Abstract
Patients with active inflammatory bowel disease (IBD) have elevated and activated myeloid leucocytes which infiltrate the colonic mucosa in vast numbers. Myeloid leucocytes such as the CD14+CD16+ monocytes are major sources of tumour necrosis factor (TNF)-α, and therefore selective granulocyte/monocyte (GM) adsorption (GMA) should promote remission or enhance efficacy of pharmacological therapy. However, studies in IBD have reported both impressive as well as disappointing efficacy outcomes, indicating that patients' demographic factors might determine responders or non-responders to GMA. Nonetheless, this non-drug intervention has an excellent safety profile, and therapeutic GMA is expected to expand. In this review, attempts have been made to compile an update on the mode of actions (MoA) of the Adacolumn GMA. The MoA of GMA appears to be more than adsorption of excess neutrophils and TNF-producing CD14+CD16+ monocytes per se. Adsorbed GMs release interleukin (IL)-1 receptor antagonist, hepatocyte growth factor and soluble TNF receptors, which are anti-inflammatory. Additionally, a sustained increase in lymphocytes including the regulatory CD4+CD25+ T cells (lymphocyte sparing) is seen post-GMA. The impact of GMA on the immune system is potentially very interesting in the context of treating immune-related diseases. Future studies are expected to add intriguing insights to the MoA of GMA.
Keywords: adsorptive leucocytapheresis, hepatocyte growth factor, IL-1 receptor antagonist, inflammatory bowel diseases, proinflammatory CD14+CD16+ monocytes
Introduction
Patients with active inflammatory bowel disease (IBD) harbour elevated and activated granulocytes and monocytes (GMs) in the presence of compromised lymphocytes [1–5]. Similarly, neutrophils in patients with IBD show activation behaviour [1] and prolonged survival time [6]. Factors that are known to promote neutrophil survival in IBD include inflammatory cytokines [7] and, paradoxically, corticosteroids [8], which are commonly used to treat patients with IBD or other immune-related conditions.
It is now known that IBD is exacerbated by inflammatory cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8 and others [9,10]. Accordingly, anti-cytokine antibodies, notably anti-TNF antibodies such as infliximab (IFX), are being used and new antibodies are under development for the treatment of IBD [11–14]. Indeed, the efficacy of anti-TNF, notably IFX, in patients with Crohn's disease (CD) [11–13], as well as in patients with ulcerative colitis (UC) [14], has validated the role of this cytokine in the immunopathogenesis of IBD. However, enthusiasm towards biologics is increasingly being dampened by concerns about their long-term efficacy and, in particular, their safety profiles [15,16].
Mucosal biopsies from patients with active IBD reveal a spectrum of pathological manifestations, among which an abundance of neutrophils accounts not only for the morphological lesions in IBD, but also for the prevailing mucosal inflammation [17–19]. When activated, GM produce an array of pleiotropic cytokines such as TNF-α, IL-1β, IL-6, IL-12 and IL-23, which are strongly inflammatory [9,10,20–22]. Therefore, targeting myeloid leucocytes as key players in the exacerbation of IBD lies behind extracorporeal granulocyte monocyte/macrophage adsorption (GMA) with the Adacolumn (Jimro, Takasaki, Japan).
Modes of actions of GMA
Adsorption of GMs to the GMA carriers
Figure 1 shows that patients with IBD have elevated granulocytes compared with non-IBD healthy subjects (control). Figures 2 and 3 show selective adsorption of GMs to the Adacolumn GMA carriers in clinical settings. Upon adsorption to the carriers, the binding sites on the plasma immunoglobulin (Ig)G and immune complexes (IC) become available for the Fcγ receptors (FcγRs) on neutrophils and monocytes/macrophages [23,24]. Further, complement activation is triggered and generates complement fragments including C3a, C5a and the opsonins C3b/C3bi [22–25]. C3b/C3bi also adsorb onto the carriers and serve as the binding sites for the leucocyte complement receptors CR1, CR2, CR3 (Mac-1, CD11b/CD18) [24]. Accordingly, leucocyte adsorption to the carriers is governed by C3b/C3bi, FcγRs and the leucocyte complement receptors [24]. The expression of these sets of receptors are common features of neutrophils and monocytes/macrophages. Hence, the GMA carriers selectively adsorb the leucocytes from peripheral blood, with the granulocytes exhibiting the highest affinity towards the carrier beads [26]. Effects on erythrocytic components of the blood were not observed [26,27].
Fig. 1.
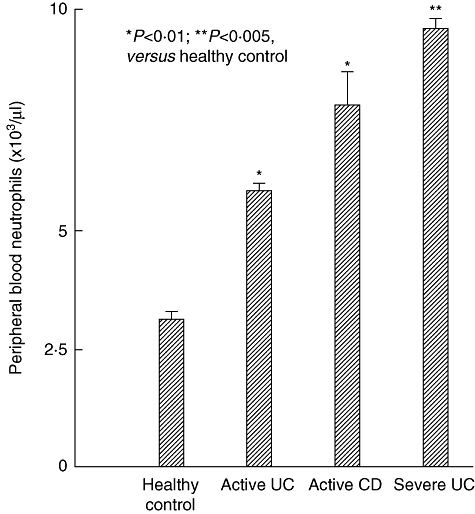
Peripheral blood neutrophil (granulocyte) counts in healthy controls and patients with inflammatory bowel disease (IBD), showing elevated neutrophils in IBD. P-values by paired t-test. Data sources are described in the text, refs [2,26,27].
