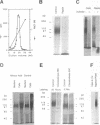
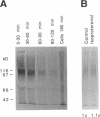
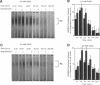
Abstract
Secretion of parathyroid hormone (PTH) is regulated in part by a classical "stimulus-secretion" pathway responsive to catecholamines. The primary physiological modulator of PTH exocytosis in parathyroid cells, however, is extracellular free Ca2+. Ca(2+)-modulated PTH release exhibits several characteristics suggestive of constitutive secretion. The aim of this work was to obtain further information about the possible intracellular origins of Ca(2+)-modulated exocytosis in parathyroid cells. Freshly dissociated bovine parathyroid cells labeled with [35S]sulfate synthesized a soluble chondroitin/dermatan sulfate proteoglycan (M(r) approximately 90-150 K) that was secreted into the medium. The export of [35S]sulfated proteoglycan satisfied several criteria that generally define constitutive release: 1) export is detected in the medium shortly (7-15 min) after a 5-min pulse, 2) there is minimal intracellular storage after equilibrium labeling (because of combined processes of rapid release and intracellular degradation), and 3) there is insensitivity to stimulation with isoproterenol, a known secretagogue in parathyroid cells. Nevertheless, the increase in extracellular Ca2+ from 0.5 to 2.0 mM reduced the export of the [35S]sulfated proteoglycan from 60% of total labeled to 30%. In addition, a secreted pool of immunoreactive PTH and [35S]sulfated proteoglycan was modulated by external Ca2+ to the same degree and sensitivity, although isoproterenol was more effective in stimulating the release of PTH than that of proteoglycan. Together, our experimental results show that in the parathyroid cell extracellular Ca2+ modulates negatively the export of both PTH and proteoglycan, a putative marker for constitutive secretion. We further suggest that a portion of newly synthesized PTH also enters this pathway, whereas another portion proceeds to an isoproterenol-releasable compartment from which the proteoglycan is largely excluded.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvan P., Castle D. Protein sorting and secretion granule formation in regulated secretory cells. Trends Cell Biol. 1992 Nov;2(11):327–331. doi: 10.1016/0962-8924(92)90181-l. [DOI] [PubMed] [Google Scholar]
- Arvan P., Castle J. D. Phasic release of newly synthesized secretory proteins in the unstimulated rat exocrine pancreas. J Cell Biol. 1987 Feb;104(2):243–252. doi: 10.1083/jcb.104.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P., Kuliawat R., Prabakaran D., Zavacki A. M., Elahi D., Wang S., Pilkey D. Protein discharge from immature secretory granules displays both regulated and constitutive characteristics. J Biol Chem. 1991 Aug 5;266(22):14171–14174. [PubMed] [Google Scholar]
- Barr F. A., Leyte A., Mollner S., Pfeuffer T., Tooze S. A., Huttner W. B. Trimeric G-proteins of the trans-Golgi network are involved in the formation of constitutive secretory vesicles and immature secretory granules. FEBS Lett. 1991 Dec 9;294(3):239–243. doi: 10.1016/0014-5793(91)81438-e. [DOI] [PubMed] [Google Scholar]
- Blair E. A., Castle A. M., Castle J. D. Proteoglycan sulfation and storage parallels storage of basic secretory proteins in exocrine cells. Am J Physiol. 1991 Nov;261(5 Pt 1):C897–C905. doi: 10.1152/ajpcell.1991.261.5.C897. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Brent G. A., LeBoff M. S., Seely E. W., Conlin P. R., Brown E. M. Relationship between the concentration and rate of change of calcium and serum intact parathyroid hormone levels in normal humans. J Clin Endocrinol Metab. 1988 Nov;67(5):944–950. doi: 10.1210/jcem-67-5-944. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Carroll R. J., Aurbach G. D. Dopaminergic stimulation of cyclic AMP accumulation and parathyroid hormone release from dispersed bovine parathyroid cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4210–4213. doi: 10.1073/pnas.74.10.