Abstract
In a co-culture of osteoclast precursor cells and synovial cells, interleukin-6 (IL-6) induces osteoclast formation. In contrast, in a monoculture of osteoclast precursor cells, IL-6 directly suppresses receptor activator for nuclear factor κB ligand (RANKL)-induced differentiation of osteoclast precursor cells into osteoclasts. In the present study, we explored why the effect of IL-6 differed between the monoculture and the co-culture systems. In the monoculture, mouse osteoclast precursor cell line, RAW 264·7 (RAW) cells were cultured with soluble RANKL (sRANKL) for 24 h or 3 days. sRANKL increased both expression of osteoclastogenesis marker, tartrate-resistant acid phosphatase isoform 5b (TRAP5b) and nuclear factor of activated T cells cytoplasmic 1 (NFATc1), whereas the co-addition of IL-6 decreased them both in a dose-dependent manner. In the co-culture, RAW cells and human synovial cell line, SW982 cells were cultured with IL-6 + soluble IL-6 receptor (sIL-6R) for 3 days. TRAP5b and NFATc1 expression reduced by IL-6 was increased by the addition of SW982 cells in a manner dependent upon the number of added cells. IL-6 + sIL-6R treatment significantly induced RANKL production in SW982 cells, and anti-RANKL antibody inhibited IL-6 + sIL-6R-induced osteoclastogenesis. SW982 cells expressed high levels of ICAM-1 originally, and ICAM-1 expression was increased significantly by IL-6 + sIL-6R. Anti-ICAM-1 antibody suppressed IL-6-induced osteoclastogenesis. Finally, in the monoculture system, addition of sICAM-1 dose-dependently restored the expression of TRAP5b reduced by IL-6. Similar results were obtained when the formation of TRAP-positive multi-nuclear cells were examined using mouse bone marrow cells. In conclusion, IL-6 gave different results in the co-culture and monoculture systems because in the co-culture, ICAM-1 from the synovial cells restored osteoclastogenesis suppressed by IL-6.
Keywords: ICAM-1, IL-6, osteoclastogenesis, sIL-6R, synovial cell
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune inflammatory disorder that affects many tissues and organs throughout the body, but the joints are usually the most severely affected. Chronic synovitis often leads to irreversible destruction of the articular cartilage and periarticular bone. Because osteoclasts are often seen in RA synovium at sites of bone erosion [1,2], it is thought that osteoclasts are responsible for the erosion of periarticular bone in RA patients.
Receptor activator of nuclear factor-κB ligand (RANKL) is an essential factor in osteoclastogenesis. It differentiates precursor cells of the myeloid lineage into mature osteoclasts by binding to the signalling receptor, RANK [3,4]. RANKL also stimulates osteoclast migration, fusion, activation, and survival; i.e. it acts at all stages of osteoclast formation and activity. RANKL is expressed at sites of bone erosion in RA synovial membranes [2,5].
Interleukin (IL)-6 is a proinflammatory cytokine, and excessive IL-6 production is observed in many autoimmune inflammatory diseases. For example, IL-6 levels in serum and synovial fluid of RA patients are higher than in osteoarthritis patients or healthy subjects [6,7]. In a previous study, we demonstrated that co-addition of IL-6 and soluble IL-6R (sIL-6R) induces RANKL expression in fibroblast-like synovial cells from RA patients (RA-FLS) and induces the differentiation of osteoclast precursor cells, RAW 264·7 (RAW) cells, into osteoclasts [8]. In contrast, it has been reported that IL-6 acts directly on osteoclast precursor cells and suppresses their differentiation by regulating the transcription of specific genes related to mitogen-activated protein kinase (MAPK) phosphatases and the ubiquitin pathway [9]. Therefore, induction of RANKL by IL-6 cannot explain fully why osteoclast formation was induced by IL-6 in a co-culture of synovial cells and osteoclast precursor cells.
In the present study, we explored the reasons for the discrepancy between the effects of IL-6 on osteoclast differentiation in a monoculture system of osteoclast precursor cells and the effects of IL-6 in a co-culture system of osteoclast precursor cells and synovial cells.
Materials and methods
Reagents, cells and animals
Recombinant human IL-6, human sIL-6R and anti-mouse IL-6R antibody (MR16-1) were prepared in our laboratories [10–12]. Recombinant human soluble RANKL (sRANKL) and recombinant human macrophage-colony-stimulating factor (M-CSF) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Recombinant human soluble ICAM-1 (sICAM-1), anti-human RANKL antibody and anti-human ICAM-1 antibody were purchased from R&D Systems (Minneapolis, MN, USA).
The human synovial sarcoma cell line SW982 was obtained from the American Type Culture collection (ATCC, Manassas, VA, USA) and cultured using Leibovitz's l-15 medium supplemented with 10% fetal bovine serum (FBS). Mouse macrophage-like cell line RAW 264·7 (RAW) was purchased from DS Pharma Biomedical Co. (Osaka, Japan) and cultured using RPMI-1640 medium supplemented with 10% FBS.
