Abstract
Previous studies have shown that neutralization of macrophage migration inhibitory factor (MIF) by anti-MIF antibody reduces intestinal inflammation in mice. In this study we tested whether or not anti-MIF autoantibody induced by DNA vaccine targeting MIF protects mice against experimental colitis. Mice were administered a MIF-deoxyribonucleic acid (DNA) vaccine by introducing oligonucleotides encoding helper T epitope into the cDNA sequence of murine MIF by in vivo electroporation. Preventive effects of this method against dextran sulphate sodium-induced (DSS) colitis were evaluated. Mice administered with MIF-DNA vaccine raised values of autoantibody significantly. The clinical and histological findings of colitis induced by 3·0% DSS solution were ameliorated significantly in mice treated with MIF-DNA vaccine compared with saline or pCAGGS-treated mice given DSS. Myeloperoxidase activity, infiltration of F4/80-positive staining cells and the levels of proinflammatory cytokines were suppressed in the colon of MIF-DNA vaccine treated mice compared with saline or pCAGGS-treated mice exposed to DSS. Our results suggest that immunization with helper T epitope DNA-vaccine targeting MIF may be a useful approach for the treatment of colitis including inflammatory bowel diseases.
Keywords: colitis, inflammatory bowel disease, macrophage migration inhibitory factor, Th epitope, vaccine
Introduction
Inflammatory bowel diseases (IBDs), the major ones being ulcerative colitis (UC) and Crohn's disease (CD), are chronic and relapsing intestinal inflammatory diseases for which the aetiology has not yet been elucidated fully [1]. Recently, the involvement of some cytokines in the pathogenesis and/or exacerbation of IBDs has been described [1–3]. Moreover, neutralization of cytokines such as tumour necrosis factor (TNF)-α using antibodies has been shown to be effective for treating these diseases [4,5]. However, the use of antibodies as a therapy is sometimes limited for the following reasons: (i) the administration of a very large amount of antibodies is needed at one time; (ii) the duration of its protective effects is short, thereby requiring frequent repetition of administration; (iii) humanized antibodies are usually heterogeneous antibodies, repeated administration of which is likely to result in an anti-antibody response [6]; and (iv) the cost of treatment with antibodies is greater than that of conventional treatment. These limitations have prompted us to develop an active immunization method that enables elicitation of autoantibodies against target proteins such as cytokines or pathogens by administration of a naked or partially modified form as therapeutic vaccines.
Macrophage migration inhibitory factor (MIF) plays a key role in immune responses and inflammatory processes and is critical in the pathogenesis of inflammatory diseases [7–9]. In IBDs, circulating level and local expression of MIF have been reported to be increased in patients with IBDs [10–13]. Conversely, promoter polymorphisms in the MIF gene have been shown to be correlated with disease activity and the extent of lesions in UC and CD [14,15]. Furthermore, MIF is expressed exclusively in epithelial cells and immune cells in colonic mucosa of mice with experimental colitis, and neutralization of MIF with an anti-MIF antibody reduces considerably the severity of colitis in mice [11,12]. Consistent with the effects of anti-MIF antibody in murine colitis, it has been reported that the severity of colitis is decreased in MIF-deficient mice in colitis induced by dextran sulphate sodium (DSS) or CD45RBhigh transfer [11,16]. These findings indicated that neutralization of MIF bioactivity would be effective for treatment of colitis.
We have developed previously a DNA vaccine encoding a cDNA sequence of murine MIF (mMIF), which was replaced in part by oligonucleotides encoding the T helper (Th) epitope, and demonstrated the therapeutic effects of this vaccine in murine arthritis and dermatitis models [17,18].
DSS-induced colitis is a useful experimental model for investigating the mechanism underlying the onset of IBDs and the effectiveness of methods for treating IBDs because its pathological features are similar to those of chronic inflammatory intestinal disorders [19,20]. We have reported that MIF expression was up-regulated in serum and colon tissues in murine DSS colitis and that anti-MIF antibody treatment effectively reduced the severity of colitis [12]. Thus, in this study, we investigated the effect of MIF Th-modified DNA vaccine on DSS-induced colitis in mice.
Materials and methods
Mice
Male BALB/c mice, 4 weeks old, were purchased from Sankyou Laboratory service (Shizuoka, Japan) and maintained under specific pathogen-free conditions. All animal procedures were conducted according to the guidelines of the Hokkaido University Institutional Animal Care and Use Committee under an approved protocol. Male adult mice at 6 weeks of age were used in each experiment.
MIF DNA vaccine
For active immunization against autologous MIF, a MIF species harbouring a Th epitope was designed, and its expression plasmid was constructed as described previously [17]. Briefly, a coding region for the second loop of the mMIF (amino acids 32–37; GKPAQY) was substituted with a complementary DNA coding for tetanus toxin P30 Th epitope (TTX, FNNFTVSFWLRVPKVSASHL). This mutant MIF cDNA was cloned into a pCAGGS mammalian expression vector. For animal vaccination, plasmid DNA was purified by alkaline lysis followed by two rounds of CsCl density gradient ultracentrifugation.
Innoculation of MIF DNA vaccine
Gene transfer into muscle by electroporation was performed as described previously [17]. Briefly, mice were anaesthetized with ether and their hind legs were shaved. A pair of electrode needles (each with a 0·5-mm diameter and a 5-mm gap between the needles; NEPA GENE, Chiba, Japan) was inserted into an anterior tibial muscle, and DNA vaccine (25 µg/25 µl 0·9% saline) was injected into the portion between the needles. Electric pulses (50 V, 50 ms, three times) were applied using an electric pulse generation system (T820 and Optimizer 500; BTX, San Diego, CA, USA), followed by another three pulses with inverted polarity. The same injection and electroporation were applied to the other tibial muscle. A total of 50 µg of the naked plasmid was injected per mouse into the two tibias. A similar vaccination was repeated 3 weeks later.
