups as described above, and random effects regression models were computed for each frontal EEG asymmetry group. In the right frontal EEG asymmetry group, there was a significant main effect for affect expression, F(1, 5.8)=8.11, p<0.05. For mothers whose children had right frontal EEG asymmetry, children's low affect expression—which reflects high negative affect and low positive affect—was associated with high depressive symptoms. Correlation analyses within the right frontal EEG asymmetry group for each time point were conducted to examine this effect further. Child affect expression was strongly and negatively correlated with maternal depressive symptoms at both time 1 (r=−0.82, p<0.05) and time 2 (r=−0.71, p=0.05), indicating also that children's expression of negative affect was associated with maternal depression. These correlations correspond to large effect sizes, with Cohen's d=2.85 and 2.01, respectively. Thus, the combination of affect expression and child right frontal EEG asymmetry was related to high maternal depressive symptoms at both time points.
In the left frontal EEG asymmetry group, there was a significant time X affect expression effect for depressive symptoms, F(1, 6.38)=8.85, p<0.05. Although Fig. 1b appears to indicate that this interaction effect was due to higher depressive symptoms at time 1 in mothers of children with left frontal EEG asymmetry and low affect expression (i.e., high negative and low positive affect) than in mothers of children with left frontal EEG asymmetry and high affect expression (i.e., high positive and low negative affect), the affect expression factor was not significant in random effects models computed after the sample was split by time.
A set of random effects regression models examined whether mothers’ depressive symptoms changed with time based on the combination of affect expression and frontal EEG asymmetry. The sample was split by frontal asymmetry and affect expression based on cases at least 1 SD above and below the mean, and time was the predictor in the regression models. Results indicated that depressive symptoms did not increase with time for any combination of child affect expression and frontal EEG asymmetry.
Finally, based on Fig. 1b, a related random effects regression model was conducted to examine whether symptoms were higher at both time points in mothers whose children exhibited right frontal asymmetry and low affect expression (i.e., high negative and low positive affect) than in mothers whose children had other combinations of frontal asymmetry and affect expression. This model indicated a marginally significant effect, F(1, 13.55)=3.63, p=0.08.
As with the other three-way interaction, additional probing of this interaction using the technique proposed by Preacher et al. (2006) yielded generally similar findings to probing using traditional methods. Child affect expression behavior was considered the focal predictor variable and frontal EEG asymmetry the moderator variable. At time 1, the slope of maternal depressive symptoms regressed on child affect expression was significant for frontal EEG asymmetry values <−0.0697 and >0.259 (see Fig. 2b). As only the lower value falls within the range of frontal EEG asymmetry scores, the relation between child affect expression and maternal depressive symptoms was significant only for women whose children had extreme right frontal EEG asymmetry. That is, depressive symptoms were higher in mothers whose children had extreme right frontal EEG asymmetry and expressed high-frequency negative affect/low-frequency positive affect. As would be expected, the confidence band around this regression line was wider at more extreme values of frontal EEG asymmetry (Bauer and Curran 2005). There was no region of significance for this interaction at time 2.
Cross-Lagged Models of Child Effects on Mothers’ Depression
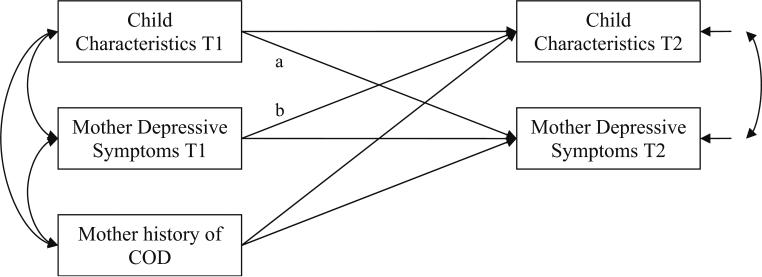
To examine whether the main findings described above could reflect a direction of effects from children to mothers, we conducted additional cross-lagged models for the subset of participants who had data for child affective characteristics and mothers’ depressive symptoms at both time points. The model for child frontal EEG asymmetry indicated a marginally significant effect from child to mother (B=−10.87, SE=6.47, β=−0.13, p<0.10), and the models for child frontal EEG asymmetry and child affect expression behavior both indicated a direction of effects from mother to child (B=0.004, SE=0.002, β=0.35, p=0.05; B=0.30, SE=0.02, β=0.26 and p<0.10, respectively). Figure 3 provides an example of the structure of each cross-lagged model. Despite limited power to detect effects (i.e., n=34 for all variables at both time points), these models provided some evidence for both parent-to-child and child-to-parent directions of effects.
