Abstract
Adiposity is more prevalent among individuals with a predominance of small, dense LDL (pattern B) than among those with larger LDL (pattern A). We tested for differences in resting energy expenditure (REE) and respiratory quotient (RQ) in overweight men with pattern A (n=36) or pattern B (n=60). Men consumed a standardized isoenergetic diet for 3 weeks after which a ~9 kg weight loss was induced by caloric deficit for 9 weeks, followed by 4 weeks of weight stabilization. REE and RQ were measured by indirect calorimetry before and after weight loss. Results were analyzed separately in pattern B men who converted to pattern A (B→A; n=35) and those who did not (B→B; n=25). At baseline, B→B men had higher trunk fat, triacylglycerol (TG) and insulin concentrations, HOMA-IR and smaller LDL particles compared to B→A men and baseline pattern A men who remained pattern A (A→A; n=35). REE normalized to fat-free mass did not change after weight loss. RQ decreased in A→A men, increased in B→A men and did not change significantly in B→B men after weight loss. Calculated fat oxidation rates paralleled the RQ results. Baseline plasma TG concentrations were positively correlated with RQ and inversely correlated with the magnitude of weight loss achieved for a given prescribed energy reduction in the entire study population. Pattern B men who converted to pattern A with weight loss may have an underlying impairment in fat oxidation that predisposes to both dyslipidemia and an impaired ability to achieve weight loss by energy restriction.
INTRODUCTION
LDL subclass pattern B, as defined by a predominance of small and dense LDL particles, is a component of an atherogenic lipoprotein phenotype that includes elevations in triglyceride, reductions in HDL cholesterol, insulin resistance and obesity (1). Although LDL subclass pattern B is in part genetically influenced (2, 3), it can also be significantly modulated by environmental factors, including dietary carbohydrate intake and adiposity (4).
We have recently shown that weight reduction and normalization of adiposity through a short-term dietary intervention led to the reversal of pattern B in a cohort of overweight men (1). Furthermore, we documented a tendency for men with pattern B to be heavier and have higher percentages of body fat at baseline as well as after weight loss relative to men with pattern A (1). This raises the hypothesis that differences in energy metabolism between men with pattern A or B may contribute to the tendency toward increased adiposity in men with pattern B.
Variations in components of energy balance, including resting energy expenditure (REE), may contribute to the efficacy of a given reduction in energy intake in achieving both weight loss and weight loss maintenance (5). In addition, fuel oxidation as measured by respiratory quotient (RQ) affects overall energy balance, and impaired fat oxidation has been associated with obesity and insulin resistance (6, 7). The aim of this study was to test whether differences in REE and/or RQ between men with LDL pattern A or B might contribute to a greater tendency toward adiposity in those with pattern B. The findings suggest that an underlying metabolic abnormality in pattern B men may contribute both to dyslipidemia and altered weight loss responsiveness to energy restriction.
METHODS AND PROCEDURES
Study design and participants
The data reported here were derived from a study designed to test whether normalization of adiposity by diet-induced weight loss could reverse the expression of LDL subclass pattern B in men with baseline BMIs ranging from 25–30 (1). After a 3 week run-in period on the study diet, weight-stable men by self-report with pattern B (n=60) and pattern A (n=36) were placed on hypoenergetic diets for 9 weeks to induce a weight loss of ~ 9 kg with the goal of achieving a BMI < 25. The acute weight loss phase was followed by a 4 week weight stabilization period.
Study participants were free-living and consumed diets designed to provide 40% carbohydrate, 40% fat (14% saturated, 19% monounsaturated and 7% polyunsaturated) and 20% protein over 6-day cycles. Diets contained ~25 g/day fiber, 150 mg per 1000 kcal cholesterol (to a maximum of 300 mg per day), and a ratio of simple:complex carbohydrates of 50%:50%. In addition, the diet contained three portions of dairy products (milk, cheese or yogurt) per day. Nutrient calculations were performed using the Nutrition Data System for Research software (version 4.06; Minneapolis, MN). Frozen, prepared entrees fortified with vitamins and minerals to meet the Recommended Dietary Allowances (Lifespring Home Nutrition, Irvine, CA) were provided for lunch and dinner. The participants prepared their own snacks and breakfasts according to individualized menus, and the participants were weighed weekly by the staff who adjusted energy intakes as necessary to ensure steady weight loss towards the goal of 9 kilograms. Adherence was promoted through frequent telephone contacts and weekly meetings with the dietitians. Study compliance was assessed using food lists and direct communication with study participants. For the purposes of the analyses presented here, only men in the weight loss arm were considered.
Study participants ranged from 28 to 63 years of age, had no history of CVD or other chronic diseases and none were taking drugs known to affect lipid metabolism. Other eligibility criteria included body mass index (BMI) between 25–30 kg/m2, total and LDL cholesterol below the 95th percentile for age and sex, TG concentration < 500 mg/dl (5.65 mmol/L), fasting glucose concentration <126 mg/dl (6.94 mmol/L), systolic blood pressure < 150 mmHg and diastolic blood pressure <90 mm Hg. None of the men smoked, and no alcohol was consumed during the study. All participants gave written informed consent under a protocol approved by the Institutional Review Board of Children's Hospital & Research Center Oakland, CA.
