Abstract
The pathogenesis of nasal polyposis remains unclear; it severely affects patients' quality of life and complicates inflammation in adjacent organs such as sinusitis and asthma. Aberrant immune regulatory function in these patients is proposed. The present study aims to examine the regulatory T cells (Treg) in nasal mucosa of patients with allergic rhinitis (AR) and nasal polyposis (NP). Patients with AR or AR/NP were treated with inferior turbinectomy for their inferior turbinate hyperplasia. Surgically removed nasal mucosa was collected to examine the Treg by immunohistochemistry and flow cytometry. The results showed that more forkhead box P3 (FoxP3)+ cells were found in AR with polyps than in those with AR alone. Further studies revealed that these FoxP3+ T cells from AR/NP group also expressed interleukin (IL)-17. In vitro study showed that staphylococcal enterotoxin B (SEB) induced CD4+ FoxP3+ T cells to become FoxP3+ IL-17+ cells via facilitating the expression of IL-6, that in synergy with transforming growth factor-beta, induce the expression of IL-17 in FoxP3+ cells. We conclude that FoxP3+ IL-17+ T cells were localized in the nasal mucosa of patients with AR and NP. SEB may play a role in converting FoxP3+ Treg to FoxP3+ IL-17+ T cells. The presence of IL-17+ FoxP3+ T cells may play a role in the remodelling of the nasal airways in certain people who develop polyps, irrespective of whether or not they are atopic.
Keywords: allergy, cytokines, nasal mucosa, polyposis, regulatory T cells
Introduction
It has been noted that a correlation exists between nasal allergy (AR) and nasal polyposis (NP) [1–3]; however, the underlying mechanism remains to be further understood. Functional deficiency or decrease in the number of regulatory T cells (Treg) plays a critical role in allergic diseases [4]. However, the properties of Treg in upper airway mucosa need to be further elucidated.
Forkhead box P3 (FoxP3) is a transcription factor in CD4+ CD25+ Treg that is regarded as a signature molecule in CD4+ Treg[5]. Recent studies indicate that there is a subset of CD4+ FoxP3+ T cells that express interleukin (IL)-17 [6,7]. Although this subset of T cell still has a suppressive function, such as inhibiting CD4+ CD25- T cell proliferation, the cells might be new proinflammatory cells because of the expression of IL-17.
IL-17 is a newly described member of a cytokine family and has several members, including IL-17A-E. IL-17A (IL-17 in brief), and enhances T cell priming and stimulates fibroblasts, endothelial cells, neutrophils, macrophages and epithelial cells to drive these cells to produce multiple proinflammatory mediators, including IL-1, IL-6, tumour necrosis factor (TNF)-α, nitric oxide synthase 2, metalloproteinases and chemokines [8]. Based on these properties, IL-17 may protect against bacterial, fungal and protozoal infection. However, IL-17 is also proposed as being involved predominantly in an array of inflammatory disorders such as systemic rheumatic diseases, multiple sclerosis, inflammatory bowel disease and asthma [9,10].
Published studies have noted that staphylococcal enterotoxin B (SEB) has a relation with allergic disorders [11,12]. SEB can induce IL-6 expression in the nasal mucosa [13]. Because the synergistic effect of IL-6 and transforming growth factor (TGF)-β induces IL-17 expression in CD4+ T cells, we speculate that SEB-induced IL-6 may be in synergy with TGF-β to initiate the expression of IL-17 in CD4+ FoxP3+ Treg to drive these cells to become CD4+ FoxP3+ IL-17+ T cells. To test the hypothesis, we analysed surgically removed nasal mucosa from patients with AR or AR/NP. Indeed, CD4+ FoxP3+ IL-17+ T cells were localized in the nasal mucosa of patients with AR/NP.
Materials and methods
Reagents
Cell culture-related reagents and Western blotting reagents were purchased from (Invitrogen, Shanghai, China). Enzyme-linked immunosorbent assay (ELISA) kits of immunoglobulin (Ig)E, IL-17, IL-6 and SEB were purchased from R&D Systems (Shanghai, China). Magnetic cell sorting reagents were purchased from (Miltenyi Biotec, Suntec City, Singapore). IL-6 siRNA and scrambled siRNA, antibodies of FoxP3, TGF-β, β-arresting 2, retinoic acid-related orphan receptor (ROR)γt and β-actin were purchased from (Santa Cruz Biotech, Santa Cruz, CA, USA).
