Introduction
The prevalence of obesity in the United States (U.S.) is increasing at an alarming rate and represents a significant burden on the U.S. healthcare system. It is estimated that approximately two thirds of adults within the U.S. are classified as being overweight (body mass index between 25 to 30 kg/m2) and that one third of adults within the U.S. are classified as being obese (body mass index ≥ 30 kg/m2) (Ogden et al., 2006). In addition, the prevalence of obesity among children is increasing and 80% of obese children will become obese adults (Serdula et al., 1993; Whitaker et al., 1997). Thus, the obesity epidemic in the U.S. represents a significant challenge to health care providers. This challenge lies in the fact that obesity is a major risk factor for the development of insulin resistance and subsequent type 2 diabetes mellitus (T2D), as well as a major risk factor for the development of the metabolic syndrome and atherosclerotic cardiovascular disease (Despres et al., 2008).
The exact etiology of insulin resistance and T2D remains an enigma. However, it is believed that adipose tissue dysfunction associated with obesity plays a major role in the formation of insulin resistance (Frayn, 2001; Goossens, 2008). Until recently, the adipose tissue was merely considered a storage depot for free fatty acids (FFA). Within the last decade, the adipose tissue and its constituents have been shown to play a major role in energy metabolism and insulin sensitivity through the production and secretion of a host of inflammatory cytokines and adipokines as well as through dysregulation of its FFA buffering capacity (Antuna-Puente et al., 2008; Boden, 1997). Expansion of the adipose tissue or obesity is associated with chronic, low-level inflammatory state induced by an increase in inflammatory mediators such as tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1) (Shoelson et al., 2006; Wellen and Hotamisligil, 2003; Xu et al., 2003). In addition to elevations of these inflammatory mediators, there are elevations in the adipokines leptin and resistin as well as decreased production of the insulin sensitizer, adiponectin (Antuna-Puente et al., 2008). Elevated plasma concentrations of these inflammatory mediators, adipokines, and FFA as well as decreased plasma concentrations of adiponectin have all been associated with the occurrence of systemic insulin resistance. Specifically, elevations in TNFα, IL-6, and FFA have been shown to decrease insulin signaling and subsequent glucose uptake in L6 skeletal muscle cells, primary adipocytes, NIH3T3-L1 adipocytes, and in vivo animal models of T2D (Rabe et al., 2008; Shulman, 2000). Thus, alterations in the ability of the adipocyte to take up and store FFA or in the production of adipokines and cytokines by the adipocyte may promote insulin resistance and result in T2D.
Until recently, exposure to environmental contaminants as a mechanism for the development of insulin resistance and T2D had not been explored. However, there is an increasing amount of epidemiological data implicating a role for exposure to certain environmental contaminants in the development of T2D. Exposure to TCDD, a chlorinated contaminant of the herbicide Agent Orange, has been significantly correlated with the occurrence of T2D in Vietnam veterans (Cranmer et al., 2000; Fujiyoshi et al., 2006; Henriksen et al., 1997; Kern et al., 2004). In addition to exposure to TCDD, retrospective analysis of data collected during the National Health and Nutrition Examination Survey from 1999 to 2002 revealed that elevated serum concentrations of the organochlorine compounds oxychlordane and DDE were significantly associated with the occurrence of diabetes (Lee et al., 2007; Lee et al., 2006). Investigations by Turyk et al. (2009) further substantiated the link between exposure to DDE and the formation of T2D. In a cohort of Great Lakes sport fishermen that were followed over ten years starting prior to the diagnosis of T2D, elevated serum concentrations of DDE were significantly correlated with the incidence of T2D (Turyk et al., 2009a; Turyk et al., 2009b).
While these epidemiological data implicate a role for exposure to environmental contaminants, especially the persistent organochlorine compounds, they do not represent a causal relationship. Recently, Ruzzin et al. (2009) demonstrated dietary supplementation with salmon oil that was contaminated with a mixture of persistent organic pollutants (POPs), including oxychlordane and DDE, in conjunction with a high fat diet feeding promotes the formation of obesity and T2D in a rodent model (Ruzzin et al., 2009). In addition to these in vivo data, it has also been demonstrated that exposure to various POPs including TCDD, PCBs, DDT, endrin, aldrin, and dieldrin can alter adipogenesis and/or adipokine/cytokine production in vitro (Arsenescu et al., 2008; Moreno-Aliaga and Matsumura, 1999, 2002; Phillips et al., 1995). Thus, exposure to the highly prevalent, bioaccumulative organochlorine compounds DDE or oxychlordane may promote the formation of T2D by altering adipogenesis and adipokine production.
