Abstract
We analyzed mortality among 201 patients with AIDS and tuberculosis in Haiti. Patients who received a diagnosis of tuberculosis during the first 3 months after the initiation of antiretroviral therapy were 3.25 times more likely to die than were other patients with AIDS and tuberculosis. Failure to recognize active tuberculosis at initiation of antiretroviral therapy leads to increased mortality.
The incidence of tuberculosis (TB) is high among patients during the 3-month period after initiation of antiretroviral therapy (ART) in resource-poor settings because of subclinical TB that is unmasked with reconstitution of the immune system and because of more-intensive clinical surveillance and care [1, 2]. TB often goes undetected before ART initiation because of the low sensitivity of available diagnostic tests and the nonspecific symptoms of patients who have advanced AIDS [3]. We hypothesized that these patients with subclinical TB at ART initiation have high mortality rates because of the delays in diagnosis of TB and in initiation of anti-TB therapy. Therefore, we conducted a study to compare the mortality of patients who received a diagnosis of TB during the first 3 months after ART initiation with the mortality of other patients with AIDS and active TB.
METHODS
Study setting
The study was conducted at the Groupe Haitien d’Etude du Sarcome de Kaposi et des Infections Opportunistes (GHESKIO) Center in Port-au-Prince, Haiti. Three percent of the adult population of Haiti is estimated to be infected with HIV; the prevalence of TB is 405 cases per 100,000 people [4]. Patients with AIDS at GHESKIO are treated according to the recommendations of the World Health Organization (WHO) [5]. ART treatment protocols and outcomes have been described elsewhere [6]. All patients who present with cough are screened for TB with acid-fast bacilli testing of 3 sputum smears and examination of a chest radiograph. If screening findings are negative but TB is suspected, mycobacterial culture of sputum is performed on solid media.
Patients with AIDS and active TB receive an efavirenz-based ART regimen and the standard 6-month TB treatment protocol recommended by the WHO (isoniazid, rifampin, ethambutol, and pyrazinamide daily for 2 months followed by isoniazid and rifampin daily for 4 months). ART is initiated 2–8 weeks after starting TB therapy for patients with CD4 cell counts <200 cells/mm3; for patients with advanced AIDS or CD4 cell counts <50 cells/mm3, ART is started at the same time as TB therapy [5]. This is earlier than some programs, which defer ART until patients receiving a TB regimen are stable.
Study population
We included all patients aged ≥13 years who received a diagnosis of active TB from 1 March 2003 through 30 June 2006 and who were already receiving ART or met WHO criteria for initiation of ART (i.e., an AIDS-defining illness or CD4 cell count <200 cells/mm3). For the diagnosis of pulmonary TB, we used the case definition of the American Thoracic Society, as described elsewhere [7]. Diagnosis of TB requires symptoms consistent with TB and microbiologic confirmation of disease or symptoms, chest radiograph findings consistent with TB, and a positive response to anti-TB therapy [8]. The diagnosis of extrapulmonary TB was based on microbiologic confirmation and/or histopathological findings.
Patients were divided into 3 groups on the basis of the relative timing of ART initiation and TB diagnosis: (1) active TB diagnosis received before ART initiation, (2) TB diagnosis received in the first 3 months after ART initiation, and (3) TB diagnosis received after 3 months of ART.
Statistical analysis
Data were analyzed using SAS, version 9.1 (SAS Institute). The primary study outcome was death while receiving TB therapy. The χ2 test or Fisher’s exact test were used to compare proportions and the Wilcoxon rank-sum test was used to compare medians. All statistical tests were 2-sided with α =0.05. Variables with P < .10 in univariate analyses were included in the multivariate analyses. Logistic regression with a stepwise selection method was used to determine predictors of death during receipt of TB therapy. We also performed Kaplan-Meier survival analysis. Patients were followed up for 1 year after starting TB therapy, and groups were compared with use of the log-rank test. The study was approved by the institutional review boards of GHESKIO, Cornell Medical College, and Brigham and Women’s Hospital.
RESULTS
From March 2003 through June 2006, 1775 patients aged ≥13 years received a diagnosis of TB at GHESKIO; 895 (50%) of these patients were HIV infected. Of the HIV-infected patients with TB, 201 patients had AIDS at the time of TB diagnosis. One hundred four patients met the WHO criteria for initiation of ART but had not yet started ART when the TB diagnosis was made. There were 49 patients who had received ART for <3 months when the TB diagnosis was made and 48 patients who had received ART for ≥3 months at the time of TB diagnosis.
Characteristics and TB treatment outcomes of the 3 treatment groups are listed in table 1. Of note, patients who received a diagnosis within 3 months after ART initiation (the group with the highest mortality) had CD4 cell counts that were higher (median CD4 cell count, 134 cells/mm3; interquartile range, 55–223 cells/mm3) than CD4 cell counts of those with TB diagnosed before ART initiation (median CD4 cell count, 70 cells/mm3; interquartile range, 28–146 cells/mm3; P =.018).