Fig. 2.
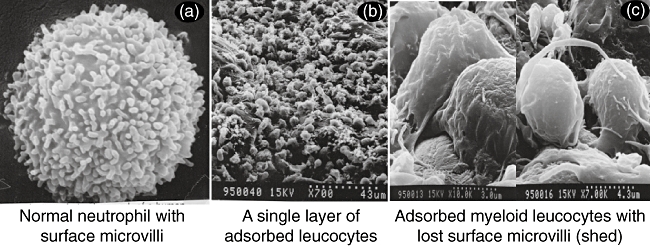
Selective adsorption of myeloid leucocytes to the granulocyte/monocyte adsorption (GMA) carriers. The high magnification view in (c) reveals that the adsorbed leucocytes are primarily neutrophils and monocytes/macrophages (myeloid linage leucocytes). For further details see ref [24].
Lymphocyte sparing
In contrast, lymphocytes are spared, as seen in Fig. 3, and in fact increase subsequently in absolute numbers [4,5,26]. Lymphocytes are not known to express complement receptors except on small subsets of B, T and natural killer (NK) cells. Similarly, FcγRs are not expressed widely on lymphocytes except on small subsets of CD19+ B cells and CD56+ NK cells [23]. These basic phenomena lend the carriers selectivity for GM [23,24]. To our knowledge, GMA is the only therapeutic intervention that enhances the lymphocyte population; this is expected to impact the dysregulated immunity in patients with IBD and other immune-related diseases (see below).
Fig. 3.
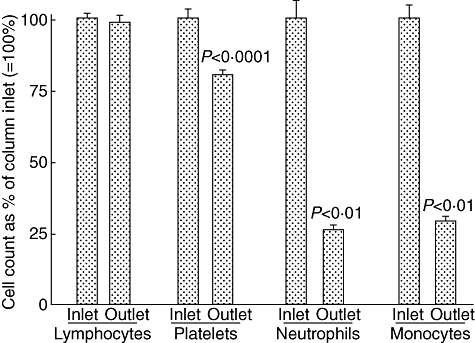
Selective depletion of neutrophils, monocytes and platelets by the Adacolumn granulocyte/monocyte adsorption (GMA) in patients with inflammatory bowel disease (IBD). In each case, the column inflow count was expressed as 100% and the column outflow count (blood return to patient) as a % of the inflow count, taken 30 min (peak fall) during a 60-min GMA session. Mean ± standard deviation values are presented; P-values by paired t-test for n = 17 and 153 (for platelets).
Modification of surface antigens on post-column granulocytes
In clinical settings, on passing through the Adacolumn the expression of l-selectin was down-regulated and Mac-1 was up-regulated [24,26]. Besides these, a decreased granulocyte chemotactic activity to the anaphylatoxin, C5a, and IL-8 was observed [28]. This reduction of leucocyte adhesion activity was not seen in subjects who were given sham GMA (without carriers) [29].
Depletion of proinflammatory CD14+CD16+DR++ monocytes
Two monocyte populations are known in the blood: CD14++CD16-, known as the classical monocyte phenotype, and CD14+CD16+DR++, known as the proinflammatory monocyte phenotype, which accounts for about 10% of total monocytes, but can expand and promote inflammatory conditions by producing large amounts of inflammatory cytokines, including TNF-α and IL-1β[30,31]. Therefore, a potentially very significant action of GMA is the adsorptive depletion of elevated proinflammatory CD14+CD16+DR++ monocyte phenotype (Fig. 4) [31]. Hanai and colleagues found that the number of CD14+CD16+ monocytes decreased dramatically in patients with IBD post-GMA [31].
Fig. 4.
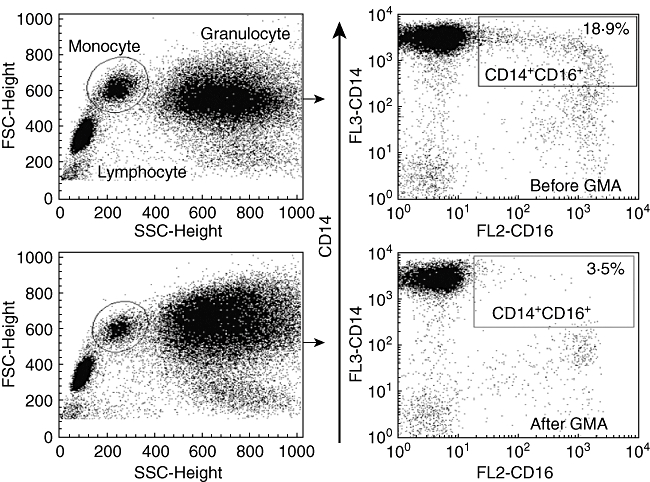
Typical flow cytometry patterns showing depletion of proinflammatory CD14+CD16+DR++ monocytes in patients with inflammatory bowel disease (IBD) by the Adacolumn granulocyte/monocyte adsorption (GMA). Source: ref. [31].
Mobilization of bone marrow CD10- neutrophils
Data from flow cytometry analysis of peripheral blood during the Adacolumn GMA procedure showed a fall in peripheral neutrophils, mainly old and activated neutrophils which are CD10+, and the emergence of CD10-, immature naive neutrophils from the bone marrow, during a 60-min GMA procedure (Fig. 5). As shown, maximum mobilization of CD10- neutrophils was seen within 30 min of GMA. The CD10- neutrophil phenotype is believed not to be proinflammatory [32].