Male C57BL/6 mice were purchased from Charles River Laboratory Japan (Yokohama, Japan). Mice were specific pathogen-free and were kept in cages in a room maintained at 20–26°C and 35–75% relative humidity. The experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Chugai Pharmaceutical Co. Ltd (Shizuoka, Japan).
Osteoclast formation culture of RAW cells
In the co-culture system, RAW cells (4 × 104 cells/well) and SW982 (4 × 105 cells/well) were cultured with IL-6 + sIL-6R for 3 days in a six-well culture plate using RPMI-1640 medium.
In the monoculture system, RAW cells (2 × 105 cells/well) were cultured with sRANKL (50 ng/ml) for 24 h or 3 days in a six-well culture plate using RPMI-1640 medium. After culture, supernatants and cell lysates were collected.
Osteoclast formation culture of mouse bone marrow cells
Bone marrow cells were isolated from male C57BL/6 mice (8–12 weeks old) and cultured in α-modified Eagle's medium (MEM) supplemented with 10% FBS for 5 h. Non-adherent cells were harvested and cultured for 3 days in α-MEM supplemented with 10% FBS, and M-CSF (30 ng/ml). After culture, adherent cells were harvested as osteoclast precursor cells. Osteoclast precursor cells (1 × 104 cells/well) were seeded into 96-well plates and cultured for 3 days in α-MEM supplemented with 10% FBS, M-CSF (30 ng/ml) and sRANKL (50 ng/ml) in the presence or absence of IL-6 (100 ng/ml). Cultured cells were fixed with 10% formalin in phosphate-buffered saline (PBS) for 10 min at room temperature. After treatment with ethanol/acetone (50:50 vol/vol) for 1 min, the well surface was air-dried and incubated for 30 min at room temperature with tartrate-resistant acid phosphatase isoform (TRAP)-staining solution: 0·1 M sodium acetate (pH 5·0) containing 0·01% naphthol AS-MX phosphate (Sigma-Aldrich, St Louis, MO, USA) as a substrate, and 0·03% red violet LB salt (Sigma-Aldrich) as a stain for the reaction product in the presence of 50 mM sodium tartrate. TRAP-positive multinuclear cells containing more than three nuclei were counted as osteoclasts.
Culture of SW982 cells
SW982 cells (4 × 105 cells/well) were cultured with IL-6 (100 ng/ml) and sIL-6R (100 ng/ml) for 24 h in a six-well culture plate. After culture, supernatants and cell lysates were collected.
Quantitative real-time PCR for TRAP5b and NFATc1
Total RNA was extracted using an RNeasy kit (Qiagen, Valencia, CA, USA). Synthesis of cDNA was performed using an Omniscript RT kit (Qiagen) with random 9-mer primers (Takara Bio, Shiga, Japan), according to the manufacturer's protocol.
Quantitative real-time PCR was performed by running TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA) targeting mouse TRAP5b, nuclear factor of activated T cells cytoplasmic 1 (NFATc1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and human ICAM-1, RANKL and GAPDH on an ABI PRISM 7500 system (Applied Biosystems), according to the manufacturer's protocol.
Measurement of TRAP5b protein
The concentration of TRAP5b protein in the supernatants was measured with an enzyme-linked immunosorbent assay (ELISA) kit (MouseTRAP Assay Kit; Immunodiagnostic Systems, Boldon, UK), according to the manufacturer's instructions.
Western blotting for RANKL
After cells were lysed, protein concentrations were determined using a bicinchoninic acid (BCA) protein assay reagent kit (Pierce, Rockford, IL, USA). Cell lysates (10 µg protein) were separated by 15% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA). After blocking with PVDF blocking reagent (Toyobo, Osaka, Japan) for 1 h at room temperature, the membranes were incubated overnight at 4°C either with goat anti-human RANKL polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:500 in Can Get Signal Solution 1 (Toyobo) or with mouse anti-human β-actin antibody (Sigma-Aldrich) diluted 1:2000, and then incubated with either alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) or donkey anti-goat IgG (Santa Cruz Biotechnology) diluted 1:2000 in Can Get Signal Solution 2 (Toyobo) for 1 h at room temperature. Binding was visualized by using SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific, Waltham, MA, USA) and the ChemiDoc XRS imaging system (Bio-Rad Laboratories, Hercules, CA, USA) and analysed using Quantity One software (Bio-Rad Laboratories).
