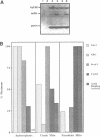
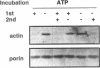
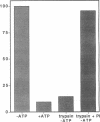
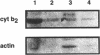
Abstract
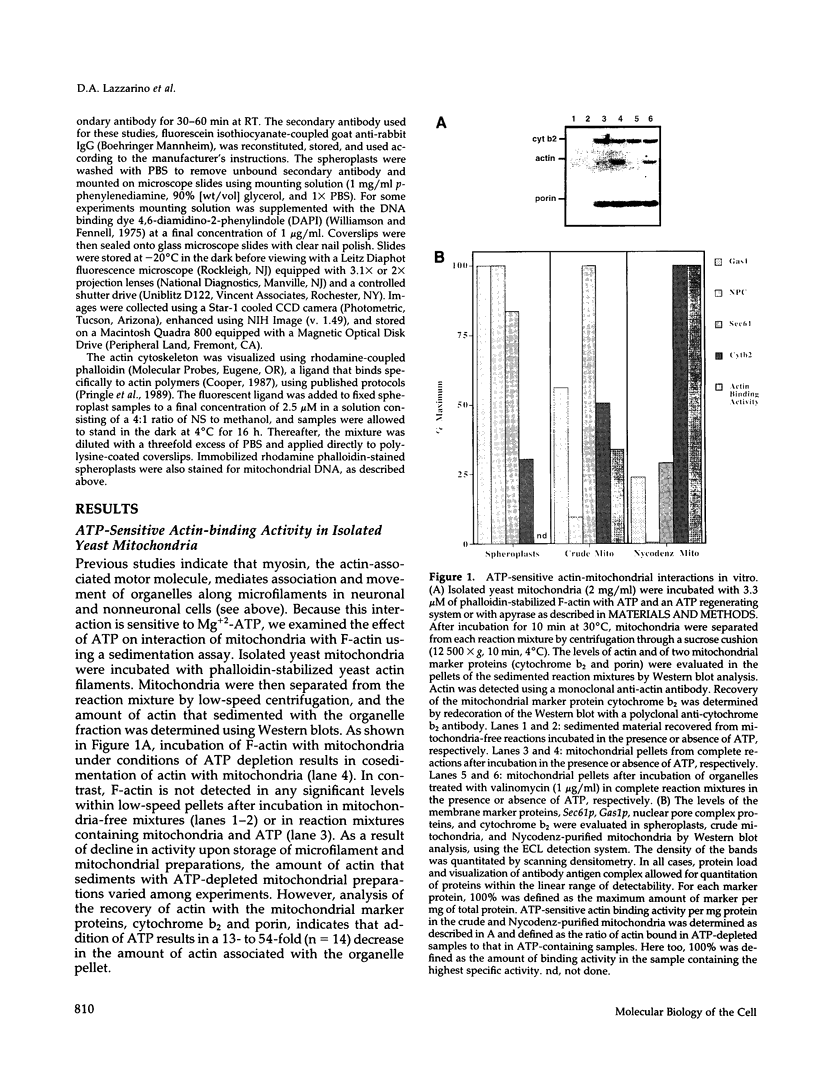
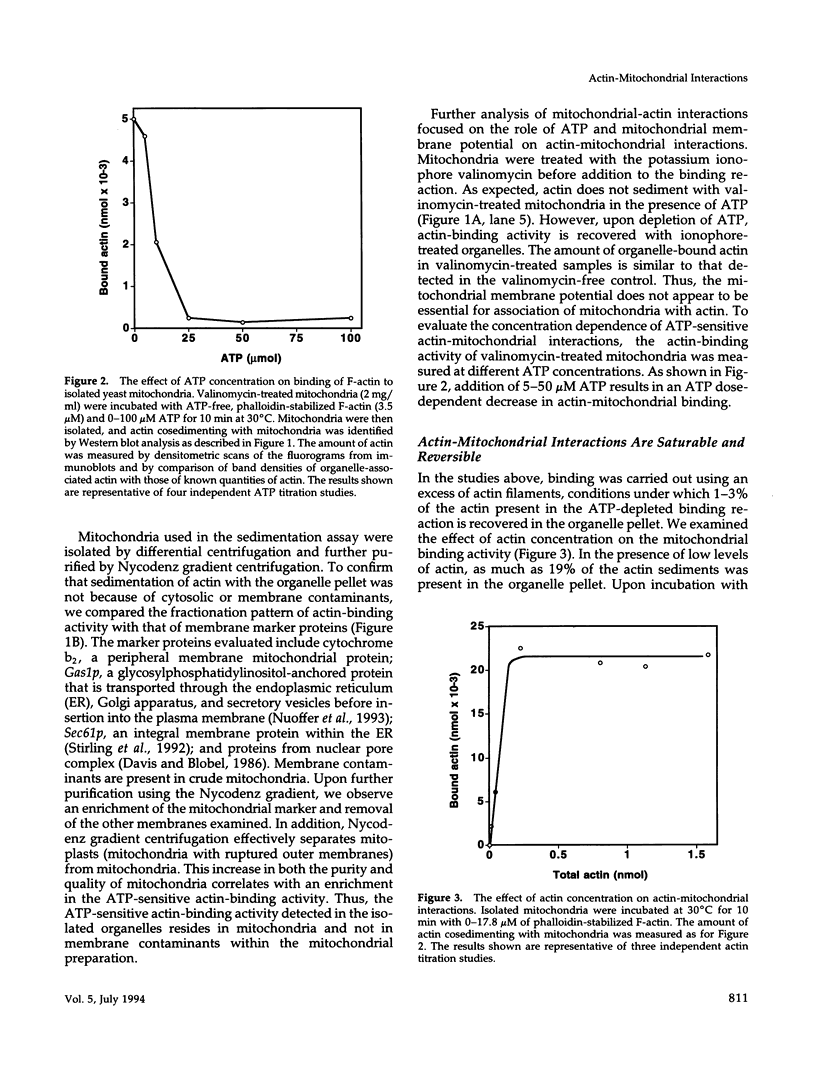
Sedimentation assays were used to demonstrate and characterize binding of isolated yeast mitochondria to phalloidin-stabilized yeast F-actin. These actin-mitochondrial interactions are ATP sensitive, saturable, reversible, and do not depend upon mitochondrial membrane potential. Protease digestion of mitochondrial outer membrane proteins or saturation of myosin-binding sites on F-actin with the S1 subfragment of skeletal myosin block binding. These observations indicate that a protein (or proteins) on the mitochondrial surface mediates ATP-sensitive, reversible binding of mitochondria to the lateral surface of microfilaments. Actin copurifies with mitochondria during subcellular fractionation and is released from the organelle upon treatment with ATP. Thus, actin-mitochondrial interactions resembling those observed in vitro may also exist in intact yeast cells. Finally, a yeast mutant bearing a temperature-sensitive mutation in the actin-encoding ACT1 gene (act1-3) displays temperature-dependent defects in transfer of mitochondria from mother cells to newly developed buds during yeast cell mitosis.
