Abstract
Six individuals with probable Alzheimer’s disease (AD) participated in a phase 1 study employing a repeated measures, parallel baseline design testing the hypothesis that error-free experience during word production practice combined with an acetyl cholinesterase inhibitor would improve confrontation naming ability. While acetyl cholinesterase inhibitors are safe and delay cognition decline associated with AD, improvement over baseline cognition is less evident; clinically significant cognitive deficits persist and progress. Both animal and clinical research strongly implicate acetylcholine in learning, a form of neuroplasticity. In clinical practice, however, people with AD are given cholinergic medications without concomitant systematic/targeted retraining. In this study six participants with probable AD and taking donepezil participated in targeted word production practice using an errorless learning strategy. Results showed that combining behavioral enrichment training and an acetyl cholinesterase inhibitor resulted in significant improvements in verbal confrontation naming of trained items for three of six participants. Differences in baseline dementia severity, living conditions, and medications may have influenced the training response. Detection of substantial treatment effects in 50% of subjects suggests further language treatment studies in AD in combination with an acetyl cholinesterase inhibitor are warranted and provide useful information on inclusion/exclusion criteria for use in subsequent studies.
Keywords: Dementia, Rehabilitation, Anomia, Cholinesterase inhibitors, Language therapy, Verbal learning
INTRODUCTION
The relentlessly progressive cognitive deterioration caused by Alzheimer’s disease (AD) is a major cause of disability and suffering, with the total health-related costs estimated to exceed $100 billion per year in the U.S. alone (Ernst & Hay, 1997). According to Petersen et al. (2001) there are currently 4 million people in the United States with AD and by 2050 this number is expected to increase to 14 million people. Although the most important goal is primary prevention, until the time that a cure is found it will be important to reduce the disability associated with AD. Individuals with AD often suffer from verbal communication disorders, the most frequent being anomia. This Phase I study was designed to learn if subjects with AD given errorless word production practice paired with a cholinergic medication can learn new words and retain that knowledge.
While multiple double-blind placebo-controlled studies have demonstrated that acetyl cholinesterase inhibitors are safe and delay cognitive decline in people with AD to a statistically significant degree (Burns et al., 1999), the clinical effects of these drugs are less impressive (Doody et al., 2001). The question therefore arises: Can the impact of acetyl cholinesterase inhibitors on the trajectory of cognitive decline in AD be amplified, perhaps through a behavioral therapy? This is the question we test empirically in this study. The hypothesis motivating this experiment is that because acetyl cholinesterase inhibitors partially correct the deficient cortical and hippocampal acetylcholine levels observed in AD, and because acetylcholine is essential to learning, acetyl cholinesterase inhibitors work by potentiating capacity for learning, a form of neuroplasticity. Learning is unlikely to occur in the absence of an environment that is rich in information to be learned. Behavioral treatment can potentially provide that information.
There is considerable convergent evidence that the effect of acetyl cholinesterase inhibitors on cognitive function in AD is mediated through neuroplastic mechanisms rather than through simple enhancement of cortical function. In animal studies, immunotoxic injury to the nucleus basalis (the source of cortical acetylcholine) or the administration of anticholinergic drugs impairs the modifications of neural synapses that underlie learning, modifications of the central nervous system in response to peripheral nerve injury, and recovery from central nervous system injury (Baskerville et al., 1997;Butt & Hodge, 1995; Conner et al., 2003; Juliano et al., 1991; Saponjic et al., 1998; Webster et al., 1991). In contrast, stimulation of cholinergic basal forebrain structures or administration of cholinomimetic agents in adult animals greatly enhances neural plasticity, learning derived from experience, and recovery from brain injury (Kilgard & Merzenich, 1998; 2002; Saponjic et al., 1998).
Findings from human studies are quite comparable. Administration of anticholinergic agents impedes learning (Drachman & Leavitt, 1974; Wezenberg et al., 2005), whereas the administration of acetyl cholinesterase inhibitors promotes learning and memory (Benke et al., 2005; Crowell et al., 2006; Grön et al., 2005; Krupp et al., 2004; Nadeau et al., 2004; Wezenberg et al., 2005). These studies in animals and human subjects provide strong support for the concept that the beneficial effects of acetyl cholinesterase inhibitors in AD are mediated through their effect on learning. Long-term, open-label, extension studies of randomized trials of donepezil in subjects with AD provide further support for this concept. If the effect of this drug were purely symptomatic, one would expect that: (1) the cognitive benefits of the drug would decline rapidly during the post-randomization trial drug-free washout period, closely following drug clearance; and (2) after the washout period, when all patients are given the drug, the cognitive function of subjects, whether previously in the drug or placebo arms of the randomized trial, would rapidly improve to the level of drug-treated subjects before the washout period. Neither hypothesis is supported by trial results (Doody et al., 2001). After three weeks of no drug (i.e., during drug washout), when >99% of the drug has been cleared, formerly drug-treated patients continue to exhibit higher AD Assessment Scale-Cognitive scores than formerly placebo-treated patients. When donepezil is given to all subjects at the end of three weeks of washout, formerly placebo-treated patients do not catch up to the level of formerly drug-treated patients. It takes a full six weeks of washout for the cognitive benefit of drug treatment during the randomized phase of the trial to be lost (Burns et al., 2007; Doody et al., 2001). Furthermore, long-term, open-label follow-up studies suggest that the rate of cognitive decline in patients on central acetyl cholinesterase inhibitors is less than that of comparable untreated subjects (Burns et al., 2007; Rogers et al., 2000; Small et al., 2005). In summary, the result of long-term, open-label follow-up trials point strongly to an effect on neuroplasticity, not just enhancement of existing cognitive functions.
We have reviewed the evidence that centrally acting acetyl cholinesterase inhibitors act by potentiating learning, which presumably might occur during daily life. However, in most people with AD given cholinomimetic medications, learning is left to chance, and no adjunctive behavioral training is provided. Previous studies have suggested that rehabilitation can stabilize or improve cognitive function in mild to moderate AD (Clare et al., 2000; 2002), and that rehabilitation paired with an acetyl cholinesterase inhibitor may boost the positive drug effects and enhance their clinical impact (Bottino et al., 2005; Lowenstein et al., 2004; Rozzini et al., 2007). In this study, we test the potentiating effect of rehabilitation on a specific domain of language impairment in AD: anomia.
The “errorless learning” method used in this study represents a paradigmatic change from traditional language therapy in that it focuses on preventing or greatly reducing the opportunity to practice incorrect responses. This method discourages “guessing” by either instructing participants to make a response only if they feel confident they will be correct or by providing participants with the correct model prior to their response. The goal is to maximize the occurrence of correct productions while avoiding incorrect responses. Errorless learning has been successfully utilized in rehabilitation for individuals with AD in learning face-name associations, personal information, and object naming (Bottino et al., 2005; Haslam et al., 2006; Lowenstein et al., 2004).