Fig. 3.
Example of a cross-lagged model for the relation between a child affective characteristic and maternal depressive symptoms at time 1 (T1) and time 2 (T2). Path a reflects child effects on mother, and path b reflects mother effects on child. In the actual analyses, two separate models were estimated. Both models contained the same maternal variables. In one model child frontal EEG asymmetry was included, and in the other child affect expression was included
Discussion
This study contributes to the understanding of the association of child factors with parents’ psychopathology by testing hypotheses about the relation of children's behavioral and physiological affective characteristics to mothers’ depressive symptoms. By including a subgroup of parents with a history of childhood-onset mood disorder, the study also examined whether women's history of depression moderates the relation between child affective characteristics and mothers’ recurring depressive symptoms.
Children's negative affect expression and frontal EEG asymmetry, considered together and in conjunction with maternal history of COD, were associated with severity and persistence of maternal depressive symptoms. Symptoms tended to increase with time in mothers with a history of COD whose children exhibited right frontal EEG asymmetry. Despite this longitudinal finding based on traditional interaction probing, the relation between COD, frontal EEG asymmetry, and depressive symptoms was more notable at time 1 than at time 2. In mothers whose children exhibited right frontal EEG asymmetry and expressed higher-frequency negative affect/lower-frequency positive affect, symptoms were high at both time points. For this subgroup, the association between child characteristics and maternal depressive symptoms was quite strong. Exploratory cross-lagged models indicated some preliminary support for the interpretation that the association between child affective characteristics and mother depression could be partly attributed to child effects on mothers, in addition to corroborating previous research documenting maternal effects on children's subsequent affective characteristics. These results are consistent with a transactional perspective (Sameroff and Chandler 1975), suggesting that children's negative affect and mothers’ depressive symptoms could exert mutual influence, with each potentially serving to exacerbate the presence of the other.
We obtained some surprising results about change in mothers’ symptoms over approximately one year. First, we had predicted that mothers’ history of COD, in combination with either child negative affect or child right frontal EEG asymmetry, would be related to mothers’ depressive symptoms at both time points. Instead, we found that the combination of maternal COD and child right frontal EEG asymmetry was associated with an increase in mothers’ depressive symptoms. Second, in the case of affective behavior and frontal EEG asymmetry, we found that mothers whose children exhibited both high negative affect/low positive affect and right frontal EEG asymmetry had high depressive symptoms at both time points. In general, we interpret these findings as indicating that, in combination, child right frontal asymmetry and affect expression are associated with maternal depressive symptoms. In the case of mother history of COD and child frontal EEG asymmetry, there could be an exacerbation of maternal depressive symptoms over time. For instance, mothers’ history of COD could exert an influence on their mood and behavior over time, creating vulnerability to increased symptoms in the context of the typical affective style of children with right frontal EEG asymmetry. In the case of the combination of child right frontal EEG asymmetry and high rates of child negative affect, the presence of these two factors together could play a role in high, stable maternal symptoms. Ideally, we would follow the course of all of these factors—maternal depression, child frontal EEG asymmetry, and child affective behavior—over a period of years before drawing firm conclusions about our interpretation of the current data.
We note, however, that while a newer and more precise technique for probing interactions generally produced results consistent with traditional techniques, it also led to different results regarding the relation of mother and child variables at the two time points. For example, while both techniques indicated that relations among mother and child variables were stronger at time 1 than at time 2, the newer technique indicated that the combination of child affective expression and child frontal EEG asymmetry was related to maternal depressive symptoms only at time 1. An interpretation of this finding is that the child factors we have examined may not create stable risks for mothers or play a critical role in symptom changes.