Given the growing body of epidemiological data that implicate exposure to the organochlorine compounds DDE and oxychlordane in the formation of T2D and that adipocyte dysfunction may be a key contributing factor to the progression of this disease, the current study seeks to determine whether exposure to either DDE or oxychlordane may promote T2D by altering adipocyte function including adipogenesis, lipolysis, and cytokine/adipokine production. The murine NIH3T3-L1 cell line was employed as a model of adipocyte function. This cell line has been well characterized and displays many physiological similarities to primary adipocytes. NIH3T3-L1 cells are maintained in culture as preadipocytes and are induced to differentiate into mature adipocytes as indicated by intracellular lipid accumulation as well as expression of adipocyte specific markers (Ntambi and Young-Cheul, 2000).
Materials and Methods
Chemicals
Oxychlordane (Vesicol, Inc.), DDE (Chem Service), and dieldrin (Chem Service) stock solutions (40 mM in DMSO) were prepared and diluted to the working concentration immediately prior to use. Dexamethasone, insulin, 3-isobutyl-1-methylxanthine (IBMX), and isoproterenol were all purchased from Sigma-Aldrich.
Cell Culture
NIH3T3-L1 preadipocytes were purchased (ATCC) and cultured as previously described (Moreno-Aliaga and Matsumura, 1999, 2002; Phillips et al., 1995; Robinson and James, 1992). Briefly, preadipocytes were maintained in Dulbecco’s modified essential medium (DMEM) supplemented with glucose (4.5 g/L), L-glutamine (4 mM), sodium pyruvate (1 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% calf serum. Preadipocytes were passaged when approximately 80% confluent. To initiate differentiation into mature adipocytes, preadipocytes were allowed to grow for two days post confluence in DMEM supplemented with 10% calf serum. Two days after reaching confluency (d0), the media was removed and replaced with DMEM supplemented with glucose (4.5 g/L), L-glutamine (4 mM), sodium pyruvate (1 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), dexamethasone (1 μM), insulin (1 μg/ml), IBMX (500 μM) and 10% fetal bovine serum. After two days (d2), the media was removed and DMEM with 10% FBS and insulin (1 μg/ml) was added for an additional two days. Four days following the initiation of differentiation (d4), the media containing insulin was removed and the cells were maintained in DMEM containing 10% FBS until reaching maturity (8 days from the initiation of differentiation). On day 8 (d8) following the initiation of differentiation, approximately 90–95% of cells were mature adipocytes as determined visually by the appearance of intracellular lipid accumulation and by increased Oil Red O staining.
Adipogenesis assay
To determine the effect of exposure to the organochlorine compounds oxychlordane, DDE, or dieldrin on adipogenesis, NIH3T3-L1 preadipocytes were exposed to vehicle (DMSO 0.05%), oxychlordane (2 or 20 μM), DDE (2 or 20 μM), or dieldrin (2 or 20 μM) 24 hours prior to the initiation of differentiation (d-1) and throughout the differentiation process upon media exchange every 48 hours. Exposure to dieldrin (30 μM) has been shown to decrease adipogenesis (Moreno-Aliaga and Matsumura, 1999). Therefore, it was used as a negative control. On d6, intracellular lipid accumulation was measured as an index of adipocyte maturation. Intracellular lipid accumulation was determined by Oil Red O staining as previously described (Moreno-Aliaga and Matsumura, 1999; Phillips et al., 1995). To quantify the differences in Oil Red O staining between each treatment group, Oil Red O was extracted from the confluent cell monolayer and the absorbance was measured at 520 nm.
