Abstract
OBJECTIVE
IL-7 is a multifunctional cytokine and a promising immunotherapeutic agent. However, since a transient T-cell depletion is an immediate outcome of IL-7 administration at supraphysiological doses, we investigated the mechanism by which the IL-7 proliferative signal transduced through Cdc25A, a key activator of cyclin dependent kinases (CDKs), could modulate lymphocyte movement.
METHODS
Employing novel methods of manipulating Cdc25A gene expression, combined with in vitro and in vivo evaluation of IL-7 application, we assessed the expression of activation and homing markers and identified the mechanism by which IL-7 could induce T-cell expansion and alter lymphocyte motility.
RESULTS
Constitutively active Cdc25A drove T-cell proliferation independently of IL-7 and resulted in an activated phenotype (CD69hi, CD44hi). Conversely, inhibition of Cdc25A resulted in decreased proliferation, reduced expression of activation markers and the up regulation of the lymph node homing molecule, CD62L, which promoted cell adhesion when engaged by ligand. We found that IL-7 prevented the nuclear translocation of the transcription factor, Foxo1, in a manner dependent on the activity of Cdc25A, resulting in decreased levels of CD62L. In vivo administration of IL-7 decreased lymph node cellularity, while treatment with IL-7, premixed with a neutralizing IL-7 antibody (M25), increased total lymph node cells – with more nuclear Foxo1 detected in cells from mice receiving IL-7 + M25.
CONCLUSION
These results are consistent with the model that IL-7 drives Cdc25A-mediated T-cell proliferation, which prevents the nuclear translocation of Foxo1, leading to reduced expression of CD62L and the migration of T-cells into circulation.
Keywords: Cytokines, T-lymphocytes, DNA replication, Cell Migration, Immunotherapy
INTRODUCTION
As effective agents of immunity, T-cells transit to widely dispersed areas of the body. To enter a lymph node from the blood, naïve T-cells must express the adhesion molecule, L-selectin (CD62L), which binds to its peripheral node addressins on the high endothelial venules (HEVs) of the lymph nodes [1]. This interaction promotes “rolling” and facilitates the transmigration of naïve T-cells into the lymph nodes. Antigen encounter and activation proceeds with T-cells stimulated to undergo massive clonal expansion. During this process, the newly activated, effector T-cells down regulate CD62L to prevent activated T-cells from re-entering the lymph nodes and enable migration of activated T-cells to sites of infection. Hence, CD62L controls the entry or re-entry of T-cells into the lymph nodes, and its expression is linked, in a manner still to be fully understood, with their activation and proliferative expansion.
One of the essential cytokines that T-cells encounter upon entering a lymph node is Interleukin-7 (IL-7), likely presented to them by fibroblastic reticular cells (FRC) which express detectable levels of IL-7 mRNA [2;3]. IL-7 is an important regulator of T-cell development as well as the survival of peripheral T-cells and maintenance of long-term memory T-cells [4;5]. The receptor for IL-7 (IL-7R) is expressed by T-cells and consists of the IL-7Rα chain and the common cytokine γ chain (γc) [6]. Upon binding of IL-7, the two receptor chains heterodimerize and initiate signaling events through the JAK/STAT pathway (reviewed in [5]). Mutations in IL-7, its receptor (IL-7R) or components of its signaling pathway lead to severe immunodeficiency [7], demonstrating that this cytokine is a potent mediator of the homeostatic mechanisms that maintain populations of naïve and memory T-cells in the peripheral immune system [8-10].
The mechanism by which IL-7 supports the expansion of T-cells is partially characterized. We reported that the activity of the cdk inhibitor p27kip1 and the cdk activating phosphatase, Cdc25A, was regulated by IL-7[11;12]. Over-expression of p27kip1 induced G1 arrest in the presence of IL-7 and deletion could partially restore proliferation of T-cells from IL-7-/- mice[12]. Cdc25A levels declined upon cytokine loss due to p38 MAP kinase (MAPK)-targeted degradation[11;13]. Expression of a constitutively active form of Cdc25A promoted cell cycling of lymphocytes in the absence of IL-7, even in the presence of elevated levels of p27kip1 [11;14]. Hence in the absence of IL-7, expression of Cdc25A could support cell proliferation, suggesting that Cdc25A is a critical transducer of the IL-7 replicative signal.
Survival, proliferative and metabolic[15] activities have all been ascribed to IL-7, revealing the potential therapeutic applications of this cytokine. However, the effect of IL-7-mediated proliferation upon lymphocyte trafficking is poorly understood. To this end, we examined the functionality of IL-7 under conditions in which proliferative activity was regulated by genetic manipulation of Cdc25A and detected phenotypic changes that could alter lymphocyte migration. Specifically, we observed the up regulation of activation markers like CD69 and the down regulation of adhesion molecules like CD62L on Cdc25A-expressing T-cells. Expression of constitutively active Cdc25A indicated that proliferation driven through IL-7 could significantly alter lymphocyte trafficking in the absence of any antigen stimulation.
MATERIALS AND METHODS
Mice and cell isolation
Bim deficient (Bim-/-) mice, on a C57Bl/6 background, were housed at the National Cancer Institute, Frederick, Maryland. C57Bl/6 mice were purchased from Jackson Labs. Mice were housed in the animal facility at the University of Central Florida. Lymph node and spleen cells were isolated by gentle crushing through a 70μM pore filter (BD Falcon) and pooled. Spleen cells were further treated with ACK lysis buffer (Quality Biological, Inc.). T-cells were enriched by negative selection with the Mouse T-lymphocyte Enrichment kit according to the manufacturer's protocol (BD Biosciences). For CD62Lhi and CD62Llo enrichment experiments, T-cells were purified from spleen and enriched for CD62Lhi or CD62Llo cells with biotinylated anti-CD62L (clone MEL-14; BD Biosciences) according to manufacturer's protocol.
In vitro culture
Cells were maintained at a density of 3-5×106 cells in complete medium (RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone), 2-βmercaptoethanol, and penicillin/ streptomycin. Recombinant human IL-7 (rhIL-7) (Peprotech) was added at a concentration of 10 ng/mL or 150 ng/mL, except for IL-7 withdrawal experiments in which cell cultures were maintained in complete medium.
Plasmids and nucleofection of T-cells
The constitutively active HA-tagged Cdc25A (Cdc25A-DP) plasmid was previously described [11]. To inhibit Cdc25A activity, a catalytically inactive Cdc25A (Cdc25A-DN) was generated in the background of the Cdc25A-DP plasmid by targeting the active site Cys-(X)5-Arg motif [16]. Cys-430 was mutated to serine and Arg436 was mutated to alanine by site-directed mutagenesis (QuikChange II site directed mutagenesis kit, Stratagene). To transiently express Cdc25A-DP, Cdc25A-DN, GFP or the empty vector (pcDNA), T-cells were nucleofected with 4μg of plasmid DNA using the Mouse T-cell Nucleofection kit (Amaxa) according to the manufacturer's protocol. Based on GFP expression, nucleofection efficiency was determined to be 30-40%. GFP+ nucleofected cells appear in the FSC/SSC gate as a separate population of large viable cells. This information was used for gating on the 30-40% of nucleofected cells in subsequent analyses. In the surface staining experiments, nucleofected cells were maintained in IL-7 overnight and then washed the next day and cultured an additional 24 hrs with or without IL-7 prior to analysis. For the BrdU experiments, nucleofected cells were immediately cultured with or without IL-7 and pulsed with 10μM BrdU (BD Biosciences) and analyzed 24 hrs later as described above.
Surface phenotyping
Surface expression on T-cells was assessed with PE-conjugated anti-CD4, PerCP-conjugated anti-CD8, FITC-conjugated anti-CD44, FITC-conjugated anti-CD69, PE-conjugatedd anti-CCR7 and PE-conjugated anti-L-selectin (CD62L) (BD Biosciences). Cells were incubated with saturating amounts of the appropriate antibodies for 20 minutes on ice and analyzed by flow cytometry using the C6 flow cytometer (Accuri). The data was analyzed using FCS Express software (Ontario, Canada).