Flow cytometry analysis for ICAM-1
SW982 cells (1 × 106/well) were cultured for 24 h in a culture dish with a combination of human IL-6 and sIL-6R using Leibovitz's l-15 medium. After culturing, cells were treated with trypsin-ethylenediamine tetraacetic acid (EDTA) (Gibco, Grand Island, NY, USA) to obtain a single cell suspension, and cells were then stained with mouse anti-human ICAM-1 antibody (R&D Systems) and Alexa Fluor 488 conjugated anti-mouse IgG (Molecular Probes, Eugene, OR, USA). After staining, ICAM-1 expression on the cell surface was measured with a fluorescence-activated cell sorter (FACSCanto II; BD Biosciences, Franklin Lakes, NJ, USA), using FACSDiva software (BD Biosciences) for data acquisition and FlowJo software (Tree Star, Ashland, OR, USA) for data analysis.
Statistical analysis
Statistical significances were analysed by unpaired t-test and Dunnett's multiple comparison test using a software package (SAS Institute Japan, Tokyo, Japan), with the significance level set to 5%.
Results
IL-6 inhibited RANKL-induced osteoclast differentiation
First, we examined the effect of IL-6 on RANKL-induced differentiation of RAW cells into osteoclasts. RAW cells were cultured with sRANKL (50 ng/ml) in the presence or absence of IL-6 for 24 h or 3 days. In cell lysate from the 24 h culture, the expression of TRAP5b and NFATc1 mRNA was increased by sRANKL, whereas the co-addition of IL-6 decreased sRANKL-induced TRAP5b and NFATc1 mRNA expression in a concentration-dependent manner (Fig. 1a). Moreover, in cell-free supernatant from the 3-day culture, production of TRAP 5b protein was also suppressed by IL-6, and anti-IL-6R antibody blocked IL-6-induced suppression (Fig. 1b).
Fig. 1.
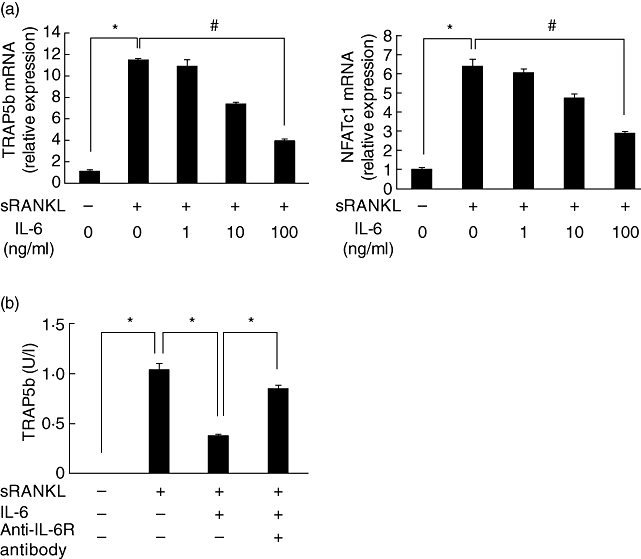
Interleukin (IL)-6 suppressed receptor activator of nuclear factor-κB ligand (RANKL)-induced differentiation of RAW cells into osteoclasts. RAW cells were cultured with soluble RANKL (sRANKL) (50 ng/ml) and various concentrations of IL-6 for 24 h. Cell lysate was collected from 24 h culture and the expression of mRNA for tartrate-resistant acid phosphatase isoform 5b (TRAP5b) and nuclear factor of activated T cells cytoplasmic 1 (NFATc1) was measured by real-time polymerase chain reaction (PCR) (a). RAW cells were cultured with sRANKL (50 ng/ml), IL-6 (100 ng/ml) and anti-IL-6R antibody (100 µg/ml) for 24 h or 3 days. Cell-free supernatant was collected and the concentration of TRAP5b was measured by enzyme-linked immunosorbent assay (b). Statistical significances were analysed by t-test (*: P < 0·05) or Dunnett's multiple comparison test (#P < 0·05).
IL-6 induced differentiation of RAW cells into osteoclasts in co-culture with synovial cells
We have reported previously that the osteoclastogenesis marker, NFATc1, expression in RAW cells was induced by co-culture with RA-FLS in the presence of IL-6 + sIL-6R [8]. In this study, we used SW982 cells instead of RA-FLS. RAW cells (4 × 104/well) were co-cultured with various quantities of SW982 cells (0, 1·6 × 104/well, 8 × 104/well, 4 × 105/well or 2 × 106/well) in the presence of IL-6 + sIL-6R for 3 days. IL-6 + sIL-6R reduced RANKL-induced TRAP5b expression in RAW cells again. By the addition of SW982 cells, the TRAP5b expression reduced by IL-6 + sIL-6R was restored in a manner dependent upon cell numbers (Fig. 2a). A similar result was obtained in the case of NFATc1 mRNA expression (Fig. 2b).
Fig. 2.