Errorless learning has not yet been thoroughly studied; however, it is clear that the mechanisms underlying its effects are complex and may vary with the type of material being learned (Haslam et al., 2006; Nadeau et al., 2008). There may be circumstances in which the effects of errorless learning are beneficial and other circumstances in which they are harmful. Because in lexical learning (as in the current study) there is typically one and only one correct response, there is no evident reason why allowing the participant to struggle to overcome a word retrieval problem (“errorful learning”) should be beneficial, and such struggling may significantly impede the learning process. On the other hand, there may be learning situations (e.g., involving treatment of semantic deficits or apraxia of speech), not assessed in the current study, in which redifferentiation of dedifferentiated representations is likely to be important and errorless learning might theoretically be harmful.
The purpose of this Phase I study was to learn if adding behavioral practice aimed at improving naming ability to treatment with an acetyl cholinesterase inhibitor (donepezil) might boost the drug effect reported in individuals with AD, thereby determining whether the clinical impact of this commonly used drug might be enhanced and disability reduced through combined treatment (Clare et al., 2002). Phase I studies are intended to investigate whether a treatment has a measurable therapeutic effect, to establish safety, and to determine the best outcome measures, candidates for treatment, and optimal intensity and/or duration of treatment (Rodriguez & Rothi, 2008; Rothi, 2006). In phase I studies, such as the one reported here, a within-subject design is an efficient way to address these early questions (McReynolds & Kearns, 1983). Group designs require a control group in addition to the experimental group to maintain control; a single subject design with multiple baselines allows a subject to be used as his or her own control. Our study employed an N of 1, repeated probes, parallel baseline design in which all subjects received the acetyl cholinesterase inhibitor (donepezil) and each subject was serially trained to name sets of semantically orthogonal items. Because performance on all items, trained and untrained, was monitored during training and no-training periods, performance on trained and untrained items, as well as performance with and without treatment, could be systematically contrasted. Because the naming sets were semantically orthogonal, performance on the untrained “generalization” set (List 3, below) provides the requisite experimental control measure: how subjects perform on the drug without behavioral treatment. Because interruption or delay of drug treatment has been shown to have enduring adverse effects and would therefore be unethical, our study did not incorporate a drug placebo component. Thus, we could not determine the impact of donepezil alone on naming performance, or potential donepezil by behavioral treatment interaction effects.
METHODS
Participants
Twenty-eight individuals with probable AD, ranging from 40–91 years of age, were considered for participation in this study and seven who met the inclusion/exclusion criteria were enrolled in the training protocol. Based on review of medical records and examination by a study neurologist, all seven participants met the National Institute of Neurological and Communicative Disorders and Stroke criteria (McKhann et al., 1984) for probable degenerative dementia of the Alzheimer’s type. All participants had brain imaging studies (e.g., MRI), thyroid function tests, B12 levels, and a serological test for syphilis (MHA-TP). All participants were native English speakers and based on the results of the Boston Naming Test (Kaplan et al., 1983), all were anomic. None of the participants had a history of prior strokes, head trauma, learning disabilities (including dyslexia), co-existing chronic neurological disorders, significant drug or alcohol abuse, or depression.
While the use of potentially anti-neuroplastic drugs (including neuroleptics, α-1 noradrenergic antagonists, α-2 noradrenergic agonists, anticonvulsants, benzodiazepines, and tricyclic antidepressants) represented a relative contraindication to study participation, and the use of anticholinergic medications represented an absolute contraindication (Goldstein, 1998), the use of such drugs is so ubiquitous that exclusion of all participants receiving even one of them would have seriously compromised recruitment. All participants entered into the study had been on a dosage of either 5 or 10 mg of donepezil for at least 10 weeks prior to the start of their participation in this study. Demographic data for all participants who completed the study can be found in Table 1 .
Table 1.
Participant demographics
| Subject | Age | Gender | Education | Time post diagnosis (years + months) |
Antineuroplastic medications |
|---|---|---|---|---|---|
| S1 | 72:9 | F | High school | 1:4 | None |
| S2 | 81:6 | M | 1yr college | 1:2 | None |
| S3 | 85:3 | M | Masters | 4:0 | None |
| S4 | 73:9 | M | High school | 1:2 | Oxazepam |
| S5 | >90 | M | High school | 1:11 | None |
| S6 | 76:0 | M | Unknown | 0:8 | Gabapentin |
| Haloperidol |
This study was approved and monitored by the Institutional Review Board of the University of Florida Health Science Center and the Subcommittee for Clinical Investigation of the Malcom Randall VA Medical Center. All participants provided informed consent to participate.
All participants were given a battery of tests before training began and after training ended. This included the Mini Mental State Exam (MMSE) (Folstein et al., 1975) to assess severity of dementia, and the California Verbal Learning Test (CVLT) (Delis et al., 1987) to assess free recall, which is often affected adversely by AD. The Rey Osterrieth Complex Figure Test (Rey-O) (Rey, 1941) was used to assess visuospatial perceptual ability as well as visuospatial construction, as visuoperceptual deficits are common in AD. We also included language tests such as the Western Aphasia Battery (WAB) (Kertesz, 1982) to provide a picture of the participant’s overall language function, the Boston Naming Test (BNT) (Kaplan et al., 1983) to assess confrontation naming as confrontation naming may be affected by semantic or perceptual errors, and the Controlled Oral Word Association Test (COWAT) (Spreen & Benton, 1969) to assess verbal generation, which is often reduced in AD even in the early stages. Scores for all participants can be found in Table 2.
Table 2.
Pre- and post-training scores for all participants
| MMSE | CVLT | BNT | WAB | REY-O | COWAT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant# | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| S1 | 15 | 12 | 11 | 8 | 39 | 41 | 71 | 71 | 2.5 | 2 | 10 | 7 |
| S2 | 30 | 29 | 35 | 24 | 32 | 35 | 96 | 99 | 23 | 29 | 14 | 17 |
| S3 | 19 | 18 | 8 | 13 | 43 | 50 | 95 | 98 | 15 | 18 | 19 | 26 |
| S4 | 11 | 7 | 10 | 3 | 11 | 14 | 67 | 56 | 19 | 9 | 8 | 9 |
| S5 | 11 | 11 | 1 | 2 | 14 | 11 | 69 | 54 | 5 | 3.5 | 10 | 8 |
| S6 | 10 | 11 | 6 | 5 | 11 | 11 | 74 | 67 | 2.5 | 0 | 9 | 8 |
Note. MMSE = Mini Mental State Exam, CVLT = California Verbal Learning Test, BNT = Boston Naming Test, WAB = Western Aphasia Battery, REY-O = Rey Osterrieth Complex Figure Test, COWAT = Controlled Oral Word Association Test.