Intracellular BrdU labeling
Cells were pulsed with BrdU (10μM) for 48 hours and BrdU incorporation was detected with a commercially available kit (BD Biosciences) according to the manufacturer's protocol. Briefly, cells were surface stained as described above, washed, fixed, and permeabilized prior to incubation with a FITC-conjugated anti-BrdU antibody. Staining with 7AAD was performed following manufacturer's protocol. Cells were analyzed by flow cytometry using the C6 flow cytometer (Accuri). The data was analyzed using FCS Express software (Ontario, Canada).
Intracellular staining of Cdc25A or Foxo1
For detection of intracellular or nuclear Cdc25A or nuclear Foxo1, we used an optimized protocol designed to enhance detection of these intracellular proteins [17]. Nuclei were isolated in extraction buffer (320 mM sucrose, 5mM MgCl2, 10mM HEPES, 1% Triton-X100). Prior to fixation, intact cells or nuclei were stained with Foxo1 antibody (Cell Signaling) or Cdc25A antibody (Santa Cruz). Cells or nuclei were washed, fixed, and permeabilized with the Fix & Perm Cell Permeabilization kit (Caltag) following the manufacturer's protocol. Cells or nuclei were stained with PE-conjugated anti-rabbit secondary antibody. The secondary antibody alone or isotype matched PE-conjugated antibodies (BD Biosciences) were used as controls. Cells were analyzed by flow cytometry using the C6 flow cytometer (Accuri) described above. The data was analyzed using FCS Express software (Ontario, Canada).
IL-7 injections of mice
C57Bl/6 mice were injected once, intraperitoneally, with 10μg recombinant human IL-7 (rhIL-7, Peprotech), with 100 μg of M25, an anti-IL-7 antibody (a kind gift from Amgen), or a premixed combination of 10 μg rhIL-7 and 100 μg M25 antibody in 200 μl PBS. Mice were euthanized after 72 hours and lymphoid organs were harvested for analysis of T-cell content as previously described.
T-cell movement assay
CD62Lhi lymph node T-cells or CD62Llo spleen T-cells were enriched as previously described. T-cells were cultured overnight in complete medium supplemented with IL-7 (150 ng/mL), and DMSO (vehicle control) or 10μM of Cdc25 inhibitor I (EMD Biosciences) on fibronectin plates (BD Biosciences). Next day, cells were resuspended in serum free medium with IL-7 and DMSO or 10μM Cdc25 inhibitor and T-cell movement was monitored for 2 hours using the UltraView spinning disc confocal system (Perkin Elmer) with AxioObserver Z1 (Carl Zeiss) stand and a Plan-Neofluar 10X objective. For the final hour, 1 μg of PSGL-1/Fc chimera (R & D Systems), the CD62L ligand, was added to the cultures. Tracking of individual T cell movement and calculation of T cell velocity over the two hour experimental period was performed with Volocity Workstation software (Perkin Elmer).
RESULTS
Previously, we reported that the phosphatase, Cdc25A, was an essential transducer of cytokine-mediated proliferation in lymphocytes [11]. Using cytokine-dependent T and B cell lines, we found that a constitutively active form of Cdc25A (Cdc25A-DP), lacking the p38 MAPK phosphorylation sites that target the phosphatase for degradation, could promote cell division even when levels of the cell cycle inhibitor, p27kip1, were elevated [14].
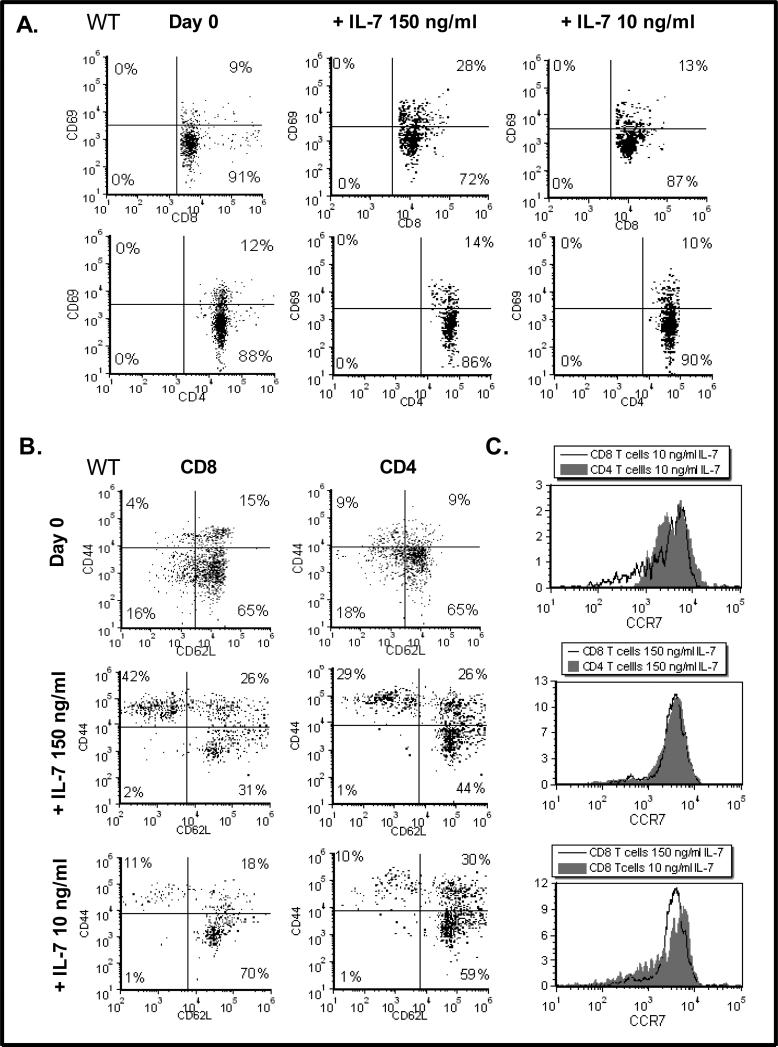
To examine the function of Cdc25A in a biologically relevant scenario, we used primary T-cells freshly isolated from the lymph nodes of C57Bl/6 mice. Our initial observation, using a cell culture method that we optimized to expand IL-7 dependent T-cells [18], was that the dose of IL-7 used for in vitro culture had differential effects upon the T-cell subsets expanded. We found that culture of lymph node T-cells with high dose IL-7 (150 ng/mL), as compared to low dose IL-7 (10 ng/mL), for 2 weeks, up regulated the expression of the CD69 activation marker (a marker typically found increased upon antigen-activation [19]) on CD8 T-cells (28% compared to 13%) (Fig. 1A). The activation and memory marker, CD44, was also elevated on CD8 T-cells grown with high dose IL-7 (150 ng/mL) (Fig. 1B). These results confirmed our published findings that CD8 T-cells optimally respond to high dose IL-7 [20] and that the expression of activation/ memory markers is also enhanced in CD8 T-cells cultured with high doses of IL-7 [18].
Figure 1. High dose IL-7 Promotes Expression of CD69 and CD44 and Down Regulates CD62L.
(A). Lymph node T-cells were isolated from wild type (WT) C57Bl/6 mice (Day 0) and cultured with 150 or 10 ng/mL of IL-7 for 14 days as described in Materials and Methods. Dot plots display CD69 surface expression on CD8 and CD4 T-cells as determined by staining with a FITC-conjugated CD69 antibody, a PE-conjugated anti-CD4 antibody or a PerCP-conjugated anti-CD8 antibody and analyzed by flow cytometry as described in Materials and Methods. Results shown were acquired from the viable cell gate. Quadrants were established using control antibodies. (B). Lymph node T-cells were isolated from WT C57Bl/6 mice (Day 0) and cultured with 150 or 10 ng/mL of IL-7 for 14 days as described in Materials and Methods. Dot plots display CD44 and CD62L surface expression as determined by staining with a FITC-conjugated CD44 antibody and a PE-conjugated anti-CD62L antibody and analyzed by flow cytometry as described in Materials and Methods. Gating was performed on CD4 or CD8-expressing cells using aPerCP-conjugated anti-CD4 antibody or a PerCP-conjugated anti-CD8 antibody. Results shown were acquired from the viable cell gate. Quadrants were established using control antibodies. (D) Following the methodology described above, histograms display the levels of CCR7 observed on CD8 or CD4 T-cells cultured with 10 or 150 ng/mL IL-7. Representative experiments of six performed are shown.