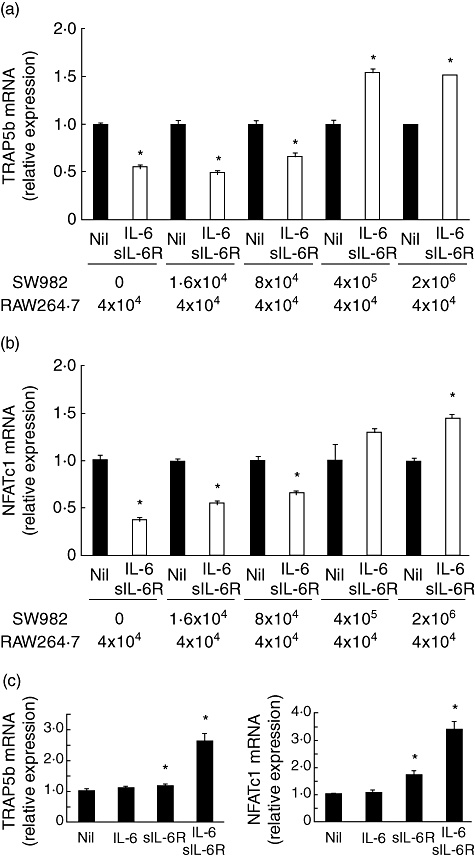
Interleukin (IL)-6 + sIL-6R induced osteoclastogenesis in a co-culture system of RAW cells and SW982 cells. RAW cells (4 × 104/well) were co-cultured with various quantities of SW982 cells (0, 1·6 × 104/well, 8 × 104/well, 4 × 105/well or 2 × 106/well) in the presence of IL-6 (100 ng/ml) and sIL-6R (100 ng/ml) for 3 days. After culture, cell lysate was collected and the expression of mRNA for tartrate-resistant acid phosphatase isoform 5b (TRAP5b) and nuclear factor of activated T cells cytoplasmic 1 (NFATc1) was measured by real-time polymerase chain reaction (PCR). mRNA expression without IL-6 + sIL-6R was defined as 1·0 in each case (a,b). RAW cells (4 × 104/well) were co-cultured with SW982 cells (4 × 105/well) in the presence of IL-6 (100 ng/ml), sIL-6R (100 ng/ml) or IL-6 + sIL-6R for 3 days. After culture, cell lysate was collected and the expression of mRNA for TRAP5b and NFATc1 was measured by real-time PCR. mRNA expression without IL-6 + sIL-6R was defined as 1·0 in each case (c). Statistical significances between Nil and IL-6 + sIL-6R were analysed by t-test (*P < 0·05).
We examined the effect of IL-6 or sIL-6R alone on osteoclastgenesis in the co-culture system. RAW cells (4 × 104/well) and SW982 cells (4 × 105/well) were co-cultured with IL-6, sIL-6R or IL-6 + sIL-6R for 3 days. IL-6 alone did not induce expression of mRNA for TRAP5b and NFATc1, whereas sIL-6R and IL-6 + sIL-6R significantly induced osteoclastgenesis. However, IL-6 + sIL-6R induced osteoclastgensis more potently than sIL-6R alone (Fig. 2c).
Involvement of RANKL in co-culture system
To confirm the involvement of RANKL in the co-culture system, RANKL expression in SW982 cells and the influence of anti-RANKL antibody on osteoclast formation were examined. IL-6 + sIL-6R induced both RANKL mRNA expression and protein production significantly in SW982 cells (Fig. 3a). Furthermore, TRAP5b mRNA expression in RAW cells was inhibited by the addition of anti-RANKL antibody (Fig. 3b).
Fig. 3.
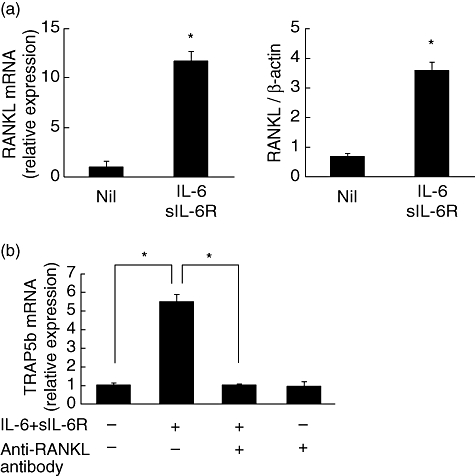
Involvement of receptor activator of nuclear factor-κB ligand (RANKL) in co-culture system. SW982 cells were cultured with interleukin (IL)-6 (100 ng/ml) + sIL-6R (100 ng/ml) for 24 h and RANKL mRNA expression and RANKL protein production was measured (a). RAW cells were co-cultured with SW982 cells in the presence of IL-6 + sIL-6R with or without anti-RANKL antibody (10 µg/ml) for 24 h and tartrate-resistant acid phosphatase isoform 5b (TRAP5b) mRNA expression was measured by real-time polymerase chain reaction (PCR) (b). Statistical significances were analysed by t-test (*P < 0·05).