Full text
PDF


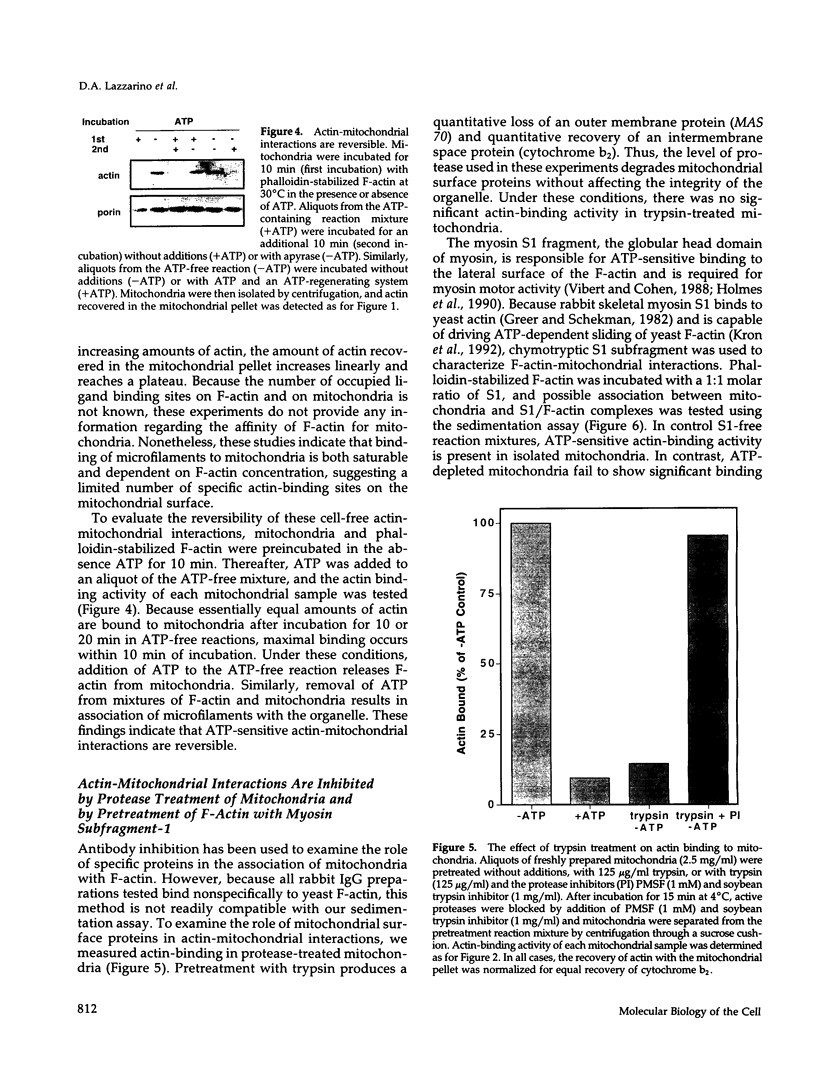
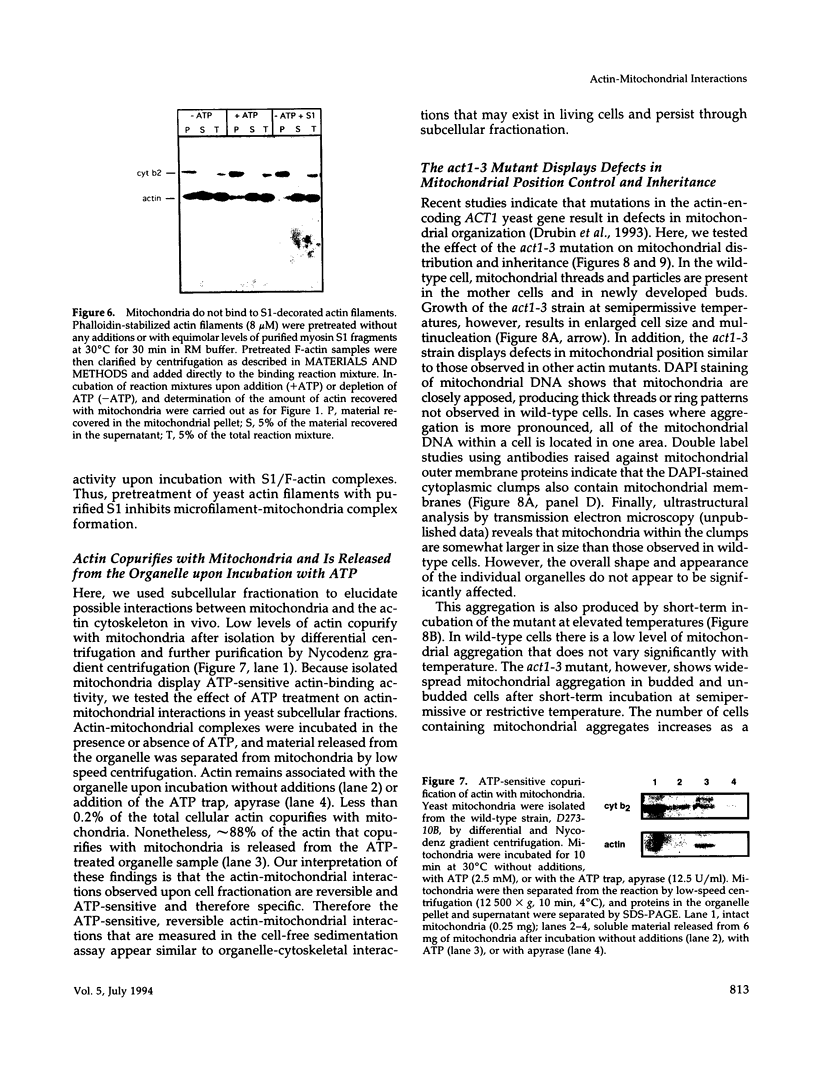
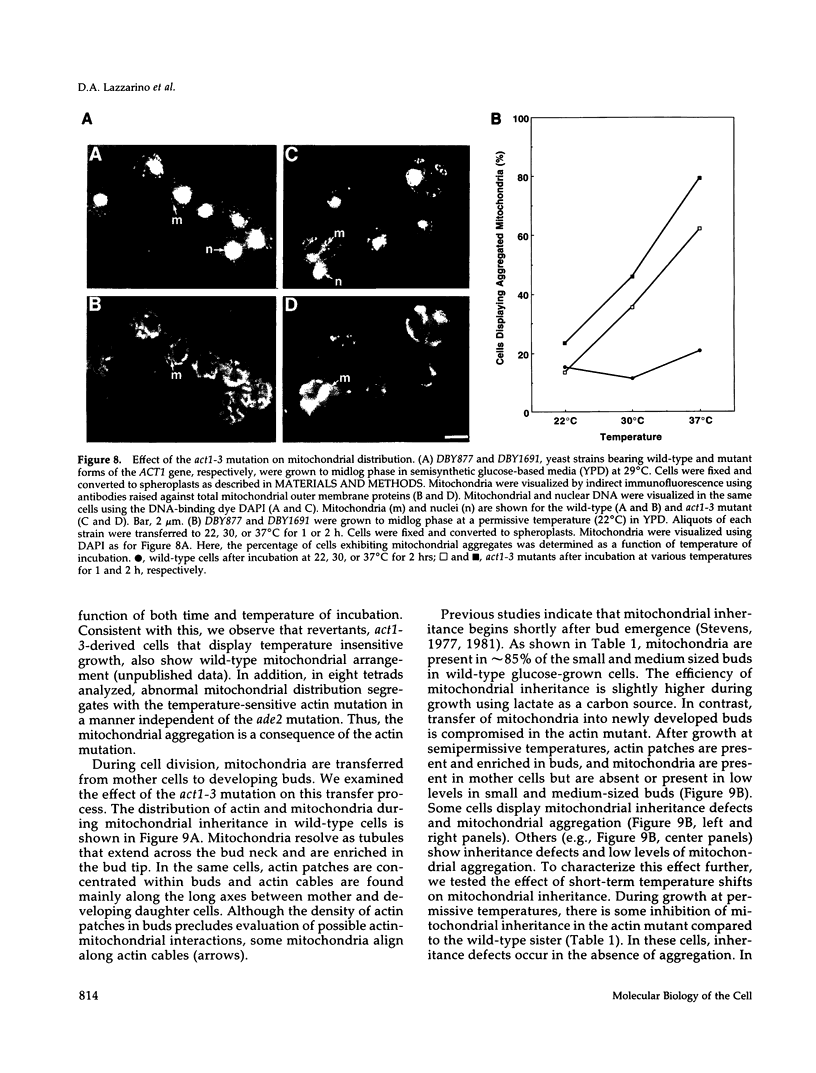
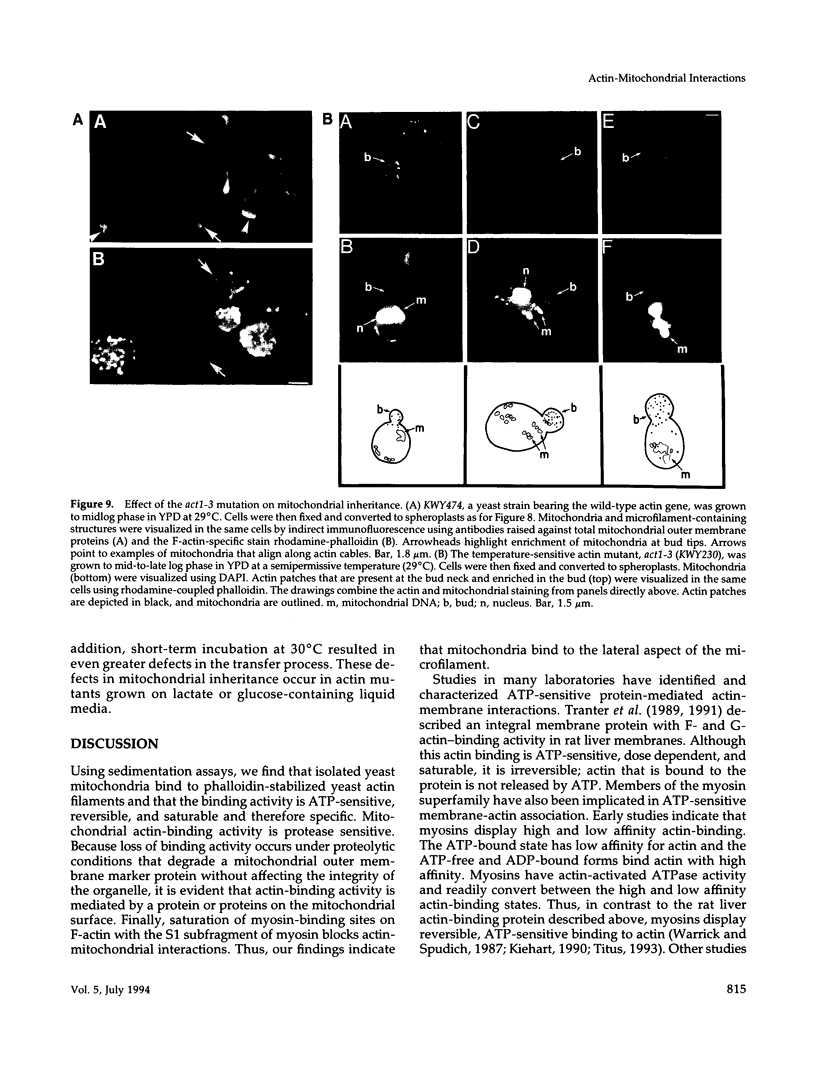



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. E., Pringle J. R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984 Mar;98(3):934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R. J., Pollard T. D. Propulsion of organelles isolated from Acanthamoeba along actin filaments by myosin-I. Nature. 1986 Aug 21;322(6081):754–756. doi: 10.1038/322754a0. [DOI] [PubMed] [Google Scholar]
- Albanesi J. P., Fujisaki H., Hammer J. A., 3rd, Korn E. D., Jones R., Sheetz M. P. Monomeric Acanthamoeba myosins I support movement in vitro. J Biol Chem. 1985 Jul 25;260(15):8649–8652. [PubMed] [Google Scholar]
- Baker D., Schekman R. Reconstitution of protein transport using broken yeast spheroplasts. Methods Cell Biol. 1989;31:127–141. doi: 10.1016/s0091-679x(08)61605-2. [DOI] [PubMed] [Google Scholar]
- Burridge K., Phillips J. H. Association of actin and myosin with secretory granule membranes. Nature. 1975 Apr 10;254(5500):526–529. doi: 10.1038/254526a0. [DOI] [PubMed] [Google Scholar]
- Chowdhury S., Smith K. W., Gustin M. C. Osmotic stress and the yeast cytoskeleton: phenotype-specific suppression of an actin mutation. J Cell Biol. 1992 Aug;118(3):561–571. doi: 10.1083/jcb.118.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Davis L. I., Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986 Jun 6;45(5):699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- Drubin D. G., Jones H. D., Wertman K. F. Actin structure and function: roles in mitochondrial organization and morphogenesis in budding yeast and identification of the phalloidin-binding site. Mol Biol Cell. 1993 Dec;4(12):1277–1294. doi: 10.1091/mbc.4.12.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin D. G., Miller K. G., Botstein D. Yeast actin-binding proteins: evidence for a role in morphogenesis. J Cell Biol. 1988 Dec;107(6 Pt 2):2551–2561. doi: 10.1083/jcb.107.