Table 1.
Characteristics and treatment outcomes of 201 patients with AIDS and active tuberculosis (TB) in Haiti.
| Characteristic or outcome | TB diagnosed before ART (n = 104) | TB diagnosed in months 0–3 of ART (n = 49) | TB diagnosed after 3 months of ART (n = 48) | All patients with TB and AIDS (n = 201) |
|---|---|---|---|---|
| Characteristic at TB diagnosis | ||||
| Female sex | 53 (51) | 31 (63) | 28 (58) | 112 (56) |
| Age, median years | 38 | 36 | 36 | 37 |
| Weight, median pounds (IQR) | ||||
| Male patients | 114 (106–128) | 121 (101–137) | 122 (106–130) | 116 (106–130) |
| Female patients | 99 (86–109) | 101 (94–112) | 102 (96–118) | 100 (90–112) |
| CD4 cell count, median cells/mm3 (IQR) | 70 (28–146) | 134 (55–223) | 221 (150–363) | 117 (44–219) |
| Prior TB | 44 (42) | 22 (42) | 26 (54) | 92 (46) |
| Microbiologic confirmation by smear or culture | 35 (34) | 20 (41) | 16 (33) | 71 (35) |
| Extrapulmonary TB | 22 (21) | 15 (31) | 15 (31) | 52 (26) |
| TB treatment outcome | ||||
| Cured/completed treatment | 82 (79) | 34 (69) | 43 (90) | 159 (79) |
| Died | 11 (10) | 13 (27) | 1 (2) | 25 (12) |
| Failure | 3 (3) | 2 (4) | 4 (8) | 9 (5) |
| Lost to follow-up | 8 (8) | 0 (0) | 0 (0) | 8 (4) |
NOTE. Data are no. (%) of patients, unless otherwise indicated. ART, antiretroviral therapy; IQR, interquartile range.
Among the 49 patients who received a diagnosis of TB within 3 months after initiating ART, 13 (27%) died. Among the other 152 patients with AIDS and TB, 12 (8%) died (OR, 3.36; 95% CI, 1.64–6.87; P < .001). In multivariate analysis of the 201 patients, predictors of death while receiving TB treatment included receipt of TB diagnosis during the first 3 months after ART initiation (OR, 3.25; 95% CI, 1.23–8.55; P =.017) and a CD4 cell count <50 cells/mm3 (OR, 3.05; 95% CI, 1.16–8.00; P =.023). Figure 1 shows the Kaplan-Meier curves comparing survival among the 49 patients who received a TB diagnosis during the first 3 months after ART initiation with the survival among the other 152 patients with AIDS and TB.
Figure 1.
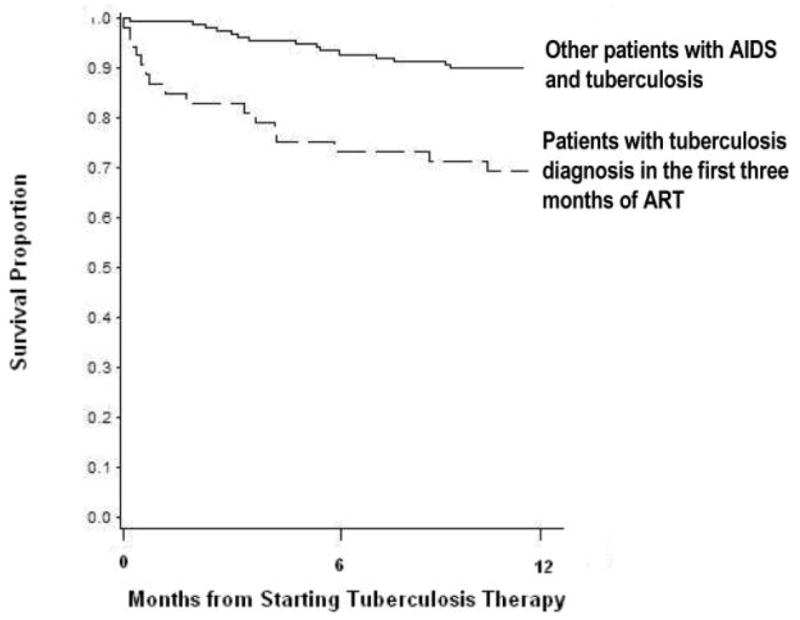
Kaplan-Meier survival estimate of patients with AIDS and active tuberculosis. Patients are stratified by whether or not they received a diagnosis of active tuberculosis in the first 3 months after initiation of antiretroviral therapy (ART). P < .001, by log-rank test.