Study Measurements
REE in kilocalories per day and RQ were determined by indirect calorimetry (ParvoMedics, Sandy, UT). Study measurements were taken at baseline and at the end of the intervention period during a morning visit after participants had fasted overnight for at least 12 and no more than 15 hours. Participants rested supine for 30 to 45 minutes before measurements were begun. Measurements of oxygen consumption and expired CO2 for the determination of REE were then taken over a period of at least 40 minutes, so as to generate at least 30 minutes of usable steady state data.
Energy derived from carbohydrate (CHO) and lipid oxidation was calculated from the pulmonary gas exchange (8), where VO2 is in liters per minute. Calculation of these parameters rely on the assumption that protein oxidation is constant. Further, because carbon dioxide output was measured under stable conditions and acid-base balance was therefore not disturbed, we have considered the respiratory exchange ratio (RER) equivalent to the RQ.
% Energy from CHO = [( RER – 0.707)/ 0.293] (100)
% Energy from lipid = 100 - [( RER – 0.707)/ 0.293] (100)
Energy from CHO oxidation (kcal/min) = [(%CHO/100)( VO2)] (5.05 kcal/l O2)
Energy from lipid oxidation (kcal/min) = [(1 - %CHO/100)( VO2)] (4.7 kcal/l O2)
Energy expenditure (kcal/min) = [(%CHO/100)(VO2)] (5.05 kcal/l O2)+ [(1 - %CHO/100)( VO2)] (4.7 kcal/l O2)
Total body fat and body fat distribution were measured by dual energy X-ray absorptiometry (DXA; Hologic Delphi-A, software version 11.2, Bedford, MA).
Plasma samples were prepared within 2 h of collection from venous blood collected in tubes containing Na2EDTA (1.4 g/L) and a preservative cocktail of protease and bacterial inhibitors. Blood and plasma were kept at 4 °C throughout processing. Plasma total cholesterol and triglyceride concentrations were determined by enzymatic procedures on an Express 550 Plus analyzer (Ciba Corning, Oberlin, OH). These measurements were consistently in control ranges as monitored by the Centers for Disease Control and Prevention - National Heart, Lung and Blood Institute standardization program. Nondenaturing polyacrylamide gradient gel electrophoresis with lipid staining of plasma was performed as described previously for the determination of LDL subclass patterns A and B (9). Men with LDL diameters >257.5 angstroms were defined as having LDL subclass pattern A whereas those with LDL diameters <257.5 angstroms were defined as having LDL subclass pattern B (9).
Glucose concentrations were measured enzymatically (Cat. No 07157965, Siemens and Express 550 Plus Analyzer). Insulin concentrations were measured with commercially available enzyme linked immunoassay kits (cat. no. EXHI-14K, Millipore). The Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) was calculated using the following equation (10): (Insulin (μU/ml) * Glucose (mg/dl) * 0.055)/22.5.
Weight loss effectiveness
The effectiveness of weight loss was a constructed variable that was based on the amount of weight lost for a given reduction in calories as prescribed by the study dietitian. Specifically,
Calories were initially prescribed based on a modified version of the Harris Benedict equation, i.e. [66 + (13.8 × Wt kg) + (5 × Ht cm) - (6.8 × Age yr)] × 1.3 for men. During the run-in period, calories were adjusted as necessary to maintain weight. The calorie levels at the end of the baseline and weight loss phases that were used in the above equation were an average of the 3-week and 9-week periods, respectively.
Statistical Analysis
Differences in changes of LDL subclass pattern were tested by Chi-squared analyses and the likelihood ratio test in men who were pattern A or B at baseline. Chi-squared tests were repeated according to LDL subclass conversion pattern, i.e. A→A (n=35), B→A (n=35) and B→B (n=25). Since there was only one man with A→B change, he was eliminated from statistical analyses. Significant differences between pattern A and pattern B men were assessed by t-test. Differences by change pattern were assessed by analysis of variance and post-hoc Tukey's tests. Linear regression models were used to determine significant associations between various parameters. Log transformations of plasma triglyceride concentrations were performed to attain normal distributions. All statistical procedures were performed using JMP statistical software (version 6.0.2; SAS Institute Inc, Cary, NC). Graphs were created in PRISM (version 4.0; GraphPad, San Diego, CA). Group averages and changes are reported as means ± standard deviations (SD).
RESULTS
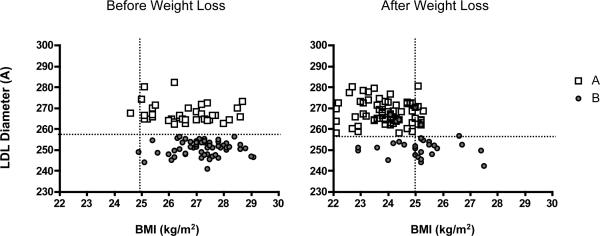
Weight loss was effectively induced by energy restriction in pattern A (n=36) and pattern B (n=60) men (−8.9 ± 2.2 and −8.2 ± 2.7 kg, respectively; p= 0.12) who began the intervention at similar weights and BMIs (85 ± 8 and 85 ± 8 kg, respectively; p= 0.57; 26.7 ± 1.1 and 27.1 ± 1.0, respectively; p=0.14) (1). However, fewer men with pattern B at baseline reached the study goal of achieving BMI less than 25 compared to pattern A men (61.7% vs 83.3%; χ2: p=0.02; Figure 1).
Figure 1.
Pre- and post-weight loss body mass indices are plotted in relation to LDL diameter for pattern A (n=36; open square) and pattern B men (n=60; closed circle). Where 257.5 angstroms defines the threshold above which LDL subclass pattern is defined as pattern B and below which LDL subclass pattern is defined as pattern A (horizontal dotted line), a BMI of 25 was the pre-specified study goal (vertical dotted line) in this weight loss intervention (1). A higher percentage of pattern A compared to pattern B men was able to meet the study goal.