Patients
Fifty patients were recruited into this study, comprising 20 NP/AR, 20 AR and 10 CR (chronic rhinitis). The diagnosis of AR followed the established criteria in our department, which has also been published elsewhere [14]. All patients were treated with conventional medical intervention that did not respond well and asked for inferior turbinectomy, NP resection and some with endoscopic sinus surgery if the patient complicated with chronic sinusitis. Another five nasal or sinus cancer patients were recruited into this study. Marginal non-cancer nasal mucosa was collected and used as control (Con). Informed consent was obtained from each patient. The study protocol was approved by the Human Research Ethic Committee at Shanxi Medical University. No subjects had used any medicines during the past 2 weeks.
ELISA
Levels of IgE, IL-17, IL-6 and SEB in the serum or extracted proteins were determined by ELISA with commercial reagent kits, following the manufacturer's instructions.
Flow cytometry
Isolated immune cells were incubated with primary antibodies (fluorescence labelled, 1 µg/ml; isotype IgG was used as control) on ice for 30 min (for the intracellular staining, cells were fixed with 1% paraformaldehyde on ice for 30 min and incubated with permealization reagents for 30 min on ice). The stained cells were analysed using a fluorescence activated cell sorter (FACSarray; BD Bioscience, San Jose, CA, USA). Data were analysed with FlowJo software.
Immunohistochemistry
Nasal mucosal cryosections were fixed with acetone for 20 min. After blocking with 2% bovine serum albumin for 30 min, the sections were incubated with primary antibodies (1 µg/ml, or isotype IgG as control) at 4°C overnight. Sections were incubated with horseradish peroxidase-labelled secondary antibodies (1:300) for 1 h at room temperature. Washing with phosphate-buffered saline (PBS) was performed after incubation. Sections were observed under a microscope.
Immune cell isolation
Surgically removed nasal tissue was cut into small pieces (2 × 2 × 2 mm) and treated with predigestion solution [1 × Hanks's balanced salt solution (HBSS) containing 5 mm ethylenediamine tetraacetic acid (EDTA) and 1 mm dithiothreitol (DTT)] at 37°C for 30 min under slow rotation. The tissue was collected by centrifugation (300 g for 10 min) and incubated in digestion solution (0·05 g of collagenase D, 0·05 g of DNase I and 0·3 g of dispase II in 100 ml of 1 × PBS) at 37°C for 60 min under slow rotation. Cells were filtered with a cell strainer. Isolation of CD4+ T cells was performed with commercial magnetic cell sorting kits. The purity of the isolated CD4+ T cells was more than 95%, as checked by flow cytometry.
Statistics
Data are presented as the means ± standard deviation. Differences between two groups were evaluated with Student's t-test; data among three or more groups were evaluated with analysis of variance (anova). Bonferroni adjustment was applied to post-hoc group comparisons when required. Two-variable correlation analysis was performed when necessary. A P < 0·05 was accepted as a significant criterion.
Results
Increase in Treg numbers in AR nasal mucosa with NP
Emerging evidence indicates that Treg functional deficiency or a decrease in Treg numbers plays a critical role in the pathogenesis of allergic disorders [15,16]. However, an increase in Treg numbers in allergic patients has also been reported [17]. Considering that the difference might result from allergic patients complicating with other disorders, 40 AR patients with or without NP (20 AR/NP, 20 AR; male 20, female 20; age: 22–58 years) were recruited into this study. Ten patients with chronic non-allergic rhinitis (CR) were recruited as a control group. All the AR patients showed a positive response to the challenge with mite antigen Der p1 (Der, in brief) and high serum Der-specific IgE levels (Fig. S1). These 50 patients also had inferior turbinate hyperplasia that did not respond well to conventional medical treatment; turbinatectomy was performed for these 50 patients. The surgically removed nasal mucosal tissue was collected and analysed as described below.