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. M. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev. 1991 Apr;71(2):371–411. doi: 10.1152/physrev.1991.71.2.371. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Gamba G., Riccardi D., Lombardi M., Butters R., Kifor O., Sun A., Hediger M. A., Lytton J., Hebert S. C. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993 Dec 9;366(6455):575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Hurwitz S., Aurbach G. D. Preparation of viable isolated bovine parathyroid cells. Endocrinology. 1976 Dec;99(6):1582–1588. doi: 10.1210/endo-99-6-1582. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Leombruno R., Thatcher J., Burrowes M. The acute secretory response to alterations in extracellular calcium concentration and dopamine in perifused bovine parathyroid cells. Endocrinology. 1985 Mar;116(3):1123–1132. doi: 10.1210/endo-116-3-1123. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Redgrave J., Thatcher J. Effect of the phorbol ester TPA on PTH secretion. Evidence for a role for protein kinase C in the control of PTH release. FEBS Lett. 1984 Sep 17;175(1):72–75. doi: 10.1016/0014-5793(84)80572-4. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Sorting and secretion of adrenocorticotropin in a pituitary tumor cell line after perturbation of the level of a secretory granule-specific proteoglycan. J Cell Biol. 1984 Dec;99(6):2223–2230. doi: 10.1083/jcb.99.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan M. J., Stow J. L., Newman A. P., Madri J., Anderson H. C., Farquhar M. G., Palade G. E., Jamieson J. D. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987 Oct 15;329(6140):632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- Christy K. G., Jr, LaTart D. B., Osterhoudt H. W. Modifications for SDS-PAGE of proteins. Biotechniques. 1989 Jul-Aug;7(7):692–693. [PubMed] [Google Scholar]
- Chu L. L., MacGregor R. R., Hamilton J. W. Effects of isoproterenol and cycloheximide on parathyroid secretion. Mol Cell Endocrinol. 1983 Dec;33(2-3):157–168. doi: 10.1016/0303-7207(83)90164-8. [DOI] [PubMed] [Google Scholar]
- Cifonelli J. A., King J. The distribution of 2-acetamido-2-deoxy-D-glucose residues in mammalian heparins. Carbohydr Res. 1972 Feb;21(2):173–186. doi: 10.1016/s0008-6215(00)82144-8. [DOI] [PubMed] [Google Scholar]
- Cohn D. V., MacGregor R. R. The biosynthesis, intracellular processing, and secretion of parathormone. Endocr Rev. 1981 Winter;2(1):1–26. doi: 10.1210/edrv-2-1-1. [DOI] [PubMed] [Google Scholar]
- Emura S., Shoumura S., Utsumi M., Hayakawa D., Yamahira T., Terasawa K., Tamada A., Arakawa M., Isono H. Effects of short-term treatment with calcium on the parathyroid glands in golden hamsters of different ages, with special reference to large vacuolar bodies. Acta Anat (Basel) 1992;143(3):223–230. doi: 10.1159/000147252. [DOI] [PubMed] [Google Scholar]
- Gorr S. U., Hamilton J. W., Cohn D. V. Sulfated secreted forms of bovine and porcine parathyroid chromogranin A (secretory protein-I). J Biol Chem. 1991 Mar 25;266(9):5780–5784. [PubMed] [Google Scholar]
- Gowda D. C., Hogue-Angeletti R., Margolis R. K., Margolis R. U. Chromaffin granule and PC12 cell chondroitin sulfate proteoglycans and their relation to chromogranin A. Arch Biochem Biophys. 1990 Sep;281(2):219–224. doi: 10.1016/0003-9861(90)90435-2. [DOI] [PubMed] [Google Scholar]