Baseline parameters by conversion phenotype
After weight loss, all but one of the men who were pattern A at baseline remained pattern A, and 59% of pattern B men converted to pattern A (1). Due to limited statistical power, the one man who changed from pattern A to pattern B with weight loss was eliminated from data analyses in this study.
An evaluation of baseline parameters by conversion pattern groups, or LDL subclass pattern at baseline versus the end of the intervention, i.e. A→A, B→A or B→B, showed that all three groups of men began the study at similar weights, BMIs, waist circumferences and percentage body fat (Table 1). B→B men had higher trunk fat as a percentage of total fat compared to the other two groups at baseline. Total cholesterol, LDL cholesterol and glucose concentrations were similar in all three groups at baseline. HDL cholesterol was higher in A→A men compared to the two other groups. Plasma TG levels were significantly different in all three groups, with concentrations highest in the B→B group and lowest in the A→A group. LDL peak particle diameters were also different among the three groups, with the B→B group having the smallest diameters and the A→A group having the largest. Insulin concentrations as well as HOMA-IR were significantly lower at baseline in the A→A group compared to the B→B group.
TABLE 1.
Baseline, end and change data by LDL subclass conversion pattern groups
| A→ARx (n=35) | B→ARx (n=35) | B→BRx (n=35) | ANOVA p-value | ||
|---|---|---|---|---|---|
| Weight (kg) | Baseline | 85 ± 7.9 | 85 ± 8.4 | 86 ± 8.3 | NS |
| End | 76 ± 7.0 | 75 ± 7.6 | 79 ± 8.0 | NS | |
| % Change | −10.6 ± 2.3a | −11.0 ± 1.7a | −7.7 ± 3.1b | <.0001 | |
| BMI (kg/m2) | Baseline | 26.7 ± 1.12 | 27.0 ± 0.98 | 27.3 ± 0.93 | NS |
| End | 23.9 ± 0.86a | 24.0 ± 0.84a | 25.2 ± 1.2b | <.0001 | |
| % Change | −10.6 ± 2.3a | −11.0 ± 1.7a | −7.7 ± 3.1 b | <.0001 | |
| Waist (cm) | Baseline | 95 ± 6.2 | 96 ± 5.6 | 98 ± 5.5 | NS |
| End | 87 ± 5.4 a,b | 87 ± 6.0a | 91 ± 5.6b | 0.02 | |
| % Change | −8.2 ± 3.4a,b | −10.0 ± 3.6a | −7.2 ± 4.3b | 0.01 | |
| % Body fat | Baseline | 24.5 ± 3.7 | 24.6 ± 3.8 | 25.0 ± 2.4 | NS |
| End | 20.2 ± 4.2a | 20.0 ± 3.4a | 22.6 ± 2.7b | 0.01 | |
| % Change | −17.9 ± 8.3a | −18.5 ± 6.4a | −9.4 ± 8.9b | <0.0001 | |
| Trunk fat as % total fat | Baseline | 52 ± 4.5a | 53 ± 3.8a | 56 ± 3.9b | 0.0003 |
| End | 47.6 ± 5.4a | 48.4 ± 4.4a | 53.3 ± 3.8b | <0.0001 | |
| % Change | −7.7 ± 6.3 | −7.9 ± 6.7 | −4.7 ± 4.5 | NS | |
| Fat(kg) | Baseline | 20.8 ± 4.2 | 20.8 ± 4.0 | 21.5 ± 3.5 | NS |
| End | 15.3 ± 3.8 | 15.1 ± 3.3 | 17.9 ± 3.1 | 0.004 | |
| % Change | −26.6 ± 7.9a | −27.5 ± 6.4a | −16.2 ± 10.4b | <0.0001 | |
| Fat-free mass (kg) | Baseline | 64.4 ± 6.0 | 64.3 ± 6.9 | 64.7 ± 5.9 | NS |
| End | 61.0 ± 5.6 | 60.9 ± 6.2 | 61.7 ± 6.2 | NS | |
| % Change | −5.2 ± 2.6 | −5.2 ± 2.5 | −4.6 ± 2.5 | NS | |
| TC (mg/dl) | Baseline | 192 ± 32 | 203 ± 35 | 206 ± 35 | NS |
| End | 187 ± 25 | 192 ± 30 | 202 ± 30 | NS | |
| % Change | −1.6 ± 12.3 | −4.7 ± 11 | −0.90 ± 12 | NS | |
| TG (mg/dl) | Baseline | 117 ± 58a | 173 ± 58b | 259 ± 120c | <0.0001 |
| End | 81 ± 28a | 106 ± 29b | 182 ± 63C | <0.0001 | |
| % Change | −22.4 ± 27.4 | −35.8 ± 17.8 | −20.7 ± 38.8 | NS | |
| LDL-C (mg/dl) | Baseline | 124 ± 28 | 132 ± 32 | 118 ± 25 | NS |
| End | 120 ± 23 | 125 ± 26 | 128 ± 26 | NS | |
| % Change | −0.99 ± 19.8a,b | −2.7 ± 15.3a | 11.0 ± 26.5b | 0.027 | |
| HDL-C (mg/dl) | Baseline | 45 ± 6.5a | 37 ± 8.1b | 34 ± 5.3b | <.0001 |
| End | 51 ± 8.5a | 45 ± 10b | 38 ± 7.4c | <.0001 | |
| % Change | 14.3 ± 13.2a | 25.0 ± 15.5b | 11.1 ± 17.5b | 0.0013 | |
| LDL particle size (Å) | Baseline | 265 ± 7.4a | 254 ± 6.8b | 249 ± 5.3c | <.0001 |
| End | 270 ± 6a | 265 ± 5 b | 250 ± 4c | <.0001 | |
| % Change | 1.7 ± 2.8a | 4.5 ± 2.8b | 0.78 ± 2.8a | <0.0001 | |
| Glucose (mg/dl) | Baseline | 95 ± 6 | 94 ± 6 | 98 ± 9 | NS |
| End | 91 ± 5 | 91 ± 6 | 94 ± 7 | NS | |