Evaluation of plasma MIF-reactive antibody
Anti-MIF antibody titres in plasma were determined with a direct enzyme-linked immunosorbent assay (ELISA) kit, as described previously [17]. Briefly, plasma samples from vaccinated mice were collected from the tail vein and diluted with 0·1% bovine serum albumin (BSA)/phosphate-buffered saline (PBS)/0·05% Tween 20. Small aliquots of diluted plasma (1:200) were added to 96-well flat-bottomed plates precoated with recombinant mMIF. The serum anti-MIF antibodies that reacted with the precoated MIF were detected by goat anti-mouse antibody conjugated with horseradish peroxidase, followed by colour development with a substrate reagent (Techne, Minneapolis, MN, USA). The absorbance was measured with an ELISA plate reader (Model 3550; Bio-Rad Laboratories, Hercules, CA, USA).
Induction and assessment of DSS colitis
Eight weeks after the first immunization, experimental colitis was induced by administration of 3·0% (wt/vol) DSS (molecular weight, approximately 40 000; MP Biochemicals, Solon, OH, USA) from days 0–7 in drinking water. Mice were weighed and inspected visually for rectal bleeding and diarrhoea on days 0 and 7. The percentage weight change from day 0 for each mouse was calculated. Seven days after DSS treatment, mice were euthanized by intraperitoneal injection of thiopental. The colon was removed and stored until use. The severity of colitis was evaluated by assessment of colon length and histological examination on day 7 after the first DSS administration. The disease activity index (DAI) was used to assess the grade of colitis based on a previously published scoring system [19] (Table 1). The score ranges from 0 to 4 (total score) and represents the sum of the scores for body weight loss, stool consistency and rectal bleeding divided by 3.
Table 1.
Scoring system for the disease activity index (DAI).
| Score | Weight loss (%) | Stool consistency | Occult/gross rectal bleeding |
|---|---|---|---|
| 0 | <1 | Normal | Negative |
| 1 | 1–5 | ||
| 2 | 5–10 | Loose stool | Haemo-occult positive |
| 3 | 10–20 | ||
| 4 | >20 | Diarrhea | Gross bleeding |
Scores were tailed for each category and then divided by three to obtain the DAI. These clinical criteria were used to evaluate the severity of colitis in mice.
Histological evaluation of DSS colitis
The colon tissues were opened longitudinally, fixed with 10% neutral buffered formalin and embedded in paraffin. After deparaffinizing thin tissue sections on glass slides, the samples were stained with haematoxylin and eosin (H&E). Histological findings were evaluated microscopically, as reported previously [12]. Briefly, the tissue damage was categorized into six grades: grade 0, normal mucosa; grade 1, infiltration of inflammatory cells; grade 2, shortening of the crypt by less than half; grade 3, shortening of the crypt by more than half; grade 4, crypt loss; and grade 5, destruction of epithelial cells (ulceration and erosion). The extent of inflammatory lesions was also examined. The extent of the lesions in the total colon was classified into six grades: grade 0, 0%; grade 1, 1–20%; grade 2, 21–40%; grade 3, 41–60%; grade 4, 61–80%; and grade 5, 81–100%. In grading histological scores, a pathologist evaluated each section in a blinded fashion.
Measurement of tissue myeloperoxidase activity
Tissue myeloperoxidase (MPO) activity was measured by a standard enzymatic procedure, as described previously [21]. In brief, the tissue specimen was homogenized in 50 mM potassium phosphate buffer (pH 6·0) with 0·5% hexadecyltrimethylammonium bromide using a Polytron-type homogenizer three times for 30 s each on ice. The sample was centrifuged at 20 000 g for 10 min at 4°C, and the supernatant was collected. This sample (100 µl) was added to 2·9 ml of 50 mM phosphate buffer (pH 6·0) containing 0·167 mg/ml O-dianisidine hydrochloride and 0·0005% hydrogen peroxide. The absorbance at 460 nm in the sample was measured using a spectrometer at 25°C. The protein concentration of the supernatant was determined using a Bradford assay kit (Bio-Rad Laboratories) for calibration, and the values were standardized using MPO purified from human leucocytes (Sigma, St Louis, MO, USA). One unit of change in MPO level was defined as the value that can degrade 1 µM H2O2 per min at 25°C.
Cytokine assay in colon tissues
The sample of colon tissue in PBS with a protease inhibitor cocktail (Sigma) was homogenized, and supernatant was collected. The levels of TNF-α, interferon (IFN)-γ and interleukin (IL)-1β in the supernatant were measured using a multiplex bead array (Upstate Biotechnology, Lake Placid, NY, USA) and analysed with the Bioplex workstation and associated software, according to the manufacturer's procedure.
Immunohistochemistry
Immunohistochemical analysis for F4/80 was performed using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA), as described previously [22]. In brief, the paraffin-embedded colon tissues were cut into 4-µm-thick sections. The sections were incubated with 3% H2O2 for 10 min at 4°C, and then treated with 10% normal goat serum for 30 min at room temperature followed by overnight incubation with the anti-F4/80 antibody (diluted 100:1; Biosource, Camarillo, CA, USA) at 4°C. F4/80-positive staining was visualized with diaminobenzidine as a chromogen. After F4/80 staining, the number of positively stained cells was counted in the colonic mucosa per mm2 with a microscope. Three areas of mucosa in each mouse were evaluated in five mice in each group.
Moreover, the expression of MIF was examined by immunohistochemistry as described previously [12].
Statistics
All data are presented as the mean ± standard error (s.e.). The results were analysed statistically using analysis of variance (anova) for ranks and post-hoc tests (StatView; SAS Institute, Cary, NC, USA). P < 0·05 was considered statistically significant.