Given that we found that mothers’ depressive symptoms were related to (1) the interaction of maternal history of COD and child frontal EEG asymmetry and (2) the interaction of child affective expression and child frontal EEG asymmetry, we propose that the mechanism for both patterns of interaction is the mother–child relationship. Several factors may account for the association of child affective characteristics and maternal depressive symptoms across time.These include the stability of mothers’ interpersonal functioning, the development of affect regulation in children, and the mutual influence of children's and mothers’ affective style. For instance, the combination of right frontal EEG asymmetry and expression of negative affect by children could result in withdrawn, threat-sensitive behavior in children and may contribute to a mother–child relationship that is characterized by consistently high, dysregulated negative affect or interpersonal disengagement. Alternatively, from another perspective on laterality in brain function, the attentional process of monitoring is localized in right prefrontal areas (Stuss and Alexander 2007). As a result, right frontal EEG asymmetry in children could also be associated with vigilance. This vigilance could include a focus on mothers’ mood and behavior, so that when combined with negative affective reactivity, it could contribute to mothers’ depressive symptoms.
Of course, mothers do not respond to children's patterns of brain activity per se, but mothers could be sensitive to the types of behavior that are associated with the motivational and affective characteristics indexed by right frontal EEG asymmetry. Additionally, given that frontal asymmetry and affective behavior were uncorrelated and therefore likely to represent fairly independent constructs, it appears that frontal EEG asymmetry represents elements of affective behavior that were not captured by our behavior observations. The authors of a study reporting that frontal EEG asymmetry and child affect interacted to predict child social behavior reached similar conclusions (Henderson et al. 2001). Right frontal EEG asymmetry is thought to predispose children to a style of affect regulation involving withdrawal from aversive or threatening stimuli, fearfulness, and negative affect (Fox 1994). Thus, frontal EEG asymmetry may be more relevant to threatening contexts than our measure of child affective behavior. Perhaps under conditions involving both response to perceived threat (indexed by frontal EEG asymmetry) and children's tendency to display high negative affect and low positive affect during interactions with their mothers (indexed by our behavioral measure), mothers’ depressive symptoms are especially sensitive to child affect. To elucidate the many aspects of children's affect that could play a role in mothers’ depression, it will be important for future studies to include more complex measures of behavior and to use converging methods such as resting EEG, observation of the mother–child relationship over time, the observation of behavior in natural environments, and diary or experience sampling measures of affect.
We do not interpret these findings as indicating a detrimental, direct effect of frontal EEG asymmetry in a single child—as one of possibly many important family members in a woman's social network—on maternal depression. Rather, we view these findings as suggesting an association of greater relative right frontal brain activity (and its accompanying behaviors) with maternal symptoms. This association is not likely to be unique to one child, could be shared by other children in a family, and could be shaped by other important interpersonal and affective factors. Furthermore, frontal EEG asymmetry itself was not associated with maternal depression as a main effect: It was only related in combination with either mother history of depression or child affect expression. Thus, a child's right frontal EEG asymmetry could be a potent variable in the larger constellation of social and affective factors that contribute to maternal depression.
To some extent, our results support claims that greater relative right frontal brain activity is an affective characteristic associated (in this case, indirectly) with depressed mood. In contrast to previous studies, however, the current study examined change in mothers’ depressive symptoms in relation not to their own frontal EEG asymmetry, but to their children's frontal EEG asymmetry. The mechanisms for the relation between children's frontal EEG asymmetry and mothers’ depression are likely to involve children's affect-related behavior and its effects on the quality of the mother–child relationship. From a traditional perspective, children of depressed mothers could have inherited a tendency toward right frontal EEG asymmetry along with a vulnerability to depression. Even so, from a child effects perspective, frontal EEG asymmetry may impact mothers’ mood or symptoms. The style of affect regulation indexed by right frontal EEG asymmetry may be especially troubling to mothers with a history of depression because these mothers may be sensitive to social rejection, biased toward thinking of themselves as ineffective parents, and prone to withdraw from social contact (Hammen et al. 1990; Lyons-Ruth et al. 2002). For example, if a child with right frontal EEG asymmetry withdraws physically in response to a socially ambiguous event (e.g., a mother's expression of frustration), a mother prone to depression might interpret that behavior as evidence that her child does not love her, that she is incompetent as a parent, or that it is better for her to avoid interacting with her child.