Fig. 5.
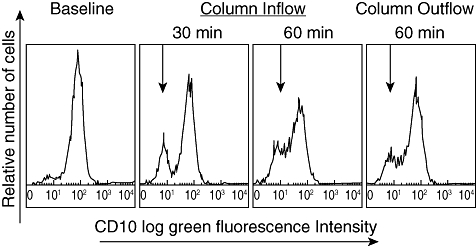
Fall in neutrophils and emergence of CD10-negative neutrophils (naive, immature) during Adacolumn granulocyte/monocyte adsorption (GMA). Source: Kashiwagi et al. Dig Dis Sci 2002;47:1334–41, ref. [32].
Release of anti-inflammatory substances
Takeda et al. [33] incubated human whole blood with the GMA carriers in in-vitro settings and measured significant amounts of IL-1 receptor antagonist (IL-1ra) and hepatocyte growth factor (HGF) released from granulocytes and monocytes that adhered to the carriers (Fig. 6); the amounts released were directly proportional to the number of cells that adhered to the carriers. Similarly, Hiraishi et al. [23] reported release of soluble TNF receptors I and II (sTNF RI and RII) during incubation of human blood with the GMA carriers. Similar data on IL-1ra and sTNF RI and RII were reported by Hanai et al. in clinical settings [34,35].
Fig. 6.
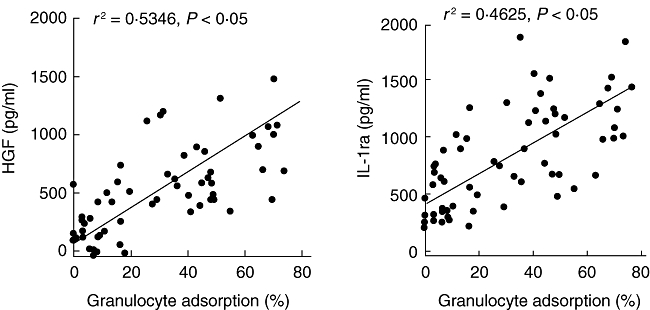
Adsorption-dependent release of interleukin (IL)-1ra and hepatocyte growth factor (HGF) from myeloid leucocytes. Source: ref. [33].
Rise in regulatory CD4+CD25+ T cells
The CD4+CD25+ T cell phenotype has been recognized as the naturally occurring regulatory T cell (Treg), with an essential role in the regulation of intestinal immune function [36–38]. Animal models of colitis showed that depletion of CD4+CD25+ T cells from the circulation leads to an inflammatory colitis similar to the ulcerative colitis (UC) seen in human IBD [36–38]. In humans, the functional Treg cells express high levels of the IL-2 receptor (CD25), and are also characterized by expression of the transcription factor forkhead box P3 (FoxP3) (CD4+CD25high+/FoxP3 phenotype) [39,40]. Both IL-10 and transforming growth factor (TGF)-β are reported to be involved in the regulatory activities of CD4+CD25high+ Treg[41,42]. Two research groups have reported independently a significant increase in circulating levels of the CD4+CD25high+/FoxP3 phenotype following a course of GMA [43,44].
Impact on the mucosal cytokine levels
GMA significantly impacts mucosal healing [45] and decreases the mucosal levels of neutrophils [18]. Muratov et al. [46] and Yamamoto et al. [47] reported a striking decrease in mucosal tissue levels of inflammatory cytokines. Muratov et al. reported depletion of interferon (IFN)-γ- or TNF-α-positive T cells in clinical responders after GMA (Fig. 7). In parallel, significantly lower levels of IFN-γ-producing lymphocytes were detected in the peripheral blood. IFN-γ-positive T cells in pretreatment biopsies disappeared completely or decreased in post-treatment biopsies sampled 2 weeks after the last GMA session in responders, and appeared to predict the maintenance of long-term remission up to 12 months [46]. Similarly, according to Yamamoto et al. [47], at entry the mucosal concentrations of inflammatory cytokines, including IL-1β, IL-6 and TNF-α, were significantly high compared with the control levels, while the IL-1ra/IL-1β ratio was significantly low. In patients with quiescent UC, but not in those with active UC, the mucosal tissue concentrations of IL-1β, IL-6, IL-8 and TNF-α decreased significantly, whereas the IL-1ra/IL-1β ratio increased significantly. Further, endoscopic remission was associated with a decline in mucosal IL-1β, IL-1ra, IL-6, IL-8, TNF-α and an increase in the IL-1ra/IL-1β ratio.
Fig. 7.
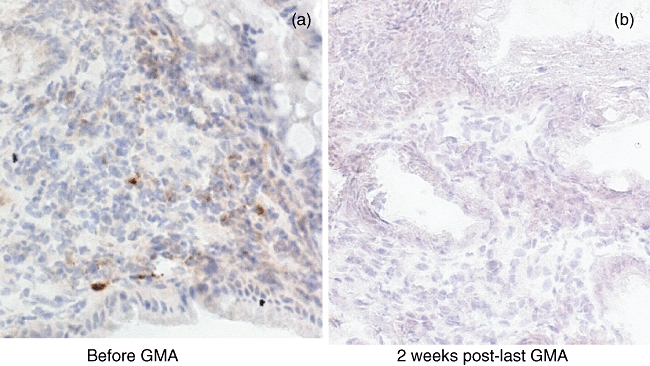
Typical immunohistochemistry staining for tumour necrosis factor (TNF)-α in colonic biopsies of a patient with inflammatory bowel disease (IBD) before (a) granulocyte/monocyte adsorption (GMA) and (b) 2 weeks post-last GMA session. For additional information, see text and the report by Muratov et al., ref [46].