Results
MIF/TTX DNA vaccination protects mice against DSS-induced colitis
Mice administered the DNA vaccine for MIF/TTX showed high levels of antibody that reacted to MIF 8 weeks after the first vaccination (Fig. 1). Conversely, the mice treated with the vaccine encoding wild-type MIF or the pCAGGS vector did not show an increase of antibody reactive to MIF 8 weeks after the first vaccination (Fig. 1). There was a statistically significant difference in OD values for anti-MIF antibody in sera of mice treated with MIF/TTX and in plasma of mice treated with pCAGGS or wild-type MIF (0·202 ± 0·037 versus 0·032 ± 0·001 and 0·041 ± 0·002, respectively) (Fig. 1). Referring to the results from our previous study [17,18], the current data indicated that polyclonal antibodies were induced by MIF/TTX DNA vaccination.
Fig. 1.

Effect of macrophage migration inhibitory factor (MIF)/tetanus toxoid (TTX) DNA vaccine on elicitation of autoantibodies recognized native MIF protein. Optical density (OD) at 490 nm against anti-MIF antibody in plasma of mice vaccinated with 50 µg of endotoxin-free pCAGGS, wild-type MIF and MIF/TTX in 0·9% sterile saline. Each group consisted of five mice. Results are given as means ± standard error. Statistical significance was assessed compared with pCAGGS-treated mice. *P < 0·05.
We next examined the effects of MIF/TTX DNA vaccine in DSS-induced colitis. BALB/c mice were immunized with MIF/TTX DNA vaccine or with either saline or pCAGGS vector as controls. Eight weeks after the first immunization, the mice were given 3·0% DSS solution for 7 days. The DAI values in mice treated with saline or pCAGGS vector were increased on day 7 (3·4 ± 0·2 and 2·8 ± 0·5, respectively) (Fig. 2a). Treatment with MIF/TTX DNA vaccine significantly suppressed increase of the DAI values in mice given DSS compared with mice treated with saline or pCAGGS vector on day 7 (1·2 ± 0·4, P < 0·05 versus saline- or pCAGGS-treated mice) (Fig. 2a). Similar to the DAI, body weight loss was inhibited in mice treated with MIF/TTX DNA vaccine compared with the mice treated with saline or pCAGGS vector 7 days after initial DSS administration (−3·2 ± 0·6%, −24·0 ± 4·3% and −15·2 ± 6·2%, respectively, P < 0·05 versus saline- or pCAGGS-treated mice) (Fig. 2b).
Fig. 2.

(a) Scores of the disease activity index (DAI) in dextran sulphate sodium (DSS)-induced colitis in mice. (b) Body weight changes of mice. Mice were subjected to administration of migration inhibitory factor (MIF)/tetanus toxoid (TTX) DNA vaccine. For control, mice were provided with the saline or pCAGGS vector alone. Eight weeks after the immunization, all mice were administered by 3·0% DSS. DAI was examined by an observer blind to the experimental protocol. Percentage in body weight change at day 7 was calculated in comparison with body weight at day 0 (100%). (c) Effect of MIF/TTX DNA vaccine on shortening of colon length in DSS-induced colitis in mice. Colon length was measured by an observer blind to the experimental protocol. Results are given as means ± standard error. Statistical significance was assessed compared with saline and DSS-treated mice. *P < 0·05; n = 5 in each group. Normal: non-treated mice. Saline: saline-treated mice given 3% DSS for 7 days. pCAGGS: pCAGGS-treated mice given 3% DSS for 7 days. MIF/TTX: MIF/TTX DNA vaccine-treated mice given 3% DSS for 7 days.
Shortening of colon length is useful for the assessment of severity of DSS-induced colitis. Non-treated mice had a colon length of 9·2 ± 0·1 cm (Fig. 2c). Conversely, saline-treated mice and pCAGGS-treated mice had colon lengths of 6·5 ± 0·5 and 6·1 ± 0·3 cm when administered 3·0% DSS solution for 7 days (Fig. 2c). In contrast, immunization with MIF/TTX DNA vaccine inhibited the DSS-induced colon shortening (8·0 ± 0·4 cm, P < 0·05 versus saline treatment and P < 0·01 versus pCAGGS treatment) (Fig. 2c).
Immunization with MIF/TTX DNA vaccine decreased significantly the histological scores of tissue damage and extent of lesions in colon tissues compared with those saline-treated mice and pCAGGS-treated mice (tissue damage scores: 2·0 ± 0·4, 4·2 ± 0·4 and 3·8 ± 0·6, respectively; extent of lesion: 1·6 ± 0·3, 3·6 ± 0·2 and 3·4 ± 0·5, respectively) (Fig. 3). Colon tissues of mice treated with pCAGGS or saline showed marked pathological changes, including infiltration of inflammatory cells with epithelial cell destruction (Fig. 4b and c). In contrast, histological sections of colon tissues of MIF/TTX DNA-vaccinated mice showed mild infiltration of inflammatory cells without epithelial cell destruction (Fig. 4d).
Fig. 3.