Design
This study utilized a within-participant, multiple-baselines-across-behaviors design, with generalization probes. All participants received donepezil 10 mg daily throughout the study. Stability of performance on the naming task was assessed via the C-statistic (Tryon, 1982) prior to initiating the errorless naming practice. Participants underwent an initial baseline phase for a minimum of 8 sessions as specified by Tryon (1982) as a means to accurately establish the steady-state level or trend of performance before training and to provide a sufficient dataset for inference employing the C-statistic. The baseline phase assessed each participant’s ability to produce the correct word when presented with a picture from the corpus of 100 pictures. Each picture was shown to the participant with the examiner saying, “I’m going to show you a picture and I want you to name it.” Participants were allowed up to 30 seconds to attempt to name the picture. Responses were scored immediately as correct/incorrect and if no attempt was made, it was scored as incorrect. Upon treatment termination, this same procedure was followed with all participants at three time points (immediately post-treatment, and 1-month and 3-months post-treatment) (See Table 3 for timetable).
Table 3.
Timeline for training
| Baseline | Training stimuli subset 1 | Training stimuli subset 2 | Post-test (Immediate, 1 month, and 3 months) |
||
|---|---|---|---|---|---|
| Drug only | Condition 1 (Immediate cue) | Condition 2 (Delayed cue) | Condition 1 (Immediate cue) | Condition 2 (Delayed cue) | Drug only |
Stimuli
Stimuli for training included a corpus of 100 (50 high and 50 low frequency) words (Francis & Kucera, 1982). Black and white line drawings were matched to each word. The corpus included pictures of animals, body parts, people, household items, clothing, means of transportation, fruit, and musical instruments. The stimuli were chosen such that all items in any given category had less than 0.10 relatedness to all items in any other category, as determined using the University of South Florida Word Relatedness Database (Nelson et al., 1998); that is, the word lists were semantically orthogonal.
Training Procedures
The training involved instructing participants to name stimulus items using an errorless learning technique. Each participant was assigned three uniquely designed stimulus subsets selected from the entire corpus, corresponding to three item categories. Because balance in difficulty, as demonstrated by performance accuracy, was necessary so that no subset was more difficult for any one participant than the others, and because each participant performed uniquely on the various individual stimuli, accuracy in naming each picture during baseline performances was used to select stimulus subsets uniquely for each subject.
Training sessions
Participants received the errorless learning training in 60 min sessions, 4 times a week. Ability to provide an accurate name for all items in the participant’s three uniquely designed stimulus subsets (Lists 1–3) was probed at the start of each training session to establish training outcome measurement. Naming training began by targeting only one of the stimulus subsets (List 1) while Lists 2 and 3 remained untrained but were probed to allow ongoing evaluation of experimental control and also as a test of generalization. If the participant was able to reach a criterion of 90% accuracy in naming objects in List 1 over three consecutive sessions, the focus of the training switched to a second subset (List 2) (see Table 3). However, if the participant was not able to reach criterion on naming performance for the first trained list, training for the second list was never initiated, and outcome measurements were completed. List 3 was never trained and was used throughout the experiment as an untrained control.
During the training, two sequential conditions were employed, the first reflecting maximal therapist support, the second somewhat reduced support. In the first condition (simultaneous condition) the therapist spoke the name of the picture as it was presented. The clinician would say, “I’m going to show you a picture and tell you the name of it. I want you to repeat the name after me.” During the second condition (delayed condition), the therapist named the picture three seconds after it was presented. During the delay the participant was allowed to provide a verbal response if she/he felt capable of producing it. The clinician would say, “I’m going to show you a picture. If you know the name of it, go ahead and say it. If you’re not sure I will tell you the name and then you can repeat it after me.” The criterion for transition from the simultaneous to the delayed condition was the same as the criterion for switching lists (i.e., 90% correct naming performance on the subset targeted by the training on three consecutive sessions). If this criterion was not met within 20 sessions training was discontinued.
Training outcome measurement
The data provided by the probes obtained at the start of each training session for each participant’s three stimulus subsets were plotted graphically with separate lines reflecting performance on Lists 1, 2, and 3. Participants were given a weekly task of drawing clocks as a control measure. Performance on this task was not expected to change because it was not trained and the effects of training ability to name were highly unlikely to generalize to this very different domain.
Analysis
Training outcome data were statistically analyzed in two complementary ways: (1) using piecewise linear regression, and (2) through calculation of effect sizes. The piecewise linear regression analysis explicitly tests the knowledge acquisition process. The effect size calculations give us a “before-retention” snapshot. Piecewise linear regression models were fitted to the probe data to test whether there were significant changes in performance on probes of all three lists between baseline and the List 1 training period, and between the baseline plus List 1 training period and the List 2 training period. To control for multiple testing we conducted each test at the .01 significance level. Effect sizes were calculated using a variation of Cohen’s d-statistic (Cohen, 1988) as calculated by Busk and Serlin (1992) (for overview on calculation of effect sizes in cognition rehabilitation research, see Beeson & Robey, 2006; Robey, 2004). Effect sizes for all participants were calculated by subtracting mean performance on baseline probes from mean performance on all post-test probes (1-week, 1-month, and 3-months) and dividing by the standard deviation of the baseline probes (Busk & Serlin, 1992). Although no benchmarks have been established for the magnitude of effect sizes in rehabilitation of degenerative disease such as AD, benchmarks for magnitude estimates have been provided for single-subject studies in aphasia rehabilitation (Robey et al., 1999) with first, second, and third quartiles corresponding to small, medium, and large effect sizes being 2.6, 3.8, and 5.8, respectively.
The control task of clock drawing was scored by two independent raters (both licensed neuropsychologists) who were blind to subject identity and date/sequence of the clock draw sample acquisition. Scoring criteria described by Rouleau and colleagues (1992) were employed. This scale allows quantification on a 10-point scale across three facets of performance: (1) integrity of the clock face (0–2 points), (2) presence and sequencing of numbers (0–4 points), and (3) presence and placement of hands (0–4 points). Although there are simpler and more time efficient strategies for quantitative clock scoring (e.g.,Roth et al., 1986; Royall et al., 1999; Shulman et al., 1993), it was decided to use this more detailed and comprehensive method to maximize sensitivity to change in these moderately demented patients. The results of the scoring were also analyzed via Tryon’s C-statistic (Tryon, 1982), which tests trends in the data in relation to point-to-point variability.