| % Change | −4.0 ± 6.2 | −3.3 ± 5.0 | −3.3 ± 6.3 | NS | |
| Insulin (μU/ml) | Baseline | 7.4 ± 5.01a | 9.0 ± 5.4a,b | 10.6 ± 4.0b | 0.04 |
| End | 5.28 ± 1.68a | 6.11 ± 2.63a,b | 7.4 ± 3.7b | 0.01 | |
| % Change | −14.7 ± 32.4 | −23.0 ± 38.8 | −24.9 ± 37.1 | NS | |
| HOMA-IR | Baseline | 1.75 ± 1.3a | 2.1 ± 1.3ab | 2.5 ± 0.9b | 0.04 |
| End | 1.18 ± 0.37a | 1.37 ± 0.62ab | 1.71 ± 0.87b | 0.007 | |
| % Change | −17.4 ± 33.1 | −25.6 ± 37.3 | −26.8 ± 38.5 | NS |
Values given are means ± standard deviations. Statistical comparisons were performed by ANOVA. Statistical analysis of triglyceride data was performed with log-transformed data. Insulin concentrations and HOMA-IR in the AA group were derived from 34 men. Values not sharing the same letter (a, b or c) are significantly different by post-hoc Tukey's test. To convert cholesterol and triacylglycerol concentrations to SI units (mmol/L), multiply by 0.0259 and 0.0113, respectively. BMI: body mass index; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; TC: total cholesterol; TG: triacylglycerol; HOMA-IR: homeostasis model assessment of insulin resistance.
Effects of weight loss
The B→B group lost significantly less weight in response to energy restriction than groups A→A and B→A (Table 1). Similarly, the B→B group reduced their percentage body fat to a lesser extent compared to the A→A and B→A groups with weight loss. Weight loss was associated with a greater decrease in waist circumference in the B→A group compared to the B→B group. Trunk fat was not reduced significantly in any of the groups. Notably, fewer men in the B→B group (28%) achieved the study goal of BMI<25 relative to groups A→A and B→A (86% in both groups) (χ2: p<0.0001).
Total cholesterol and TG concentrations were not significantly reduced with weight loss (Table 1). At the end of the intervention, group B→B had the highest and group A→A men had the lowest concentrations of TG. LDL cholesterol concentrations changed significantly after weight loss, with the decrease in the B→A group significantly different from the increase in the B→B group. HDL cholesterol was increased significantly with weight loss. At the end of the intervention, HDL cholesterol was highest in the A→A group and lowest in the B→B group. By design, the greatest increase in peak LDL particle diameter was in the B→A group. Weight loss was associated with no changes in glucose concentrations but lower insulin concentrations and HOMA-IR in all three groups.
REE
REE was not different between men with pattern A and B at baseline or at the end of the intervention whether they were categorized by baseline or by conversion pattern (Table 2). With weight loss, REE was significantly reduced in all groups. However, normalization of REE to fat-free mass showed no significant change in REE with weight loss (Table 2).
Table 2.
REE and RQ by LDL subclass pattern
| LDL or Conversion Pattern | REE (kcal/day) | REE (kcal/day/kg) | RQ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | End | Percent Change | Baseline | End | Percent Change | Baseline | End | Percent Change | |
| A | 35 | 1641 ± 236 | 1529 ± 224¶ | −6.1 ± 13.5 | 25.6 ± 3.2 | 25.1 ± 3.6 | −0.99 ± 13.4 | 0.84 ± 0.04 | 0.82 ± 0.04 | −1.8 ± 5.6 |
| B | 60 | 1674 ± 270 | 1561 ±216 ¶ | −5.1 ± 17.4 | 26.0 ± 3.4 | 25.5 ± 2.9 | −0.12 ± 18.4 | 0.83 ± 0.04 | 0.84 ± 0.05* | 1.5 ± 7.5* |
| A→A | 35 | 1641 ± 236 | 1529 ± 224¶ | −6.1 ± 13.5 | 25.5 ± 3.2 | 25.1 ± 3.6 | −0.99 ± 13.4 | 0.84 ± 0.04 | 0.82 ± 0.04 | −1.8 ± 5.6a |
| B→A | 35 | 1654 ± 295 | 1526 ± 218¶ | −5.5 ± 21 | 25.7 ± 3.3 | 25.1 ± 3.1 | −0.25 ± 22 | 0.82 ± 0.04 | 0.85 ± 0.05 | 3.1 ± 6.2b |
| B→B | 25 | 1702 ± 235 | 1610 ± 208¶ | −4.7 ± 11.0 | 26.4 ± 3.6 | 26.1 ± 2.4 | −0.07 ± 11.4 | 0.84 ± 0.05 | 0.83 ± 0.05 | -0.78±8.5a,b |
Values given are means ±standard deviations.
p< 0.05 relative to pattern A by t-test
p< 0.05 relative to baseline by t-test.