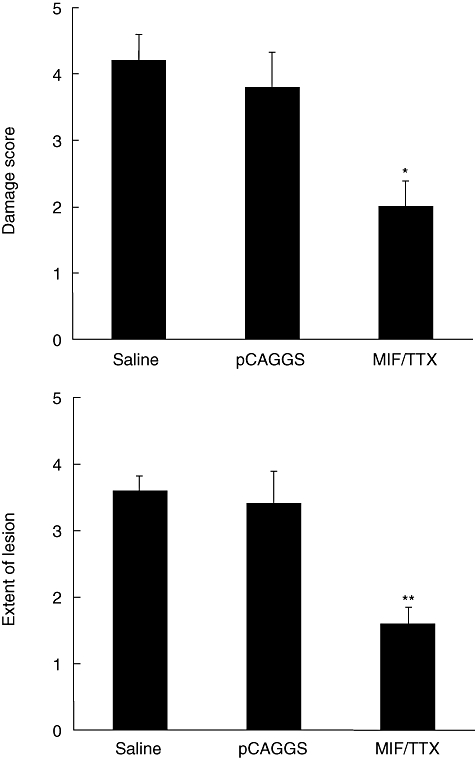
Migration inhibitory factor (MIF)/tetanus toxoid (TTX) DNA vaccine treatment ameliorates histological findings with colitis in mice. Histological score was determined on a scale of 0–5 as explained in Materials and methods. Results are given as means ± standard error. Statistical significance was assessed compared with saline and dextran sulphate sodium (DSS)-treated mice on day 7. *P < 0·05, **P < 0·01; n = 5 in each group. Normal: non-treated mice. Saline: saline-treated mice given 3% DSS for 7 days. pCAGGS: pCAGGS-treated mice given 3% DSS for 7 days. MIF/TTX: MIF/TTX DNA vaccine-treated mice given 3% DSS for 7 days.
Fig. 4.
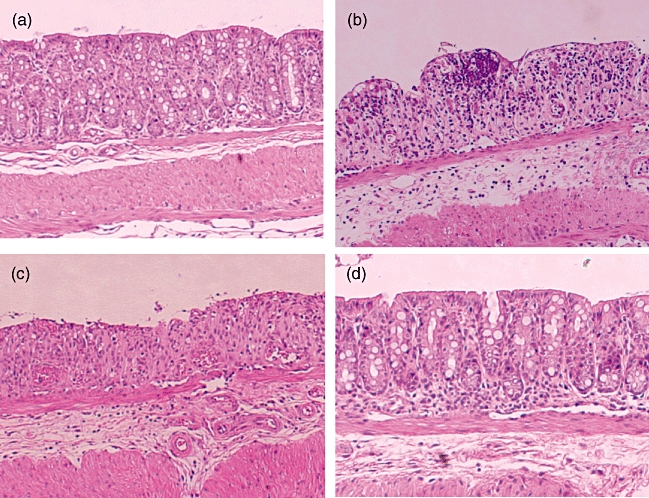
Histological findings of colon in mice. (a) The section of the colon from non-treated mice without dextran sulphate sodium (DSS) treatment shows normal crypts and no inflammatory infiltrate. (b) The sections of saline-treated mice and (c) pCAGGS-treated mice show crypt loss, destruction of the epithelial cells and severe inflammatory infiltrate at 7 days after DSS treatment. (d) Migration inhibitory factor (MIF)/tetanus toxoid (TTX)-vaccinated mice show no crypt damage with slight infiltration of inflammatory cells at 7 days after DSS treatment. Original magnification × 100. Representative pictures are shown. Similar appearances were observed in the colons of the other mice.
MIF/TTX DNA vaccination suppresses the levels of inflammatory mediators in DSS-induced colitis
MPO is an enzyme produced mainly by polymorphonuclear leucocytes and is associated with granulocyte contents of tissues. The levels of MPO activity in colon of mice treated with saline or pCAGGS were increased 7 days after initial administration of DSS (3·0 ± 0·4 and 3·4 ± 0·7 U/g tissue, respectively) (Fig. 6). Immunization with MIF/TTX DNA vaccine significantly suppressed the increase in the level of MPO activity in mice colons compared with saline- or pCAGGS-treatment 7 days after initial DSS administration (1·6 ± 0·4 U/g tissue, P < 0·05 versus saline- or pCAGGS-treated mice, respectively) (Fig. 5).
Fig. 6.
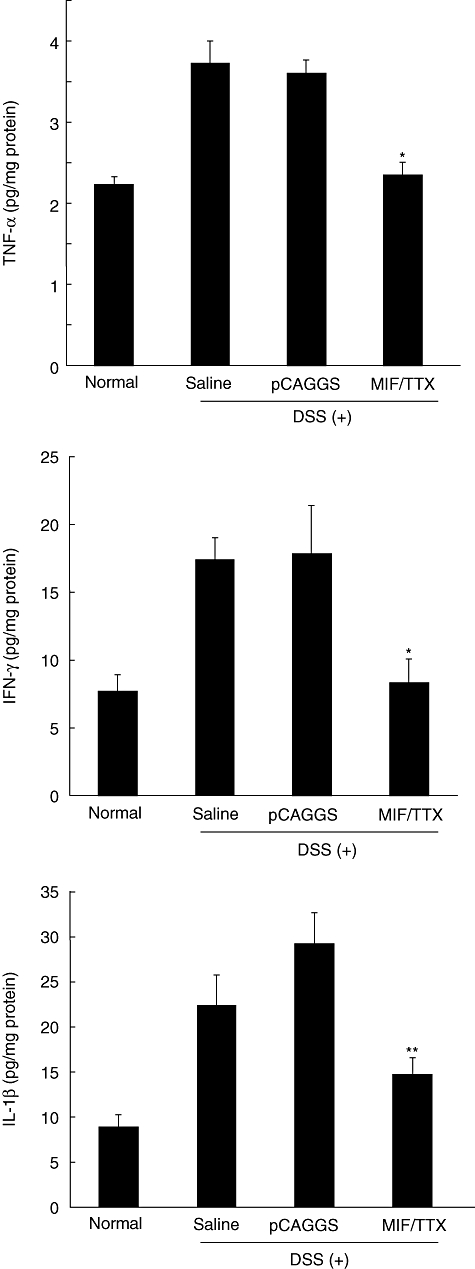
Migration inhibitory factor (MIF)/tetanus toxoid (TTX) DNA vaccine treatment inhibits increase in proinflammatory cytokines in colon of mice with dextran sulphate sodium (DSS)-induced colitis. Results are given as means ± standard error. Statistical significance was assessed compared with saline and DSS-treated mice. *P < 0·05, **P < 0·01; n = 4–5 in each group. Normal: non-treated mice. Saline: saline-treated mice given 3% DSS for 7 days. pCAGGS: pCAGGS-treated mice given 3% DSS for 7 days. MIF/TTX: MIF/TTX DNA vaccine-treated mice given 3% DSS for 7 days.
