Abstract
Strong evidence exists for an age-related impairment in associative processing under intentional encoding and retrieval conditions, but the status of incidental associative processing has been less clear. Two experiments examined the effects of age on rapid response learning – the incidentally learned stimulus-response association that results in a reduction in priming when a learned response becomes inappropriate for a new task. Specifically, we tested whether priming was equivalently sensitive in both age groups to reversing the task-specific decision cue. Experiment 1 showed that cue inversion reduced priming in both age groups using a speeded inside/outside classification task, and in Experiment 2 cue inversion eliminated priming on an associative version of this task. Thus, the ability to encode an association between a stimulus and its initial task-specific response appears to be preserved in aging. These findings provide an important example of a form of associative processing that is unimpaired in older adults.
Keywords: rapid response learning, aging, repetition priming, associative priming
The cognitive aging literature has established strong evidence of a disproportionate age-related impairment in memory for associative information relative to item information (see Chalfonte & Johnson, 1996, Old & Naveh-Benjamin, 2008; Spencer & Raz, 1995). This pattern has been formalized by Naveh-Benjamin (2000) as the Associative Deficit Hypothesis (ADH). However, not all forms of associative processing appear to be equally affected by aging. In particular, the status of incidental associative processing has been controversial. Critically, examining whether incidental associative processing is preserved in aging can speak to the importance of impaired strategic processes affecting associative encoding and retrieval, a topic of much recent interest (e.g., Cohn, Emrich & Moscovitch, 2008; Naveh-Benjamin, Brav & Levy, 2007; Naveh-Benjamin, Craik, Guez, & Kreuger, 2005; Naveh-Benjamin, Shing, Kilb, Werkle-Bergner, Lindenberger & Li, 2009; for earlier discussions see also Light, 1991; Smith, Park, Earles, Shaw, & Whiting, 1998). To date, incidental associative processing in aging has been studied in two primary ways. First, a number of studies have compared the effects of incidental versus intentional encoding on explicit associative memory. Second, several studies have examined the status of implicit memory for new associations. Collectively, these studies have used widely varying paradigms and have offered conflicting results.
Using tests of explicit memory, age differences have been found for a wide variety of associations, using both verbal and pictorial materials (Bastin & Van der Linden, 2003; Chalfonte & Johnson, 1996; Naveh-Benjamin, 2000; Naveh-Benjamin, Hussain, Guez, & Bar-On, 2003; Naveh-Benjamin, Guez, Kilb & Reedy 2004). However, the magnitude of age differences is affected by whether encoding is incidental or intentional (see meta-analysis by Old & Naveh-Benjamin, 2008). For instance, Chalfonte and Johnson (1996) found a significant age difference in recognition memory for item-color combinations under intentional instructions, whereas under incidental instructions older adults' performance was numerically but not significantly lower than young adults. Naveh-Benjamin (2000) observed an associative deficit under incidental encoding instructions, but the impairment was magnified under intentional instructions. In contrast, Naveh-Benjamin et al. (2009) more recently found that although young adults outperformed older adults overall following incidental encoding, there was not a larger effect of age on memory for face-name associations relative to memory for individual faces or names. That is, incidental encoding was linked with general age differences in explicit memory but not with a disproportionate decline for associative relative to item memory. Taken altogether, these mixed findings are difficult to interpret. Age differences under incidental conditions appear to be consistently smaller than under intentional conditions. Yet, explicit memory is known to operate as a combined function of encoding and retrieval operations; thus, it is uncertain whether any explicit memory impairments that follow incidental encoding are a function of age-related declines in incidental encoding or in the explicit access of that information.