RESULTS
One of the seven participants who signed the informed consent for this study expired after screening but prior to the start of the training for reasons unrelated to his/her dementia or the screening exam. Of the six participants who did enter the training protocol, all completed the training protocol within a range of 20–35 sessions. No adverse events were noted during this project, demonstrating the safety (tolerability) and the feasibility of completing this training protocol.
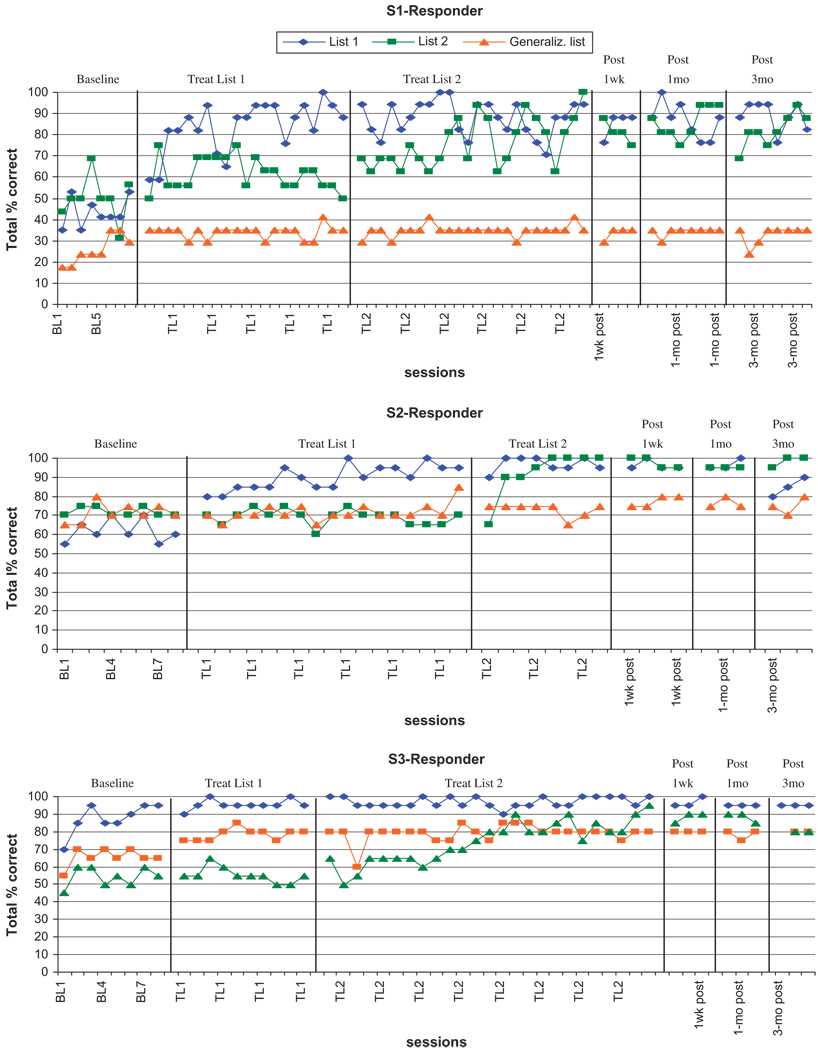
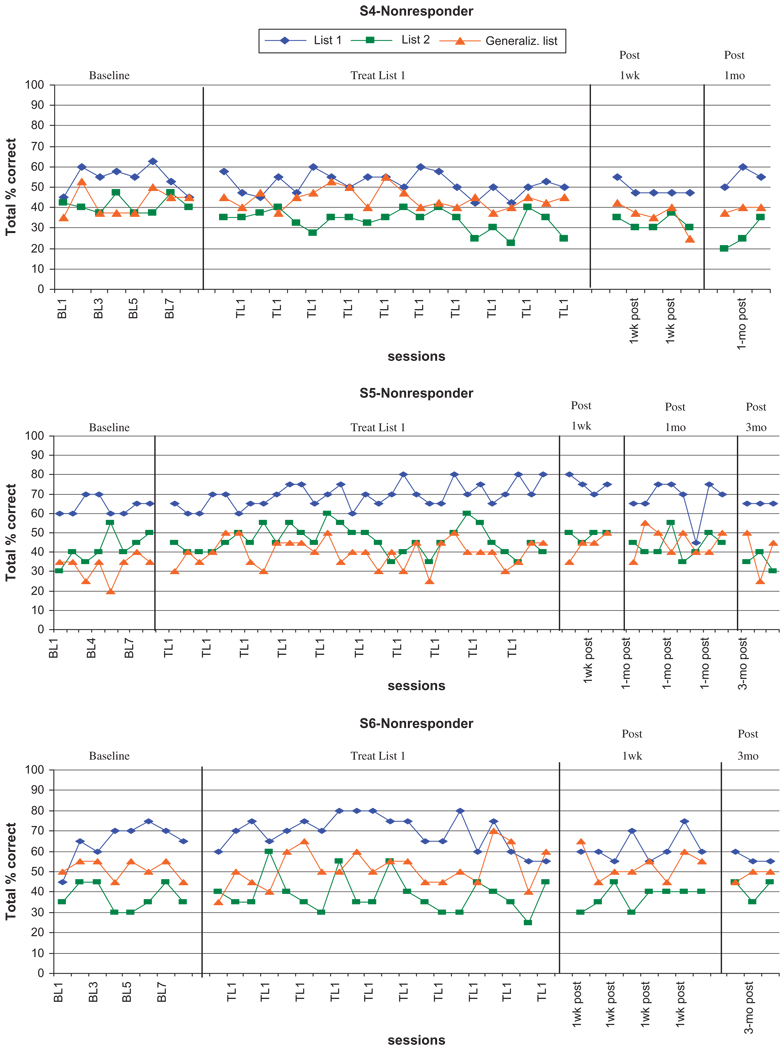
The piece-wise linear regression analysis indicated that S1, S2, and S3 were responders, and S4, S5, and S6 were nonresponders. The analyses showed that for S1, S2, and S3, performance on List 1 probes was significantly better after the list had been trained, compared to baseline, with F-statistics and p-values of F(1, 57) = 117.2, p < .0001; F(1, 26) = 112.6, p < .0001; and F(1, 37) = 19.7, p < .0001, respectively. Similarly, these three subjects (S1, S2, and S3) showed significant improvement in performance on List 2 probes after training compared with performance before List 2 had been trained, with F-statistics and p-values of F(1, 57) = 9.7, p = .0029; F(1, 26) = 45.5, p < .0001, and F(1, 37) = 5.0, p = .0032, respectively. Two of the three responders showed evidence of significant improvement on the generalization probe: S1 during training of List 1, relative to baseline (F(1, 57) = 33.3, p < .0001), and S3 during training of List 2, relative to the baseline plus List 1 training period (F(1, 37) = 30.5, p < .0001). In contrast, the nonresponders (S4, S5, and S6) showed no significant improvement in performance for any of the lists.