Fig. 5.

Treatment with migration inhibitory factor (MIF)/tetanus toxoid (TTX) DNA vaccine inhibits increase in myeloperoxidase (MPO) activity in the colon of mice exposed to 3% DSS for 7 days. MPO level was determined as described in the Materials and methods. Results are given as means ± standard error. Statistical significance was assessed compared with saline and dextran sulphate sodium (DSS)-treated mice. *P < 0·05; n = 5 in each group. Normal: non-treated mice. Saline: saline-treated mice given 3% DSS for 7 days. pCAGGS: pCAGGS-treated mice given 3% DSS for 7 days. MIF/TTX: MIF/TTX DNA vaccine-treated mice given 3% DSS for 7 days.
Conversely, the contents of TNF-α, IFN-γ and IL-1β in colon were increased in mice treated with saline or pCAGGS 7 days after initial DSS administration (TNF-α: 3·7 ± 0·3 and 3·6 ± 0·7 pg/mg protein, IFN-γ: 17·4 ± 1·6 and 17·8 ± 3·6 pg/mg protein, IL-1β: 22·4 ± 3·4 and 29·1 ± 3·5 pg/mg protein, respectively), whereas the levels of these cytokines were significantly lower in mice treated with MIF/TTX DNA vaccine (TNF-α: 2·4 ± 0·2, IFN-γ: 8·3 ± 1·7, IL-1β: 14·7 ± 2·0 pg/mg protein) (Fig. 6).
MIF/TTX DNA vaccination reduces F4/80-macrophages infiltrating and MIF expression in colonic mucosa in mice with DSS-induced colitis
Immunohistochemical analysis of F4/80-positive cells was performed in the colon tissues of mice. F4/80-positive staining cells were found in the colons of non-treated mice (18 ± 2 cells/mm2) (Figs 7a and 8). The number of these positive cells increased markedly in the lamina propria of colons in saline- or pCAGGS-treated mice 7 days after initial DSS administration (76 ± 8 cells/mm2 and 74 ± 7 cells/mm2) (Figs 7b,c and 8). Conversely, there were fewer F4/80-positive cells in the colons of MIF/TTX DNA-vaccinated mice given DSS (45 ± 3 cells/mm2, P < 0·01 versus saline- or pCAGGS-treated mice) (Figs 7d and 8). Additionally, the expression of MIF was enhanced in the epithelial cells and immune cells of colons from saline- or pCAGGS-treated mice with DSS-induced colitis (Fig. 9b and c). In MIF/TTX DNA-vaccinated mice, the expression of MIF in colonic mucosa was attenuated compared with saline- or pCAGGS-treated mice with DSS-induced colitis (Fig. 9d).
Fig. 7.
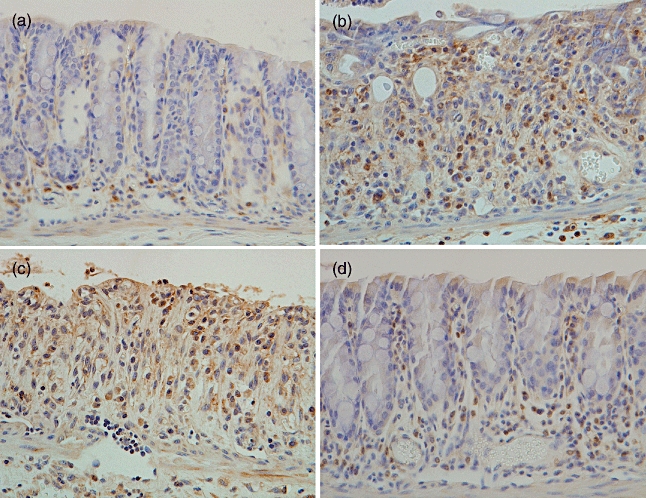
Immunohistochemical analysis for F4/80 in the colon. F4/80-positive staining was seen mainly in the mononuclear cells infiltrating the colon mucosa. (a) Non-treated mice. (b) Saline-treated mice given 3% dextran sulphate sodium (DSS) for 7 days. (c) pCAGGS-treated mice given 3% DSS for 7 days. (d) Migration inhibitory factor (MIF)/tetanus toxoid (TTX) DNA-vaccinated mice given 3% DSS for 7 days. Original magnification × 200. Representative pictures are shown. Similar appearances were observed in the colons of the other mice.
Fig. 8.
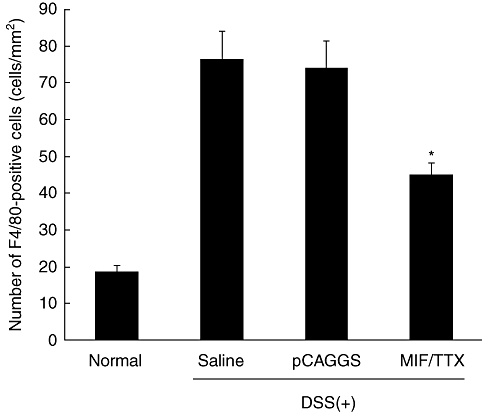
Number of infiltrating F4/80-positive cells. The number of cells with F4/80-positive staining was counted in three areas of colonic mucosa and evaluated in five mice in each group. Average numbers of cell counts were caluculated. Statistical significance was assessed compared with saline and dextran sulphate sodium (DSS)-treated mice. *P < 0·01. Normal: non-treated mice. Saline: saline-treated mice given 3% DSS for 7 days. pCAGGS: pCAGGS-treated mice given 3% DSS for 7 days. migration inhibitory factor (MIF)/tetanus toxoid (TTX): MIF/TTX DNA vaccine-treated mice given 3% DSS for 7 days.