Fatty acid uptake
The effect of organochlorine compound exposure on fatty acid uptake was determined in mature (d10) NIH3T3-L1 adipocytes as previously described with minor modifications (Liao et al., 2005). NIH3T3-L1 cells were maintained in culture and induced to differentiate into mature adipocytes as described above. Ten days following the initiation of differentiation (d10), DMEM containing 10% FBS was removed and cells were incubated in DMEM containing 0.2% bovine serum albumin (BSA) for 6 hours for acclimation. After 6 hours, cells were treated with DMEM containing 0.2% fatty acid free BSA and 2.5 μM of 4,4-difluoro-5-methyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoic acid (BODIPY 500/510 C12; Molecular Probes) with vehicle (DMSO 0.05%), oxychlordane (2 or 20 μM), DDE (2 or 20 μM), or dieldrin (2 or 20 μM) for 24 hours to determine basal fatty acid uptake over a 24 hour time period. To determine whether exposure to organochlorine compounds effected insulin stimulated fatty acid uptake, mature adipocytes (d10) were treated for 24 hours with DMSO (0.05%), oxychlordane (2 or 20 μM), DDE (2 or 20 μM), or dieldrin (2 or 20 μM) in DMEM containing 0.2% BSA following a 6 hour acclimation period. At the end of 24 hours, cells were washed and incubated in DMEM containing 0.2% BSA, BODIPY-FA (2.5 μM), and insulin (100 nM) or vehicle (PBS) for 60 minutes. Cellular uptake of BODIPY-FA after either 24 hour exposure or 1 hour insulin stimulation was determined by washing the cells three times with cold PBS prior to lysis in 0.2% SDS. Fluorescent intensity within the cell lysates was determined at an excitation of 490 nm and emission of 510 nm (Molecular Devices SpectraMax).
Lipolysis assay
To determine the effect of organochlorine compound exposure on 24 hour basal lipolysis or isoproterenol (2 μM) stimulated lipolysis, mature NIH3T3-L1 adipocytes (d10) were acclimated for 4 hours in DMEM containing 0.2% BSA then treated for 24 hours in DMEM containing 0.2% BSA with vehicle (DMSO 0.05%), oxychlordane (2 or 20 μM), DDE (2 or 20 μM), or dieldrin (2 or 20 μM). Following exposure for 24 hours, the media was removed, cells washed, and incubated for 3 hours in DMEM containing 0.2% BSA and either vehicle or isoproterenol (2 μM). At the end of isoproterenol stimulation, media was removed and the free glycerol content (Free glycerol reagent; Sigma-Aldrich) of both the 24-hour exposure media and the 3-hour stimulation media was determined per the manufacturer’s protocol as an index of triglyceride metabolism.
Cytokine/adipokine multiplex immunoassay
The ability of organochlorine exposure to alter adipokine/cytokine release was determined in mature NIH3T3-L1 adipocytes (d8). Mature adipocytes were treated with vehicle (DMSO 0.05%), oxychlordane (2 or 20 μM), DDE (2 or 20 μM), or dieldrin (2 or 20 μM) in DMEM containing 10% FBS for 48 hours. Following the 48 hour exposure, media was removed and the concentrations of leptin, IL-6, TNFα, MCP-1, adiponectin, and resistin were measured by multiplex immunoassay (Adipocyte 6-plex Milliplex; Millipore) per the manufacturer’s protocol.
Real-time PCR measurement of leptin, resistin, and adiponectin expression
To determine if enhanced adipokine release was coupled with increased expression, alterations in mRNA levels of leptin, resistin, and adiponectin were determined by real-time PCR using the ΔΔCt method as previously described with minor modifications (Howell et al., 2009). Briefly, total RNA was isolated (RNeasy Mini Prep kit, Qiagen) from mature adipocytes that were treated for 48 hours with DDE (20 μM). Complementary DNA (cDNA) was synthesized (Affinityscript cDNA synthesis, Stratagene) from 1 μg of total RNA and subjected to real-time PCR (Stratagene MxP3005) with Sybr green detection (Brilliant Sybr green master mix, Stratagene). DDE mediated changes in leptin, resistin, or adiponectin expression were normalized to cyclophilin D and expressed as the fold change from vehicle (DMSO 0.05%). Primer sequences were as follows: leptin (forward, 5′-CAGGATCAATGACATTTCACACA-3′; reverse, 5′-GCTGGTGAGGACCTGTTGAT-3′), resistin (forward, 5′-TTCCTTGTCCCTGAACTGCT-3′; reverse, 5′-CCAATGTTCTTTATTGCATTTGG-3′), and adiponectin (forward, 5′-GGAGAGAAAGGAGATGCAGGT-3′; reverse, 5′-CTTTCCTGCCAGGGGTTC-3′).
Statistical analysis
All data are expressed as the means ± the standard error of the mean. In experiments with more than two groups, data were analyzed with a one-way ANOVA followed by a Dunnett’s post hoc test (SigmaStat 2.0) to determine statistically significant differences between organochlorine and vehicle treatments. In experiments with only two groups, data were analyzed with a student’s t-test to determine statistically significant differences between organochlorine and vehicle treatments. A P-value of less than 0.05 was considered the threshold of statistical significance.