Examining implicit associative memory is an alternative way to assess the status of incidental associative processing and theoretically removes the potentially confounding variable of strategic or explicit retrieval. Implicit memory is a nonconscious, unintentional form of memory retrieval, and can be measured by priming, in which task performance is facilitated for repeated relative to new stimuli. Priming of new associations (also called episodic priming, see McKoon & Ratcliff, 1979) is typically measured as facilitated task performance for items presented in their studied context relative to a new context. To date, mixed findings have emerged on the effects of age on associative priming (for a review see Dew, Bayen & Giovanello, 2007). While some studies have found no age difference in the extent of associative priming (Gibson, Brooks, Friedman & Yesavage, 1993; Laver, 2009; Light, LaVoie, Valencia-Laver Albertson-Owens & Mead, 1992; Lloyd-Jones, 2005; Wiggs & Martin, 1994), other studies have found proportionately lower or even no evidence of associative priming in older adults (Ergis, Van der Linden, & Deweer, 1998; Howard, Fry, & Brune, 1991; Howard, Heisey & Shaw, 1986; Monti et al., 1997; O'Hanlon, Wilcox & Kemper, 2001; Spieler & Balota, 1996). One factor that may account for the variability in findings is whether the manipulations inadvertently involve explicit processes (see Laver, 2009; Wegesin, Ream & Stern, 2004). Indeed, standard paradigms of implicit associative memory, such as the associative version of word stem completion (WSC), have been shown to be sensitive to the same variables that affect explicit retrieval processes, including divided attention (e.g., Kinoshita, 1999), and awareness of the study-test connection (e.g., Bowers & Schacter, 1990). Thus, it is difficult to discern the source of age differences that have been observed using the associative WSC paradigm (e.g., in Ergis et al., 1998; Howard et al., 1991; O'Hanlon et al., 2001). More generally, it has been an ongoing challenge in the memory and aging literatures to distinguish between automatic and controlled contributions to associative priming.
An alternative way to assess the status of incidental associative processing in aging is to examine rapid response learning. Evidence of rapid response learning has emerged from several recent studies using speeded classification tasks (Denkinger & Koustaal, 2009; Dobbins, Schnyer, Verfaillie, & Schacter, 2004; Horner & Henson, 2008; Horner & Henson, 2009; Poldrack & Cohen, 1997; Schnyer, Dobbins, Nicholls, Davis, Verfaillie & Schacter, 2007; Schnyer, Dobbins, Nicholls, Schacter & Verfaillie, 2006). In these tasks, subjects encode objects by classifying them according to a criterion of interest (e.g., whether the object is bigger than a shoebox). Later, two speeded tests are presented. In one version, subjects make the same classification decision as previously. In the second version, the decision cue is changed or reversed. For instance, subjects may be instructed to decide whether each object is smaller than a shoebox. If a mapping is formed between the initial stimulus and its task-specific response, a reduction in priming will result when the previously learned response becomes inappropriate, even if the perceptual as well as abstract knowledge representation of the stimulus remain the same. Of note, this phenomenon is observed during the subset of priming tasks in which prior responses are relevant to the task at test (Dobbins et al., 2004; Schacter, Dobbins, & Schnyer, 2004).
The idea of rapid response learning is heavily modeled on Logan's (1988; 1990) instance theory, a prominent account of priming and automaticity of processing. According to instance theory, priming occurs as a function of a recorded instance, or stored representation, of a previously encountered stimulus. Task-specific responses on repeated items become automatic if they are driven by a memory phenomenon in which the initial conclusion is recovered, rather than by a recalculation of the original algorithm. Automaticity thus occurs in task environments that are consistent, such that practice with a specific solution to a specific problem forms a mapping between them. An important feature of this learning mechanism is that it is both incidental and associative. As such, the study of rapid response learning can be used to evaluate the status of incidental associative learning as well as automatic associative retrieval in older adults.
To date, evidence for the mechanism of rapid response learning has been studied primarily in healthy young adults (e.g., Dobbins, et al., 2004; Horner & Henson, 2008; Horner & Henson, 2009; Schnyer, et al., 2007), as well as patient populations (Schnyer et al., 2006). Rapid response learning has been the subject of considerable theoretical interest in the recent memory literature, in part because it presents a challenge to prominent theoretical frameworks of implicit memory. For instance, research on the neural basis of implicit memory has shown that repetition priming is associated with decreased neural activation for repeated relative to new stimuli. According to a model by Wiggs and Martin (1998), this decreased activation reflects a neural tuning, or sharpening, mechanism, in which only the neurons that respond best to the stimulus are recruited for re-processing that stimulus at a later time. This model has been successful in explaining many instances of repetition priming, in particular visual specificity effects in which perceptual priming is reduced by changes to the physical features of the stimulus (for further discussion of this model, see Grill-Spector, Henson & Martin, 2006; Schacter, Wig & Stevens, 2007).