Immunohistochemistry was performed with nasal mucosal specimens from all patients to detect FoxP3+ Treg in nasal mucosa. FoxP3+ Treg were detected in the nasal mucosa of the Con group that were compatible with the CR group; fewer FoxP3+ Treg were observed in the AR group. However, the number of FoxP3+ Treg was significantly greater in the AR/NP group than the Con and CR groups (Fig. 1). The results indicate that Treg numbers are fewer in patients with AR, but greater in patients with AR/NP compared with the Con group.
Fig. 1.
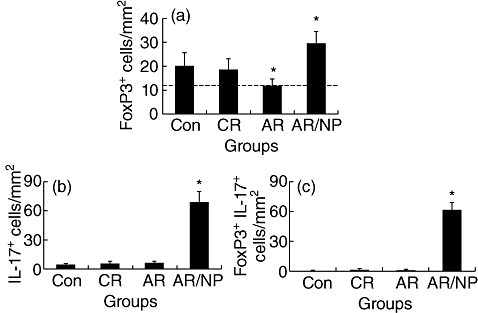
Interleukin (IL)-17+forkhead box P3 (FoxP3)+ cells in allergic nasal mucosa. Surgically removed nasal mucosa was collected from the 50 subjects (see text for detail) and observed by immunohistochemistry to detect FoxP3+ cells. Bars indicate the immune staining positive cell counts of FoxP3+ cells (a), IL-17+ cells (b) and both FoxP3+ IL-17+ cells (c) in nasal mucosa (× 200). Data were presented as mean ± standard deviation from 20 fields per sample. *P < 0·05, compared with control (Con) group. Isotope immunoglobulin (Ig)G staining was used as a negative control; no positive cells were observed (data not shown). The broken line in (a) is to contrast data between groups.
FoxP3+ Treg in nasal mucosa with AR/NP express high levels of IL-17
It is accepted that Treg have an immune regulatory function in suppression of aberrant immune responses. However, our results showed that FoxP3+ Treg numbers were even higher in the nasal mucosa of patients with AR/NP, but a lower number of Treg was detected in patients with AR (Figs 1 and S2). We questioned whether the Treg properties in the nasal mucosa of these two groups of patients were somehow different from each other. Based on recent reports that some FoxP3+ Treg express IL-17, which have a different function from IL-17- Treg[6,18], we therefore hypothesize that those Treg in AR/NP nasal mucosa may be also IL-17+. We isolated CD4+ T cells from surgically removed nasal mucosa. Indeed, as detected by flow cytometry, CD4+ FoxP3+ cells were detected in all four groups (Fig. 2a), with a tendency similar to that observed with immunohistochemistry (Fig. 1). Using the gating technique, we revealed that FoxP3+ CD4+ T cells from the AR/NP group were also IL-17+ (Fig. 2b). Few IL-17+ cells were detected in those FoxP3+ CD4+ T cells from the AR, CR and Con groups.
Fig. 2.

Forkhead box P3 (FoxP3)+ CD4+ T cells express interleukin (IL)-17 in allergic rhinitis/nasal polyposis (AR/NP) nasal mucosa. CD4+ T cells were isolated from nasal mucosa collected in this study and analysed by flow cytometry. A1-4: flow cytometry dot plots show FoxP3+ cells (gated cells). B1-4: histograms indicate IL-17+ cells in gated cell population in A1-4. Summarized data are also presented in Fig. S3. Cells stained with isotype immunoglobulin (Ig)G did not show any positive staining (data not shown). Each group consisted of samples from six patients.