Because psychopathology in families is determined by a multiplicity of factors and processes (Dickstein et al. 1998; Seifer et al. 1996), it is valuable to take a multifactor, multimethod approach to investigating family members’ influences on parent psychopathology. For example, we found in a related study that children's depressive and anxiety problems were associated with affect expression only in combination with frontal EEG asymmetry (Forbes et al. 2006). In the current study, children's frontal EEG asymmetry and behavior did not contribute to mothers’ symptoms when considered in isolation. When considered in combination with each other, with time, and with maternal history of depression, however, both were found to be important factors.
Our findings raise many questions about possible child effects on parent psychopathology. First, how might relations among children's frontal EEG asymmetry, children's affect expression, and parents’ depressive symptoms represent a shared genetic diathesis toward depression? Second, how might children's affective behaviors in other interpersonal contexts, such as sibling or peer play, influence parent psychopathology? Third, are other types of parent psychopathology comparably sensitive to children's affective characteristics? Additional studies are also needed to explore family process factors that may mediate the relation between child characteristics and parent depressive symptoms. Longitudinal and genetically informed designs will be essential, as will studies focusing on fathers and other caregivers.
Certainly, it is possible that our findings reflect maternal influence on child affective characteristics rather than child effects on maternal functioning. Our methods and our sample size do not allow us to disentangle these sources of influence definitively. However, cross-lagged models involving both time points indicated some support for child effects on mothers’ symptoms and the more heavily documented association from mothers to children. Our findings are also consistent with the child effects view insofar as a transactional perspective on developmental psychopathology allows for bidirectional influence within parent–child dyads. Incorporating a more experimental design into future studies of families at risk for affective disorders could provide opportunities for testing hypotheses about the direction of effects in maternal depression. For instance, in a study of the effects of child behavior on adults’ distress, child confederates were trained to behave defiantly during interactions with adults (Pelham et al. 1997).
Although we were able to address an understudied topic within the area of adult depression, our study has several limitations. Our sample was fairly small, the developmental range of the children included was broad, and we focused on a particular subtype of depression. Because we assessed children whose ages ranged from 3 to 9 years, we adapted our methods developmentally, and this resulted in the use of two definitions of alpha EEG activity and the inclusion of different sets of toys for the mother–child interaction task. Idiosyncrasies of the sample include the recruitment of families from multiple sources and the inclusion of multiple children in COD families. In addition, although the study focused on a subtype of maternal depression, the COD group included diverse types of depression. As a result of these limitations, findings may not be generalizable to populations with adult-onset depression, other affective disorders, or other childhood-onset disorders. At the very least, future research should address these questions in parents with other subtypes of depression. Also, we focused our investigation on child effects on mothers’ symptoms, rather than on other important caregivers. Finally, we focused on children's affect as a source of variability in mothers’ symptoms, but other domains such as children's academic functioning, antisocial behavior (i.e., problems with school and the law), and cognitive ability may be equally important factors that could predict mothers’ symptoms.
Despite these limitations, the current study represents an initial step toward understanding how child attributes may be associated with parents’ ongoing adjustment. The findings contribute to the child effects literature by indicating that children's affective characteristics may be one of several contributors to parents’ depressive symptoms over time.
Acknowledgments
This work was supported by NIMH grants (P01 MH56193, K01 MH74769) and a NARSAD Young Investigator Award. We thank Emily Skuban, Rachel Chung, and Tonya Lane for assistance with coding, processing, and managing data. We are also grateful to the families who participated.
Footnotes
We have previously used the term affect regulation for this factor because we conceptualize it as a proxy, in some ways, for appropriate affect regulation in a social context. Upon reconsideration of the construct captured by this combination of behaviors in this behavioral paradigm, we have decided that affect expression is a more appropriate term.
Contributor Information
Erika E. Forbes, WPIC–Loeffler 319, Department of Psychiatry, University of Pittsburgh, 3811 O'Hara Street, Pittsburgh, PA 15213, USA forbese@upmc.edu
Daniel S. Shaw, Department of Psychology, University of Pittsburgh, Sennott Square, 210 South Bouquet Street, Pittsburgh, PA 15260, USA
Jennifer S. Silk, Department of Psychiatry, University of Pittsburgh, 3811 O'Hara Street, Pittsburgh, PA 15213, USA
Xin Feng, Department of Psychiatry, University of Pittsburgh, 3811 O'Hara Street, Pittsburgh, PA 15213, USA.