However, the recent evidence of rapid response learning on speeded classification tasks is not well-accommodated by the framework of neural tuning. If priming reflects a neural sharpening mechanism, then the re-presentation of the stimulus should be sufficient to produce neural priming, regardless of the decision cue. In contrast to this prediction, Dobbins et al. (2004) found in an fMRI study that cue reversal reduced neural priming in both fusiform gyrus as well as in left prefrontal cortex (PFC). The neural priming effects in fusiform and PFC regions were restored when a final classification task recapitulated the original cue. Thus, neural priming was dependent on the learned association between the objects and their task-specific responses, even in a region that might have been assumed to reflect object processing (e.g., fusiform gyrus) and thus be independent from the classification task. These results provide evidence against a neural sharpening mechanism, and instead suggest a mechanism of priming in which relying on a prior response reduces executive processes required for the task in favor of a more automated response mechanism (Dobbins et al., 2004; Schacter et al., 2004; see also Horner & Henson, 2008).
Of note, strong evidence that an S-R associative learning mechanism can account for priming on speeded classification tasks comes from several studies (Dobbins et al., 2004; Horner & Henson, 2009; Schnyer et al., 2007) that have compared items presented multiple times at encoding (high-primed items) relative to items that were presented only once (low-primed items). Theoretically, stimulus-response learning should be strengthened for high-primed items, given their additional presentations and thus opportunities for S-R learning. In turn, cue reversal should have a larger impact (i.e., a larger reduction in priming) on high-primed relative to low-primed items. Consistent with these predictions, Dobbins et al. (2004) found a larger priming reduction for high-primed relative to low-primed items, although significant slowing accompanied cue reversal for both high-and low-primed trials. Likewise, Horner and Henson (2009) found a significant priming reduction for high-primed items in three experiments, and a trend toward a larger priming reduction in high-primed items in their remaining experiments. These differences between the cue conditions (i.e., same versus inverted) indicate that multiple repetitions do not simply improve access to item-specific information but rather strengthen the relationship between the item and its associated response.
Critically, evidence that rapid response learning reflects an associative binding process has also emerged from the neuropsychological literature. Schnyer et al. (2006) tested the effect of cue reversal on patients with damage to the medial temporal lobes (MTL), which are critical for relational processing (Cohen, Poldrack, & Eichenbaum, 1997). MTL-amnesic patients and healthy age-matched controls demonstrated equivalent overall levels of priming for studied objects relative to novel (i.e., unstudied) objects. However, the two groups responded differently to the variables of same- versus reverse-cue classification conditions. Cue inversion had a larger impact on high-primed than low-primed items in the controls, but this interaction was not apparent in the patient group. These findings indicate that priming on the speeded classification task is likely the result of multiple mechanisms. In part, priming likely reflects a facilitation of object knowledge that results in the faster overall reaction time for studied versus new trials, a process that is unimpaired in amnesia and operates independently from the MTL. In addition, priming also reflects a stimulus-response associative learning mechanism that is impaired in amnesia and that is linked with the MTL.
Taken together, these described studies establish the study of rapid response learning as critical both for evaluating the status of incidental associative encoding and retrieval in aging as well as for extant theories of implicit memory more generally. Research that highlights general age-related declines in binding processes (e.g., Chalfonte & Johnson, 1996) leads to the prediction that older adults will be less likely than young adults to demonstrate rapid response learning. In contrast, research that highlights a more specific contribution of impaired strategic processes to age-related associative memory declines (e.g., Naveh-Benjamin et al., 2009) leads to the prediction that an age-equivalent reduction in priming will follow decision cue reversal.