Results
Exposure to dieldrin attenuates adipocyte differentiation
Intracellular lipid accumulation was determined by Oil Red O staining as a marker of adipocyte maturation (Figure 1A). Extraction and subsequent quantification of Oil Red O staining (Figure 1B) revealed that exposure to oxychlordane (2 or 20 μM) or DDE (2 or 20 μM) had no significant effect on lipid accumulation within differentiated adipocytes when compared to DMSO. However, exposure to the higher concentration of dieldrin (20 μM) decreased intracellular lipid accumulation by 2.8 fold compared to DMSO exposure (4.23 vs. 11.92 normalized absorbance at 520 nm). Thus, as previously reported (Moreno-Aliaga and Matsumura, 1999), exposure to dieldrin during adipocyte maturation decreased adipogenesis while exposure to oxychlordane or DDE at comparable concentrations had no effect on adipogenesis.
Figure 1.
Effect of exposure to oxychlordane, DDE, or dieldrin on adipocyte maturation. NIH3T3-L1 preadipocytes were exposed to vehicle (DMSO 0.05%), oxychlordane (Oxy), DDE, or dieldrin prior to and throughout the differentiation process then subjected to Oil Red O staining. Intracellular lipid accumulation was confirmed visually (1A) followed by extraction and quantification by absorbance at 520 nm (1B). Data represent mean ± SEM of six individual replicates per group (n = 6). * P < 0.05 vs. DMSO (0.05%)
Organochlorine compounds increase basal free fatty acid uptake in mature adipocytes
Basal fatty acid accumulation was determined over a 24 hour time period with or without exposure to oxychlordane, DDE, or dieldrin (Figure 2A). Each organochlorine compound significantly increased fatty acid uptake over this time period. While the lower concentration of oxychlordane had no effect on fatty acid uptake over the 24 hour exposure period, exposure to oxychlordane (20 μM) for 24 hours significantly increased fatty acid uptake in mature adipocytes compared to DMSO (6112.8 vs. 5407.9 fluorescence at 510 nm). Unlike the effect of oxychlordane, exposure to the lower concentration of DDE (2 μM) significantly increased fatty acid uptake over 24 hours when compared to DMSO (5994.1 vs. 5407.9 fluorescence at 510 nm) while the higher concentration of DDE (20 μM) had no effect. Lastly, exposure to dieldrin at both concentrations tested (2 and 20 μM) significantly increased fatty acid uptake over 24 hours compared to DMSO (6051.4 and 6052 vs. 5407.9 fluorescence at 510 nm, respectively). It should be noted that there was no significant effect of DMSO on basal fatty acid uptake compared to naïve cells (data not shown).
Figure 2.
Exposure to organochlorine pesticides increase basal fatty acid uptake. Mature adipocytes (d10) were treated with vehicle (DMSO 0.05%), oxychlordane (Oxy), DDE, or dieldrin in serum free media for 24 hours with fluorescently labeled (BODIPY) dodecanoic acid (2.5 μM) (2A) or with vehicle (DMSO 0.05%), oxychlordane (Oxy), DDE, or dieldrin for 24 hours then treated with vehicle (PBS; open bars) or insulin (100 nM; black bars) in the presence of labeled dodecanoic acid (2.5 μM) for 60 minutes (2B). Intracellular fluorescence was determined as performed with basal fatty acid uptake. Data represent the mean ± SEM of seven to eight individual replicates per group (n = 7–8). * P < 0.05 vs. DMSO (0.05%)
In addition to effects on basal fatty acid uptake, the ability of organochlorine compounds to alter insulin-stimulated free fatty acid uptake in mature adipocytes was explored (Figure 2B). Exposure to oxychlordane (2 or 20 μM), DDE (2 or 20 μM), or dieldrin (2 or 20 μM) for 24 hours prior to insulin stimulation had no significant effect on insulin-stimulated fatty acid uptake in the mature adipocytes. Thus, basal fatty acid uptake and not insulin-stimulated fatty acid uptake appears to be increased by organochlorine compound exposure.