Clinical efficacy
So far the focus of this review has been on the MoA of GMA without reference to the clinical efficacy of this non-drug-based therapeutic intervention. In recent years, there has been a large number of reports from Japan [4,18,45,48–51], as well as from Europe [46,52–56] and the United States [57–60] on the efficacy of GMA in IBD. The clinical data show that GMA in patients with steroid-dependent or steroid-refractory UC was associated with significant clinical efficacy and corticosteroid tapering. Similarly, in steroid-naive patients, GMA spared patients from exposure to corticosteroids. There is no shortage of contrasting efficacy reports. Efficacy rates vary from an 85% [4,27,59] to a statistically insignificant level [60]. However, it is not within the scope of this review paper to comment on the merit of hitherto studies, except that the study by Suzuki et al. [50] and a very recent study by Tanaka et al. [61] found an interesting association between patients' entry demography and clinical response to GMA. Both reports state that, at entry, candidate patients should have a fair level of intact mucosal tissue; patients who have extensive and deep colonic lesions are not likely to benefit, while the best responders appeared to be the first-episode cases followed by steroid-naive patients. Further, Sakuraba et al. [51] reported that intensive GMA (two sessions per week) was superior to weekly GMA both in efficacy rate and time to clinical remission. The report by Tanaka et al. [61] is the most thorough so far on identifying the responders and non-responders to GMA. The authors have progressed as far as immunohistochemically examining colonic biopsies to see that GMA does, in fact, reduce the concentrations of myeloid leucocytes within the colonic mucosa, where they can exacerbate IBD.
Other immune disorders that should respond to GMA
Given that the Adacolumn GMA can reduce the number of myeloid leucocytes both in the blood [27], as well as in tissues [18,61], GMA should have indications in other immune disorders that bear association with elevated and activated myeloid leucocytes. Several conditions which should potentially respond to GMA are the followings.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is associated with activated neutrophils; up to 5 × 109 neutrophils are found in one joint during active RA, but very few lymphocytes [62]. Similarly, the joint fluid from patients with RA contains large amounts of macrophage and neutrophil cytokines (IL-1β, TNF-α, IL-6), but few lymphocyte cytokines (IL-2, IL-3, IL-4). Among the infiltrated leucocytes in RA joints, the neutrophil is considered to have the greatest potential to cause inflammation and tissue injury [62]. Accordingly, RA might respond to GMA [28].
Patients with HIV infection
Monocytes/macrophages are known to serve as reservoirs for human immunodeficiency virus (HIV) replication and dissemination. Additionally, these leucocytes produce large amounts of cytokines such as TNF-α, which is a major pathological factor in patients with HIV [63]. Additionally, as stated above, GMA increases lymphocytes, which are depleted by HIV. Therefore, in patients with HIV, GMA is expected to selectively deplete monocytes/macrophages, which are major cellular reservoirs of HIV, and to increase CD4+ T cells as the most desirable effect [63].
Pyoderma gangrenosum and psoriasis
It is interesting to know that pyoderma and psoriasis-like skin lesions are reported to be the best responders to Adacolumn GMA [64–66]. Every treated case appears to have responded, yet this seems to have occurred by chance. A patient with IBD-associated pyoderma was treated for IBD, but the response of pyoderma lesions was more striking than anything expected from GMA [65]. The chemokine receptor, CXCR3, is known to have an active role in the initiation and perpetuation of inflammatory skin lesions by mediating leucocyte trafficking [67,68] and is down-modulated strongly by GMA [24]. This action, if duplicated by follow-up studies, can serve as a major breakthrough in our efforts to understand the immunopathology of these often drug-refractory conditions.
Hepatitis C infection
In patients with hepatitis C virus (HCV) infection, granulocytes and monocytes/macrophages are known to be extrahepatic sites of HCV replication and dissemination [69,70]; hence, reduction of these cells should contribute to the clearance of HCV. Sawada et al. [71] reported successful treatment of a patient with HCV–UC co-morbidity by applying GMA. In their report, the authors suggest that in patients with HCV, GMA should have the following actions: (a) reduce HCV-infected leucocytes; (b) directly eliminate HCV probably by complement generated in the column (see Fig. 3); and (c) serve as an adjunct to enhance drug efficacy.
Behçet's disease
Biopsies from active lesions of Behçet's disease (BD) show the presence of large numbers of neutrophils in the absence of infection. Neutrophil hyperactivity is a feature in BD as judged by enhanced chemotaxis and excessive release of granular enzymes, TNF-α, IL-1β and IL-8 [72–74]. Accordingly, BD should respond to therapeutic reduction of neutrophils [75].
Advanced cancer
The Adacolumn was developed to treat a subgroup of cancer patients whose cancer was thought to reflect inadequate cytotoxic T lymphocyte (CTL) function [76,77]. Accordingly, the first studies were in cancer patients. The perception that led to selective leucocytapheresis as a treatment strategy, now called GMA, was as follows. According to the theory of anti-cancer immune defence, CTL can eliminate newly formed tumours. Hence, the presence of tumour reflects inadequate anti-tumour CTL function in tumour-bearing hosts. Physicians had noticed that some cancer patients had raised granulocyte counts but low lymphocyte counts. It was thought that lymphocytes were depleted by activated myeloid leucocytes. The Adacolumn was developed to take away the excess and activated myeloid cells, hoping that this might lead to enhanced lymphocyte function [26,76,77].