One man converted from pattern A to B with weight loss; his data were excluded from analysis due to the lack of statistical power. Statistical comparisons by LDL conversion pattern were performed by ANOVA and post-hoc Tukey's test. Values not sharing the same letter (a or b) are significantly different by post-hoc Tukey's test.
RQ
At the beginning of the intervention, RQ measured in the fasting state was not different in groups of men categorized by baseline LDL subclass pattern or by conversion pattern (Table 2). At the end of the intervention, the men who were pattern B at baseline had a higher RQ than men who were pattern A at baseline. Pattern B had men had a significant increase in RQ relative to pattern A men when the data were expressed as percent change from baseline (Table 2).
Among conversion phenotypes in men achieving BMI<25, there were no differences in RQ at baseline or at the end of the intervention, but men in the B→A group showed increases in their RQ while men in the A→A group had a decrease in RQ.
Changes in RQ and fat oxidation rates
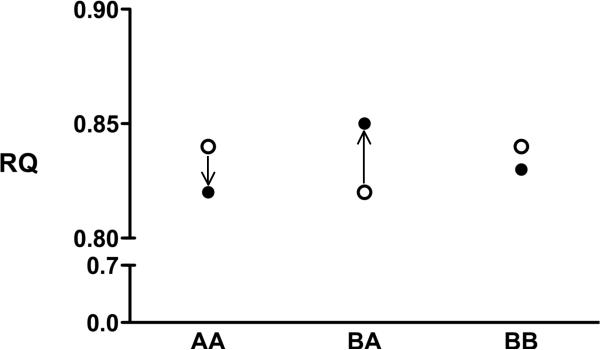
Weight loss was associated with a significant reduction in RQ in men who were pattern A, but not pattern B, at baseline (−0.02 ± 0.05 versus 0.01 ± 0.062, respectively; p=0.03), which was explained by a significant increase in RQ in men who converted from B→A versus a decrease in those who retained the initial pattern A (0.03 ± 0.05 and −0.02 ± 0.05, respectively; p=0.007) (Figure 2). These relationships remained significant after adjustment for change in body weight and/or change in plasma triglyceride concentrations (data not shown). The disparate changes in RQ with weight loss according to conversion phenotype were also not affected after adjustment for percentage body fat or steps per day as a marker of physical activity in logistic regression models (data not shown).
Figure 2.
RQ measurements before (open circle) and after (closed circle) weight loss as a function of conversion phenotype (A→A, B→A or B→B). RQ was decreased after weight loss in A→A men and increased in B→A men as determined by analysis of variance and post-hoc Tukey's tests (p=0.007). The change in the B→B group was not significantly different from the two other groups.
The percentages of energy derived from carbohydrate versus lipids were not different at baseline or after weight loss among the three conversion phenotype groups (Table 3). Similarly, the energy from carbohydrate or lipid oxidation was not different at baseline or at the end of the intervention among the three conversion groups. However, changes in the percentage of energy from lipid and the energy from lipid oxidation in response to the weight loss intervention were significantly different, with the A→A group showing increases and the B→A group showing decreases in these parameters (Table 3). Changes in the percentage of energy from carbohydrate and the energy from carbohydrate oxidation from baseline were not different among the conversion phenotypes (Table 3).
Table 3.
Fuel Oxidation Parameters by Conversion Phenotype
| A→A (n=35) | B→A (n=35) | B→B (n=25) | ANOVA | |
|---|---|---|---|---|
| Percentage energy from CHO | ||||
| Baseline | 44.0 ± 15.2 | 39.2 ± 13.0 | 45.5 ± 15.7 | NS |
| End | 38.4 ± 13.6 | 47.5 ± 16.6 | 42.4 ± 16.9 | NS |
| % Change from Baseline | −0.6 ± 55 | 36 ± 72 | 15 ± 118 | 0.18 |
| Percentage energy from lipid | ||||
| Baseline | 56.0 ± 15.2 | 60.8 ± 13.0 | 54.5 ± 15.7 | NS |
| End | 61.6 ± 13.6 | 52.5 ± 16.6 | 57.6 ± 16.9 | NS |
| % Change from Baseline | 16.4 ± 38a | −11.6 ± 33b | 19.4 ± 62.5a,b | 0.01 |
| Energy from CHO oxidation (kcal/min) | ||||
| Baseline | 0.52 ± 0.19 | 0.47 ± 0.18 | 0.55 ± 0.21 | NS |
| End | 0.42 ± 0.16 | 0.52 ± 0.19 | 0.49 ± 0.22 | NS |
| % Change from Baseline | −0.8 ± 49 | 41 ± 136 | 3.9 ± 86 | 0.11 |
| Energy from lipid oxidation (kcal/min) | ||||
| Baseline | 0.62 ± 0.19 | 0.67 ± 0.16 | 0.62 ± 0.23 | NS |
| End | 0.63 ± 0.17 | 0.54 ± 0.20 | 0.61 ± 0.17 | NS |
| % Change from Baseline | 9.7 ± 42a | −19 ± 27b | 13 ± 57a,b | 0.004 |
Values given are means ± standard deviations. One man converted from pattern A to B with weight loss; his data were excluded from analysis due to the lack of statistical power. Statistical comparisons by LDL conversion pattern were performed by ANOVA. Values not sharing the same letter (a, b or c) are significantly different by post-hoc Tukey's test.