Fig. 9.
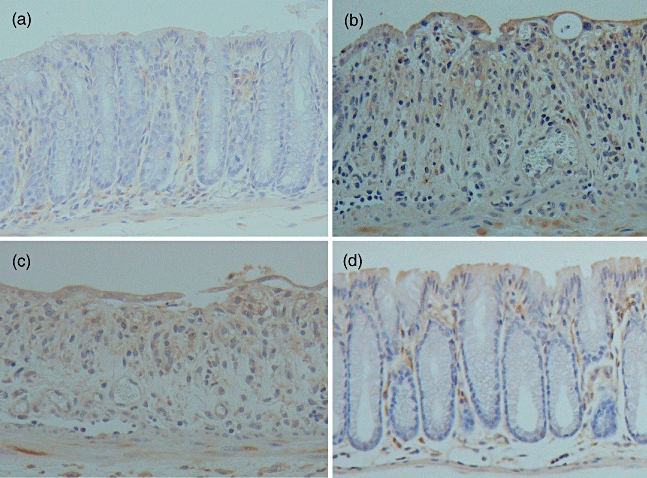
Immunohistochemical analysis for migration inhibitory factor (MIF) in the colon. (a) Non-treated mice. (b) Saline-treated mice given 3% dextran sulphate sodium (DSS) for 7 days. (c) pCAGGS-treated mice given 3% DSS for 7 days. (d) MIF/tetanus toxoid (TTX) DNA-vaccinated mice given 3% DSS for 7 days. Original magnification × 200. Representative pictures are shown. Similar appearances were observed in the colons of the other mice.
Discussion
MIF is a key up-stream regulator of inflammation and immune response, and neutralization of MIF bioactivity results in amelioration of disease activity in animal disease models [7–9,23–25]. These findings led us to speculate that inhibition of MIF bioactivity by anti-MIF autoantibody induced by MIF/TTX DNA vaccine might be effective for the treatment of colitis.
Administration of DSS has been shown to cause bloody diarrhoea, weight loss, shortening of the colon and mucosal damage with inflammatory cell infiltration in the colon [12,19,20]. Although the mechanism behind DSS-induced colitis is not well understood, we have demonstrated previously that MIF is essential for the induction of DSS colitis in mice [12,16,26]. Based on our previous investigations, it is thought that MIF is a useful target for the development of a method for treatment of colitis. In this study, clinical findings and shortening of the colon was suppressed markedly by MIF/TTX DNA vaccination. Furthermore, treatment with MIF/TTX DNA vaccine ameliorated remarkably the histological features of colitis in mice. Interestingly, these findings are comparable to the results of our previous study showing that an anti-MIF antibody effectively suppressed DSS-induced colitis in mice [12]. The results of this study suggest that MIF/TTX DNA vaccine is useful and cost-effective for the induction and maintenance of remission in patients with IBDs.
Local MPO activity increases in the colon of mice with DSS-induced colitis [22]. MPO activity is useful for the assessment of neutrophil accumulation in tissues because MPO activity is consistent with granulocyte, but not other immunocyte, content in the tissues [27]. Our previous studies show that MPO activity is related positively to MIF activity in mice with DSS-induced colitis [22,26]. Consistent with these data, in this study MIF/TTX DNA vaccination inhibited the increase in MPO activity in mice with DSS-induced colitis.
MIF enhanced infiltration of macrophages and monocytes in the inflammatory lesion. We have shown previously marked infiltration of F4/80-positive staining cells reflecting macrophages in the livers of mice with acute hepatitis induced by bacille Calmette–Guerin and lipopolysaccharide [25]. Moreover, neutralization by anti-MIF antibody suppressed infiltration of F4/80-positive staining cells into the livers of mice with acute hepatitis. In addition, we have shown previously that infiltration of F4/80-positive staining cells into colons was suppressed in MIF-deficient mice [26]. Consistent with our previous findings, in this study we have demonstrated that infiltration of F4/80-positive staining cells into colonic mucosa was suppressed by MIF/TTX DNA vaccination in mice with DSS-induced colitis. Moreover, we have demonstrated that MIF/TTX DNA vaccination reduces MIF expression in the epithelial and immune cells of colon. These findings suggest that autoantibody induced by MIF/TTX DNA vaccine reduces infiltration of MIF positive stained cells into the colon tissue. Further study is needed to clarify these issues. Conversely, the levels of proinflammatory cytokines were increased in colons of mice with DSS-induced colitis [12,22]. In this study, consistent with these findings, we found that the levels of TNF-α, IFN-γ and IL-1β were reduced by treatment with MIF/TTX vaccine in colons of mice with DSS-induced colitis. Taken together, these results indicate that MIF/TTX DNA vaccination suppresses the up-regulation of inflammatory mediators such as MPO activity, macrophage infiltration and production of cytokines in mice with DSS-induced colitis.
The DSS model, however, may not be the most suitable for evaluation of the therapeutic effect of MIF-DNA vaccination after the establishment of chronic colitis. Moreover, the precise mechanism by which MIF/TTX DNA vaccination reduces colitis has not been resolved. In this study, we did not examine the effect of MIF/TTX DNA vaccination on acquired immune response in colitis. To resolve this problem, the use of other experimental colitis models such as IL-10-deficient mouse models, trinitrobenzene sulphonate-induced colitis and CD45RBhigh transfer-induced colitis should be considered. Experiments using such model animals are under way in our laboratory. In addition, there is an important problem in the duration between vaccination and induction of colitis. In this study, we tested the effect of MIF/TTX DNA vaccine on experimental colitis 8 weeks after inoculation of MIF/TTX DNA vaccine because MIF-reactive antibody was increased significantly in the plasma of mice 8 weeks after vaccination. Further study is needed.