- Grimes M., Kelly R. B. Intermediates in the constitutive and regulated secretory pathways released in vitro from semi-intact cells. J Cell Biol. 1992 May;117(3):539–549. doi: 10.1083/jcb.117.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes M., Kelly R. B. Sorting of chromogranin B into immature secretory granules in pheochromocytoma (PC12) cells. Ann N Y Acad Sci. 1992 Dec 31;674:38–52. doi: 10.1111/j.1749-6632.1992.tb27475.x. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Amherdt M., Ravazzola M., Orci L. Parathyroid hormone biosynthesis. Correlation of conversion of biosynthetic precursors with intracellular protein migration as determined by electron microscope autoradiography. J Cell Biol. 1979 Mar;80(3):715–731. doi: 10.1083/jcb.80.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J. F., Kemper B., Potts J. T., Jr Calcium-dependent intracellular degradation of parathyroid hormone: a possible mechanism for the regulation of hormone stores. Endocrinology. 1975 Aug;97(2):431–441. doi: 10.1210/endo-97-2-431. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Kimura J. H. Proteoglycans: isolation and characterization. Methods Enzymol. 1982;82(Pt A):769–800. doi: 10.1016/0076-6879(82)82102-2. [DOI] [PubMed] [Google Scholar]
- Huttner W. B. Determination and occurrence of tyrosine O-sulfate in proteins. Methods Enzymol. 1984;107:200–223. doi: 10.1016/0076-6879(84)07013-0. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. Pathways of protein secretion in eukaryotes. Science. 1985 Oct 4;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Kuliawat R., Arvan P. Protein targeting via the "constitutive-like" secretory pathway in isolated pancreatic islets: passive sorting in the immature granule compartment. J Cell Biol. 1992 Aug;118(3):521–529. doi: 10.1083/jcb.118.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leyte A., Barr F. A., Kehlenbach R. H., Huttner W. B. Multiple trimeric G-proteins on the trans-Golgi network exert stimulatory and inhibitory effects on secretory vesicle formation. EMBO J. 1992 Dec;11(13):4795–4804. doi: 10.1002/j.1460-2075.1992.tb05585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini A., De Matteis M. A. Receptor-mediated regulation of constitutive secretion. Trends Cell Biol. 1993 Sep;3(9):290–292. doi: 10.1016/0962-8924(93)90002-i. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Hamilton J. W., Cohn D. V. The by-pass of tissue hormone stores during the secretion of newly synthesized parathyroid hormone. Endocrinology. 1975 Jul;97(1):178–188. doi: 10.1210/endo-97-1-178. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Jilka R. L., Hamilton J. W. Formation and secretion of fragments of parathormone. Identification of cleavage sites. J Biol Chem. 1986 Feb 5;261(4):1929–1934. [PubMed] [Google Scholar]
- MacGregor R. R., Sarras M. P., Jr, Houle A., Cohn D. V. Primary monolayer cell culture of bovine parathyroids: effects of calcium, isoproterenol and growth factors. Mol Cell Endocrinol. 1983 Jun;30(3):313–328. doi: 10.1016/0303-7207(83)90067-9. [DOI] [PubMed] [Google Scholar]
- Matsuuchi L., Kelly R. B. Constitutive and basal secretion from the endocrine cell line, AtT-20. J Cell Biol. 1991 Mar;112(5):843–852. doi: 10.1083/jcb.112.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer G. P., Habener J. F., Potts J. T., Jr Parathyroid hormone secretion in vivo. Demonstration of a calcium-independent nonsuppressible component of secretion. J Clin Invest. 1976 Mar;57(3):678–683. doi: 10.1172/JCI108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. J., Cohn D. V. Regulation of secretion of parathormone and secretory protein-I from separate intracellular pools by calcium, dibutyryl cyclic AMP, and (1)-isoproterenol. J Cell Biol. 1979 Jul;82(1):93–102. doi: 10.1083/jcb.82.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. J., Cohn D. V. Secretion and degradation of parathormone as a function of intracellular maturation of hormone pools. Modulation by calcium and dibutyryl cyclic AMP. J Cell Biol. 1979 Dec;83(3):521–528. doi: 10.1083/jcb.83.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Perrelet A., Powell S. K., Quinn D. L., Moore H. P. The trans-most cisternae of the Golgi complex: a compartment for sorting of secretory and plasma membrane proteins. Cell. 1987 Dec 24;51(6):1039–1051. doi: 10.1016/0092-8674(87)90590-3. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Pimplikar S. W., Simons K. Regulation of apical transport in epithelial cells by a Gs class of heterotrimeric G protein. Nature. 1993 Apr 1;362(6419):456–458. doi: 10.1038/362456a0. [DOI] [PubMed] [Google Scholar]