Effect of exposure to organochlorine compounds on lipolysis
Lipolysis was determined by measuring the amount of free glycerol, a marker of triglyceride metabolism, in the cell culture media. Exposure to the organochlorine compounds for 24 hours had no significant effect on the media concentrations of free glycerol when compared to DMSO (Figure 3A). Incubation of mature adipocytes for 24 hours with the test compounds followed by stimulation of lipolysis by isoproterenol (2 μM) for 3 hours (Figure 3B) resulted in significant increases in lipolysis compared to the corresponding vehicle controls. However, isoproterenol stimulation of lipolysis was not altered by exposure to the organochlorine compounds. These data indicate that neither basal nor isoproterenol-stimulated lipolysis is affected by exposure to test compounds at concentrations that alter basal fatty acid uptake and adipogenesis.
Figure 3.
Effect of exposure to oxychlordane, DDE, or dieldrin on basal and isoproterenol-stimulated lipolysis. Mature adipocytes (d10) were exposed to vehicle (DMSO 0.05%), oxychlordane (Oxy), DDE, or dieldrin for 24 hours in serum free media to determine if exposure to these compounds altered basal lipolysis. At the end of 24 hours, free glycerol content of the media was determined as an index of triglyceride metabolism (3A). To determine if exposure to these compounds altered isoproterenol-stimulated lipolysis, mature adipocytes (d10) were exposed to vehicle (DMSO 0.05%), oxychlordane (Oxy), DDE, or dieldrin for 24 hours in serum free media and then stimulated with vehicle (PBS; open bars) or isoproterenol (2 μM; black bars) for 3 hours (3B). At the end of 3 hours, media was collected and free glycerol content measured. Data represent the mean ± SEM of eight individual replicates per group (n = 8). * P < 0.05 vs. DMSO (0.05%)
DDE exposure increases adipokine release from mature adipocytes
The effect of exposure to organochlorine compounds on release of adipokines and cytokines was determined in mature NIH3T3-L1 adipocytes (d8). Exposure to DDE (2 and 20 μM) significantly increased the media concentrations of adiponectin (50,464.4 and 50,831.1 pg/ml; Figure 4A) compared to DMSO (46,376.6 pg/ml) and resistin (13,907.9 and 16781.44 pg/ml; Figure 4B) compared to DMSO (10,100.9 pg/ml). In addition, exposure to the lower concentration of dieldrin (2 μM) significantly elevated the media concentration of adiponectin. The highest concentration of DDE (20 μM) also significantly increased the release of leptin from mature adipocytes compared to DMSO (445.5 vs. 234.3 pg/ml; Figure 4C). Release of the pro-inflammatory cytokines IL-6 and MCP-1 was not altered following exposure to the organochlorine compounds and media concentrations of TNFα were below the limit of detection for the assay (data not shown). Therefore, these data indicate that exposure to DDE significantly increases the release of the adipokines leptin, adiponectin, and resistin from mature adipocytes.
Figure 4.
Exposure to DDE alters adipokine release in mature adipocytes. To determine if exposure to oxychlordane vehicle (DMSO 0.05%), oxychlordane (Oxy), DDE, or dieldrin alters adipokine/cytokine release, mature adipocytes (d8) were exposed to the organochlorine compounds in serum containing media for a total of 48 hours. The media concentration of the adiponectin (4A), resistin (4B), and leptin (4C) were determined by multiplex immunoassay during the last 24 hours of OC compound exposure. Data represent the mean ± SEM of at least five replicates per group (n 5). * P < 0.05 vs. DMSO (0.05%)
DDE-mediated alterations in adipokine expression
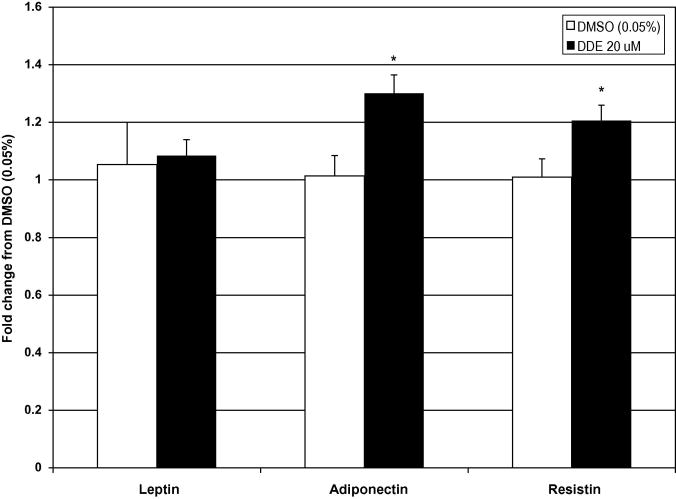
In order to determine if increased adipokine release following DDE exposure was a result of increased production, the expression of leptin, adiponectin, and resistin was determined after 48 hours of exposure to DDE (20 μM) by real-time PCR in the adipocytes that were used to determine adipokine release. The expression of adiponectin (1.3 fold) and resistin (1.21 fold) were slightly, but significantly increased in DDE (20 μM) treated adipocytes compared to DMSO (Figure 5). These data correspond with increased release of the two adipokines. Although there was a significant increase in leptin release following 48 hours of DDE exposure, expression of leptin mRNA was not altered by DDE treatment when compared to vehicle. Thus, increased leptin release does not appear to be mediated by increased leptin mRNA expression at 48 hours.