Next we examined the effect of high and low dose IL-7 on the expression of the adhesion molecule, CD62L, which, along with CD44, distinguishes memory T-cells from naïve T-cells. We found that high dose IL-7 (150 ng/mL) supported the growth CD44hiCD62Llo CD8 T-cells (42%), while low dose IL-7 (10 ng/mL) favored naïve CD8 T-cells that were CD44loCD62Lhi (70%) (summarized in Table 1). Although not as striking, CD4 T-cells followed a similar trend (Table 1), indicating that the phenotypic changes observed were not cell-type specific but dependent on the dose of IL-7 used. Note that CD4 and CD8 T-cells freshly isolated from murine lymph nodes (Day 0) displayed low levels of CD69 and CD44 and high levels of CD62L, typical of naïve T-cells (Figs. 1A and 1B).
Table 1.
Summary of Data from Dot Plots in Figure 1
| Phenotype | 150 μg/mL IL-7 | 10 μg/mL IL-7 | |
|---|---|---|---|
| Memory-like | CD4 CD44hiCD62Llo | 29% | 10% |
| CD8 CD44hiCD62Llo | 42% | 11% | |
| Naïve-like | CD4 CD44loCD62Lhi | 44% | 59% |
| CD8 CD44loCD62Lhi | 31% | 70% |
The implication of these findings is that the strength of the IL-7 signal may not only drive proliferation and up regulation of activation/ memory markers but could also affect T-cell lymph node homing by altering the expression of CD62L. To determine whether the dose of IL-7 could alter expression of other important mediators of T-cell movement, we examined expression levels of the chemokine receptor, CCR7, required for the subsequent steps of arrest during lymphocyte extravasation. Shown in Figure 1C, are the results revealing that the levels of CCR7 did not appreciably change under conditions of high or low dose IL-7 cultures. Because the doses of IL-7 being used for testing in human clinical trials are supraphysiological (> 10μg/kg/dose [21]), we focused our investigation on the mechanisms by which IL-7 modulates the levels of CD62L using the conditions of high dose IL-7 that lead to the expansion of CD8 T-cells bearing activation/ memory markers.
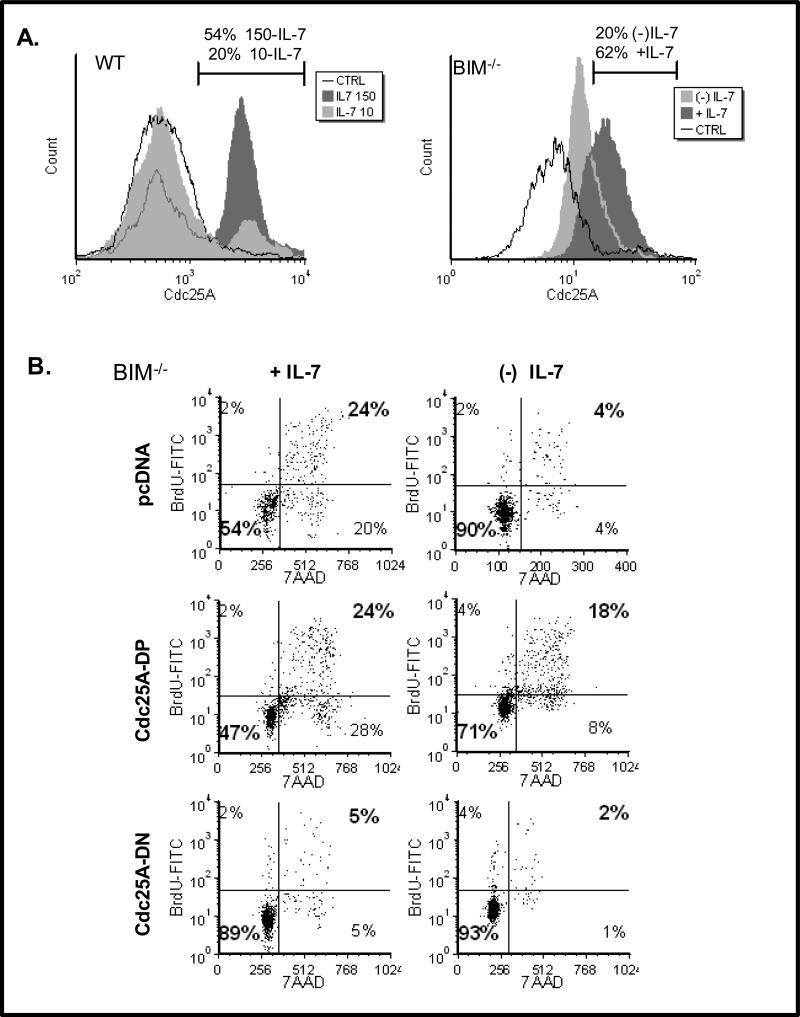
We next examined the intracellular levels of Cdc25A in response to IL-7. Figure 2A shows that lymph node T-cells, isolated from wild type (WT) C57Bl/6 mice and cultured with IL-7, contained more nuclear Cdc25A when maintained with high dose IL-7 (150 ng/mL) compared to low dose IL-7 (10 ng/mL) (Fig. 2A, left histogram). These findings showed that high dose IL-7 was a strong proliferative stimulus. We and others noted, however, that primary T-cells from WT mice rapidly die in the absence of IL-7 [18;22]. Hence examination of the effects of IL-7 deprivation is not possible using WT mice. Instead, we used lymph node T-cells from mice deficient in the pro-apoptotic protein, Bim. Others have shown that mice deficient in both the IL-7 receptor and Bim displayed partial recovery of T-cell numbers [23], indicating that T-cells from these mice are resistant to death when deprived of IL-7 [24]. Use of T-cells from Bim-/- mice enabled us to perform complex experiments requiring conditions of IL-7 deprivation with minimal T-cell loss due to apoptosis. Similar to the results achieved with WT T-cells (Fig. 2A, left histogram), we found that in lymph node T-cells from Bim-/- mice the total levels of Cdc25A were dependent on IL-7 - elevated in the presence of IL-7 and decreased in the absence of IL-7 (Fig. 2A, right histogram).
Figure 2. Cdc25A Transduces IL-7 Proliferative Signals.
(A). Lymph node T-cells from WT C57BL/6 mice (left histogram) or Bim-/- mice (right histogram) were isolated and cultured with low dose (10 ng/mL) or high dose (150 ng/mL) IL-7 (left histogram) or with or without IL-7 (right histogram). Intracellular levels of nuclear Cdc25A (left histogram) or total Cdc25A (right histogram) were determined by intracellular staining using a specific Cdc25A antibody followed by a PE-conjugated secondary antibody and analyzed by flow cytometry as described in Materials and Methods. Percentages shown represent the population of cells indicated by the marker. CTRL represents use of an isotype matched PE-conjugated secondary antibody control. (B). Nucleofection was used to transiently express the cDNA for Cdc25A-DP, Cdc25A-DN or vector only (pcDNA) in lymph node T-cells from Bim-/- mice that were cultured with (+) or without (-) IL-7 for 24 hours. Cell cycling was assessed by measuring the incorporation of BrdU into replicating DNA with a FITC-conjugated BrdU antibody and DNA content was measured by 7AAD staining using flow cytometry as described in Materials and Methods. Quadrants were established using control antibodies. Percentages in bold indicate cells that had incorporated BrdU and were in the G2/S/M phases of the cell cycle. A representative experiment of four performed is shown.