Figure 8 summarizes the actions which ideally should be expected from therapeutic GMA in patients with IBD and other immune-related complications.
Fig. 8.
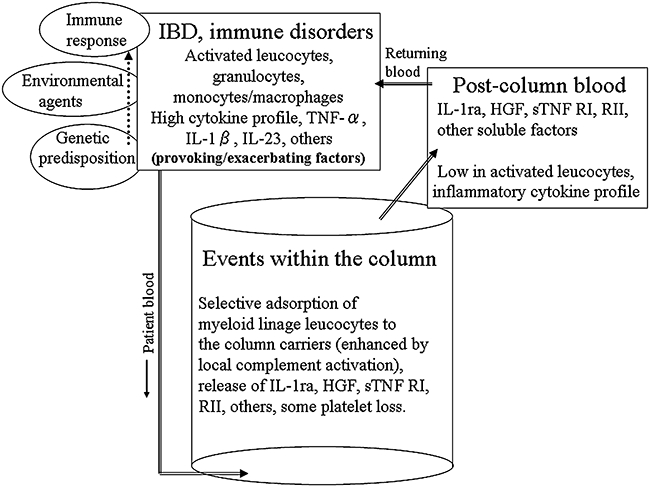
A tentative, but idealistic view summarisng the events attributed to therapeutic granulocyte/monocyte adsorption (GMA) in patients with inflammatory bowel disease (IBD) and other immune-related disorders.
Concluding remarks
Active immune disorders such as IBD reflect high inflammatory cytokine profile, and based on this knowledge the major treatment option currently is to intercept the inflammatory cytokines, notably TNF-α. As major sources of inflammatory cytokines include activated myeloid leucocytes, which in patients with IBD are elevated with activation behaviour, selective depletion of these proinflammatory leucocytes by GMA appears to be an attractive strategy to alleviate inflammation or enhance the efficacy of pharmacological therapy. However, clinical studies have hitherto reported both impressive as well as disappointing efficacy outcomes, indicating that patients' demographic factors might determine responders or non-responders to GMA. Further, as this non-drug-based treatment intervention has an excellent safety profile, it is very much favoured by patients and our impression is that the use of therapeutic GMA will expand rather than diminish. The MoA of GMA appears to be more than physical depletion of excess and activated GM per se; the release of IL-1ra, HGF, sTNF RI, RII and the rise of regulatory CD4+CD25+ T cells are typical effects which are potentially very interesting in the context of treating conditions that reflect a dysregulated immune profile. It is expected that future studies will add intriguing insights to the MoA of GMA.
Acknowledgments
No external funding was received for this study.
Disclosure
No competing interests to declare.
References
- 1.McCarthy DA, Rampton DS, Liu Y-C. Peripheral blood neutrophils in inflammatory bowel disease: morphological evidence of in vivo activation in active disease. Clin Exp Immunol. 1991;86:489–93. doi: 10.1111/j.1365-2249.1991.tb02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saniabadi AR, Hanai H, Fukunaga K, et al. Therapeutic leucocytapheresis for inflammatory bowel disease. Transfus Apher Sci. 2007;37:191–200. doi: 10.1016/j.transci.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Heimann TM, Aufses AH., Jr The role of peripheral lymphocytes in the prediction of recurrence in Crohn's disease. Surg Gynecol Obstet. 1985;160:295–8. [PubMed] [Google Scholar]
- 4.Suzuki Y, Yoshimura N, Saniabadi AR, Saito Y. Selective neutrophil and monocyte adsorptive apheresis as a first line treatment for steroid naïve patients with active ulcerative colitis: a prospective uncontrolled study. Dig Dis Sci. 2004;49:565–71. doi: 10.1023/b:ddas.0000026299.43792.ae. [DOI] [PubMed] [Google Scholar]
- 5.Aoki H, Nakamura K, Suzuki Y, et al. Adacolumn selective leukocyte adsorption apheresis in patients with active ulcerative colitis: clinical efficacy, effects on plasma IL-8 and the expression of Toll like receptor 2 on granulocytes. Dig Dis Sci. 2007;52:1427–33. doi: 10.1007/s10620-006-9406-8. [DOI] [PubMed] [Google Scholar]
- 6.Brannigan AE, O'Connell PR, Hurley H. Neutrophil apoptosis is delayed in patients with inflammatory bowel disease. Shock. 2000;13:361–6. doi: 10.1097/00024382-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Lee A, Whyte M, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54:283–8. [PubMed] [Google Scholar]
- 8.Meagher LC, Cousin JM, Seckl JR, Haslett C. Opposing effects of glucocorticoids on the rate of appoptosis in neutrophilic and eosinophilic granulocytes. J Immunol. 1996;156:4422–8. [PubMed] [Google Scholar]
- 9.Schreiber S, Nikolaus S, Hampe J. Tumour necrosis factor alpha and interleukin 1beta in relapse of Crohn's disease. Lancet. 1999;353:459–61. doi: 10.1016/S0140-6736(98)03339-X. [DOI] [PubMed] [Google Scholar]
- 10.Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–98. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 11.van Dullemen HM, van Deventer SJ, Hommes DW, et al. Treatment of Crohn's disease with anti-tumour necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–35. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 12.Targan SR, Hanauer SB, van Deventer SJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 13.Hanauer SB, Feagan BG, Lichtenstein GR. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 14.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 15.Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151–8. doi: 10.1002/art.10679. [DOI] [PubMed] [Google Scholar]
- 16.Atzeni A, Ardizzone S, Sarzi-Puttini P. Autoantibody profile during short-term infliximab treatment for Crohn's disease: a prospective cohort study. Aliment Pharmacol Ther. 2005;22:453–61. doi: 10.1111/j.1365-2036.2005.02576.x. [DOI] [PubMed] [Google Scholar]