Involvement of ICAM-1 in co-culture system
Involvement of ICAM-1 in osteoclast formation has been reported [13,14]. Therefore, we examined the expression of ICAM-1 in SW982 cells and the effect of anti-ICAM-1 antibody. IL-6 + sIL-6R increased expression of ICAM-1 mRNA significantly, but SW982 cells originally expressed high levels of ICAM-1, and IL-6 + sIL-6R increased ICAM-1 expression slightly (Fig. 4a). Moreover, anti-ICAM-1 antibody suppressed IL-6-induced TRAP5b mRNA expression in RAW cells (Fig. 4b).
Fig. 4.
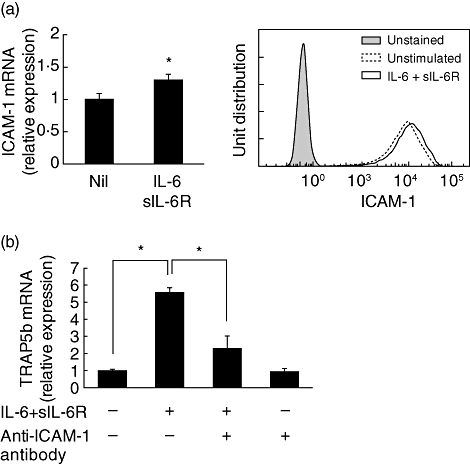
Involvement of intercellular adhesion molecule-1 (ICAM-1) in co-culture system. SW982 cells were cultured with interleukin (IL)-6 (100 ng/ml) + sIL-6R (100 ng/ml) for 24 h. The expression of ICAM-1 mRNA was measured with real-time polymerase chain reaction (PCR), and ICAM-1 expression on the cell surface was analysed by flow cytometry (a). RAW cells were co-cultured with SW982 cells in the presence of IL-6 + sIL-6R with or without anti-ICAM-1 antibody (10 µg/ml) for 24 h, and tartrate-resistant acid phosphatase isoform 5b (TRAP5b) mRNA expression was measured by real-time PCR (b). Statistical significances were analysed by t-test (*P < 0·05).
sICAM-1 attenuated IL-6-induced inhibition of osteoclast differentiation
We investigated whether sICAM-1 would reverse IL-6-induced suppression of TRAP5b expression induced by RANKL. The addition of sICAM-1 increased dose-dependently the expression of TRAP5b mRNA reduced by IL-6 (Fig. 5a). Moreover, sICAM-1 did not affect sRANKL-induced TRAP5b mRNA expression, and sICAM-1 alone did not up-regulate TRAP5b mRNA expression (Fig. 5b).
Fig. 5.
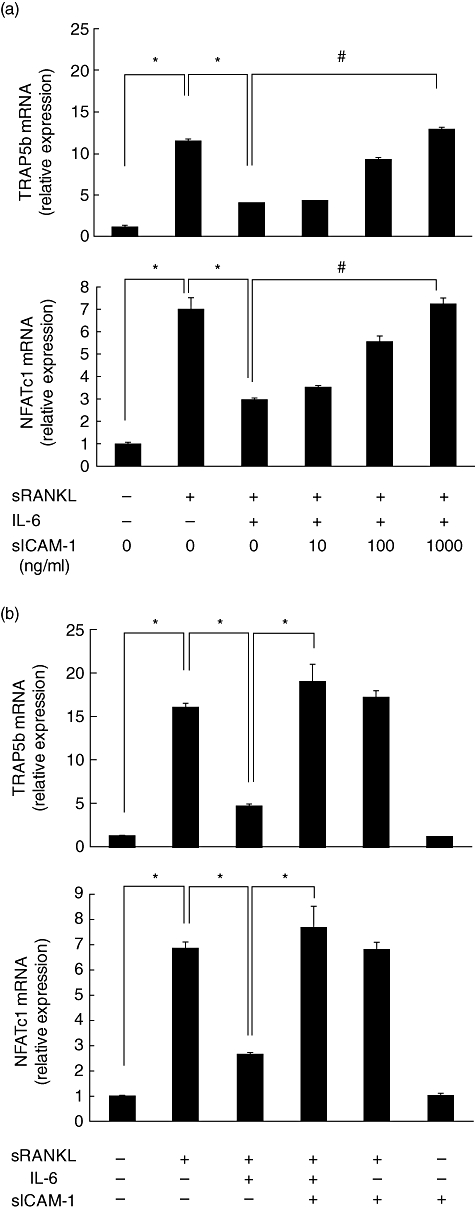
Intercellular adhesion molecule-1 (ICAM-1) reversed suppression of osteoclast differentiation of RAW cells by interleukin (IL)-6. RAW cells were stimulated by soluble receptor activator of nuclear factor-κB ligand (sRANKL) (50 ng/ml) in the presence of IL-6 (100 ng/ml) and sICAM-1 (10, 100, 1000 ng/ml) for 24 h (a). RAW cells were stimulated by sRANKL (50 ng/ml) in the presence of IL-6 (100 ng/ml) and sICAM-1 (1000 ng/ml) for 24 h (b). After culture, tartrate-resistant acid phosphatase isoform 5 (TRAP5) band nuclear factor of activated T cells cytoplasmic 1 (NFATc1) mRNA expression was measured by real-time polymerase chain reaction (PCR). Statistical significances were analysed by t-test (*P < 0·05).