Effect sizes for S1, S2, and S3 (the responders) for treated List 1 were 6.0, 5.8, and 1.0, respectively. Effect sizes for S1, S2, and S3 for treated List 2 were 3.2, 9.5, and 6.0, respectively. Most of these qualify as large effects. Effect sizes for S1, S2, and S3 for the untreated generalization list were 1.2, 1.0, and 2.75, respectively. Only the generalization effect for S3 is noteworthy, and it qualifies as a small effect. Effect sizes for S4, S5, and S6, the nonresponders, for treated List 1 were −4.3, 1.9, and −0.3, respectively. Effect sizes for S4, S5, and S6 for the untreated generalization list were −0.8, 1.8, and 0.4, respectively. The results of effect size calculations were substantially congruent with the results of the piece-wise linear regression analysis.
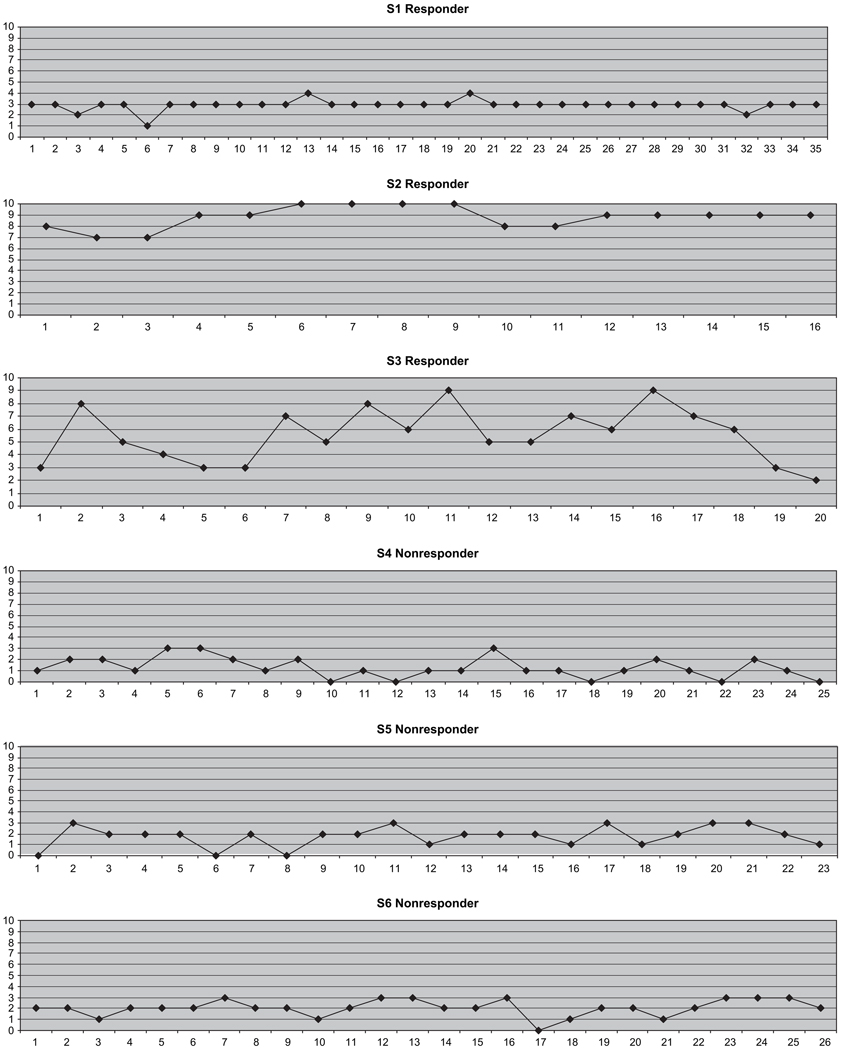
Results for the clock drawings showed no changes for 5 of the 6 participants. One participant (S2) did reach significance on the C-statistic suggesting that his clock drawing performance had improved (see Appendix).
APPENDIX.
Responders and Nonresponders Performance on Clock-Drawing Control Task
DISCUSSION
In this study we report on the proof-of-concept, feasibility, and safety of treating six participants with probable AD using behavioral enrichment in the form of language training in combination with an acetyl cholinesterase inhibitor. The language-training program focused on the accuracy of lexical retrieval utilizing a method called errorless learning (Evans et al., 2000; Jones & Eayrs, 1992). The training (all of which occurred while subjects were on donepezil) effectively eliminated word-finding struggles and perseverative responses in three of the six participants; each showed little evidence of frustration.
Our experimental design, now widely employed in speech-language treatment studies (Kearns, 2000) addresses three major challenges to behavioral therapies in general: (1) behavioral treatment research is very costly because it requires an enormous investment of time and effort in each subject; (2) there is often enormous variability in individual response to treatment; and (3) treatment may have nonspecific effects that improve outcome but obscure the mechanisms of effect. For these reasons, pilot studies are not even feasible unless they can employ exceptionally efficient designs. The N of 1, repeated probe, multiple baseline design employed in the present study is an example. It eliminates the effects of between subject variance by using each subject as their own control. The use of each subject as their own control was made possible by the use of semantically orthogonal training sets in which knowledge gained in the training of one set would not provide linguistic advantage to another set. The use of N of 1, repeated probe, multiple baseline designs anticipates that there are likely to be responders and nonresponders, and by virtue of the design it is possible to detect effects of potentially clinical importance in responders while distinguishing responders from nonresponders. Additionally, in this design, each subject has the same experience through every phase of treatment, thereby minimizing the potential for nonspecific treatment effects differentially impacting treated items. Furthermore, the design provides a means for testing for the presence of such nonspecific effects. In contrast, in a parallel group design, statistical effects of treatment would be washed out by heterogeneity of response in the treated group and by interindividual variability between the two groups. Consequently, a very large and expensive study would likely be needed simply to detect the probable presence of a treatment effect. Finally, controlling adequately for nonspecific treatment effects in parallel group designs can be extremely difficult in neurorehabilitation research.