- 17.Allison MC, Dhillon AP, Lewis WG, Pounder RE, editors. Inflammatory bowel disease. London: Mosby; 1998. pp. 9–95. [Google Scholar]
- 18.Tanaka T, Okanobu H, Murakami E, et al. In patients with ulcerative colitis, adsorptive depletion of granulocytes and monocytes impacts mucosal level of neutrophils and clinically is most effective in steroid naive patients. Dig Liver Dis. 2008;40:731–6. doi: 10.1016/j.dld.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Tibble JA, Sigthorsson G, Bridger D, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 20.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–6. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 21.Nikolaus S, Bauditz J, Gionchetti P. Increased secretion of pro-inflammatory cytokines by circulating polymorphonuclear neutrophils and regulation by interleukin-10 during intestinal inflammation. Gut. 1998;42:470–6. doi: 10.1136/gut.42.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neurath MF. IL-23: a master regulator in Crohn's disease. Nat Med. 2007;13:26–8. doi: 10.1038/nm0107-26. [DOI] [PubMed] [Google Scholar]
- 23.Hiraishi K, Takeda Y, Saniabadi A, et al. Studies on the mechanisms of leukocyte adhesion to cellulose acetate beads: an in vitro model to assess the efficacy of cellulose acetate carrier-based granulocyte and monocyte adsorptive apheresis. Ther Apher Dial. 2003;7:334–40. doi: 10.1046/j.1526-0968.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 24.Saniabadi AR, Hanai H, Lofberg R, et al. Adacolumn for selective leukocytapheresis as a non-pharmacological treatment for patients with disorders of the immune system: an adjunct or an alternative to drug therapy? J Clin Apher. 2005;20:171–84. doi: 10.1002/jca.20046. [DOI] [PubMed] [Google Scholar]
- 25.D'Arrigo C, Candal-Couto JJ, Greer M, Veale DJ, Woof JM. Human neutrophil Fc receptor mediated adhesion under flow: a hollow fibre model of intravascular arrest. Clin Exp Immunol. 1994;100:173–9. doi: 10.1111/j.1365-2249.1995.tb03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saniabadi AR, Hanai H, Bjarnason I, et al. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. 2003;7:48–59. doi: 10.1046/j.1526-0968.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 27.Hanai H, Watanabe F, Takeuchi K, et al. Leukcocyte adsorptive apheresis for the treatment of active ulcerative colitis: a prospective uncontrolled pilot study. Clin Gastroenterol Hepatol. 2003;1:28–35. doi: 10.1053/jcgh.2003.50005. [DOI] [PubMed] [Google Scholar]
- 28.Kashiwagi N, Hirata I, Kasukawa R. A role for granulocyte and monocyte apheresis in the treatment of rheumatoid arthritis. Ther Apher. 1998;2:134–41. doi: 10.1111/j.1744-9987.1998.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 29.Ramlow W, Emmrich J. In vitro and in vivo evaluation of Adacolumn cytapeheresis in healthy subjects. J Clin Apher. 2005;20:72–80. doi: 10.1002/jca.20053. [DOI] [PubMed] [Google Scholar]
- 30.Belge KU, Ziegler-Heitbrock HW. The proinflammatory CD14+CD16+ monocytes are a major source of TNF. J Immunol. 2002;168:3536–42. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 31.Hanai H, Iida T, Takeuchi K, et al. Adsorptive depletion of elevated proinflammatory CD14+CD16+DR++ monocytes in patients with inflammatory bowel disease. Am J Gastroenterol. 2008;103:1210–6. doi: 10.1111/j.1572-0241.2007.01714.x. [DOI] [PubMed] [Google Scholar]
- 32.Kashiwagi N, Sugimura K, Saniabadi AR, et al. Immunomodulatory effects of granulocyte and monocyte adsorption apheresis as a treatment for patients with ulcerative colitis. Dig Dis Sci. 2002;47:1334–41. doi: 10.1023/a:1015330816364. [DOI] [PubMed] [Google Scholar]
- 33.Takeda Y, Shiobara N, Saniabadi AR, Adachi M, Hiraishi K. Adhesion dependent release of hepatocyte growth factor and interleukin-1 receptor antagonist from human blood granulocytes and monocytes: evidence for the involvement of plasma IgG, complement C3 and β2 integrin. Inflamm Res. 2004;53:277–83. doi: 10.1007/s00011-004-1253-5. [DOI] [PubMed] [Google Scholar]
- 34.Hanai H, Iida T, Watanabe F, et al. Effects of Adacolumn selective leukocytapheresis on plasma cytokines during active disease in patients with ulcerative colitis. World J Gastroenterol. 2006;12:3393–9. doi: 10.3748/wjg.v12.i21.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanai H, Watanabe F, Takeuchi K, et al. Correlation of serum soluble TNF-alpha receptors I and II levels with disease activity in patients with ulcerative colitis. Am J Gastroenterol. 2004;99:1532–8. doi: 10.1111/j.1572-0241.2004.30432.x. [DOI] [PubMed] [Google Scholar]