Subgroup analysis
When only those men who reached the study goal of BMI<25 were evaluated, pattern B men who remained pattern B (n=7) achieved the same magnitude of weight loss and similar BMIs compared to the B→A (n=30) and A→A (n=30) groups (Supplementary Tables S1). However, the B→B group lost significantly less body fat than the two other groups, less trunk fat as a percentage of total fat relative to the A→A group and less total fat mass relative to the B→A group.
As expected, pattern B men at baseline had higher triglyceride, lower HDL cholesterol and smaller LDL particle sizes relative to pattern A men (Supplementary Table S1). With weight loss, triglyceride concentrations decreased in all groups to a similar extent, but at the end of the intervention, the B→A group had significantly higher triglyceride concentrations than the A→A group and lower triglyceride concentrations than the B→B group. Weight loss also led to increases in HDL cholesterol in all three groups, with the B→A group showing the greatest percentage increase; this resulted in comparable HDL cholesterol concentrations in the B→A and A→A group. LDL particle size was also increased with weight loss in all three groups, with the B→A group showing the greatest percentage increase; at the end of the intervention, the LDL particle size in the B→A group was larger than the B→B group but smaller than the A→A group.
There were no differences in REE or REE normalized to fat-free mass at baseline or at the end of the intervention among conversion phenotypes in men who achieved BMI < 25 with weight loss (Supplementary Table 1). REE was significantly reduced with weight loss in the B→A group, but not the two other groups. REE normalized to fat-free mass was not significantly reduced with weight loss in any of the three conversion phenotype groups. As with the entire population, RQ was significantly increased in the B→A group and decreased in the A→A group. In the B→B group, there was no apparent change in RQ with weight loss. Finally, in the B→A group, decreases in the percentage from lipid and energy from lipid oxidation were decreased relative to the A→A group.
Correlations of Lipids, Weight Loss Effectiveness and Insulin Resistance
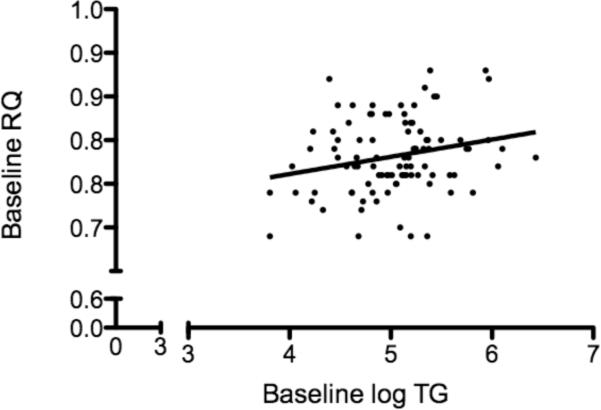
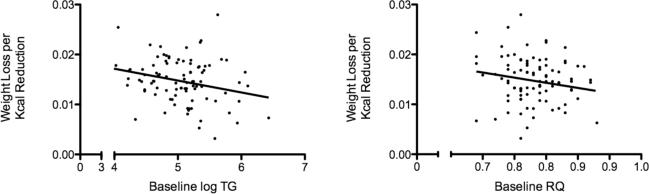
Baseline plasma triglyceride was positively associated with baseline RQ (r2 = 0.06; p=0.02) (Figure 3), whereas baseline LDL diameter was not significantly associated with baseline RQ (r2=0.003; p=0.60) by linear regression analysis. Larger baseline LDL diameter was significantly associated with weight loss effectiveness, i.e. greater weight loss per calorie reduction (r2=0.09; p=0.003; weight loss effectiveness = −0.024 + 0.00015*LDL diameter) as was lower baseline plasma triglyceride (r2=0.07; p=0.01; Figure 4a). HOMA-IR was not associated with weight loss effectiveness (data not shown), and its inclusion in a regression model with LDL diameter did not affect the significant positive relationship of LDL diameter with weight loss effectiveness (data not shown). Finally, lower baseline RQ was associated with a trend for improved weight loss effectiveness (r2=0.04; p=0.07; Figure 4b).
Figure 3.
Linear regression analysis of baseline triglyceride and baseline respiratory quotient in men enrolled in weight loss intervention (n=95; r2=0.05; p=0.02).
Figure 4.
Figure 4a: Linear regression analysis of baseline triglyceride and weight loss per calorie reduction in men undergoing weight loss (n=95; r2= 0.07; p=0.01). Figure 4b: Linear regression analysis of baseline RQ and weight loss per calorie reduction in weight loss group (n=95) at baseline (r2= 0.035; p=0.069).
DISCUSSION
LDL pattern B is a component of the dyslipidemia of the metabolic syndrome, which includes increased adiposity as one of its hallmark characteristics. Despite the well-established association of dyslipidemia with higher adiposity, the possible proximal role of lipid dysregulation in the determination of energy balance has not been well investigated. In this study, the change in the type of fuel oxidized under fasting conditions was different in pattern A men who remained pattern A compared to men with pattern B who converted to pattern A after weight loss, such that only men in the A→A group exhibited an increase in fasting fat oxidation. The men with pattern B who converted to pattern A with weight loss decreased fasting fat oxidation in response to weight loss, suggesting a possible impairment in the response to weight loss that could contribute to the known tendency for men with pattern B to have increased adiposity relative to men with pattern A (1). Furthermore, despite achieving metabolic profiles very similar to men in the A→A group, men in the B→A group who achieved a BMI less than 25 maintained higher triglyceride concentrations and smaller LDL particles than men of comparable weight and adiposity in the A→A group. Although it is possible that persistence of excess visceral adiposity in the B→A group may have contributed to these results, the findings are consistent with common metabolic pathways influencing both dyslipidemia and impaired fat oxidation.