Treatment with Th-modified vaccine has therapeutic potential against pathogenic self-proteins. By incorporating a promiscuous foreign Th epitope into self-proteins, thus providing sufficient T cell help, immunological tolerance to self-proteins can be bypassed [28–30]. DNA vaccine represents a novel means of expressing antigens in vivo for the generation of antibodies and cell-mediated immune responses. Thus, the efficacy of DNA vaccine in preclinical animal models has been well documented [31]. The advantage of a DNA vaccine against a recombinant protein is the simplicity of construction of vectors for DNA vaccination and the generic purification technology for plasmid DNA. This approach increases the speed and decreases the effort required to develop a novel protein vaccine, providing a tool for rapid screening of potential protein immunogens and making effective vaccines available more widely.
It has been reported that naked cDNA encoding C-C-chemokines without insertion of Th for eliciting autoantibodies prevents experimental adjuvant encephalitis [32]. In addition, the oligonucleotide sequences included in the plasmid sequences, such as unmethylated cytosine–guanine dinucleotide (CpG) motifs, act as an immune adjuvant, accelerating antigen-specific immune responses [33]. In contrast, Hertz et al. have reported that unmodified murine wild-type IL-5 cDNA fails to elicit antibodies against IL-5 [34]. Our previous and current studies also show that wild-type murine MIF cDNA vaccination failed to elicit antibodies against MIF [17]. Thus, the development of a modified DNA vaccine is useful for the induction of autoantibodies against cytokines. Proper selection of a Th epitope and its insertion into proper sites of cDNA may be a key to the success of treatment with DNA vaccine.
The delivery system is important for the success of vaccination. Electroporation is a promising method to enhance DNA delivery and DNA vaccine potency. Selby et al. have shown a 7·3-fold increase of luciferase expression and eight- to 20-fold enhanced antibody titres in mice by electroporation compared to simple intramuscular injection of plasmids without electric currency [35]. Thus, in this study, we adopted this method for inoculation of Th-modified MIF-DNA vaccine.
Active immunization against proinflammatory cytokines using vaccine inhibits the activity of these cytokines for a long time; thus, we were concerned about adverse effects such as infection and carcinogenesis. In our preliminary safety studies, there were no significant differences between unvaccinated and MIF/TTX DNA-vaccinated mice under normal conditions (data not shown). Further study is needed to confirm the safety of MIF/TTX vaccine.
Previously, the failure of clinical application has been shown in developed vaccines. Girard et al. have reported that the vaccine against human immunodeficiency virus (HIV) prevents chimpanzees, but not humans, from HIV [36]. Similar results were seen in vaccine against human papilloma virus [37]. Thus, although we found an effect of active vaccination against proinflammatory cytokines in inflammatory diseases in this and previous studies, it may be difficult to apply Th-modified MIF DNA vaccine to human inflammatory diseases. Additional studies are needed for the development of Th-modified MIF vaccine for clinical application.
In conclusion, in this study we provide evidence that Th-modified MIF-DNA vaccine is an effective method for the treatment of colitis in mice. Active vaccination against MIF has a potential therapeutic approach for the treatment of IBDs.
Acknowledgments
This research was supported partly by a Grant-in-Aid for research from the Japanese Ministry of Health, Welfare and Labor (H. T. and M. A.), and partly by Grant-in-Aid from Ministry of Science and Education (18590665) (T. O.), and by the Program for Promotion of Fundamental Studies in Health Science of the National Institutute of Biomedical Inovation (NIBIO) (J. N.).
Disclosure
There is no conflict of interest.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92(Suppl 12):S5–11. [PubMed] [Google Scholar]
- 3.Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–98. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 4.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 5.van Dullemen HM, van Deventer SJ, Hommes DW, et al. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–35. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 6.Clark M. Antibody humanization: a case of the ‘Emperor's new clothes’? Immunol Today. 2000;21:397–402. doi: 10.1016/s0167-5699(00)01680-7. [DOI] [PubMed] [Google Scholar]
- 7.Bucala R. MIF rediscovered: pituitary hormone and glucocorticoid-induced regulator of cytokine production. FASEB J. 1996;7:19–24. doi: 10.1016/1359-6101(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 8.Nishihira J. Macrophage migration inhibitory factor (MIF): its essential role in the immune system and cell growth. J Interferon Cytokine Res. 2000;20:751–62. doi: 10.1089/10799900050151012. [DOI] [PubMed] [Google Scholar]
- 9.Bucala R, Lolis E. Macrophage migration inhibitory factor: a critical component of autoimmune inflammatory diseases. Drug News Perspect. 2005;18:417–26. doi: 10.1358/dnp.2005.18.7.939345. [DOI] [PubMed] [Google Scholar]
- 10.Murakami H, Akbar SM, Matsui H, Onji M. Macrophage migration inhibitory factor in the sera and at the colonic mucosa in patients with ulcerative colitis: clinical implications and pathogenic significance. Eur J Clin Invest. 2001;31:337–43. doi: 10.1046/j.1365-2362.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- 11.de Jong YP, Abadia-Molina AC, Satoskar AR, et al. Development of chronic colitis is dependent on the cytokine MIF. Nat Immunol. 2001;2:1061–6. doi: 10.1038/ni720. [DOI] [PubMed] [Google Scholar]