Figure 5.
Increased adipokine expression following DDE exposure in mature adipocytes. Expression of leptin, resistin, and adiponectin was determined by real-time PCR with Sybr green detection using cyclophilin D expression for normalization following exposure to vehicle (DMSO 0.05%), oxychlordane (Oxy), DDE, or dieldrin for 48 hours. Data are expressed as the fold change from vehicle (DMSO 0.05%; open bars) and represent the mean ± SEM of six replicates per group (n = 6). * P < 0.05 vs. DMSO (0.05%)
Discussion
Although chlordane and DDT have been banned for use in the U.S., the metabolites of these compounds, oxychlordane and DDE, respectively, persist in the environment and bioaccumulate up the food chain. Due to the lipophilic nature of these compounds, they are sequestered and stored within the adipose tissue. This is illustrated by the 400:1 adipose to blood partition coefficient of DDE (Muhlebach et al., 1991). Therefore, localized adipose tissue concentrations of these compounds greatly exceed their concentrations in the blood. Data from the latest NHANES study (2003–2004) has revealed that the highest concentration of DDE (95th percentile) in human serum is approximately 12.1 parts per million (whole serum) or approximately 40 nM. Given a 400:1 partition coefficient for the adipocyte, this would put the adipose exposure at approximately 16 μM, which is within the concentration range used in the present studies. Due to the fact that these compounds are concentrated within the adipose tissue, namely the adipocyte, it is reasonable to conclude that they may affect adipocyte function (Mullerova and Kopecky, 2007). Therefore, the present study was conducted to determine the effect of adipose tissue relevant concentrations of oxychlordane, DDE, and dieldrin on adipocyte maturation, lipid flux, and adipokine production using a well-characterized in vitro model, the NIH3T3-L1 adipocyte.
Adipose tissue expansion results in obesity and has been shown to promote insulin resistance and T2D. With regards to the adipocyte, adipose tissue expansion is a result of adipocyte hyperplasia or adipogenesis and adipocyte hypertrophy. Exposure to other POPs has been shown to alter adipogenesis as determined by intracellular lipid accumulation. Arsenescu et al. (2008) demonstrated that low concentrations of TCDD promoted adipogenesis in NIH3T3-L1 preadipocytes whereas high concentrations had a deleterious effect on adipogenesis (Arsenescu et al., 2008). In addition to TCDD, exposure to PCB-77, endrin, aldrin, or dieldrin has been shown to decrease adipocyte maturation in vitro (Arsenescu et al., 2008; Moreno-Aliaga and Matsumura, 1999). In the present study, we determined that exposure to either DDE or oxychlordane (2 or 20 μM) had no significant effect on adipogenesis while dieldrin (20 μM) significantly decreased adipocyte maturation as previously reported (Moreno-Aliaga and Matsumura, 1999). Thus, the tested concentrations of DDE and oxychlordane do not appear to alter adipocyte maturation while a comparable concentration of dieldrin significantly decreased adipogenesis.
The mature white adipocyte is a unique cell type that plays a critical role in the storage and release of free fatty acids. Dysregulation of fatty acid storage in the adipose tissue can contribute to the elevated plasma levels of free fatty acids observed in obesity and T2D (Boden, 1997; Frayn, 2001; Monajemi et al., 2007). Therefore, we wanted to determine whether exposure to bioaccumulative organochlorine compounds would promote adipocyte fatty acid storage dysfunction. The present data indicate that exposure to oxychlordane (20 μM), DDE (2 μM), or dieldrin (2 or 20 μM) promotes fatty acid uptake under non-stimulated conditions in mature adipocytes. This increase in fatty acid uptake may result from increased diffusion mediated fatty acid uptake due to altered membrane composition (Donato et al., 1997a; Donato et al., 1997b). Exposure to these compounds had no effect on insulin-stimulated fatty acid uptake. Additionally, these compounds did not alter either non-stimulated or isoproterenol-stimulated lipolysis. Thus, exposure to these compounds may promote adipocyte hypertrophy due to increased fatty acid storage.