This study lacked the statistical power to determine whether there were significant changes in RQ and fuel oxidation rates after weight loss in the B→B group relative to the B→A and the A→A groups. Notably, B→B men had increased trunk fat (as an indicator of visceral fat), triglyceride and insulin concentrations, and HOMA-IR, and smaller LDL particles compared to the two other groups at baseline. Furthermore, subset analyses in men who achieved the study goal of BMI<25 showed the B→B group lost less body fat than the two other groups and less trunk fat compared to the A→A group after weight loss. Visceral fat is highly innervated, and the mobilization of free fatty acids from its depots may drive the dyslipidemia known to be associated with the metabolic syndrome, i.e. elevated triglycerides, low HDL cholesterol and smaller and more dense LDL particles, by increasing the hepatic secretion of triglyceride enriched VLDL particles which are readily metabolized to smaller and more dense LDL particles (11). The increased trunk fat and insulin resistance in the B→B group suggests a fundamental physiological difference in phenotypes that may make it more difficult for some men with pattern B to convert to pattern A. Although men in the B→B group may have a genetic basis for their phenotype independent of body weight, further weight loss could promote conversion to pattern A.
In the B→A group, weight loss led to significant improvements in anthropometric measurements, lipid profiles and glucose and insulin parameters, such that the end phenotypes of these men were comparable to the A→A group, as has been previously reported for men with pattern A and B (1). However, the reliance on carbohydrate metabolism after acute weight loss suggests an underlying impairment in fasting fat oxidation in the B→A group. Indeed, impaired fat oxidation has been associated with insulin resistance and obesity (6, 7) as well as an increased propensity to re-gain weight (12). Adjustment for HOMA-IR did not alter the significant positive relationship observed between LDL diameter (where larger LDL diameters define pattern A compared to pattern B) and the ability to lose weight effectively, suggesting that the relationship between pattern B and weight loss effectiveness was not mediated by HOMA-IR. Whether men in the B→A group are more likely to regain weight compared to their A→A counterparts could not be determined from this study.
Weight loss has been shown to improve fasting fat oxidation, in part by improving skeletal muscle substrate oxidation, in some (13), but not all studies (14, 15). In addition, those with low rates of fat oxidation have been shown to have increased intramyocellular triglyceride (16). It is possible that men in the B→A group were unable to upregulate fat oxidation in response to weight loss due to an intrinsic metabolic defect. Whether this defect exists at the level of mobilization of fat stores in adipose tissue, oxidation of fat in skeletal muscle and/or liver fuel oxidation and secretory processes requires further investigation.
The significant associations of LDL subclass pattern and plasma triglycerides with the ability to lose weight for a given energy reduction may represent novel links between lipid profiles and energy balance. Indeed, the data from our study suggest that these lipid measurements, particularly elevations in triglyceride, which are thought to drive LDL subclass pattern B (17), might be used as a predictive tool in assessing a person's capacity to lose weight efficiently, in part because higher plasma triglycerides may be an indicator of defective fat oxidation. Of note, morning fasting RQ was not shown to be significantly associated with weight loss effectiveness in this study, and as such, the type of fuel consumed probably does not account entirely for a person's ability to lose weight. Where LDL-1 and LDL-2 were positively associated with weight loss effectiveness (data not shown), waist-to-hip ratio was found to be inversely associated with weight loss effectiveness (data not shown). Larger LDL particles are generally indicative of relatively efficient hepatic secretory processes (18) and increased waist-to-hip ratios have been shown to be associated with increased visceral adiposity and a higher prevalence of dyslipidemia (19).
Weight loss effectiveness may also be due to variations in energy metabolism. REE normalized to fat-free mass was not different in men with pattern A versus those with pattern B before or after weight loss in this study. However, our post-weight loss measurements were made after stabilization from acute weight loss, and changes in REE may vary depending on whether an individual is actively losing weight. Furthermore, there may be differences in other contributors to overall energy expenditure, including the thermic effect of feeding and non-resting energy expenditure (NREE). Decreases in NREE have been shown to represent a significant component of the decrease in total energy expenditure known to occur with weight loss (20). The post-exercise recovery period has also been shown to be an important contributor to energy expenditure with a significant contribution of lipid oxidation during this period (21). Either of these mechanisms may be operational in individuals with pattern B.
There are several limitations to this study, including the post-hoc nature of the analyses concerning weight loss effectiveness. In addition, we measured REE and RQ during a morning visit in study participants in the fasting state. Whether 24-hour fuel oxidation varies in men with pattern A versus pattern B who achieve comparable weight loss remains to be determined. Finally, we were unable to establish definitively whether the differences in weight loss effectiveness observed in the A→A and B→A groups versus the B→B group were attributable to differences in compliance to the prescribed dietary regimen rather than a fundamental metabolic defect. Nonetheless, due to limited statistical power, the B→B group was not included in our observations related to RQ and the comparable amounts of weight lost in the B→A and A→A groups suggest that adherence to the prescribed regimen was similar in these two groups.