SEB induces IL-6 production by DCs
It is reported that SEB is related to the pathogenesis of nasal polyps [19], in which IL-6 plays a critical role [13]. Because IL-6 in synergy with TGF-β induces the expression of IL-17 in CD4+ T cells, we considered whether there is an association between SEB and IL-17 expression in FoxP3+ T cells in nasal mucosa. To prove the hypothesis, we examined the SEB level in surgically removed nasal mucosa. The data showed that significantly higher SEB levels were detected in the AR/NP group (Fig. 3). In another approach, we generated Der-specific CD4+ FoxP3+ Treg in vitro following published procedures [20]; the cells were exposed to SEB in culture in the presence of dendritic cells (DCs) for 48 h. As expected, abundant IL-17+ FoxP3+ T cells were generated (Fig. 4). IL-6 levels were increased in the culture media, but not increased in the culture without DCs, which indicates that IL-6 was derived from DCs (Fig. 5). As RORγt is the transcription factor of IL-17, we speculated whether exposure to SEB can also increase RORγt expression in generated CD4+ FoxP3+ Treg. Indeed, a marked increase in RORγt protein was detected in SEB-treated CD4+ FoxP3+ Treg in the presence of DCs compared with those not stimulated CD4+ FoxP3+ Treg (Fig. S3).
Fig. 3.
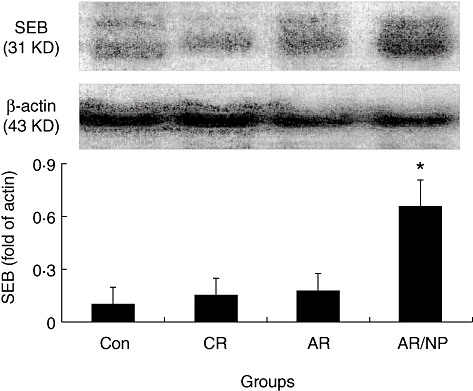
Staphylococcal enterotoxin B (SEB) level in nasal mucosa. Protein was extracted from surgically removed nasal mucosa collected in this study and processed to determine the level of SEB by Western blotting. The immune blots indicate SEB protein in nasal mucosa. Bars indicate the densitometry analysis results that were normalized to fold of β-actin. Each group consists of samples from six patients.
Fig. 4.
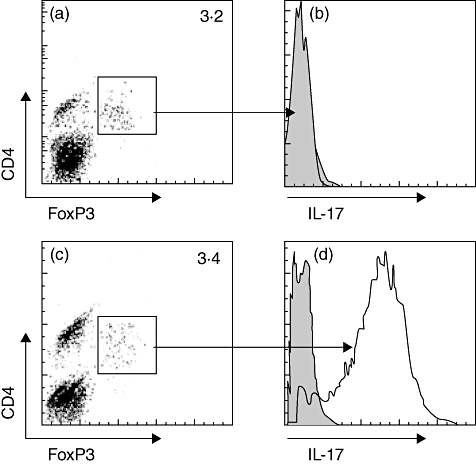
Staphylococcal enterotoxin B (SEB) induces CD4+ forkhead box P3 (FoxP3)+ regulatory T cells (Treg) to express interleukin (IL)-17. In vitro-generated CD4+ FoxP3+ T cells were generated in vitro (see supplementary material) and cultured in the presence of SEB for 2 days. Cells were then collected and analysed for the expression of IL-17 by flow cytometry. The flow cytometry histograms in (b) and (d) indicate IL-17+ cell population in gated CD4+FoxP3+ T cells in (a) (not stimulated) and (c) (stimulated by SEB). Data were from six separate experiments.
Fig. 5.
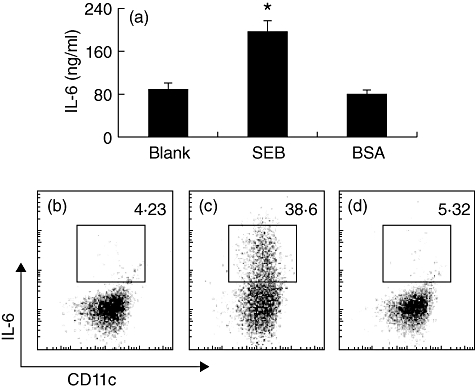
Staphylococcal enterotoxin B (SEB) induces dendritic cells (DCs) to produce interleukin (IL)-6. Generated bone marrow-derived DCs (bmDCs) were co-cultured in the presence of SEB (10 µg/ml) in culture for 48 h and analysed by flow cytometry. (a) Bars indicate levels of IL-6 in culture media were determined by enzyme-linked immunosorbent assay. (b–d) Flow cytometry dot plots indicate CD11c+ IL-6+ DCs (the gated cell population) under conditions of naive (b), stimulated by SEB (c) or bovine serum albumin (c). Isotype immunoglobulin (Ig)G did not result in any positive staining (data not shown). Data were from six separate experiments.