The results of our study revealed that with the combination of training and use of an acetyl cholinesterase inhibitor, three of the six participants improved in verbal confrontation naming of trained items. In contrast to the three responders, two of the participants did not respond to the training and one had a minimal response (S5). Two of the responders showed evidence of generalization to untreated items in the piece-wise linear regression analysis. For S1, this can be attributed entirely to instability in baseline performance because there was absolutely no further gain during the List 1 and List 2 treatment epochs. S3, however, showed steady slow improvement during both baseline and the List 1 treatment epochs, an improvement that was also born out in the small effect size of 2.75. Overall, the results of our statistical analysis, when considered in conjunction with the graphic display of the data (Figures 1 and 2) suggest preservation of experimental control. However, our results also reflect a reality of language rehabilitation, that is, generalization to untreated stimuli is substantially unpredictable and the mechanisms are poorly understood (Nadeau et al., 2008). Because the training sets were semantically orthogonal, a mechanism specifiscally involved in language processing cannot be invoked. However, there exist generalization mechanisms that may impact language without involving mechanisms of language processing, such as that thought by some to underlie the effect of constraint-induced language therapy: an enhancement of the intentional predisposition to use language notwithstanding its defects.
Fig. 1.
Graphs of responses per session for participants S1–S3 (all responders).
Fig. 2.
Graphs of responses per session for participants S4–S6 (all nonresponders).
There were notable differences between responders and nonresponders in scores on the various tests administered before and after training. All of the responders had higher scores on the MMSE, indicating less severe dementia. All of the responders scored better on the BNT than nonresponders. Free recall, as shown in performance on the CVLT, was also higher for two of the responders. Performance on the Rey-O complex figure task did not differ as strongly between the two groups.
In a post hoc review of medical/social records, we noted that the participants who responded dramatically (S1, S2, and S3) lived at home with a caregiver and the three participants who did not respond dramatically (S4, S5, and S6), lived in an institutional facility specially designed for participants with memory disorders. In addition, two of the nonresponders (S4 and S6) were taking potentially antineuroplastic drugs (see Table 1).
The differences between responders and nonresponders in living arrangements and medications might have influenced the training response. Institutionalization might not provide participants with sufficient opportunities to interact and communicate with people and this environment might adversely influence brain plasticity and learning. Animal studies suggest that enriched environments and exercise, besides providing rich sources of information and inducement to skill learning, may have direct neurotrophic effects, possibly mediated by such agents as brain derived neurotrophic factor (Lazarov et al., 2005; Molteni et al., 2002; van Praag et al., 1999; 2005). Benzodiazepines (e.g., oxazepam) and neuroleptics (e.g., haloperidol) have been shown in animal studies to interfere with brain plasticity and learning (Goldstein, 1998). Gabapentin is an antagonist at the α2δ site of voltage gated calcium channels (Dooley et al., 2007); therefore, it could plausibly impede neuroplasticity. The errorless learning therapy primarily targeted lexical retrieval, but the confrontation naming deficits associated with AD might in some participants be related to deficits in other systems (e.g., semantics) (Rogers et al., 2006). This, in particular, may have accounted for the outcome in our nonresponders. Other studies have also suggested that individuals in the earlier stages of the disease are most amenable to errorless learning rehabilitation efforts (Bottino et al., 2005).
Our participants were taking cholinergic medication before we instituted therapy and they maintained a stable behavioral baseline on untrained lexical retrieval and (for 5 out of 6) on clock drawing performances while they remained on this medication. Thus, the improvement we observed in the three participants could not be solely accounted for by this medication. It is likely, however, that this medication was critical in the improvement we observed in that without it, the rate of learning during the naming practice sessions we provided might have been much lower. Unfortunately, without a controlled acetyl cholinesterase inhibitor trial, which would be contrary to the current standard of care and was barred by our Institutional Review Board, this question cannot be answered.
Because of the sharp focus of the training and the low potential for generalization of lexical knowledge (Nadeau et al., 2008), we did not expect or find change on untrained measures (e.g., BNT, COWAT; Table 2), nor did we expect training to impact daily communicative behavior. However, the capacity for language learning demonstrated in our study suggests that treatments with a potential for generalization to daily communicative behavior may be feasible. The errorless practice strategy described in this study, in combination with the acetyl cholinesterase inhibitor, was intended as a proof of concept that learning can occur. We do not propose this training as a treatment package at this stage. However, by demonstrating a large treatment effect in 50% of our subjects, our study at once suggests that further studies of language treatment and drug combinations in AD are warranted and provides useful information on inclusion and exclusion criteria that should be used in subsequent studies.
Future studies might seek to address a number of questions: Can the results reported here be replicated in a larger sample of subjects? Will subjects on donepezil who respond to the intervention described here also respond to language therapies that might generalize to daily communication? Could the present intervention, if it employed a sufficiently large number of words and targeted responsive subjects, achieve improvement in daily communication? Would this even be feasible? Phase II studies employing parallel group designs could contrast a generalizing therapy with the present treatment, thereby testing efficacy while controlling for nonspecific effects, including the Hawthorne effect. Phase II single or parallel group studies could further test predictors of response and the impact of treatment modifications.
ACKNOWLEDGMENT
This work was supported by grant P50 DC 03888 from the National Institute on Deafness and Other Communication Disorders and by the Department of Veterans Affairs Rehabilitation Research and Development Center of Excellence Grant (F2182C). David Burks, M.D., was critical to the identification of participants for this study.