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath K. R., Burgess D. R. Golgi-derived vesicles from developing epithelial cells bind actin filaments and possess myosin-I as a cytoplasmically oriented peripheral membrane protein. J Cell Biol. 1993 Jan;120(1):117–127. doi: 10.1083/jcb.120.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer C., Schekman R. Actin from Saccharomyces cerevisiae. Mol Cell Biol. 1982 Oct;2(10):1270–1278. doi: 10.1128/mcb.2.10.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolig F., Williamson R. E., Parke J., Miller C., Anderton B. H. Myosin and Ca2+-sensitive streaming in the alga Chara: detection of two polypeptides reacting with a monoclonal anti-myosin and their localization in the streaming endoplasm. Eur J Cell Biol. 1988 Oct;47(1):22–31. [PubMed] [Google Scholar]
- Hegmann T. E., Lin J. L., Lin J. J. Probing the role of nonmuscle tropomyosin isoforms in intracellular granule movement by microinjection of monoclonal antibodies. J Cell Biol. 1989 Sep;109(3):1141–1152. doi: 10.1083/jcb.109.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegmann T. E., Schulte D. L., Lin J. L., Lin J. J. Inhibition of intracellular granule movement by microinjection of monoclonal antibodies against caldesmon. Cell Motil Cytoskeleton. 1991;20(2):109–120. doi: 10.1002/cm.970200204. [DOI] [PubMed] [Google Scholar]
- Holmes K. C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Huffaker T. C., Thomas J. H., Botstein D. Diverse effects of beta-tubulin mutations on microtubule formation and function. J Cell Biol. 1988 Jun;106(6):1997–2010. doi: 10.1083/jcb.106.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Prendergast J. A., Singer R. A. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991 May;113(3):539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B. Direct visualization of organelle movement along actin filaments dissociated from characean algae. Science. 1985 Mar 15;227(4692):1355–1357. doi: 10.1126/science.4038817. [DOI] [PubMed] [Google Scholar]