- 12.Ohkawara T, Nishihira J, Takeda H, et al. Amelioration of dextran sulfate sodium-induced colitis by anti-macrophage migration inhibitory factor antibody in mice. Gastroenterology. 2002;123:256–70. doi: 10.1053/gast.2002.34236. [DOI] [PubMed] [Google Scholar]
- 13.Murakami H, Akbar SM, Matsui H, Horiike N, Onji M. Macrophage migration inhibitory factor activates antigen-presenting dendritic cells and induces inflammatory cytokines in ulcerative colitis. Clin Exp Immunol. 2002;128:504–10. doi: 10.1046/j.1365-2249.2002.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nohara H, Okayama N, Inoue N, et al. Association of the −173 G/C polymorphism of the macrophage migration inhibitory factor gene with ulcerative colitis. J Gastroenterol. 2004;39:242–6. doi: 10.1007/s00535-003-1284-7. [DOI] [PubMed] [Google Scholar]
- 15.Dambacher J, Staudinger T, Seiderer J, et al. Macrophage migration inhibitory factor (MIF) −173G/C promoter polymorphism influences upper gastrointestinal tract involvement and disease activity in patients with Crohn's disease. Inflamm Bowel Dis. 2007;13:71–82. doi: 10.1002/ibd.20008. [DOI] [PubMed] [Google Scholar]
- 16.Ohkawara T, Nishihira J, Ishiguro Y, et al. Resistance to experimental colitis depends on cytoprotective heat shock proteins in macrophage migration inhibitory factor null mice. Immunol Lett. 2006;107:148–54. doi: 10.1016/j.imlet.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Onodera S, Ohshima S, Toyama H, et al. A novel DNA vaccine targeting macrophage migration inhibitory factor protects joints from inflammation and destruction in murine models of arthritis. Arthritis Rheum. 2007;56:521–30. doi: 10.1002/art.22407. [DOI] [PubMed] [Google Scholar]
- 18.Hamasaka A, Abe R, Koyama Y, et al. DNA vaccination against macrophage migration inhibitory factor improves atopic dermatitis in murine models. J Allergy Clin Immunol. 2009;124:90–9. doi: 10.1016/j.jaci.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 20.Cooper HS, Murthy SNS, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–50. [PubMed] [Google Scholar]
- 21.Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998;16:867–70. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 22.Ohkawara T, Miyashita K, Nishihira J, et al. Transgenic over-expression of macrophage migration inhibitory factor renders mice markedly more susceptible to experimental colitis. Clin Exp Immunol. 2005;140:241–8. doi: 10.1111/j.1365-2249.2005.02771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makita H, Nishimura M, Miyamoto K, et al. Effect of anti-macrophage migration inhibitory factor antibody on lipopolysaccharade- induced pulmonary neutrophil accumulation. Am J Respir Crit Care Med. 1998;158:573–9. doi: 10.1164/ajrccm.158.2.9707086. [DOI] [PubMed] [Google Scholar]
- 24.Calandra T, Echtenacher B, Roy DL, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–70. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi S, Nishihira J, Watanabe S, Todo S. Prevention of lethal acute hepatic failure by antimacrophage migration inhibitory factor antibody in mice treated with bacille Calmette–Guerin and lipopolysaccharide. Hepatology. 1999;29:1752–9. doi: 10.1002/hep.510290610. [DOI] [PubMed] [Google Scholar]
- 26.Ohkawara T, Mitsuyama K, Takeda H, Asaka M, Fujiyama Y, Nishihira J. Lack of macrophage migration inhibitory factor suppresses innate immune response in murine dextran sulfate sodium-induced colitis. Scand J Gastroenterol. 2008;43:1497–504. doi: 10.1080/00365520802273017. [DOI] [PubMed] [Google Scholar]
- 27.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 28.Dalum I, Jensen MR, Hindersson P, Elsner HI, Mouritsen S. Breaking of B cell tolerance toward a highly conserved self protein. J Immunol. 1996;157:4796–804. [PubMed] [Google Scholar]
- 29.Dalum I, Jensen MR, Gregorius K, Thomasen CM, Elsner HI, Mouritsen S. Induction of cross-reactive antibodies against a self protein containing a foreign T helper epitope. Mol Immunol. 1997;34:1113–20. doi: 10.1016/s0161-5890(97)00147-8. [DOI] [PubMed] [Google Scholar]
- 30.Dalum I, Butler DM, Jensen MR, et al. Therapeutic antibodies elicited by immunization against TNF-α. Nat Biotechnol. 1999;17:666–9. doi: 10.1038/10878. [DOI] [PubMed] [Google Scholar]
- 31.Donnelly JJ, Ulmer JB, Liu MA. DNA vaccines. Life Sci. 1997;60:163–72. doi: 10.1016/s0024-3205(96)00502-4. [DOI] [PubMed] [Google Scholar]
- 32.Youssef S, Wildbaum G, Maor G, et al. Long-lasting protective immunity to experimental autoimmune encephalomyelitis following vaccination with naked DNA encoding C-C chemokines. J Immunol. 1998;161:3870–9. [PubMed] [Google Scholar]
- 33.Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol Rev. 2004;99:201–16. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- 34.Hertz M, Mahalingam S, Dalum I, et al. Active vaccination against IL-5 bypasses immunological tolerance and ameliorates experimental asthma. J Immunol. 2001;167:3792–9. doi: 10.4049/jimmunol.167.7.3792. [DOI] [PubMed] [Google Scholar]
- 35.Selby M, Goldbeck C, Pertile T, Walsh R, Ulmer J. Enhancement of DNA vaccine potency by electroporation in vivo. J Biotechnol. 2000;83:147–52. doi: 10.1016/s0168-1656(00)00308-4. [DOI] [PubMed] [Google Scholar]
- 36.Girard M, Yue L, Barré-Sinoussi F, et al. Failure of a human immunodeficiency virus type 1 (HIV-1) subtype B-derived vaccine to prevent infection of chimpanzees by an HIV-1 subtype E strain. J Virol. 1996;70:8229–33. doi: 10.1128/jvi.70.11.8229-8233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandepapeliere P, Barrasso R, Meijer CJ, et al. Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J Infect Dis. 2005;192:2099–107. doi: 10.1086/498164. [DOI] [PubMed] [Google Scholar]