It follows that Cdc25A is needed to transduce the IL-7-proliferative signal in dependent T-cells. We examined this by expressing either a constitutively active form of Cdc25A (Cdc25A-DP), in which the p38 MAPK target sites (Ser75, Ser123) were mutated to alanines, or a dominant negative form (Cdc25A-DN) in which, in addition to the Ser75A and Ser123A mutations, the enzyme active site was mutated, but all activating phosphorylation sites, were retained. The expectation was that Cdc25A-DP would remain stable and induce proliferation, while Cdc25A-DN would inhibit proliferation independently of IL-7. Using Bim-/- lymph node T-cells that were resistant to IL-7-withdrawal induced death, we employed the method of Nucleofection (Amaxa) to transiently express Cdc25A-DP or Cdc25A-DN in cells grown either with high dose IL-7 (150 ng/mL) or deprived of IL-7 (0 ng/mL). Using FSC/SSC analysis guided by GFP expression as described in Methods, we gated on the population of cells that were nucleofected. In Figure 2B we showed that BrdU incorporation was inhibited in IL-7-maintained T-cells upon expression of Cdc25A-DN, and that BrdU incorporation was induced in IL-7-deprived cells upon expression of Cdc25A-DP.
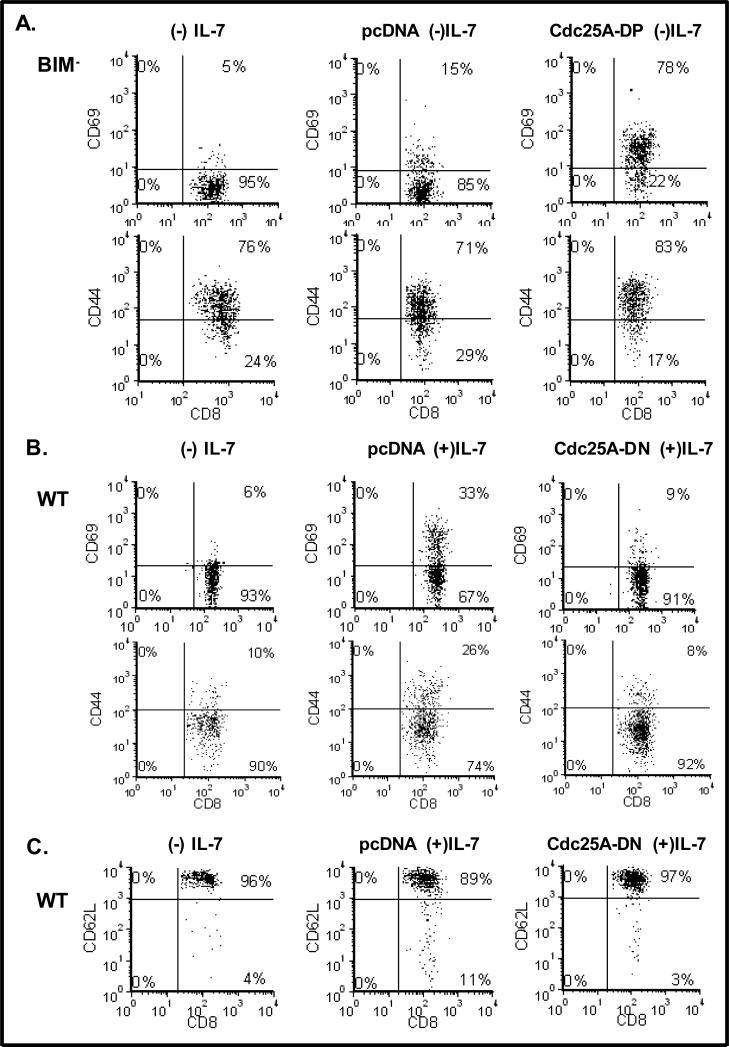
Subsequently, we determined whether Cdc25A activity could mimic the effect of IL-7 upon the levels of activation/ memory markers displayed by T-cells (shown in Fig.1). Figure 3 shows representative experiments in which Bim-/- T-cells (initially expanded in IL-7) were nucleofected with Cdc25A-DP and cultured without IL-7 (Fig. 3A), or WT T-cells (freshly isolated) were nucleofected with Cdc25A-DN and cultured with high dose (150 ng/mL) IL-7. Expression of Cdc25A-DP increased the surface levels of both CD69 and CD44 in T-cells deprived of IL-7 (Fig. 3A), while expression of Cdc25A-DN decreased CD69 and CD44 levels in T-cells maintained with high dose IL-7 (Fig. 3B). Note that CD44 levels were already elevated in Bim-/- T-cells due to initial maintenance in IL-7-containing medium. Furthermore, we observed a trend towards up regulation of CD62L due to Cdc25A inhibition through expression of Cdc25A-DN (Fig. 3C). Collectively the results shown in Figures 2 and 3 indicate that the proliferative and activating effects of high dose IL-7 upon T-cells can be replicated by inducing Cdc25A or blocked by inhibiting Cdc25A.
Figure 3. Expression of Cdc25A Mimics the Effect of High Dose IL-7, Promoting Activated T-cells that Down Regulate CD62L.
(A). Lymph node T-cells were isolated from Bim-/- mice that were expanded with IL-7 (150 ng/mL) for 48 hours. Nucleofection was used to transiently express the cDNA for Cdc25A-DP or vector only (pcDNA) and T-cells were cultured without (-) IL-7 for 24 hours. Untransfected cells are also shown for comparison. Dot plots display CD69 or CD44 surface expression on CD8 T-cells as determined by staining with a FITC-conjugated CD69 antibody and a PerCP-conjugated anti-CD8 antibody and analyzed by flow cytometry as described in Materials and Methods. (B-C). Lymph node T-cells were isolated from WT C57BL/6 mice, and nucleofection was used to transiently express the cDNA for Cdc25A-DN or vector only (pcDNA). WT T-cells were cultured with (+) IL-7 for 24 hours. Untransfected cells cultured without (-) IL-7 are also shown for comparison. Dot plots display CD69 or CD44 surface expression on CD8 T-cells as determined by staining with FITC-conjugated CD69 and CD44 antibodies (B) or PE-conjugated CD62L antibody (C) and a PerCP-conjugated anti-CD8 antibody and analyzed by flow cytometry as described in Materials and Methods. A representative experiment of three performed is shown.
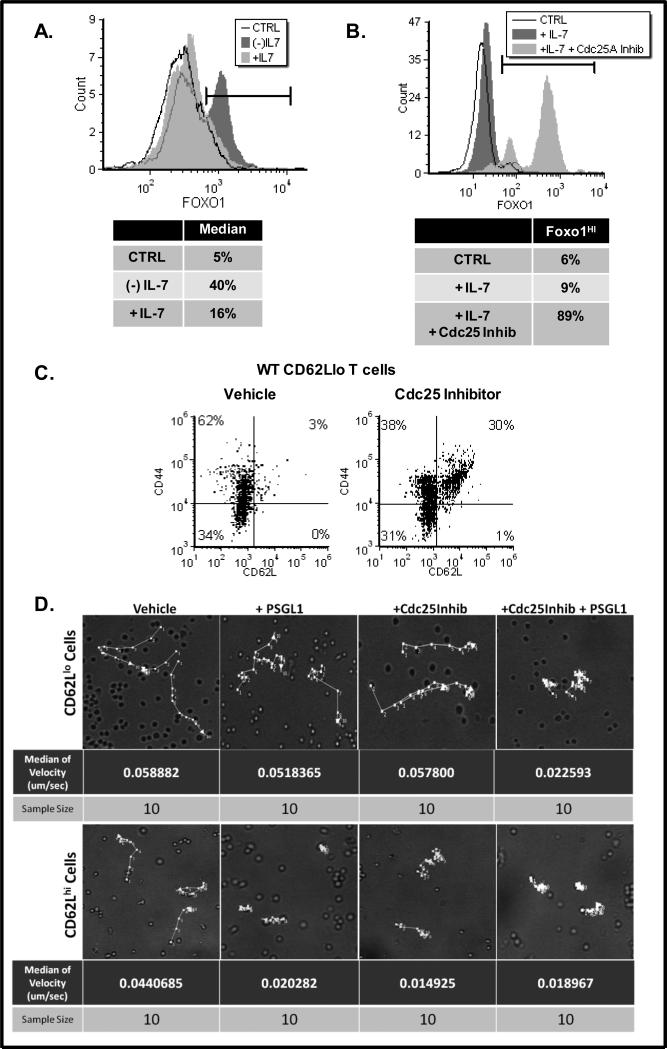
We next examined the mechanism by which IL-7 down regulated the expression of CD62L on T-cells. In Fig 4A we show a representative histogram in which the nuclear levels of the transcription factor, Foxo1, in 40% of the T-cells examined, increased in the absence of IL-7. Previous studies showed that Foxo1 controls the expression of CD62L [25;26]. We inferred, from our findings (Fig. 4A), that IL-7 controlled the nuclear translocation of Foxo1, retaining the transcription factor in the cytosol when IL-7 engaged its receptor and enabling nuclear localization in the absence of IL-7 signaling. We then determined whether the IL-7 signal transduced through Cdc25A was responsible for modulating the intracellular localization of Foxo1. Because the expression of Cdc25A-DN by nucleofection was limited to 30-40% of the cells, we examined nuclear Foxo1 levels in WT lymph node T-cells treated with a Cdc25 pharmacological inhibitor. Figure 4B shows the striking results that nuclear Foxo1 levels greatly increased in cells maintained with IL-7 when Cdc25 was inhibited.