- Kachar B., Reese T. S. The mechanism of cytoplasmic streaming in characean algal cells: sliding of endoplasmic reticulum along actin filaments. J Cell Biol. 1988 May;106(5):1545–1552. doi: 10.1083/jcb.106.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart D. P. Molecular genetic dissection of myosin heavy chain function. Cell. 1990 Feb 9;60(3):347–350. doi: 10.1016/0092-8674(90)90583-z. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Adams A. E. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984 Mar;98(3):922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron S. J., Drubin D. G., Botstein D., Spudich J. A. Yeast actin filaments display ATP-dependent sliding movement over surfaces coated with rabbit muscle myosin. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4466–4470. doi: 10.1073/pnas.89.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Langford G. M., Weiss D. G. Actin-dependent organelle movement in squid axoplasm. Nature. 1992 Apr 23;356(6371):722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lessard J. L. Two monoclonal antibodies to actin: one muscle selective and one generally reactive. Cell Motil Cytoskeleton. 1988;10(3):349–362. doi: 10.1002/cm.970100302. [DOI] [PubMed] [Google Scholar]
- McConnell S. J., Stewart L. C., Talin A., Yaffe M. P. Temperature-sensitive yeast mutants defective in mitochondrial inheritance. J Cell Biol. 1990 Sep;111(3):967–976. doi: 10.1083/jcb.111.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell S. J., Yaffe M. P. Intermediate filament formation by a yeast protein essential for organelle inheritance. Science. 1993 Apr 30;260(5108):687–689. doi: 10.1126/science.8480179. [DOI] [PubMed] [Google Scholar]
- McConnell S. J., Yaffe M. P. Nuclear and mitochondrial inheritance in yeast depends on novel cytoplasmic structures defined by the MDM1 protein. J Cell Biol. 1992 Jul;118(2):385–395. doi: 10.1083/jcb.118.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R., Abelson J. Isolation and sequence of the gene for actin in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3912–3916. doi: 10.1073/pnas.77.7.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985 Feb;40(2):405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Nuoffer C., Horvath A., Riezman H. Analysis of the sequence requirements for glycosylphosphatidylinositol anchoring of Saccharomyces cerevisiae Gas1 protein. J Biol Chem. 1993 May 15;268(14):10558–10563. [PubMed] [Google Scholar]