Discussion
Polyposis is an end disorder of mucosa that stems from various inflammations in the nasal cavity, such as nasal allergy, chronic sinusitis and aspirin-exacerbated respiratory disease. The pathogenesis is not yet fully understood. Published data indicate that AR is involved in the pathogenesis of nasal polyposis [21]. However, not all patients with AR have polyposis, or vice versa. Recent studies indicate that there is a subpopulation of T cells in peripheral blood and lymphoid tissue that expresses both FoxP3 and IL-17 [6]. Our data are in line with these pioneer studies by providing evidence that a subset of T cells in the nasal mucosa expresses both FoxP3 and IL-17. Whether this T cell subset plays a role in the pathogenesis of nasal polyposis needs further investigation. However, we found that FoxP3+ IL-17+ T cells had a close relation with the specific pathogenic condition of both AR and NP, but not in patients with AR alone. This implies that FoxP3+ IL-17+ T cells may be one of the aetiologies in the pathogenesis of both AR and NP. Previous studies also indicate that IL-17 plays a critical role in nasal polyposis [13].
It is proposed that IL-6 in synergy with TGF-β induces the generation of T helper type 17 (Th17) cells [22]. The FoxP3+ IL-17+ T cells we observed in the present study may be developed from FoxP3+ Treg in an environment with high levels of IL-6. Guided by published data that SEB has a close relation with NP [19], we detected high levels of IL-6 and SEB in collected nasal mucosal specimens of the AR/NP group. Thus, IL-6 may co-operate with intracellular TGF-β to induce the FoxP3+ Treg to become FoxP3+ IL-17+ T cells. Subsequent experimental results have confirmed this inference. In vitro study showed that SEB increases IL-6 production by DC. The concurrent presence of IL-6 and TGF-β induced expression of RORγt in CD4+ FoxP3+ T cells, resulting in the expression of IL-17.
In summary, the present study reports that a new subset of T cells, FoxP3+ IL-17+ T cells, has been detected in the nasal mucosa of patients with AR and NP.
Acknowledgments
This study was supported by grants from the Shanxi Provincial Health Research Grant (no. 200703), Shanxi Medical University Innovation Grant (no. 01200807) and grants from the Canadian Institutes of Health Research (CIHR, no. 191063) and the Natural Sciences, Engineering Research Council of Canada (NSERC, no. 371268). Dr PC Yang holds a New Investigator Award of CIHR (no. 177843).
Disclosure
The authors do not have any conflict of interest to declare.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Serum levels of immunoglobulin (Ig)E antibodies against Der. IgE antibodies against Der in the sera of patients in this study were measured by enzyme-linked immunosorbent assay (ELISA). Data were expressed in ELISA units. Isotype control wells did not show any positive results (data not shown).
Fig. S2. Forkhead box P3 (FoxP3)+ cells in nasal mucosa. Surgically removed nasal mucosa was obtained (see text), observed by immunohistochemistry to detect FoxP3+ cells. (a,b) Representative nasal mucosal images show FoxP3+ cells (in brown). (a) Samples were taken from patients with allergic rhinitis/nasal polyposis (AR/NP); (b), samples were taken from patients with AR. Negative control sections were stained with isotype immunoglobulin (Ig)G that resulted in no positive staining (data not shown) (samples were observed from all the patients described in the text).