Given that alterations in adipokine/cytokine production may cause local and systemic inflammation and insulin resistance, we examined the effect of exposure to oxychlordane, DDE, and dieldrin on adipokine/cytokine production and expression in mature adipocytes. Following 48 hours of cumulative DDE (2 or 20 μM) exposure, media concentrations of the adipokines leptin, resistin, and adiponectin were significantly increased. The increased release of resistin and adiponectin were associated with slight but significant increases in expression of these two genes. These data indicate that the increased release of resistin and adiponectin may be due, in part, to increased expression of these genes. Following 48 hours of DDE (20 μM) exposure, leptin expression was unchanged. Thus, increased expression of leptin does not appear to explain the increase in leptin release. Previous studies have shown that fluctuations in leptin release cannot be exclusively attributed to increased production due release from preformed, intracellular leptin storage pools (Kirchgessner et al., 1997). However, the possibility that leptin expression increased and returned to normal prior to the 48-hour measurement time point cannot be excluded given the present data. In addition, it should be noted that release of the pro-inflammatory cytokines TNFα, Il-6, and MCP-1 was not altered following exposure to the test compounds. Therefore, exposure to these organochlorine compounds does not appear to induce an inflammatory response in mature adipocytes as reported with PCB-77 and TCDD (Arsenescu et al., 2008).
Taken together, the current data indicate that exposure to the bioaccumulative organochlorine compounds oxychlordane, DDE, or dieldrin may cause adipocyte hypertrophy by increasing basal free fatty acid accumulation in mature adipocytes. In addition, exposure to DDE may promote systemic insulin resistance by increasing leptin and resistin release from mature adipocytes. These data reinforce recent epidemiological and empirical studies that indicate a significant association between POPs exposure, including DDE, and T2D through the promotion of adipocyte dysfunction. Future in vivo studies will be utilized to confirm that exposure to organochlorine pesticide metabolites, namely DDE, will promote obesity through increased fatty acid uptake in the adipose tissue and promote T2D by increasing serum leptin and resistin levels.
Acknowledgments
This work was supported in part by the National Institutes of Health (1P20-RR17661 and 1K01ES019182), by the Center for Environmental Health Sciences at Mississippi State University College of Veterinary Medicine (MSU-CVM), and by a Department of Basic Sciences (MSU-CVM) Preliminary Data Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34:2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008;116:761–768. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- Cranmer M, Louie S, Kennedy RH, Kern PA, Fonseca VA. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is associated with hyperinsulinemia and insulin resistance. Toxicol Sci. 2000;56:431–436. doi: 10.1093/toxsci/56.2.431. [DOI] [PubMed] [Google Scholar]
- Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodes-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- Donato MM, Antunes-Madeira MC, Jurado AS, Madeira VM. Partition of DDT and DDE into membranes and extracted lipids of Bacillus stearothermophilus. Bull Environ Contam Toxicol. 1997a;59:696–701. doi: 10.1007/s001289900536. [DOI] [PubMed] [Google Scholar]
- Donato MM, Jurado AS, Antunes-Madeira MC, Madeira VM. Effects of a lipophilic environmental pollutant (DDT) on the phospholipid and fatty acid contents of Bacillus stearothermophilus. Arch Environ Contam Toxicol. 1997b;33:341–349. doi: 10.1007/s002449900263. [DOI] [PubMed] [Google Scholar]
- Frayn KN. Adipose tissue and the insulin resistance syndrome. Proc Nutr Soc. 2001;60:375–380. doi: 10.1079/pns200195. [DOI] [PubMed] [Google Scholar]
- Fujiyoshi PT, Michalek JE, Matsumura F. Molecular epidemiologic evidence for diabetogenic effects of dioxin exposure in U.S. Air force veterans of the Vietnam war. Environ Health Perspect. 2006;114:1677–1683. doi: 10.1289/ehp.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94:206–218. doi: 10.1016/j.physbeh.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Henriksen GL, Ketchum NS, Michalek JE, Swaby JA. Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology. 1997;8:252–258. doi: 10.1097/00001648-199705000-00005. [DOI] [PubMed] [Google Scholar]