- Palmer R. E., Sullivan D. S., Huffaker T., Koshland D. Role of astral microtubules and actin in spindle orientation and migration in the budding yeast, Saccharomyces cerevisiae. J Cell Biol. 1992 Nov;119(3):583–593. doi: 10.1083/jcb.119.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Doberstein S. K., Zot H. G. Myosin-I. Annu Rev Physiol. 1991;53:653–681. doi: 10.1146/annurev.ph.53.030191.003253. [DOI] [PubMed] [Google Scholar]
- Pon L., Moll T., Vestweber D., Marshallsay B., Schatz G. Protein import into mitochondria: ATP-dependent protein translocation activity in a submitochondrial fraction enriched in membrane contact sites and specific proteins. J Cell Biol. 1989 Dec;109(6 Pt 1):2603–2616. doi: 10.1083/jcb.109.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle J. R., Preston R. A., Adams A. E., Stearns T., Drubin D. G., Haarer B. K., Jones E. W. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- Read E. B., Okamura H. H., Drubin D. G. Actin- and tubulin-dependent functions during Saccharomyces cerevisiae mating projection formation. Mol Biol Cell. 1992 Apr;3(4):429–444. doi: 10.1091/mbc.3.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben J. P., Brandt P. W., Berman M., Grundfest H. Regulation of tension in the skinned crayfish muscle fiber. I. Contraction and relaxation in the absence of Ca (pCa is greater than 9). J Gen Physiol. 1971 Apr;57(4):385–407. doi: 10.1085/jgp.57.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., Hay R., Gasser S., Daum G., Schneider G., Witte C., Schatz G. The outer membrane of yeast mitochondria: isolation of outside-out sealed vesicles. EMBO J. 1983;2(7):1105–1111. doi: 10.1002/j.1460-2075.1983.tb01553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H. Yeast endocytosis. Trends Cell Biol. 1993 Aug;3(8):273–277. doi: 10.1016/0962-8924(93)90056-7. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Spudich J. A. Movement of myosin-coated fluorescent beads on actin cables in vitro. Nature. 