Figure 4. Surface Expression of CD62L depends on the Nuclear Translocation of Foxo1 which is Inhibited by Cdc25A.
(A-B). Lymph node T-cells were isolated from WT C57BL/6 mice and cultured for 24 hours with 150 ng/mL IL-7 or without IL-7 (A) or with a Cdc25 inhibitor (B). Levels of nuclear Foxo1 were determined by intracellular staining using a specific Foxo1 antibody followed by a PE-conjugated secondary antibody and analyzed by flow cytometry as described in Materials and Methods. CTRL represents use of an isotype matched PE-conjugated secondary antibody control. Note that the first peak of (-)IL-7 cells appears behind and overlaps with +IL-7 cells. Percentages in the tables are indicative of the cells delineated by the markers. (C). Splenic CD62Llo T-cells, isolated from WT C57BL/6 mice, were sorted using anti-CD62L antibody and magnetic beads as described in Materials and Methods. T-cells were treated with a vehicle control (DMSO) or the Cdc25 inhibitor for 24 hours and analyzed for surface expression of CD44 and CD62L using a FITC-conjugated CD44 antibody and a PE-conjugated CD62L antibody by flow cytometry as described in Materials and Methods. Quadrants were determined using control antibodies. A representative experiment of four performed is shown. (D). Time-lapse microscopy was used to track the movement of enriched CD62Llo or CD62Lhi T-cells treated with vehicle (DMSO) or the Cdc25 inhibitor overnight as described in Materials and Methods. Cell adhesion was induced in the final hour by treatment with the CD62L ligand, PSGL-1. Tracks of cell movements are indicated by the white arrows in the images. Live cell images were obtained using the UltraView (PerkinElmer) spinning disc confocal system. Post-acquisition processing to determine T-cell velocity was done using Volocity software (PerkinElmer). A representative experiment of four performed is shown.
It follows that the cells, in which Cdc25 inhibition caused an increase in nuclear Foxo1, would also express higher levels of CD62L. Because T-cells freshly isolated from the lymph nodes are predominantly CD62Lhi, we used T-cells isolated from the spleen and sorted for CD62Llo cells. Overnight treatment of these CD62Llo cells with the Cdc25 inhibitor (in the presence of IL-7) caused a rapid increase in the expression of CD62L that was not observed upon treatment with the vehicle control (Fig. 4C). These results revealed the mechanism underlying our initial observation that high dose IL-7 caused down modulation of CD62L. We demonstrated that it is the dephosphorylating activity of Cdc25A, which activates CDKs and drives cell cycling, that prevents the nuclear translocation of Foxo1, resulting in decreased expression of CD62L.
Engagement of CD62L with ligand, such as PSGL-1, is reported to promote cell adhesion [1]. To determine whether treatment with the Cdc25 inhibitor, in the presence of IL-7, could promote CD62L expression leading to cell adhesion, we treated CD62Llo T-cells with PSGL-1. Shown in Figure 4D are the results of time-lapse microscopy tracking the movement of T-cells on fibronectin plates. We found that CD62Llo cells moved rapidly, as indicated by the velocity of movement in μm/sec and that treatment with the ligand, PSGL-1, had no effect (Fig. 4D). This is expected since these cells have low levels of the receptor for PSGL-1. In contrast, CD62Llo cells, in which inhibition of Cdc25 caused up regulation of CD62L, reduced their movement by more than half, indicating the receptor was being engaged by ligand (Fig. 4D). As anticipated, CD62Lhi cells displayed reduced movement in presence of PSGL-1 (Fig. 4D). Inhibition of Cdc25, which impairs proliferation, also reduced the movement of CD62Lhi T-cells more so than that of CD62Llo cells.
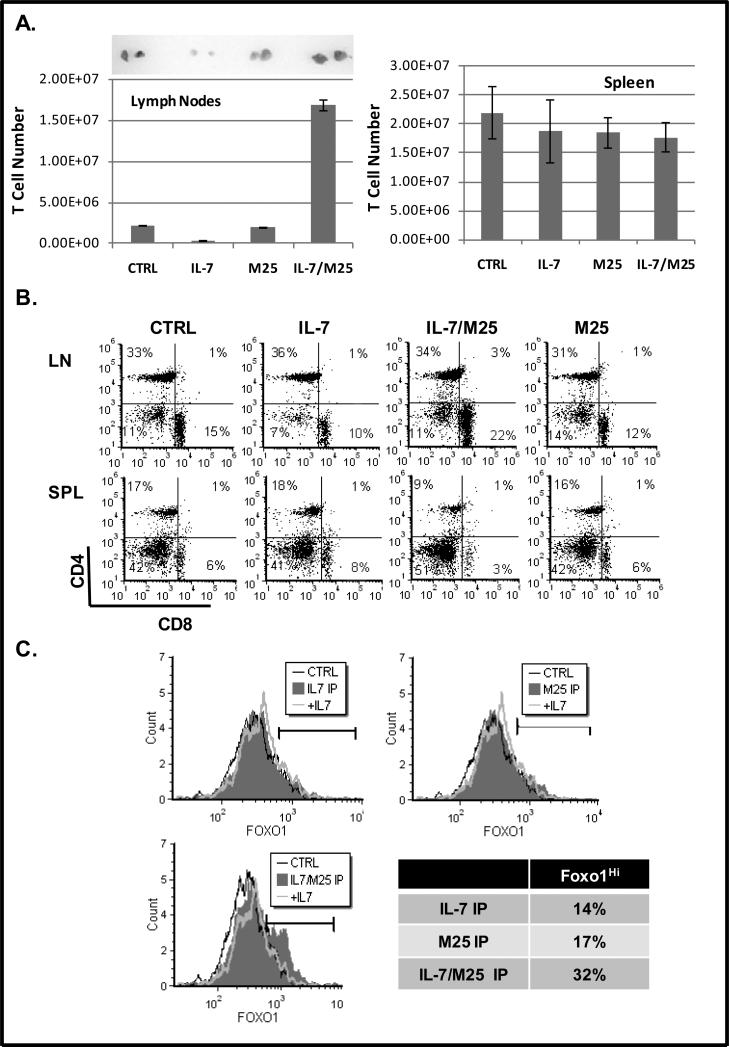
The prediction that stems from the proposed mechanism that IL-7 promotes T-cell movement by down regulating the expression of CD62L in a Cdc25A-dependent manner would be that in the lymph nodes of mice, treated with high dose IL-7, decreased T-cell retention would result. To test this, C57Bl/6 mice were injected intraperitoneally with one large dose of IL-7 (10 μg). To attenuate the IL-7 signal, we used a neutralizing antibody against IL-7 (M25). Mice were injected with one dose of IL-7 (10 μg) premixed with anti-IL-7 antibody (100 μg, M25), or, as control, one dose of M25 (100 μg) alone. We previously observed that under in vitro culture conditions the M25 antibody inhibited IL-7 signaling but had little effect in vivo (data not shown). We surmised that while IL-7 alone would provide the strongest IL-7 signal, treatment with the IL-7 neutralizing antibody, M25, together with IL-7 could generate conditions of attenuated IL-7 signaling.