Overall, this study highlights a novel link between weight loss effectiveness and lipid profiles, specifically, LDL particle diameter or plasma triglyceride concentrations. Although plasma triglyceride concentrations were less significantly associated with weight loss effectiveness than was LDL diameter, plasma triglycerides are a routine laboratory measurement that could potentially be used as a predictor for weight loss effectiveness for a given energy reduction.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Kathleen Wojnoonski and the staff of the CHORI Lipoprotein Analysis Laboratory for laboratory measurements; Linda Abe for data management; Robin Rawlings and the staff of the Cholesterol Research Center for participant recruitment and clinical study assistance; and Ellen Fung for assistance with REE and RQ measurements.
This research was supported by the National Dairy Council and was made possible by Grant Number UL1 RR024131-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. <http://www.ncrr.nih.gov/.> Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp <http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp>. In addition, a grant from the National Institutes of Health (Grant #5 T35 HL07807) Short Term Training for Minority College Students provided support for Amy Woods.
Footnotes
Reprints not available.
DISCLOSURE The authors declare no conflicts of interest.
REFERENCES
- 1.Siri-Tarino PW, Williams PT, Fernstrom HS, Rawlings RS, Krauss RM. Reversal of Small, Dense LDL Subclass Phenotype by Normalization of Adiposity. Obesity (Silver Spring) 2009 Jun 4; doi: 10.1038/oby.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin MA, Newman B, Selby JV, Edwards K, Mayer EJ, Krauss RM. Genetics of LDL subclass phenotypes in women twins. Concordance, heritability, and commingling analysis. Arterioscler Thromb. 1993 May;13(5):687–95. doi: 10.1161/01.atv.13.5.687. [DOI] [PubMed] [Google Scholar]
- 3.Campos H, Blijlevens E, McNamara JR, Ordovas JM, Posner BM, Wilson PW, et al. LDL particle size distribution. Results from the Framingham Offspring Study. Arterioscler Thromb. 1992 Dec;12(12):1410–9. doi: 10.1161/01.atv.12.12.1410. [DOI] [PubMed] [Google Scholar]
- 4.Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr. 2006 May;83(5):1025–31. doi: 10.1093/ajcn/83.5.1025. quiz 205. [DOI] [PubMed] [Google Scholar]
- 5.Stiegler P, Cunliffe A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med. 2006;36(3):239–62. doi: 10.2165/00007256-200636030-00005. [DOI] [PubMed] [Google Scholar]
- 6.Astrup A, Buemann B, Christensen NJ, Toubro S. Failure to increase lipid oxidation in response to increasing dietary fat content in formerly obese women. Am J Physiol. 1994 Apr;266(4 Pt 1):E592–9. doi: 10.1152/ajpendo.1994.266.4.E592. [DOI] [PubMed] [Google Scholar]
- 7.Blaak EE, Hul G, Verdich C, Stich V, Martinez A, Petersen M, et al. Fat oxidation before and after a high fat load in the obese insulin-resistant state. J Clin Endocrinol Metab. 2006 Apr;91(4):1462–9. doi: 10.1210/jc.2005-1598. [DOI] [PubMed] [Google Scholar]
- 8.Lusk G. Animal calorimetry: analysis of the oxidation of mixtures of carbohydrate and fat. J Biol Chem. 1924;59:41–2. [Google Scholar]
- 9.Dreon DM, Fernstrom HA, Williams PT, Krauss RM. Reduced LDL particle size in children consuming a very-low-fat diet is related to parental LDL-subclass patterns. Am J Clin Nutr. 2000 Jun;71(6):1611–6. doi: 10.1093/ajcn/71.6.1611. [DOI] [PubMed] [Google Scholar]
- 10.Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997 Jul;20(7):1087–92. doi: 10.2337/diacare.20.7.1087. [DOI] [PubMed] [Google Scholar]
- 11.Krauss RM, Siri PW. Metabolic abnormalities: triglyceride and low-density lipoprotein. Endocrinol Metab Clin North Am. 2004 Jun;33(2):405–15. doi: 10.1016/j.ecl.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990 Nov;259(5 Pt 1):E650–7. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 13.Corpeleijn E, Mensink M, Kooi ME, Roekaerts PM, Saris WH, Blaak EE. Impaired skeletal muscle substrate oxidation in glucose-intolerant men improves after weight loss. Obesity (Silver Spring) 2008 May;16(5):1025–32. doi: 10.1038/oby.2008.24. [DOI] [PubMed] [Google Scholar]
- 14.Blaak EE, Wolffenbuttel BH, Saris WH, Pelsers MM, Wagenmakers AJ. Weight reduction and the impaired plasma-derived free fatty acid oxidation in type 2 diabetic subjects. J Clin Endocrinol Metab. 2001 Apr;86(4):1638–44. doi: 10.1210/jcem.86.4.7397. [DOI] [PubMed] [Google Scholar]
- 15.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999 Dec;277(6 Pt 1):E1130–41. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 16.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006 Dec;55(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krauss RM, Siri PWb. Metabolic abnormalities: triglyceride and low-density lipoprotein. Endocrinol Metab Clin North Am. 2004 Jun;33(2):405–15. doi: 10.1016/j.ecl.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002 Sep;43(9):1363–79. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- 19.Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008 Jun;28(6):1039–49. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 20.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995 Mar 9;332(10):621–8. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 21.Kuo CC, Fattor JA, Henderson GC, Brooks GA. Lipid oxidation in fit young adults during postexercise recovery. J Appl Physiol. 2005 Jul;99(1):349–56. doi: 10.1152/japplphysiol.00997.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.