Jeffrey F. Cohn, Department of Psychology, University of Pittsburgh, Sennott Square, 210 South Bouquet Street, Pittsburgh, PA 15260, USA
Nathan A. Fox, University of Maryland, College Park, MD, USA
Maria Kovacs, Department of Psychiatry, University of Pittsburgh, 3811 O'Hara Street, Pittsburgh, PA 15213, USA.
References
- Allen NB, Badcock PBT. The social risk hypothesis of depressed mood: Evolutionary, psychosocial, and neurobiological perspectives. Psychological Bulletin. 2003;129(6):1–28. doi: 10.1037/0033-2909.129.6.887. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders (dsm–iii) (3rd ed.) American Psychiatric Association; Washington, DC: 1980. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual for mental disorders (dsm–iii–r) (3rd Revised ed.) American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual for mental disorders (DSM-IV) (4th ed.) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Bauer DJ, Curran PJ. Probing interaction sin fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behavior Research. 2005;40(3):373–400. doi: 10.1207/s15327906mbr4003_5. [DOI] [PubMed] [Google Scholar]
- Beardslee WR, Versage EM, Gladstone TR. Children of affectively ill parents: A review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37(11):1134–1141. doi: 10.1097/00004583-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988a;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the beck depression inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988b;8:77–100. [Google Scholar]
- Bell RQ. A reinterpretation of the direction of effects in studies of socialization. Psychological Review. 1968;75(2):81–95. doi: 10.1037/h0025583. [DOI] [PubMed] [Google Scholar]
- Bell RQ, Harper LV. Child effects on adults. Erlbaum; Hillsdale, NJ: 1977. [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-htt gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cole PM. Children's spontaneous control of facial expression. Child Development. 1986;57:1309–1321. [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Development. 2004;75(2):317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Cole PM, Teti LO, Zahn-Waxler C. Mutual emotion regulation and the stability of conduct problems betwen preschool and early school age. Development and Psychopathology. 2003;15:1–18. [PubMed] [Google Scholar]
- Cummings EM, Davies PT. Maternal depression and child development. Journal of Child Psychology and Psychiatry. 1994;35(1):73–112. doi: 10.1111/j.1469-7610.1994.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Development and Psychopathology. 1994;6:741–758. [Google Scholar]
- Davidson RJ. What does the prefrontal cortex “do” in affect: Perspectives on frontal EEG asymmetry research. Biological Psychology. 2004;67(1–2):219–233. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychological Bulletin. 2000a;126(6):890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Larson CL. Human electroencephalography. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2nd ed. Cambridge University Press; New York: 2000b. pp. 27–52. [Google Scholar]
- Davila J, Hammen C, Burge D, Paley B, Daley SE. Poor interpersonal problem solving as a mechanism of stress generation in depression among adolescent women. Journal of Abnormal Psychology. 1995;104(4):592–600. doi: 10.1037//0021-843x.104.4.592. [DOI] [PubMed] [Google Scholar]
- Dickstein S, Seifer R, Hayden LC, Schiller M, Sameroff AJ, Keitner G, et al. Levels of family assessment: Ii. Impact of maternal psychopathology on family functioning. Journal of Family Psychology. 1998;12(1):23–40. [Google Scholar]
- Downey G, Coyne JC. Children of depressed parents: An integrative review. Psychological Bulletin. 1990;108(1):50–76. doi: 10.1037/0033-2909.108.1.50. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Gershoff ET, Fabes RA, Shepard SA, Cumberland AJ, Losoya SH, et al. Mothers’ emotional expressivity and children's behavior problems and social competence: Mediation through children's regulation. Developmental Psychology. 2001;37(4):475–490. doi: 10.1037//0012-1649.37.4.475. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL. Emotion-related regulation: Sharpening the definition. Child Development. 2004;75:334–339. doi: 10.1111/j.1467-8624.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Field T. Fox NA, editor. The effects of mother's physical and emotional unavailability on emotion regulation. The development of emotion regulation: Biological and Behavioral Considerations. 1994;59(2–3):208–227. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for dsm-iv axis i disorders - patient edition (scid-i/d, version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Forbes EE, Shaw DS, Fox NA, Cohn JF, Silk JS, Kovacs M. Maternal depression, child frontal asymmetry, and child affective behavior as factors in child behavior problems. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47:79–87. doi: 10.1111/j.1469-7610.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- Fox NA. If it's not left, it's right: Electroencephalograph asymmetry and the development of emotion. American Psychologist. 1991;46:863–872. doi: 10.1037//0003-066x.46.8.863. [DOI] [PubMed] [Google Scholar]