- 36.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–78. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 37.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 38.Kahattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scrfin in CD4+CD25+ T regulatory cells [see Comment] Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 39.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells [Comments] Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 40.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/ winged-helix protein, scurfin, results in the fetal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 41.Huber S, Schramm C, Lehr HA, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173:6526–31. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 42.Melgar S, Yeung MM, Bas A, et al. Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clin Exp Immunol. 2003;134:127–37. doi: 10.1046/j.1365-2249.2003.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuadrado E, Alonso M, de Juan MD, Echaniz P, Arenas JI. Regulatory T cells in patients with inflammatory bowel diseases treated with Adacolumn granulocytapheresis. World J Gastroenterol. 2008;14:1521–7. doi: 10.3748/wjg.14.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoyama Y, Fukunaga K, Fukuda Y, et al. Demonstration of low CD25High+CD4+ and high CD28-CD4+ T-cell subsets in patients with ulcerative colitis: modified by selective leucocytapheresis. Dig Dis Sci. 2007;52:2725–31. doi: 10.1007/s10620-006-9560-z. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto T, Umegae S, Matsumoto K. Mucosal healing in patients with ulcerative colitis during a course of selective leukocytapheresis therapy: a prospective cohort study. Inflamm Bowel Dis. 2010;16:1905–11. doi: 10.1002/ibd.21260. [DOI] [PubMed] [Google Scholar]
- 46.Muratov V, Lundahl J, Ulfgren AK, Saniabadi A, Lofberg R. Down-regulation of interferon-γ parallels clinical response to selective leukocyte apheresis in patients with inflammatory bowel disease. A 12-month follow-up study. Int J Colorectal Dis. 2006;21:493–504. doi: 10.1007/s00384-005-0069-2. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto T, Saniabadi AR, Umegae S, et al. Impact of selective leukocytapheresis on mucosal inflammation and ulcerative colitis: cytokine profiles and endoscopic findings. Inflamm Bowel Dis. 2006;12:719–26. doi: 10.1097/00054725-200608000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Hanai H, Watanabe F, Yamada M, Saniabadi A. Adsorptive granulocyte and monocyte apheresis versus prednisolone in patients with corticosteroid dependent moderately severe ulcerative colitis. Digestion. 2004;70:36–44. doi: 10.1159/000080079. [DOI] [PubMed] [Google Scholar]
- 49.Kanke K, Nakano M, Hiraishi H. Clinical evaluation of granulocyte/monocyte apheresis therapy for active ulcerative colitis. Dig Liver Dis. 2004;36:811–7. doi: 10.1016/j.dld.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki Y, Yoshimora N, Saito Y, Saniabadi A. A retrospective search for predictors of clinical response to selective granulocyte and monocyte apheresis in patients with ulcerative colitis. Dig Dis Sci. 2006;51:2031–8. doi: 10.1007/s10620-006-9199-9. [DOI] [PubMed] [Google Scholar]
- 51.Sakuraba A, Motoya S, Watanabe K, et al. An open-label prospective randomized multicenter study shows very rapid remission of ulcerative colitis by intensive granulocyte and monocyte adsorptive apheresis as compared with routine weekly treatment. Am J Gastroenterol. 2009;104:2990–5. doi: 10.1038/ajg.2009.453. [DOI] [PubMed] [Google Scholar]
- 52.Domenech E, Hinojosa J, Esteve-Comas M. Spanish Group for the Study of Crohn's Disease and Ulcerative Colitis (GETECCU). Granulocyteaphaeresis in steroid-dependent inflammatory bowel disease: a prospective, open, pilot study. Aliment Pharmacol Ther. 2004;20:1347–52. doi: 10.1111/j.1365-2036.2004.02288.x. [DOI] [PubMed] [Google Scholar]
- 53.Maiden L, Takeuchi K, Baur R, et al. Selective white cell apheresis reduces relapse rates in patients with IBD at significant risk of clinical relapse. Inflamm Bowel Dis. 2008;14:1413–8. doi: 10.1002/ibd.20505. [DOI] [PubMed] [Google Scholar]
- 54.Caprilli R, D'Ovidio V. Leukocytapheresis as promising therapy for inflammatory bowel disease. Dig Liver Dis. 2007;39:435–7. doi: 10.1016/j.dld.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Panés J, Guilera M, Ginard D, et al. Treatment cost of ulcerative colitis is apheresis with Adacolumn cost-effective? Dig Liver Dis. 2007;39:617–25. doi: 10.1016/j.dld.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Bresci G, Parisi G, Mazzoni A, Scatena F, Capria A. Treatment of patients with acute ulcerative colitis: conventional corticosteroid therapy (MP) versus granulocytapheresis (GMA): a pilot study. Dig Liver Dis. 2007;39:430–4. doi: 10.1016/j.dld.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Schwartz D, Ferguson JR. Current pharmacologic treatment paradigms for inflammatory bowel disease and the potential role of granulocyte/monocyte apheresis. Curr Med Res Opin. 2007;23:2715–28. doi: 10.1185/030079907x233241. [DOI] [PubMed] [Google Scholar]