We further examined if a similar phenomenon was observed in mouse bone marrow cells (Fig. 6). IL-6 significantly suppressed RANKL-induced increase in the cell numbers of multi-nucleated large TRAP-positive cells. The addition of sICAM-1 restored IL-6-induced suppression of osteoclastgenesis of mouse bone marrow cells.
Fig. 6.
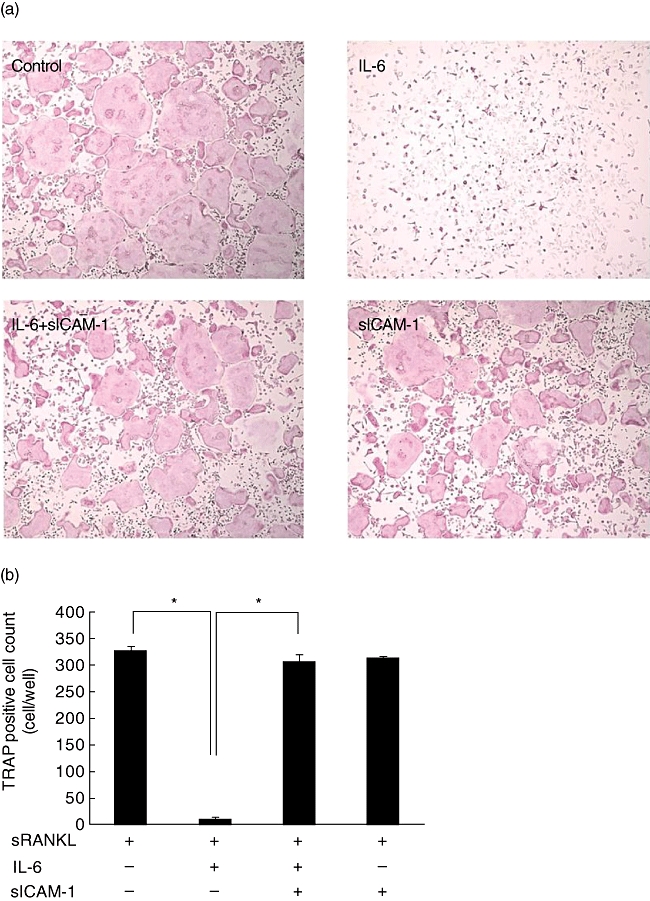
Intercellular adhesion molecule-1 (ICAM-1) reversed suppression of osteoclast differentiation of mouse bone marrow cells by interleukin (IL)-6. Mouse bone marrow cells were stimulated by soluble receptor activator of nuclear factor-κB ligand (sRANKL) (50 ng/ml) and M-CSF (30 ng/ml) in the presence of IL-6 (100 ng/ml) and sICAM-1 (1000 ng/ml) for 3 days (a), and the number of multi-nuclear tartrate-resistant acid phosphatase (TRAP)-positive cells containing more than three nuclei was scored (b). Statistical significances were analysed by t-test (*P < 0·05).
Discussion
In this study, we have demonstrated that RAW cells were differentiated into osteoclasts when they were co-cultured with SW982 cells in the presence of IL-6 and sIL-6R. In addition, we have also shown that IL-6 + sIL-6R induced RANKL expression in SW982 cells and that anti-RANKL antibody completely suppressed the differentiation of RAW cells into osteoclasts. These results indicate clearly that IL-6 + sIL-6R induced osteoclast formation in the co-culture system via induction of RANKL in SW982 cells. We have reported similar results previously [8]. Briefly, IL-6 + sIL-6R differentiated RAW cells into osteoclasts in the presence of RA-FLS. SW982 is a synovial sarcoma cell line reported to be a useful tool for studying the expression of inflammatory cytokine or matrix metalloproteinase (MMP) [15,16], and we found here that SW982 cells have properties similar to those of RA-FLS in terms of RANKL induction. Therefore, we used SW982 in this study instead of RA-FLS.
In contrast to our results, IL-6 has been shown to suppress osteoclast formation when bone marrow macrophages are stimulated with M-CSF and sRANKL [9]. In that study, it was also shown that IL-6 suppressed osteoclastogenesis because of the inhibition of I kappa-B (IkB) degradation and c-Jun N-terminal kinase (JNK) activation. In the present study, we confirmed that IL-6 suppressed the sRANKL-induced differentiation of RAW cells into osteoclasts. From these facts, it is seen that IL-6 works directly on osteoclast precursor cells as an inhibitor of differentiation.