- Rosa P., Hille A., Lee R. W., Zanini A., De Camilli P., Huttner W. B. Secretogranins I and II: two tyrosine-sulfated secretory proteins common to a variety of cells secreting peptides by the regulated pathway. J Cell Biol. 1985 Nov;101(5 Pt 1):1999–2011. doi: 10.1083/jcb.101.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig L. J., Farquhar M. G. Sites of sulfate incorporation into mammotrophs and somatotrophs of the rat pituitary as determined by quantitative electron microscopic autoradiography. Endocrinology. 1980 Aug;107(2):422–431. doi: 10.1210/endo-107-2-422. [DOI] [PubMed] [Google Scholar]
- Schubert D., Schroeder R., LaCorbiere M., Saitoh T., Cole G. Amyloid beta protein precursor is possibly a heparan sulfate proteoglycan core protein. Science. 1988 Jul 8;241(4862):223–226. doi: 10.1126/science.2968652. [DOI] [PubMed] [Google Scholar]
- Schultz G. S., Sarras M. P., Jr, Gunther G. R., Hull B. E., Alicea H. A., Gorelick F. S., Jamieson J. D. Guinea pig pancreatic acini prepared with purified collagenase. Exp Cell Res. 1980 Nov;130(1):49–62. doi: 10.1016/0014-4827(80)90041-5. [DOI] [PubMed] [Google Scholar]
- Schwartz N. B., Galligani L., Ho P. L., Dorfman A. Stimulation of synthesis of free chondroitin sulfate chains by beta-D-xylosides in cultured cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4047–4051. doi: 10.1073/pnas.71.10.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharoni Y., Eimerl S., Schramm M. Secretion of old versus new exportable protein in rat parotid slics. Control by neurotransmitters. J Cell Biol. 1976 Oct;71(1):107–122. doi: 10.1083/jcb.71.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroka C. J., Farquhar M. G. Characterization of a novel heparan sulfate proteoglycan found in the extracellular matrix of liver sinusoids and basement membranes. J Cell Biol. 1991 Jun;113(5):1231–1241. doi: 10.1083/jcb.113.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro R. C., Freeze H. H., Sampath D., Garcia J. A. Uncoupling of chondroitin sulfate glycosaminoglycan synthesis by brefeldin A. J Cell Biol. 1991 Dec;115(5):1463–1473. doi: 10.1083/jcb.115.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow J. L., de Almeida J. B., Narula N., Holtzman E. J., Ercolani L., Ausiello D. A. A heterotrimeric G protein, G alpha i-3, on Golgi membranes regulates the secretion of a heparan sulfate proteoglycan in LLC-PK1 epithelial cells. J Cell Biol. 1991 Sep;114(6):1113–1124. doi: 10.1083/jcb.114.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Sakaguchi K., Yanagishita M., Aurbach G. D., Hascall V. C. Extracellular calcium regulates distribution and transport of heparan sulfate proteoglycans in a rat parathyroid cell line. J Biol Chem. 1990 Aug 15;265(23):13661–13668. [PubMed] [Google Scholar]
- Tooze J., Kern H. F., Fuller S. D., Howell K. E. Condensation-sorting events in the rough endoplasmic reticulum of exocrine pancreatic cells. J Cell Biol. 1989 Jul;109(1):35–50. doi: 10.1083/jcb.109.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A., Flatmark T., Tooze J., Huttner W. B. Characterization of the immature secretory granule, an intermediate in granule biogenesis. J Cell Biol. 1991 Dec;115(6):1491–1503. doi: 10.1083/jcb.115.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A., Huttner W. B. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990 Mar 9;60(5):837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagishita M., Midura R. J., Hascall V. C. Proteoglycans: isolation and purification from tissue cultures. Methods Enzymol. 1987;138:279–289. doi: 10.1016/0076-6879(87)38023-1. [DOI] [PubMed] [Google Scholar]
- von Zastrow M., Castle J. D. Protein sorting among two distinct export pathways occurs from the content of maturing exocrine storage granules. J Cell Biol. 1987 Dec;105(6 Pt 1):2675–2684. doi: 10.1083/jcb.105.6.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]