REFERENCES
- Baskerville KA, Schweitzer JB, Herron P. Effects of cholinergic depletion on experience-dependent plasticity in the cortex of the rat. Neuroscience. 1997;80:1159–1169. doi: 10.1016/s0306-4522(97)00064-x. [DOI] [PubMed] [Google Scholar]
- Beeson PM, Robey RR. Evaluating single-subject treatment research: Lessons learned from the aphasia literature. Neuropsychological Review. 2006;16:161–169. doi: 10.1007/s11065-006-9013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke T, Köylü B, Delazer M, Trinka E, Kemmler G. Cholinergic treatment of amnesia following basal forebrain lesion due to aneurysm rupture – an open-label study. European Journal of Neurology. 2005;12:791–796. doi: 10.1111/j.1468-1331.2005.01063.x. [DOI] [PubMed] [Google Scholar]
- Bottino CMC, Carvalho IAM, Alvarez AM, Avila R, Zukauskas PR, Bustamante SEZ, Andrade FC, Hototian SR, Saffi F, Camargo CHP. Cognitive rehabilitation combined with drug treatment in Alzheimer’s disease patients: A pilot study. Clinical Rehabilitation. 2005;19:861–869. doi: 10.1191/0269215505cr911oa. [DOI] [PubMed] [Google Scholar]
- Burns A, Gauthier S, Perdomo CA. Efficacy and safety of donepezil over 3 years: An open-label, multicentre study of inpatients with Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2007;22:806–812. doi: 10.1002/gps.1746. [DOI] [PubMed] [Google Scholar]
- Burns A, Rossor M, Hecker J, Gauthier S, Petit H, Moller HJ, Rogers SL, Friedhoff LT the International Donepezil Study Group. The effects of donepezil in Alzheimer’s disease: Results from a multinational trial. Dementia and Geriatric Cognitive Disorders. 1999;10:237–244. doi: 10.1159/000017126. [DOI] [PubMed] [Google Scholar]
- Busk PL, Serlin R. Meta-analysis for single-case research. In: Kratochwill TR, Levin JR, editors. Single-case research design and analysis: New directions for psychology and education. Hillsdale, NJ: Erlbaum; 1992. pp. 187–212. [Google Scholar]
- Butt AE, Hodge GK. Acquisition, retention, and extinction of operant discriminations in rats with nucleus basalis magnocellularis lesions. Behavioral Neuroscience. 1995;109:699–713. doi: 10.1037//0735-7044.109.4.699. [DOI] [PubMed] [Google Scholar]
- Clare L, Wilson BA, Carter G, Breen K, Gosses A, Hodges JR. Intervening with everyday memory problems in dementia of Alzheimer type: An errorless learning approach. Journal of Clinical and Experimental Neuropsychology. 2000;22:132–146. doi: 10.1076/1380-3395(200002)22:1;1-8;FT132. [DOI] [PubMed] [Google Scholar]
- Clare L, Wilson BA, Carter G, Roth I, Hodges JR. Re-learning face-name associations in early Alzheimer’s disease. Neuropsychology. 2002;16:538–547. doi: 10.1037//0894-4105.16.4.538. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH. Lesions of the basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron. 2003;38:819–829. doi: 10.1016/s0896-6273(03)00288-5. [DOI] [PubMed] [Google Scholar]
- Crowell TA, Paramadevan J, Abdullah L, Mullan M. Beneficial effect of cholinesterase inhibitor medications on recognition memory performance in mild to moderate Alzheimer’s disease: Preliminary findings. Journal of Geriatric Psychiatry and Neurology. 2006;19:13–15. doi: 10.1177/0891988705284711. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Adult version manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Doody RS, Geldmacher DS, Gordon B, Perdomo CA, Pratt RD the Donepezil Study Group. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Archives of Neurology. 2001;58:427–433. doi: 10.1001/archneur.58.3.427. [DOI] [PubMed] [Google Scholar]
- Doody RS, Stevens JC, Beck C, Dubinski RM, Kaye JA, Gwyther L, Mohs RC, Thal LJ, Whitehouse PJ, DeKosky ST, Cummings JL. Practice parameter: Management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1154–1166. doi: 10.1212/wnl.56.9.1154. [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel alpha2delta ligands: Novel modulators of neurotransmission. Trends in Pharmacological Science. 2007;28:75–82. doi: 10.1016/j.tips.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Drachman DA, Leavitt J. Human memory and the cholinergic system. Archives of Neurology. 1974;30:113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- Ernst RL, Hay JW. Economic research on Alzheimer’s disease. A review of the literature. Alzheimer’s Disease and Associated Disorders. 1997;11 Suppl. 6:135–145. [PubMed] [Google Scholar]
- Evans JJ, Wilson BA, Schuri U, Andrade J, Baddeley AD, Bruna O, Canavan T, Sala SD, Green R, Laaksonen R, Lorenzi L, Taussik I. A comparison of “errorless” and “trial-and-error” learning methods for teaching individuals with acquired memory deficits. Neuropsychological Rehabilitation. 2000;10:67–101. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental State’. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency analysis of English usage: Lexicon and grammar. Boston: Houghton Mifflin; 1982. [Google Scholar]
- Goldstein LB. Potential impact of drugs on poststroke motor recovery. In: Goldstein LB, editor. Restorative neurology: Advances in pharmacotherapy for recovery after stroke. Armonk, NY: Future Publishing; 1998. pp. 241–256. [Google Scholar]
- Grön G, Kirstein M, Thielscher A, Riepe MW, Spitzer M. Cholinergic enhancement of episodic memory in healthy young adults. Psychopharmacology. 2005;182:170–179. doi: 10.1007/s00213-005-0043-2. [DOI] [PubMed] [Google Scholar]
- Gu Q. Contribution of acetylcholine to visual cortical plasticity. Neurobiology of Learning and Memory. 2003;80:291–301. doi: 10.1016/s1074-7427(03)00073-x. [DOI] [PubMed] [Google Scholar]
- Haslam C, Gilroy D, Black S, Beesley T. How successful is errorless learning in supporting memory for high and low-level knowledge in dementia? Neuropsycholgical Rehabilitation. 2006;16:505–536. doi: 10.1080/09602010500231867. [DOI] [PubMed] [Google Scholar]
- Jones RS, Eayrs CB. The use of errorless learning procedures in teaching people with a learning disability: A critical review. Mental Handicap Research. 1992;5:204–212. [Google Scholar]
- Juliano S, Ma W, Eslin D. Cholinergic depletion prevents expansion of topographic maps in somatosensory cortex. Proceedings of the National Academy of Sciences. 1991;88:7780–7784. doi: 10.1073/pnas.88.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kearns KP. Single-subject experimental designs in aphasia. In: Nadeau SE, Rothi LJG, Crosson B, editors. Aphasia and language: Theory to practice. New York: Guilford Press; 2000. pp. 421–441. [Google Scholar]
- Kertesz A. Western Aphasia Battery. San Antonio, TX: Psychological Corporation; 1982. [Google Scholar]