We found that treatment with IL-7/M25 resulted in the largest recovery of lymph node T-cells, while IL-7 alone caused the greatest loss of lymph node T-cells (Fig. 5A, left graph). This striking loss of cellularity was apparent when examining the actual size of the lymph nodes recovered from mice injected with IL-7 compared to IL-7/M25 (Fig. 5A). Differences in splenic cellularity among mice injected with IL-7 compared to IL-7/M25 were not as significant and no differences were observed in regards to splenic T-cell numbers (Fig. 5A, right graph). As anticipated, treatment with M25 alone did not have any notable effects and was similar to the untreated control mice (Fig. 5A).
Figure 5. In vivo Administration of IL-7 Drives Expansion of T-cells that Decrease CD62L and Exit the Lymph Nodes.
C57BL/6 mice were injected once, intraperitoneally, with PBS (CTRL), 10 μg rhIL-7 (IL-7), 100 μg M25 antibody (M25) or pre-mixed 10 μg rhIL-7 and 100 μg M25 antibody (IL-7/M25). Mice were euthanized after 72 hours and lymph nodes and spleen removed for analysis. (A) T-cell numbers recovered from lymph nodes were determined using the Accuri flow cytometer and confirmed by microscopic evaluation as described in Materials and Methods. Images display inguinal lymph nodes removed from representative mice. (B). Dot plots display ratio of CD8 and CD4 T-cells as determined by staining with a PE-conjugated anti-CD4 antibody and a PerCP-conjugated anti-CD8 antibody and analyzed by flow cytometry as described in Materials and Methods. Results shown were acquired from the viable cell gate. Quadrants were established using control antibodies. (C). Levels of nuclear Foxo1 were determined by intracellular staining using a specific Foxo1 antibody followed by a PE-conjugated secondary antibody and analyzed by flow cytometry as described in Materials and Methods. Percentages shown in the table represent the population of Foxo1hi cells indicated by the marker. CTRL represents use of an isotype matched PE-conjugated secondary antibody control. Representative data from four experiments performed is shown.
We next examined the ratio of CD4 to CD8 T-cells recovered from the lymph node and spleens of mice injected with IL-7, IL-7/M25 or M25 alone. In the lymph nodes, IL-7 treatment decreased CD8 T-cells (10%), while the IL-7/M25 injections resulted in the opposite trend, with more CD8 T-cells accumulating (22%) (Fig. 5B). CD4 T-cells did not change appreciably under any of the treatment conditions. In the spleen, we noticed that the IL-7/M25 treatment caused a loss of both CD4 (9%) and CD8 (3%) T-cells. We also observed that T-cells remaining in the lymph nodes under all treatment conditions were CD62Lhi (data not shown).
Results from the injections with IL-7 or IL-7/M25 supported our proposed mechanism by which IL-7 controls lymphocyte homing to lymph nodes through Foxo1. A strong IL-7 signal (such as that received with IL-7 alone) could cause the down regulation of CD62L, enabling T-cells to exit the lymph nodes and remain in circulation, accounting for the decreased number of lymph node T-cells observed in Fig. 5A. An attenuated IL-7 signal (such as that received with IL-7 pre-treated with the M25 neutralizing antibody) could maintain the levels of CD62L, retaining cells within the lymph nodes as seen in Figure 5A. Because we could not detect CD62Llo cells in the lymphoid organs, as these cells would exit and traffic throughout the body, we examined the levels of Foxo1 in the cells recovered from the injected mice. In correlation with our prediction that the attenuated IL-7 signal, delivered by the combination of IL-7/M25, would maintain CD62L expression, we observed the largest numbers of cells with elevated levels of nuclear Foxo1 (32%) (Fig. 5C). In contrast, cells with nuclear Foxo1 were decreased in mice receiving IL-7 or M25 alone (Fig. 5C). These in vivo studies provide strong evidence that high dose IL-7 could drive T-cell proliferation (through the activity of Cdc25A) and also down modulate CD62L through the cytosolic retention of Foxo1, enabling T-cell movement and exit but not re-entry into the lymph nodes.
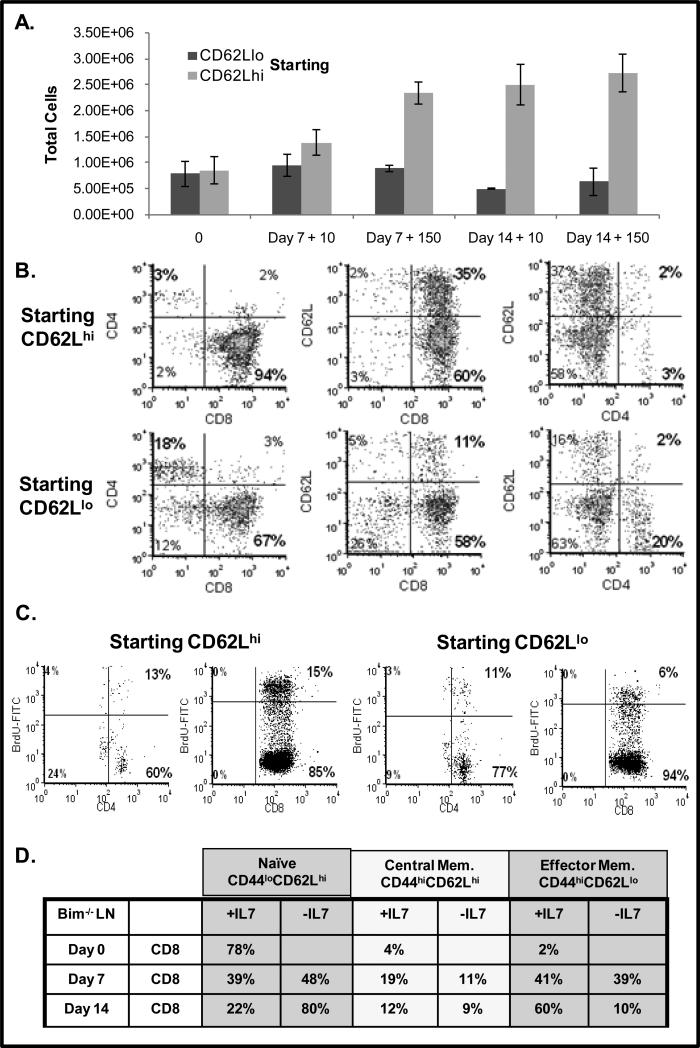
Results from the in vivo injections of mice with IL-7 were compelling but T-cells which down regulated CD62L expression could not be recovered for study. To address this problem and determine whether a strong IL-7 signal causes the down regulation of CD62L, we performed a series of long term in vitro culture experiments with high dose IL-7 to determine whether CD62Lhi cells, typically found in lymph nodes, become CD62Llo cells upon IL-7 treatment.
Using splenic T-cells from Bim-/- mice that were resistant to apoptosis, we sorted for T-cells that were either CD62Lhi or CD62Llo. These T-cells were cultured in vitro with either low dose IL-7 (10 ng/mL) or high dose IL-7 (150 ng/mL) for 7 and 14 days. Results in Figure 6A revealed that T-cells that were initially CD62Llo did not increase in cell number in culture with IL-7. In contrast, cells that started out CD62Lhi rapidly expanded, especially with high dose IL-7, more than doubling in number after 14 days of culture (Fig. 6A). These results indicate that CD62Lhi cells would be more sensitive to the effects of Cdc25A inhibition as we observed in the T-cell movement assay (Fig. 4D). We examined the phenotype of the T-cells cultured with high dose IL-7 (150 ng/mL), focusing on the expression of CD62L on CD4 and CD8 T-cells. We observed that T-cells, initially CD62Lhi, lost CD62L expression and had become predominantly CD62Llo (Fig. 6B). In contrast, CD62L was not up regulated on T-cells cultured with IL-7 that were initially CD62Llo. We further examined the proliferative status of T-cells that started out either CD62Llo or CD62Lhi and found increased BrdU incorporation, indicative of pronounced cell cycling, in the CD8+ CD62Lhi population. Note that the few remaining CD4 T-cells proliferated slightly. Thus as a consequence of a strong IL-7 signal, T-cells proliferate through the Cdc25A-driven activation of CDKs and also down regulate CD62L which promotes T-cell movement and also prevents lymph node re-entry. In support of this effect of IL-7, we isolated Bim-/- lymph node T-cells expressing heterogeneous levels of CD62L and placed them in extended in vitro culture with high dose IL-7. Table 2 shows that after 14 days, most of the T-cells cultured with IL-7 retained expression of the activation/memory marker CD44 but down regulated CD62L, while a much smaller percent maintained detectable levels of CD62L. In contrast, cells deprived of IL-7 expressed a naïve phenotype that was CD44lo and CD62Lhi.