These lines of evidence show clearly that the effect of IL-6 on osteoclast formation in a monoculture of osteoclast precursor cells differs from its effect on a co-culture of osteoclast precursor cells and synovial cells. Moreover, it suggests that osteoclast formation in a co-culture is not explained fully by only the induction of RANKL expression by IL-6.
The importance of ICAM-1 in osteoclast formation has been described [13,14]. ICAM-1 blockade inhibited IL-6-induced osteoclast formation significantly in the co-culture, but did not show any influence on osteoclast formation in a monoculture of RAW cells (unpublished data), showing that ICAM-1 of SW982 cells plays an essential role in osteoclastogenesis in the co-culture. The induction of ICAM-1 on SW982 cells by IL-6 + sIL-6R is marginal and SW982 cells originally express high levels of ICAM-1. Therefore, the original amount of ICAM-1 expression seems to be enough to influence osteoclastogenesis.
It is not yet clear how ICAM-1 participates in osteoclastogenesis. Membrane-bound ICAM-1 provides high-affinity adhesion between the osteoblast and the osteoclast precursor, thereby facilitating the interaction between RANKL and its receptor RANK [14]. However, we have shown that sICAM-1 reversed the suppressive effect of IL-6 on RANKL-induced osteoclast formation in RAW cells. Therefore, direct contact of RAW cells and SW982 cells may not always be necessary with respect to the binding of ICAM-1 to its counterpart, lymphocyte function-associated antigen 1 (LFA-1).
It is known that the soluble forms of adhesion molecules such as sICAM-1, soluble vascular adhesion molecule-1 (sVCAM-1) and sE-selectin are elevated in the serum of RA patients [17] and that tumour necrosis factor (TNF) blockade in RA patients decreases serum levels of these molecules [18]. Although the pathogenic roles of these soluble adhesion molecules in RA has not yet been elucidated, sVCAM-1 and sE-selectin have been shown to possess angiogenic activity [19], suggesting the involvement in synovitis and migration of inflammatory cells into the synovium. In the present study, we showed that sICAM-1 might play a role in bone metabolism via osteoclast formation.
LFA-1 acts not only as an adhesion molecule, but also as a signalling receptor for the reorganization of F-actin filaments in T cell-dependent processes. Although the cytoplasmic domain of LFA-1 is short and devoid of enzymatic features, it is thought that signalling is transduced by associating with adaptor proteins to couple them to the cytoskeleton, cytoplasmic kinases and G-coupled receptors [20]. For example, it is reported that LFA-1 on T cells is linked directly to a tyrosine kinase pathway that stimulates signalling by phosphatidylinositol-specific phospholipase C-gamma 1 (PLC-γ1) [21]. Yoshitake et al. reported that IL-6 suppresses RANKL-mediated JNK activation via the induction of MAPK phosphatase (MKP)1 and MKP7, which are known to inhibit JNK activity [9]. We examined the effect of sICAM-1 on the expression of MKP1 and MKP7 induced by IL-6. However, sICAM-1 did not influence the induction of these molecules (data not shown). Further studies are necessary to clarify this issue. We are currently conducting experiments to explore how signalling through ICAM-1/LFA-1 affects IL-6 signalling.
Conversely, we did not detect sRANKL in the supernatant of IL-6-stimulated SW982 cells (data not shown). Therefore, with respect to RANK/RANKL interaction, direct contact of RAW cells and SW982 cells may be necessary for osteoclast formation in co-culture.
IL-6 concentration in the synovial fluid and serum of RA patients is much higher than that of osteoarthritis patients [6,7], and therapy with tocilizumab (anti-human IL-6R antibody) significantly prevents the progress of joint destruction in RA patients [22]. Moreover, we reported that tocilizumab reduced RANKL expression and the number of osteoclasts in affected joints of monkeys with collagen-induced arthritis [23]. These lines of evidence suggest strongly that IL-6 augments osteoclast formation in vivo, although IL-6 inhibits the differentiation of osteoclast precursor cells into osteoclasts in vitro.
In conclusion, IL-6 gave different results in the co-culture and monoculture systems because, in the co-culture, ICAM-1 from the synovial cells restored osteoclastogenesis suppressed by IL-6.
Disclosure
None.