- Fox NA. Fox NA, editor. Dynamic cerebral processes underlying emotion regulation. The development of emotion regulation: Biological and behavioral considerations. 1994;59:2–3. 152–166. Serial no. 240. [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Losada MF. Positive affect and the complex dynamics of human flourishing. American Psychologist. 2005;60:678–686. doi: 10.1037/0003-066X.60.7.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T, Bacher P, Mocks J. Transformations toward the normal distribution of broadband spectral parameters of the EEG. Electroencephalography and Clinical Neurophysiology. 1982;53:119–124. doi: 10.1016/0013-4694(82)90112-2. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, Secher SM, Pearson J. Short-term outcome of major depression: Ii. Life events, family dysfunction, and friendship difficulties as predictors of persistent disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(4):474–480. doi: 10.1097/00004583-199704000-00009. [DOI] [PubMed] [Google Scholar]
- Gottman JM. Psychology and the study of marital processes. Annual Review of Psychology. 1998;49:169–197. doi: 10.1146/annurev.psych.49.1.169. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Muñoz R. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2(2):151–164. [Google Scholar]
- Grusec JE, Lytton H. Social development: History, theory, and research. Springer; New York: 1988. [Google Scholar]
- Hammen C. Interpersonal stress and depression in women. Journal of Affective Disorders. 2003;74(1):49–57. doi: 10.1016/s0165-0327(02)00430-5. [DOI] [PubMed] [Google Scholar]
- Hammen C, Burge D, Stansbury K. Relationship of mother and child variables to child outcomes in a high-risk sample: A causal modeling analysis. Developmental Psychology. 1990;26(1):24–30. [Google Scholar]
- Harrington R, Fudge H, Rutter M, Pickles A, Hill J. Adult outcomes of childhood and adolescent depression. I. Psychiatric status. Archives of General Psychiatry. 1990;47(5):465–473. doi: 10.1001/archpsyc.1990.01810170065010. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Fox NA, Rubin K. Temperamental contributions to social behavior: The moderating roles of frontal EEG asymmetry and gender. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(1):68–74. doi: 10.1097/00004583-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Hops H, Biglan A, Sherman L, Arthur J, Friedman L, Osteen V. Home observations of family interactions of depressed women. Journal of Consulting and Clinical Psychology. 1987;55(3):341–346. doi: 10.1037//0022-006x.55.3.341. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of personality and major depression in women. Archives of General Psychiatry. 1993;50(11):853–862. doi: 10.1001/archpsyc.1993.01820230023002. [DOI] [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18(2):199–214. [Google Scholar]
- Kovacs M, Devlin B, Pollock M, Richards C, Mukerji P. A controlled family history study of childhood-onset depressive disorder. Archives of General Psychiatry. 1997;54:613–623. doi: 10.1001/archpsyc.1997.01830190033004. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Feinberg TL, Crouse-Novak MA, Paulauskas SL, Finkelstein R. Depressive disorders in childhood: I—A longitudinal prospective study of characteristics and recovery. Archives of General Psychiatry. 1984a;41:229–237. doi: 10.1001/archpsyc.1984.01790140019002. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Feinberg TL, Crouse-Novak MA, Paulauskas SL, Pollock M, Finkelstein R. Depressive disorders in childhood: II—A longitudinal study of the risk for a subsequent major depression. Archives of General Psychiatry. 1984b;41:643–649. doi: 10.1001/archpsyc.1984.01790180013001. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Goldston D. Cognitive and social cognitive development of depressed children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30(3):388–392. doi: 10.1097/00004583-199105000-00006. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Gottman JM. The influence of age and gender on affect, physiology, and their interrelations: A study of long-term marriages. Journal of Personality and Social Psychology. 1994;67(1):56–68. doi: 10.1037//0022-3514.67.1.56. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Lyubchik A, Wolf R, Bronfman E. Parental depression and child attachment: Hostile and helpless profiles of parent and child behavior among families at risk. In: Goodman SH, Gotlib IH, editors. Children of depressed parents: Mechanisms of risk and implications for treatment. American Psychological Association; Washington, DC: 2002. pp. 89–120. [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Mitchell SK. Interobserver agreement, reliability, and generalizability of data collected to observational studies. Psychological Bulletin. 1979;86:376–390. [Google Scholar]