Figure 6. In Vitro Culture with High Dose IL-7 Promotes Proliferation and Down regulation of CD62L on CD8 T-cells.
(A-C). Splenic CD62Llo or CD62Lhi T-cells were isolated from Bim-/- mice and sorted using anti-CD62L antibody and magnetic beads as described in Materials and Methods. Cells were placed in culture without IL-7 or with 10 or 150 ng/mL of IL-7 and assayed after 7 or 14 days. (A). T-cell numbers from populations of cells that were initially sorted for CD62Llo or CD62Lhi expression was determined using the Accuri flow cytometer and confirmed by microscopic evaluation as described in Materials and Methods. (B). Dot plots display ratio of CD8 and CD4 T-cells and surface levels of CD62L on CD62Llo or CD62Lhi T-cells after 14 days of culture in 150 ng/mL IL-7. Phenotype was determined by staining with a FITC-conjugated anti-CD4 antibody or a PerCP-conjugated anti-CD8 antibody and a PE-conjugated CD62L antibody and analyzed by flow cytometry as described in Materials and Methods. Results shown were acquired from the viable cell gate. (C). Cell cycling of CD62Llo or CD62Lhi T-cells was assessed after 7 days of culture in 150 ng/mL IL-7 by measuring the incorporation of BrdU into replicating DNA with a FITC-conjugated BrdU antibody and CD4 or CD8 expression determined as described using flow cytometry (A). Quadrants were established using control antibodies. Percentages in bold indicate CD4 or CD8 T-cells that had incorporated BrdU. (D). Unsorted lymph node T-cells were isolated from Bim-/- mice and placed in culture without IL-7 or with high dose IL-7 (150 ng/mL) for 7 or 14 days. At the indicated time points, cells were stained with antibodies for CD4, CD8, CD44 and CD62L as described in Materials and Methods. Surface expression was analyzed by flow cytometry. Table 2 data is gated on CD8 T-cells and is presented as a percentage of total viable cells expressing the indicated phenotype. Results from a representative experiment of two experiments performed are shown.
DISCUSSION
In this study we show that Cdc25A is a critical transducer of IL-7-mediated T-cell proliferative and movement. Secondary to the effects upon cell growth, expression of either the stable or inhibitory Cdc25A altered the expression of memory and activation markers, changing the phenotype of T-cells independently of IL-7. We found that inhibition of Cdc25 enabled the translocation of Foxo1 to the nucleus and up regulated the expression of CD62L, which could promote cell adhesion upon binding to its ligand. Thus Cdc25A is not just a mediator of proliferation but also could regulates lymph node homing through control of Foxo1 localization and the Foxo1-driven expression of CD62L. Testing this mechanism both in vivo and in vitro revealed that high dose IL-7 can deplete the cellularity of lymph nodes by down regulating the expression of CD62L on responding CD8 T-cells in a Foxo1 dependent manner.
One of the hallmarks of T-cell activation and TCR stimulation is clonal expansion. A strong proliferative signal, as induced by high dose IL-7, could drive the cell cycle machinery in a manner similar to antigen stimulation. Specifically, the dephosphorylating activity of Cdc25A would result in activated CDKs, like CDK2, which can directly phosphorylate Foxo1 [27;28], causing its cytoplasmic retention and inactivation. In support of this idea, we found that the inhibition of Cdc25 resulted in the nuclear accumulation of Foxo1 protein and increased the surface expression of CD62L in the presence of IL-7.
Of significance, our data also showed that the down regulation of CD62L, as mediated by IL-7, is most prominent at the highest dose of IL-7 tested, 150 ng/mL. This indicates that merely receiving a survival signal from IL-7, which occurs at the lower dosage of IL-7 (<10ng/mL), is not sufficient to decrease expression of CD62L. Indeed, the generation of a memory effector phenotype, involving the down regulation of CD62L, the up regulation of the activation markers CD69 and CD44, and the acquisition of effector functions, results when T-cells undergo multiple cell cycles [29]. Our finding with Bim-/- T-cells supports this idea. We found that multiple rounds of IL-7-driven cell division, over a period of 7 to 14 days, resulted in the accumulation of CD62Llo T-cells, even when starting with CD62Lhi cells, while IL-7 withdrawal maintained a naïve CD44loCD62Lhi phenotype.
Our results indicate that a strong proliferative signal, as supplied by high dose IL-7 or over expressed stable Cdc25A, can down regulating CD62L, promoting T-cell movement. We found that introduction of exogenous IL-7 lead to decreased cellularity of the lymph nodes and lower T-cell counts. This is most likely an outcome of T-cell redistribution rather than massive cell apoptosis. Others have shown that injection of IL-7 into rhesus macaques did not induce cell death but rather the movement of T-cells into various organs [30], and was linked to their proliferation. Consistent with published reports [31], we found that a combination of IL-7 and M25 resulted in the highest recovery of cells from the lymph nodes. Others have attributed this accumulation of T-cells to antibody-mediated stabilization of the cytokine. However, we propose in our studies that a combination of IL-7 and M25 actually generates an attenuated IL-7 signal, which is consistent with the previously described IL-7 neutralizing activity of M25 [32]. Hence, an IL-7/M25 signal would produce a CD62Lhi phenotype, enabling T-cell retention in the lymph nodes. In support, we observed lymph node T-cells with the highest levels of nuclear Foxo1 from mice injected with IL-7 and M25.
It is difficult to determine whether T-cells in circulation normally encounter increased amounts of IL-7 comparable to that utilized in our in vitro assays or in vivo injections. The concentration of IL-7 detected in the serum of healthy individuals is low (0.3-8.4 pg/mL) [33] and reporter assays for IL-7 production did not detect the cytokine outside of primary lymphoid organs [34;35]. Other reports, however, indicate that migrating T-cells could encounter regions of higher levels of IL-7. Following injections of IL-7, increased levels of the cytokine were detected in non-lymphoid tissues such as the intestines [30]. This could be explained by the presence of heparin sulfate proteoglycans found on stromal cells within various organs that could concentrate the cytokine [36], creating localized microenvironments containing increased IL-7. More significantly, IL-7 production can be induced upon infection as was shown to occur with liver hepatocytes upon TLR engagement [37], and increases in circulating levels of IL-7 have been reported following inflammation [38]. Hence it is possible that physiological conditions exist whereby T-cells encounter elevated levels of IL-7 in tissues, yet serum levels remain undetectable. From a therapeutic standpoint, supraphysiological levels of IL-7 are currently being utilized in human clinical trials. Our findings that the action of Cdc25A upon Foxo1 and CD62L can alter the movement of T-cells exposed to high dose IL-7 provide a possible explanation for the observed effects of IL-7 application, such as the transient depletion of circulating T-cells [39], providing insight for optimizing the therapeutic potential of this essential cytokine.
ACKNOWLEDGMENTS
This study was supported by an NIH/NIGMS Predoctoral Fellowship F31GM073565 (Kittipatarin) and an NIH/NCI Grant CA109524RO1 (Khaled) from the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERST DISCLOSURE: No financial interest/relationships with financial interest relating to the topic of this article have been declared.