Of the 49 patients who received a TB diagnosis during the first 3 months after ART initiation, 23 (47%) had cough at the time that ART was started; all had smear results negative for acid-fast bacilli. Four of the patients with cough had normal chest radiograph findings. Five of the patients with cough had chest radiographs that were reported to reveal bacterial pneumonia, and they were treated with ART and antibiotics while mycobacterial culture of sputum was underway. Fourteen of the patients with cough did not have a chest radiograph obtained at ART initiation because of physician or clinic error, patient nonadherence, or radiograph equipment failure on the day that the patient was seen. Twenty (41%) of the 49 patients had fever at ART initiation, and 20 (41%) had significant weight loss. Thirteen patients (27%) had no cough, no fever, and no weight loss at ART initiation.
DISCUSSION
Patients with AIDS who receive a diagnosis of TB during the first 3 months after ART initiation had a mortality rate of 27%, which was 3 times higher than that among other patients with AIDS and TB. Some patients likely had active TB at the time of ART initiation that was only diagnosed when TB was unmasked with reconstitution of the immune system or when more-intensive clinical surveillance detected the disease [9]. We believe that the delay in diagnosis and treatment contributed to the high mortality. Active TB that is not recognized when ART is started may, in part, explain the high early mortality observed among patients with AIDS in resource-poor settings [10].
Moore et al. [11] found a similar mortality rate (26%) among patients in Uganda who received a diagnosis of TB within 3 months after starting ART, but they also reported high mortality rates among other patients with AIDS and active TB. Several other studies have reported TB outcomes among HIV-infected patients in resource-constrained settings, but they did not report rates specifically for patients who received a diagnosis within 3 months after starting ART [1, 11, 12].
Assiduous attention to the diagnosis of TB is critical when starting antiretroviral therapy in resource-poor settings. We believe that all patients starting ART in resource-poor countries should undergo a complete evaluation for TB. Patients with worsening symptoms soon after initiating ART should be promptly reevaluated. In patients with any indication or risk of active TB, empiric treatment should be considered, particularly because several studies have shown that immunological and virological responses to ART are not adversely affected by TB treatment [1, 13]. Earlier ART initiation may also decrease the occurrence of subclinical TB.
In conclusion, TB is associated with a high mortality rate when it is diagnosed in the first 3 months after ART initiation. Many of these cases are attributed to subclinical TB that is not recognized at the time of ART initiation. A diagnostic work-up for TB in all patients starting ART in resource-poor settings and empiric treatment for those who are at risk for TB is recommended.
Acknowledgments
We acknowledge Sidney Atwood, Heather Ribaudo, Hana Akselrod, Maryam Shafaee, and Liberty Reforma for their generous assistance in statistical analysis, data retrieval, and editing.
Financial support. Supported in part by the National Institutes of Health, Fogarty International Center (K01TW007142, TW006901, W006896, TW00018, and Fogarty Clinical Research Scholars Program), National Institute of Allergy and Infectious Diseases (A1058257), and by the Frank Hatch Fellowship. Patient care was supported by the Global Fund to Fight AIDS, Tuberculosis and Malaria and the US President’s Emergency Plan for AIDS Relief.
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet MM, Pinoges LL, Varaine FF, et al. Tuberculosis after HAART initiation in HIV-positive patients from five countries with a high tuberculosis burden. AIDS. 2006;20:1275–9. doi: 10.1097/01.aids.0000232235.26630.ee. [DOI] [PubMed] [Google Scholar]
- 3.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–9. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Core Health Indicators. Geneva: World Health Organization; 2007. [Google Scholar]
- 5.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: towards universal access: recommendations for a public health approach. Geneva: World Health Organization; 2006. [Google Scholar]
- 6.Severe P, Leger P, Charles M, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353:2325–34. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald DW, Desvarieux M, Severe P, et al. Effect of post-treatment isoniazid on prevention of recurrent tuberculosis in HIV-1–infected individuals: a randomised trial. Lancet. 2000;356:1470–4. doi: 10.1016/S0140-6736(00)02870-1. [DOI] [PubMed] [Google Scholar]
- 8.American Thoracic Society, Centers for Disease Control, Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52(RR-11):1–77. [PubMed] [Google Scholar]
- 9.Meinties G, Lawn S, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–23. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1–infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 11.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–9. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 12.Makombe SD, Harries AD, Yu JK, et al. Outcomes of tuberculosis patients who start antiretroviral therapy under routine programme conditions in Malawi. Int J Tuberc Lung Dis. 2007;11:412–6. [PubMed] [Google Scholar]
- 13.Breen RA, Miller RF, Gorsuch T, et al. Virological response to highly active antiretroviral therapy is unaffected by antituberculosis therapy. J Infect Dis. 2006;193:1437–40. doi: 10.1086/503437. [DOI] [PubMed] [Google Scholar]