- 58.Abreu MT, Plevy S, Sands BE, Weinstein R. Selective leukocyte apheresis for the treatment of inflammatory bowel disease. J Clin Gastroenterol. 2007;41:874–88. doi: 10.1097/MCG.0b013e3180479435. [DOI] [PubMed] [Google Scholar]
- 59.Cohen RD. Treating ulcerative colitis without medications –‘Look Mom, No Drugs!’. Gastroenterology. 2005;128:235–6. doi: 10.1053/j.gastro.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 60.Sands BE, Sandborn WJ, Feagan B, et al. A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology. 2008;135:400–09. doi: 10.1053/j.gastro.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka T, Okanobu H, Kuga Y, et al. Clinical and endoscopic features of responders and non-responders to adsorptive leucocytapheresis: a report based on 120 patients with active ulcerative colitis. Gastroenterol Clin Biol. 2010 doi: 10.1016/j.gcb.2010.08.007. [Epub ahead of print] PMID: 20934287. [DOI] [PubMed] [Google Scholar]
- 62.Edwards SW, Hallett MB. Seeing the wood for the tree: the forgotten role of neutrophils in rheumatoid arthritis. Immunol Today. 1997;18:320–4. doi: 10.1016/s0167-5699(97)01087-6. [DOI] [PubMed] [Google Scholar]
- 63.Beretta A, Hasson H, Saniabadi A, et al. Selective granulocyte/monocyte apheresis in the treatment of HIV-infected patients: short-term and long-term effects on immunological and virological parameters. Perfusion. 2002;17:47–51. doi: 10.1191/0267659102pf559oa. [DOI] [PubMed] [Google Scholar]
- 64.Kanekura T, Maruyama I. Granulocyte and monocyte adsorption apheresis for pyoderma gangrenosum. J Am Acad Dermatol. 2002;47:320–1. doi: 10.1067/mjd.2002.120597. [DOI] [PubMed] [Google Scholar]
- 65.Ohmori T, Yamagiwa A, Nakamura I, Nishikawa K, Saniabadi A. Treatment of pyoderma gangrenosum associated with Crohn's disease. Am J Gastroenterol. 2003;98:2101–102. doi: 10.1111/j.1572-0241.2003.07657.x. [DOI] [PubMed] [Google Scholar]
- 66.Kanekura T, Yoshii N, Yonezawa T, Kawabata H, Saruwatari H, Kanzaki T. Treatment of pustular psoriasis with granulocyte and monocyte adsorption apheresis. J Am Acad Dermatol. 2003;49:329–32. doi: 10.1067/s0190-9622(03)00795-3. [DOI] [PubMed] [Google Scholar]
- 67.Rottman JB, Smith TL, Ganley KG, Kikuchi T, Krueger GJ. Potential role of the chemokine receptors CXCR3, CXCR4, and the integrin alphaEbeta 7 in the pathogenesis of psoriasis vulgaris. Lab Invest. 2001;81:335–47. doi: 10.1038/labinvest.3780242. [DOI] [PubMed] [Google Scholar]
- 68.Gillitzer R. Inflammation in human skin: a model to study chemokine-mediated leukocyte migration in vivo. J Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<393::AID-PATH907>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 69.Cravatto M, Pozzato G, Zorat F, Pusini E, Sentini G. Peripheral blood neutrophils from hepatitis C virus-infected patients are replication sites for the virus. Haematologica. 2000;85:356–61. [PubMed] [Google Scholar]
- 70.Bouffard P, Hayashi PH, Acevedo R, Levy N, Zeldis JB. Hepatitis C is detected in a monocyte macrophage subpopulation of peripheral blood mononuclear cells of infected patients. J Infect Dis. 1992;166:1276–80. doi: 10.1093/infdis/166.6.1276. [DOI] [PubMed] [Google Scholar]
- 71.Sawada K, Ohnishi K, Fukunaga K, Shimoyama T. A new treatment for HCV-ulcerative colitis co-morbidity intolerant to INF-alpha. Am J Gastroenterol. 2003;98:228–9. doi: 10.1111/j.1572-0241.2003.07193.x. [DOI] [PubMed] [Google Scholar]
- 72.Sakane T, Takeno M, Suzuki N, Inaba G. Current concepts: Behçet's disease. N Engl J Med. 1999;341:1284–91. doi: 10.1056/NEJM199910213411707. [DOI] [PubMed] [Google Scholar]
- 73.Ehrlich GE. Vaculitis in Behçet's disease. Int Rev Immunol. 1997;14:81–8. doi: 10.3109/08830189709116846. [DOI] [PubMed] [Google Scholar]
- 74.Sahin S, Lawrence R, Direskeneli H, Hamuryudan V, Yazici H, Akoglu T. Monocyte activity in Behçet's disease. Br J Rheumatol. 1996;35:424–9. doi: 10.1093/rheumatology/35.5.424. [DOI] [PubMed] [Google Scholar]
- 75.Kanekura T, Gushi A, Iwata M, et al. Treatment of Behçet's disease with granulocyte and monocyte adsorption apheresis. J Am Acad Dermatol. 2004;51(Suppl 2):S83–7. doi: 10.1016/j.jaad.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 76.Tabuchi T, Ubukata H, Sato S, et al. Granulocytapheresis as a possible cancer treatment. Anticancer Res. 1995;15:985–90. [PubMed] [Google Scholar]
- 77.Tabuchi T, Ubukata H, Saniabadi AR, Soma T. Granulocyte apheresis as a possible new approach in cancer therapy: a pilot study involving two cases. Cancer Detect Prev. 1999;23:417–21. doi: 10.1046/j.1525-1500.1999.99029.x. [DOI] [PubMed] [Google Scholar]