REFERENCES
- 1.Grailer JJ, Kodera M, Steeber DA. L-selectin: role in regulating homeostasis and cutaneous inflammation. J.Dermatol.Sci. 2009;56:141–147. doi: 10.1016/j.jdermsci.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Link A, Vogt TK, Favre S, Britschgi MR, cha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat.Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 3.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat.Rev.Immunol. 2009;9:618–629. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Kittipatarin C, Khaled AR. Interlinking interleukin-7. Cytokine. 2007;39:75–83. doi: 10.1016/j.cyto.2007.07.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol.Rev. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 7.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 8.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 10.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol.Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 11.Khaled AR, Bulavin DV, Kittipatarin C, Li WQ, Alvarez M, Kim K, Young HA, Fornace AJ, Durum SK. Cytokine-driven cell cycling is mediated through Cdc25A. J.Cell Biol. 2005;169:755–763. doi: 10.1083/jcb.200409099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WQ, Jiang Q, Aleem E, Kaldis P, Khaled AR, Durum SK. IL-7 promotes T cell proliferation through destabilization of p27Kip1. J.Exp.Med. 2006;203:573–582. doi: 10.1084/jem.20051520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goloudina A, Yamaguchi H, Chervyakova DB, Appella E, Fornace AJ, Jr., Bulavin DV. Regulation of Human Cdc25A Stability by Serine 75 Phosphorylation Is Not Sufficient to Activate a S Phase Checkpoint. Cell Cycle. 2003;2:473–478. [PubMed] [Google Scholar]
- 14.Kittipatarin C, Li WQ, Bulavin DV, Durum SK, Khaled AR. Cell cycling through Cdc25A: transducer of cytokine proliferative signals. Cell Cycle. 2006;5:907–912. doi: 10.4161/cc.5.9.2693. [DOI] [PubMed] [Google Scholar]
- 15.Chehtane M, Khaled AR. Interleukin-7 Mediates Glucose Utilization in Lymphocytes through Transcriptional Regulation of the Hexokinase II Gene. Am.J.Physiol Cell Physiol. 2010 doi: 10.1152/ajpcell.00506.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fauman EB, Cogswell JP, Lovejoy B, Rocque WJ, Holmes W, Montana VG, Piwnica-Worms H, Rink MJ, Saper MA. Crystal structure of the catalytic domain of the human cell cycle control phosphatase, Cdc25A. Cell. 1998;93:617–625. doi: 10.1016/s0092-8674(00)81190-3. [DOI] [PubMed] [Google Scholar]
- 17.Van De Wiele CJ, Marino JH, Murray BW, Vo SS, Whetsell ME, Teague TK. Thymocytes between the beta-selection and positive selection checkpoints are nonresponsive to IL-7 as assessed by STAT-5 phosphorylation. J.Immunol. 2004;172:4235–4244. doi: 10.4049/jimmunol.172.7.4235. [DOI] [PubMed] [Google Scholar]
- 18.Kittipatarin C, Khaled AR. Ex vivo expansion of memory CD8 T cells from lymph nodes or spleen through in vitro culture with interleukin-7. J.Immunol.Methods. 2009;344:45–57. doi: 10.1016/j.jim.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Kittipatarin C, Tschammer N, Khaled AR. The interaction of LCK and the CD4 co-receptor alters the dose response of T-cells to interleukin-7. Immunol.Lett. 2010;131:170–181. doi: 10.1016/j.imlet.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sportes C, Babb RR, Krumlauf MC, Hakim FT, Steinberg SM, Chow CK, Brown MR, Fleisher TA, Noel P, Maric I, Stetler-Stevenson M, Engel J, Buffet R, Morre M, Amato RJ, Pecora A, Mackall CL, Gress RE. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin.Cancer Res. 2010;16:727–735. doi: 10.1158/1078-0432.CCR-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 23.Pellegrini M, Bouillet P, Robati M, Belz GT, Davey GM, Strasser A. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J.Exp.Med. 2004;200:1189–1195. doi: 10.1084/jem.20041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li WQ, Guszczynski T, Hixon JA, Durum SK. Interleukin-7 regulates Bim proapoptotic activity in peripheral T-cell survival. Mol.Cell Biol. 2010;30:590–600. doi: 10.1128/MCB.01006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat.Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C, Lazar V, Cagnard N, Dubart-Kupperschmitt A, Mangeney M, Fruman DA, Bismuth G. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J.Immunol. 2008;181:2980–2989. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- 27.Leisser C, Rosenberger G, Maier S, Fuhrmann G, Grusch M, Strasser S, Huettenbrenner S, Fassl S, Polgar D, Krieger S, Cerni C, Hofer-Warbinek R, DeMartin R, Krupitza G. Subcellular localisation of Cdc25A determines cell fate. Cell Death.Differ. 2004;11:80–89. doi: 10.1038/sj.cdd.4401318. [DOI] [PubMed] [Google Scholar]
- 28.Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–297. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- 29.Bachmann MF, Wolint P, Schwarz K, Oxenius A. Recall proliferation potential of memory CD8+ T cells and antiviral protection. J.Immunol. 2005;175:4677–4685. doi: 10.4049/jimmunol.175.7.4677. [DOI] [PubMed] [Google Scholar]
- 30.Beq S, Rozlan S, Gautier D, Parker R, Mersseman V, Schilte C, Assouline B, Rance I, Lavedan P, Morre M, Cheynier R. Injection of glycosylated recombinant simian IL-7 provokes rapid and massive T-cell homing in rhesus macaques. Blood. 2009;114:816–825. doi: 10.1182/blood-2008-11-191288. [DOI] [PubMed] [Google Scholar]
- 31.Boyman O, Ramsey C, Kim DM, Sprent J, Surh CD. IL-7/anti-IL-7 mAb complexes restore T cell development and induce homeostatic T Cell expansion without lymphopenia. J.Immunol. 2008;180:7265–7275. doi: 10.4049/jimmunol.180.11.7265. [DOI] [PubMed] [Google Scholar]
- 32.Plum J, De SM, Leclercq G, Verhasselt B, Vandekerckhove B. Interleukin-7 is a critical growth factor in early human T-cell development. Blood. 1996;88:4239–4245. [PubMed] [Google Scholar]
- 33.Sasson SC, Zaunders JJ, Kelleher AD. The IL-7/IL-7 receptor axis: understanding its central role in T-cell homeostasis and the challenges facing its utilization as a novel therapy. Curr.Drug Targets. 2006;7:1571–1582. doi: 10.2174/138945006779025365. [DOI] [PubMed] [Google Scholar]
- 34.Alves NL, Goff OR, Huntington ND, Sousa AP, Ribeiro VS, Bordack A, Vives FL, Peduto L, Chidgey A, Cumano A, Boyd R, Eberl G, Di Santo JP. Characterization of the thymic IL-7 niche in vivo. Proc.Natl.Acad.Sci.U.S.A. 2009;106:1512–1517. doi: 10.1073/pnas.0809559106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazzucchelli RI, Warming S, Lawrence SM, Ishii M, Abshari M, Washington AV, Feigenbaum L, Warner AC, Sims DJ, Li WQ, Hixon JA, Gray DH, Rich BE, Morrow M, Anver MR, Cherry J, Naf D, Sternberg LR, McVicar DW, Farr AG, Germain RN, Rogers K, Jenkins NA, Copeland NG, Durum SK. Visualization and identification of IL-7 producing cells in reporter mice. PLoS.One. 2009;4:e7637. doi: 10.1371/journal.pone.0007637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarke D, Katoh O, Gibbs RV, Griffiths SD, Gordon MY. Interaction of interleukin 7 (IL-7) with glycosaminoglycans and its biological relevance. Cytokine. 1995;7:325–330. doi: 10.1006/cyto.1995.0041. [DOI] [PubMed] [Google Scholar]
- 37.Sawa Y, Arima Y, Ogura H, Kitabayashi C, Jiang JJ, Fukushima T, Kamimura D, Hirano T, Murakami M. Hepatic interleukin-7 expression regulates T cell responses. Immunity. 2009;30:447–457. doi: 10.1016/j.immuni.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Unsinger J, McGlynn M, Kasten KR, Hoekzema AS, Watanabe E, Muenzer JT, McDonough JS, Tschoep J, Ferguson TA, McDunn JE, Morre M, Hildeman DA, Caldwell CC, Hotchkiss RS. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J.Immunol. 2010;184:3768–3779. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J.Exp.Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]