1983 May 5;303(5912):31–35. doi: 10.1038/303031a0. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Shortle D., Haber J. E., Botstein D. Lethal disruption of the yeast actin gene by integrative DNA transformation. Science. 1982 Jul 23;217(4557):371–373. doi: 10.1126/science.7046050. [DOI] [PubMed] [Google Scholar]
- Shortle D., Novick P., Botstein D. Construction and genetic characterization of temperature-sensitive mutant alleles of the yeast actin gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4889–4893. doi: 10.1073/pnas.81.15.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreter F., Holtzer S., Gergely J., Holtzer H. Some properties of embryonic myosin. J Cell Biol. 1972 Dec;55(3):586–594. doi: 10.1083/jcb.55.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling C. J., Rothblatt J., Hosobuchi M., Deshaies R., Schekman R. Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol Biol Cell. 1992 Feb;3(2):129–142. doi: 10.1091/mbc.3.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J., Tilney L. G., Portnoy D. A. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992 May 21;357(6375):257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- Thomas J. H., Botstein D. A gene required for the separation of chromosomes on the spindle apparatus in yeast. Cell. 1986 Jan 17;44(1):65–76. doi: 10.1016/0092-8674(86)90485-x. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., DeRosier D. J., Tilney M. S. How Listeria exploits host cell actin to form its own cytoskeleton. I. Formation of a tail and how that tail might be involved in movement. J Cell Biol. 1992 Jul;118(1):71–81. doi: 10.1083/jcb.118.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., DeRosier D. J., Weber A., Tilney M. S. How Listeria exploits host cell actin to form its own cytoskeleton. II. Nucleation, actin filament polarity, filament assembly, and evidence for a pointed end capper. J Cell Biol. 1992 Jul;118(1):83–93. doi: 10.1083/jcb.118.