Fig. S3. Staphylococcal enterotoxin B (SEB) increases the levels of acid-related orphan receptor (ROR)γt in forkhead box P3 (FoxP3)+ regulatory T cells (Treg). Peripheral blood mononuclear cells (PBMC) were isolated from 10 healthy subjects and cultured in the presence of SEB (10 μg/ml) for 4 days. Cells were collected at the end and analysed by flow cytometry. (a,c) Dot plots indicate CD4+ FoxP3+ Treg before (a) and after (c) culture. (b,d) Histograms indicate RORγt+ Treg (open histograms). The solid histograms indicate isotype immunoglobulin (Ig)G staining.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Verbruggen K, Van Cauwenberge P, Bachert C. Anti-IgE for the treatment of allergic rhinitis – and eventually nasal polyps? Int Arch Allergy Immunol. 2009;148:87–98. doi: 10.1159/000155739. [DOI] [PubMed] [Google Scholar]
- 2.Nelson HS. Multiallergen immunotherapy for allergic rhinitis and asthma. J Allergy Clin Immunol. 2009;123:763–9. doi: 10.1016/j.jaci.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Braunstahl GJ, Fokkens W. Nasal involvement in allergic asthma. Allergy. 2003;58:1235–43. doi: 10.1046/j.0105-4538.2003.00354.x. [DOI] [PubMed] [Google Scholar]
- 4.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–46. doi: 10.1016/j.jaci.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Miyara M, Wing K, Sakaguchi S. Therapeutic approaches to allergy and autoimmunity based on FoxP3+ regulatory T-cell activation and expansion. J Allergy Clin Immunol. 2009;123:749–55. doi: 10.1016/j.jaci.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Voo KS, Wang YH, Santori FR, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–8. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beriou G, Costantino CM, Ashley CW, et al. L-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–9. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol. 2009;123:1004–11. doi: 10.1016/j.jaci.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto T, Akiyama K, Kobayashi N, Mori A. Comparison of IL-17 production by helper T cells among atopic and nonatopic asthmatics and control subjects. Int Arch Allergy Immunol. 2005;137(Suppl. 1):51–4. doi: 10.1159/000085432. [DOI] [PubMed] [Google Scholar]
- 10.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–74. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 11.Yang PC, Xing Z, Berin CM, et al. TIM-4 expressed by mucosal dendritic cells plays a critical role in food antigen-specific Th2 differentiation and intestinal allergy. Gastroenterology. 2007;133:1522–33. doi: 10.1053/j.gastro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Lee JY, Kim HM, Ye YM, et al. Role of staphylococcal superantigen-specific IgE antibodies in aspirin-intolerant asthma. Allergy Asthma Proc. 2006;27:341–6. doi: 10.2500/aap.2006.27.2908. [DOI] [PubMed] [Google Scholar]
- 13.Xu G, Xia JH, Zhou H, et al. Interleukin-6 is essential for staphylococcal exotoxin B-induced T regulatory cell insufficiency in nasal polyps. Clin Exp Allergy. 2009;39:829–37. doi: 10.1111/j.1365-2222.2009.03218.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu T, Wang BQ, Yang PC. A possible link between sinusitis and lower airway hypersensitivity: the role of staphylococcal enterotoxin B. Clin Mol Allergy. 2006;4:7. doi: 10.1186/1476-7961-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang N, Van Zele T, Perez-Novo C, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–8. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008;121:1467–72. doi: 10.1016/j.jaci.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Yu HH, Wang LC, Yang YH, Lin YT, Chiang BL. The levels of CD4+CD25+ regulatory T cells in paediatric patients with allergic rhinitis and bronchial asthma. Clin Exp Immunol. 2007;148:53–63. doi: 10.1111/j.1365-2249.2007.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitani A, Xu L. Regulatory T cells and the induction of IL-17. Mucosal Immunol. 2008;1:S43–6. doi: 10.1038/mi.2008.51. [DOI] [PubMed] [Google Scholar]
- 19.Yu RL, Dong Z. Proinflammatory impact of Staphylococcus aureus enterotoxin B on human nasal epithelial cells and inhibition by dexamethasone. Am J Rhinol Allergy. 2009;23:15–20. doi: 10.2500/ajra.2009.23.3252. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearlman AN, Chandra RK, Chang D, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009;23:145–8. doi: 10.2500/ajra.2009.23.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annunziato F, Cosmi L, Liotta F, et al. Type 17 T helper cells-origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol. 2009;5:325–31. doi: 10.1038/nrrheum.2009.80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