- Newman DL, Moffitt TE, Caspi A, Magdol L, Silva PA, Stanton WR. Psychiatric disorder in a birth cohort of young adults: Prevalence, comorbidity, clinical significance, and new case incidence from ages 11 to 21. Journal of Consulting and Clinical Psychology. 1996;64(3):552–562. [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109(3):504–511. [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Patterson GR. A social learning approach: 3. Coercive family process. Castalia; Eugene, OR: 1982. [Google Scholar]
- Pelham WE, Lang AR, Atkeson B, Murphy DA, Gnagy EM, Greiner AR, Vodde-Hamilton M, Greenslade KE. Effects of deviant child behavior on parental distress and alcohol consumption in laboratory interactions. Journal of Abnormal Child Psychology. 1997;25:413–424. doi: 10.1023/a:1025789108958. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Neuper C. Event-related synchronization (ers) in the alpha band—An electrophysiological correlate of cortical idling: A review. International Journal of Psychophysiology. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation, and consciousness. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- Sameroff AJ, Chandler M. Reproductive risk and the continuum of caretaking casualty. In: Horowitz FD, Hetherington EM, Scarr-Salapatek S, Siegel G, editors. Review of child development research (Vol. 4) University of Chicago Press; Chicago: 1975. [Google Scholar]
- Sanson A, Oberklaid F, Pedlow R, Prior M. Risk indicators: Assessment of infancy predictors of pre-school behavioural adjustment. Journal of Child Psychology and Psychiatry. 1991;32:609–626. doi: 10.1111/j.1469-7610.1991.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype greater than environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Seifer R, Sameroff AJ, Dickstein S, Keitner G, Miller I. Parental psychopathology, multiple contextual risks, and one-year outcomes in children. Journal of Clinical Child Psychology. 1996;25:423–435. [Google Scholar]
- Shaw DS, Schonberg M, Sherrill J, Huffman D, Lukon J, Obrosky D, Kovacs M. Responsivity to offspring's expression of emotion among childhood-onset depressed mothers. Journal of Clinical Child and Adolescent Psychology. 2006;35:540–552. doi: 10.1207/s15374424jccp3504_1. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Gross H, Moilanen K. Developmental transactions between boys’ conduct problems and mothers’ depressive symptoms. In: Sameroff A, editor. Transactional processes in development. American Psychology Association; Washington D.C.: in press. [Google Scholar]
- Sheeber L, Hops H, Davis B. Family processes in adolescent depression. Clinical Child and Family Psychology Review. 2001;4(1):19–35. doi: 10.1023/a:1009524626436. [DOI] [PubMed] [Google Scholar]
- Sherrill JT, Kovacs M. Interview schedule for children and adolescents (ISCA). Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:67–75. doi: 10.1097/00004583-200001000-00018. [DOI] [PubMed] [Google Scholar]
- Silk JS, Shaw DS, Forbes EE, Lane TJ, Kovacs M. Maternal depression and child internalizing: The moderating role of child emotion regulation. Journal of Clinical Child and Adolescent Psychology. 2006;35:116–126. doi: 10.1207/s15374424jccp3501_10. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philosophical Transactions of the Royal Society of London: B, Biological Sciences. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Teti DM, Gelfand DM. Behavioral competence among mothers of infants in the first year: The mediational role of maternal self-efficacy. Child Development. 1991;62(5):918–929. doi: 10.1111/j.1467-8624.1991.tb01580.x. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Keener AD. Frontal brain asymmetry and depression: A self-regulatory perspective. Cognition and Emotion. 1998;12(3):387–420. [Google Scholar]
- Watson D. Mood and temperament. Guilford; New York: 2000. [Google Scholar]
- Weissman MM, Wolk S, Wickramaratne P, Goldstein RB, Adams P, Greenwald S, et al. Children with prepubertal-onset major depressive disorder and anxiety grown up. Archives of General Psychiatry. 1999;56(9):794–801. doi: 10.1001/archpsyc.56.9.794. [DOI] [PubMed] [Google Scholar]