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus M. A. Myosins. Curr Opin Cell Biol. 1993 Feb;5(1):77–81. doi: 10.1016/s0955-0674(05)80011-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranter M. P., Sugrue S. P., Schwartz M. A. Binding of actin to liver cell membranes: the state of membrane-bound actin. J Cell Biol. 1991 Mar;112(5):891–901. doi: 10.1083/jcb.112.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranter M. P., Sugrue S. P., Schwartz M. A. Evidence for a direct, nucleotide-sensitive interaction between actin and liver cell membranes. J Cell Biol. 1989 Dec;109(6 Pt 1):2833–2840. doi: 10.1083/jcb.109.6.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert P., Cohen C. Domains, motions and regulation in the myosin head. J Muscle Res Cell Motil. 1988 Aug;9(4):296–305. doi: 10.1007/BF01773873. [DOI] [PubMed] [Google Scholar]
- Warrick H. M., Spudich J. A. Myosin structure and function in cell motility. Annu Rev Cell Biol. 1987;3:379–421. doi: 10.1146/annurev.cb.03.110187.002115. [DOI] [PubMed] [Google Scholar]
- Weber A. Parallel response of myofibrillar contraction and relaxation to four different nucleoside triphophates. J Gen Physiol. 1969 Jun;53(6):781–791. doi: 10.1085/jgp.53.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Wessels D., Schroeder N. A., Voss E., Hall A. L., Condeelis J., Soll D. R. cAMP-mediated inhibition of intracellular particle movement and actin reorganization in Dictyostelium. J Cell Biol. 1989 Dec;109(6 Pt 1):2841–2851. doi: 10.1083/jcb.109.6.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels D., Soll D. R. Myosin II heavy chain null mutant of Dictyostelium exhibits defective intracellular particle movement. J Cell Biol. 1990 Sep;111(3):1137–1148. doi: 10.1083/jcb.111.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Fennell D. J. The use of fluorescent DNA-binding agent for detecting and separating yeast mitochondrial DNA. Methods Cell Biol. 1975;12:335–351. doi: 10.1016/s0091-679x(08)60963-2. [DOI] [PubMed] [Google Scholar]
- Zechel K. Isolation of polymerization-competent cytoplasmic actin by affinity chromatography on immobilized DNAse I using formamide as eluant. Eur J Biochem. 1980 Sep;110(2):343–348. doi: 10.1111/j.1432-1033.1980.tb04873.x. [DOI] [PubMed] [Google Scholar]