References
- 1.Fujikawa Y, Shingu M, Torisu T, Itonaga I, Masumi S. Bone resorption by tartrate-resistant acid phosphatase-positive multinuclear cells isolated from rheumatoid synovium. Br J Rheumatol. 1996;35:213–7. doi: 10.1093/rheumatology/35.3.213. [DOI] [PubMed] [Google Scholar]
- 2.Gravallese EM, Manning C, Tsay A, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–8. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 3.Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Sarosi I, Yan XQ, et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA. 2000;97:1566–71. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takayanagi H, Iizuka H, Juji T, et al. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43:259–69. doi: 10.1002/1529-0131(200002)43:2<259::AID-ANR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Houssiau FA, Devogelaer JP, Van Damme J, de Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988;31:784–8. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- 7.Hirano T, Matsuda T, Turner M, et al. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988;18:1797–801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- 8.Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxf) 2008;47:1635–40. doi: 10.1093/rheumatology/ken363. [DOI] [PubMed] [Google Scholar]
- 9.Yoshitake F, Itoh S, Narita H, Ishihara K, Ebisu S. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J Biol Chem. 2008;283:11535–40. doi: 10.1074/jbc.M607999200. [DOI] [PubMed] [Google Scholar]
- 10.Asagoe Y, Yasukawa K, Saito T, et al. Human B-cell stimulatory factor-2 expressed in Escherichia coli. Nat Biotechnol. 1988;6:806–9. [Google Scholar]
- 11.Yasukawa K, Saito T, Fukunaga T, et al. Purification and characterization of soluble human IL-6 receptor expressed in CHO cells. J Biochem. 1990;108:673–6. doi: 10.1093/oxfordjournals.jbchem.a123261. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki M, Yamada Y, Nishimoto N, Yoshizaki K, Mihara M. Characterization of anti-mouse interleukin-6 receptor antibody. Immunol Lett. 2002;84:231–40. doi: 10.1016/s0165-2478(02)00202-x. [DOI] [PubMed] [Google Scholar]
- 13.Kurachi T, Morita I, Murota S. Involvement of adhesion molecules LFA-1 and ICAM-1 in osteoclast development. Biochim Biophys Acta. 1993;1178:259–66. doi: 10.1016/0167-4889(93)90202-z. [DOI] [PubMed] [Google Scholar]
- 14.Harada H, Kukita T, Kukita A, Iwamoto Y, Iijima T. Involvement of lymphocyte function-associated antigen-1 and intercellular adhesion molecule-1 in osteoclastogenesis: a possible role in direct interaction between osteoclast precursors. Endocrinology. 1998;139:3967–75. doi: 10.1210/endo.139.9.6171. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki T, Yokoyama T, Akatsu H, Tukiyama T, Tokiwa T. Phenotypic characterization of a human synovial sarcoma cell line, SW982, and its response to dexamethasone. In Vitro Cell Dev Biol Anim. 2003;39:337–9. doi: 10.1290/1543-706X(2003)039<0337:PCOAHS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M, Hashizume M, Yoshida H, Shiina M, Mihara M. IL-6 and IL-1 synergistically enhanced the production of MMPs from synovial cells by up-regulating IL-6 production and IL-1 receptor I expression. Cytokine. 2010;51:178–83. doi: 10.1016/j.cyto.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Littler AJ, Buckley CD, Wordsworth P, Collins I, Martinson J, Simmons DL. A distinct profile of six soluble adhesion molecules (ICAM-1, ICAM-3, VCAM-1, E-selectin, L-selectin and P-selectin) in rheumatoid arthritis. Br J Rheumatol. 1997;36:164–9. doi: 10.1093/rheumatology/36.2.164. [DOI] [PubMed] [Google Scholar]
- 18.Klimiuk PA, Sierakowski S, Domyslawska I, Chwiecko J. Effect of etanercept on serum levels of soluble cell adhesion molecules (sICAM-1, sVCAM-1, and sE-selectin) and vascular endothelial growth factor in patients with rheumatoid arthritis. Scand J Rheumatol. 2009;38:439–44. doi: 10.3109/03009740903079321. [DOI] [PubMed] [Google Scholar]
- 19.Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature. 1995;376:517–9. doi: 10.1038/376517a0. [DOI] [PubMed] [Google Scholar]
- 20.van Kooyk Y, Figdor CG. Avidity regulation of integrins: the driving force in leukocyte adhesion. Curr Opin Cell Biol. 2000;12:542–7. doi: 10.1016/s0955-0674(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 21.Kanner SB, Grosmaire LS, Ledbetter JA, Damle NK. Beta 2-integrin LFA-1 signaling through phospholipase C-gamma 1 activation. Proc Natl Acad Sci USA. 1993;90:7099–103. doi: 10.1073/pnas.90.15.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–7. doi: 10.1136/ard.2006.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato A, Matsuo S, Takai H, Uchiyama Y, Mihara M, Suzuki M. Early effects of tocilizumab on bone and bone marrow lesions in a collagen-induced arthritis monkey model. Exp Mol Pathol. 2008;84:262–70. doi: 10.1016/j.yexmp.2008.03.003. [DOI] [PubMed] [Google Scholar]