- Kilgard M. Cholinergic modulation of skill learning and plasticity. Neuron. 2003;38:678–680. doi: 10.1016/s0896-6273(03)00327-1. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Order-sensitive plasticity in adult primary auditory cortex. Proceedings of the National Academy of Sciences. 2002;99:3205–3209. doi: 10.1073/pnas.261705198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevic K, Pumain R, Renaud L. The mechanism of excitation by acetylcholine in the cerebral cortex. Journal of Physiology. 1971;215:247–268. doi: 10.1113/jphysiol.1971.sp009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp LB, Christodoulou C, Melville P, Scherl WF, Mac-Allister WS, Elkins LE. Donepezil improved memory in multiple sclerosis in a randomized clinical trial. Neurology. 2004;63:1579–1585. doi: 10.1212/01.wnl.0000142989.09633.5a. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Robinson J, Tang Y-P, Hairston IS, Korade-Mirnics Z, Lee VM-Y, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces aβ levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Loewenstein DA, Acevedo A, Czaja SJ, Duara R. Cognitive rehabilitation of mildly impaired Alzheimer’s disease patients on cholinesterase inhibitors. American Journal of Geriatric Psychiatry. 2004;12:395–402. doi: 10.1176/appi.ajgp.12.4.395. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Two types of muscarinic responses to acetylcholine in mammalian cortical neurons. Proceedings of the National Academy of Sciences. 1985;82:6344–6348. doi: 10.1073/pnas.82.18.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McReynolds LV, Kearns KP. Single-subject experimental designs in communicative disorders. Austin, TX: Pro-Ed; 1983. [Google Scholar]
- Molteni R, Ying Z, Gómez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. European Journal of Neuroscience. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Nadeau SE, Behrman AL, Davis SE, Reid K, Wu SS, Stidham BS, Helms KM, Rothi LJG. Donepezil: Possibly effective adjuvant to constraint induced therapy for upper extremity dysfunction after stroke. Journal of Rehabilitation Research and Development. 2004;41:525–535. doi: 10.1682/jrrd.2003.07.0108. [DOI] [PubMed] [Google Scholar]
- Nadeau SE, Rothi LJG. Rehabilitation of language disorders. In: Ponsford J, editor. Cognitive and behavioral rehabilitation: From neurobiology to clinical practice. New York: Guilford; 2004. pp. 129–174. [Google Scholar]
- Nadeau SE, Rothi LJG, Rosenbek JC. Language rehabilitation from a neural perspective. In: Chapey R, editor. language intervention strategies in aphasia and related neurogenic communication disorders. 5th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008. pp. 689–734. [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida Word Association, Rhyme, and Word Fragment Norms. 1998 doi: 10.3758/bf03195588. http://www.usf.edu/FreeAssociation/ [DOI] [PubMed]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: Early detection of dementia. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD. The role of acetylcholine in cortical synaptic plasticity. Behavioural Brain Research. 2000;115:205–218. doi: 10.1016/s0166-4328(00)00259-x. [DOI] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Archieves de Psychologie. 1941;28:286–340. [Google Scholar]
- Robey RR. A five-phase model for clinical-outcome research. Journal of Communication Disorders. 2004;37:401–411. doi: 10.1016/j.jcomdis.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Robey RR, Schultz MC. A model for conducting clincal-outcome research: An adaptation of the standard protocol for use in aphasiology. Aphasiology. 1998;12:787–810. [Google Scholar]
- Robey RR, Schultz MC, Crawford AB, Sinner CA. Single-subject clinical-outcome research: Designs, data, effect sizes, and analyses. Aphasiology. 1999;13:445–473. [Google Scholar]
- Rodriguez AD, Rothi LJG. Principles in conducting rehabilitation research. In: Stuss DT, Winocur G, Robertson IH, editors. Cognitive neurorehabilitation: Evidence and application. Cambridge: Cambridge University Press; 2008. pp. 79–90. [Google Scholar]
- Rogers SL, Doody RS, Pratt RD, Ieni JR. Long-term efficacy and safety of donepezil in the treatment of Alzheimer’s disease: Final analysis of a multicentre open-label study. European Neuropsychopharmacology. 2000;10:195–203. doi: 10.1016/s0924-977x(00)00067-5. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Ivanoiu A, Patterson K, Hodges JR. Semantic memory in Alzheimer’s disease and the frontotemporal dementias: A longitudinal study of 236 patients. Neuropsychology. 2006;20:319–335. doi: 10.1037/0894-4105.20.3.319. [DOI] [PubMed] [Google Scholar]
- Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, Goddard R. CAMDEX – A standard instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. British Journal of Psychiatry. 1986;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- Rothi LJG. Cognitive rehabilitation: The role of theoretical rationales and respect for the maturational process needed for our evidence. Journal of Head Trauma Research. 2006;21:194–197. doi: 10.1097/00001199-200603000-00011. [DOI] [PubMed] [Google Scholar]
- Rouleau I, Salmon DP, Butters N, Kennedy C, McGuire K. Quantitative and qualitative analyses of clock drawings in Alzheimer’s and Huntington’s disease. Brain and Cognition. 1992;18:79–87. doi: 10.1016/0278-2626(92)90112-y. [DOI] [PubMed] [Google Scholar]
- Royall DR, Mulroy AR, Chiodo LK, Polk MJ. Clock drawing is sensitive to executive control: A comparison of six methods. Journal of Gerontology. 1999;54B:P328–P333. doi: 10.1093/geronb/54b.5.p328. [DOI] [PubMed] [Google Scholar]
- Rozzini L, Costardi D, Chilovi BV, Franzoni S, Trabucchi M, Padovani A. Efficacy of cognitive rehabilitation in patients with mild cognitive impairment treated with cholinesterase inhibitors. International Journal of Geriatric Psychiatry. 2007;22:356–360. doi: 10.1002/gps.1681. [DOI] [PubMed] [Google Scholar]
- Saponjic RM, Hoane MR, Barth TM. Acetylcholine and recovery of function following brain injury. In: Goldstein LB, editor. Restorative neurology : Advances in pharmacotherapy for recovery after stroke. Armonk, NY: Futura Publishing; 1998. pp. 79–89. [Google Scholar]
- Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Research Reviews. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Shulman KI, Pushkar GD, Cohen CA, Zucchero CA. Clock-drawing and dementia in the community: A longitudinal study. International Journal of Geriatric Psychiatry. 1993;8:487–496. [Google Scholar]
- Schneider LS. Training of Alzheimer’s disease with cholinesterase inhibitors. Clinics in Geriatric Medicine. 2001;17:337–358. doi: 10.1016/s0749-0690(05)70072-0. [DOI] [PubMed] [Google Scholar]
- Small GW, Kaufer D, Mendiondo MS, Quarg P, Speigel R. Cognitive performance in Alzheimer’s disease patients receiving rivastigmine for up to 5 years. International Journal of Clinical Practice. 2005;59:473–477. doi: 10.1111/j.1368-5031.2005.00524.x. [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton AL. Neurosensory Center Comprehensive Examination for Aphasia (NCCEA) Victoria: University of Victoria Neuropsychology Laboratory; 1969. [Google Scholar]
- Tryon WW. A simplified time-series analysis for evaluating treatment interventions. Journal of Applied Behavior Analysis. 1982;15:423–429. doi: 10.1901/jaba.1982.15-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster HH, Hanisch U-K, Dykes RW, Biesold D. Basal forebrain lesions with or without reserpine injection inhibit cortical reorganization in rat hindpaw primary somatosensory cortex following sciatic nerve section. Somatosensory and Motor Research. 1991;8:327–346. doi: 10.3109/08990229109144756. [DOI] [PubMed] [Google Scholar]
- Wezenberg E, Verkes RJ, Sabbe BGC, Ruigt GSF, Hulstijn W. Modulation of memory and visuospatial processes by biperiden and rivastigmine in elderly healthy subjects. Psychopharmacology. 2005;181:582–594. doi: 10.1007/s00213-005-0083-7. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Lerner A, Hedera P. Dementia. In: Heilman KM, Valenstein E, editors. Clinical neuropsychology. New York: Oxford University Press; 1993. pp. 603–645. [Google Scholar]