- Howell G, 3rd, Deng X, Yellaturu C, Park EA, Wilcox HG, Raghow R, Elam MB. N-3 polyunsaturated fatty acids suppress insulin-induced SREBP-1c transcription via reduced trans-activating capacity of LXRalpha. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbalip.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern PA, Said S, Jackson WG, Jr, Michalek JE. Insulin sensitivity following agent orange exposure in Vietnam veterans with high blood levels of 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Clin Endocrinol Metab. 2004;89:4665–4672. doi: 10.1210/jc.2004-0250. [DOI] [PubMed] [Google Scholar]
- Kirchgessner TG, Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J Clin Invest. 1997;100:2777–2782. doi: 10.1172/JCI119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR., Jr Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2007;30:622–628. doi: 10.2337/dc06-2190. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, Jacobs DR., Jr A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006;29:1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- Liao J, Sportsman R, Harris J, Stahl A. Real-time quantification of fatty acid uptake using a novel fluorescence assay. J Lipid Res. 2005;46:597–602. doi: 10.1194/jlr.D400023-JLR200. [DOI] [PubMed] [Google Scholar]
- Monajemi H, Stroes E, Hegele RA, Fliers E. Inherited lipodystrophies and the metabolic syndrome. Clin Endocrinol (Oxf) 2007;67:479–484. doi: 10.1111/j.1365-2265.2007.02906.x. [DOI] [PubMed] [Google Scholar]
- Moreno-Aliaga MJ, Matsumura F. Endrin inhibits adipocyte differentiation by selectively altering expression pattern of CCAAT/enhancer binding protein-alpha in 3T3-L1 cells. Mol Pharmacol. 1999;56:91–101. doi: 10.1124/mol.56.1.91. [DOI] [PubMed] [Google Scholar]
- Moreno-Aliaga MJ, Matsumura F. Effects of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)-ethane (p,p′-DDT) on 3T3-L1 and 3T3-F442A adipocyte differentiation. Biochem Pharmacol. 2002;63:997–1007. doi: 10.1016/s0006-2952(01)00933-9. [DOI] [PubMed] [Google Scholar]
- Muhlebach S, Moor MJ, Wyss PA, Bickel MH. Kinetics of distribution and elimination of DDE in rats. Xenobiotica. 1991;21:111–120. doi: 10.3109/00498259109039455. [DOI] [PubMed] [Google Scholar]
- Mullerova D, Kopecky J. White adipose tissue: storage and effector site for environmental pollutants. Physiol Res. 2007;56:375–381. doi: 10.33549/physiolres.931022. [DOI] [PubMed] [Google Scholar]
- Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr. 2000;130:3122S–3126S. doi: 10.1093/jn/130.12.3122S. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Phillips M, Enan E, Liu PC, Matsumura F. Inhibition of 3T3-L1 adipose differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Cell Sci. 1995;108 (Pt 1):395–402. doi: 10.1242/jcs.108.1.395. [DOI] [PubMed] [Google Scholar]
- Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, James DE. Insulin-regulated sorting of glucose transporters in 3T3-L1 adipocytes. Am J Physiol. 1992;263:E383–393. doi: 10.1152/ajpendo.1992.263.2.E383. [DOI] [PubMed] [Google Scholar]
- Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, Ma T, Pesenti S, Sonne SB, Marstrand TT, Malde MK, Du ZY, Chavey C, Fajas L, Lundebye AK, Brand CL, Vidal H, Kristiansen K, Froyland L. Persistent Organic Pollutant Exposure Leads to Insulin Resistance Syndrome. Environ Health Perspect. 2009 doi: 10.1289/ehp.0901321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature. Prev Med. 1993;22:167–177. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk M, Anderson H, Knobeloch L, Imm P, Persky V. Organochlorine exposure and incidence of diabetes in a cohort of Great Lakes sport fish consumers. Environ Health Perspect. 2009a;117:1076–1082. doi: 10.1289/ehp.0800281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk M, Anderson HA, Knobeloch L, Imm P, Persky VW. Prevalence of diabetes and body burdens of polychlorinated biphenyls, polybrominated diphenyl ethers, and p,p′-diphenyldichloroethene in Great Lakes sport fish consumers. Chemosphere. 2009b;75:674–679. doi: 10.1016/j.chemosphere.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]