Experiment 1
The primary goal of Experiment 1 was to determine whether healthy older adults show evidence of rapid response learning to the same extent as younger adults. As described previously, studies of associative memory in aging (e.g., Naveh-Benjamin, 2000) have provided strong evidence that aging impairs the ability to form and retrieve associative information, especially during intentional encoding and retrieval tasks. However, there have been mixed findings as to the status of incidentally formed associations, both within the explicit (Old & Naveh-Benjamin, 2008) and the implicit (Dew et al., 2007) memory literatures. One possible explanation for the inconsistent findings is that the pattern may depend on whether the task employed involves explicit retrieval processes (Laver, 2009; Wegesin et al., 2004). Importantly, incidentally-learned stimulus-response associations have been demonstrated to affect priming by reducing executive processes by promoting increased automaticity of retrieval (e.g., Dobbins et al., 2004; Logan, 1988). Thus, if older adults are unimpaired in associative learning and retrieval when controlling for strategic or explicit retrieval processes, then older and younger adults should demonstrate rapid response learning to an equal extent.
Method
Participants
Twenty-four younger adults (ages 18–25 years, M = 20.0, SD = 2.1; mean yrs education =13.9, SD = 1.6) participated in partial fulfillment of a requirement in an Introduction to Psychology course at the University of North Carolina at Chapel Hill. Twenty-four community-dwelling older adults (ages 64–85 years, M = 73.8, SD = 7.3; mean yrs education = 16.6, SD = 2.1) participated and were compensated $10 per hour. Participants received a general health screen and completed a battery of neuropsychological tests to assess memory, language, attention, visuo-spatial abilities, and general intellectual functioning. These tests included the Mini Mental State Examination (MMSE), American National Adult Reading Test (ANART), Trail Making Test parts A and B, Vocabulary from the WAIS-III, and the Morningness-Eveningness questionnaire. Mean scores are listed in Table 1.
Table 1.
Mean scores on battery ofneuropsychological tests.
| TMA | TMB | |||||||
|---|---|---|---|---|---|---|---|---|
| MMSE | ANART | Vocab | Trail Making A | Errors | Trail Making B | Errors | Morn/Eve | |
| Experiment 1 | ||||||||
| YA | 30.00 | 40.21 | 55.78 | 22.45 | 0.00 | 58.13 | 0.00 | 38.65 |
| OA | 29.78 | 46.28 | 59.29 | 30.78 | 0.00 | 69.11 | 0.00 | 60.17 |
|
|
||||||||
| Experiment 2* | ||||||||
| YA | 29.83 | 31.23 | 47.32 | 18.64 | 0.04 | 55.94 | 0.00 | 52.78 |
| OA | 29.09 | 41.26 | 55.96 | 34.87 | 0.00 | 74.74 | 0.00 | 60.52 |
Includes data from 29/30 OAs
Design and Materials
The stimuli consisted of line drawings of familiar objects taken from the Microsoft Online Clip-Art Database and the website http://www.clipart.com. Each object was singularly colored in one of eight plausible colors (e.g., a silver hammer, a brown shoe) using Adobe Photoshop. All objects were pilot tested for consistency in response to the classification task. Objects with less than 95% consistency were used as practice or filler trials. In total, there were 140 objects which were counterbalanced across all conditions using the Latin Square method. This counterbalance produced 4 list versions for each task. The stimuli were divided pseudo-randomly with the constraint that each list produced approximately 50% yes responses and 50% no responses to the classification task. Each of the two encoding lists began with 4 practice pairs (in which the experimenter could provide feedback to ensure adherence to the instructions), followed by 2 primacy buffers, 35 critical trials, and ended with 2 recency buffers. Each of the two retrieval lists included the 35 studied trials and 35 baseline critical new trials, plus 28 filler trials that served to decrease the connection between the study and test portions of the experiment. Order of the test pairs was randomized with the constraint that it began and ended with two filler trials. During the encoding tasks, each trial remained on the screen for 6000ms regardless of the timing of response, in order to ensure that all subjects viewed the stimuli for the same amount of time. In order to encourage speed during the implicit retrieval tasks, trials disappeared from the screen as soon as a response was pressed, and were replaced by a crosshair which remained on the screen until the onset of the next trial, with a total ISI of 4000ms. The experiment was presented on an Apple iBookG4 using the program MacStim (WhiteAnt Occasional Publishing).
Procedure
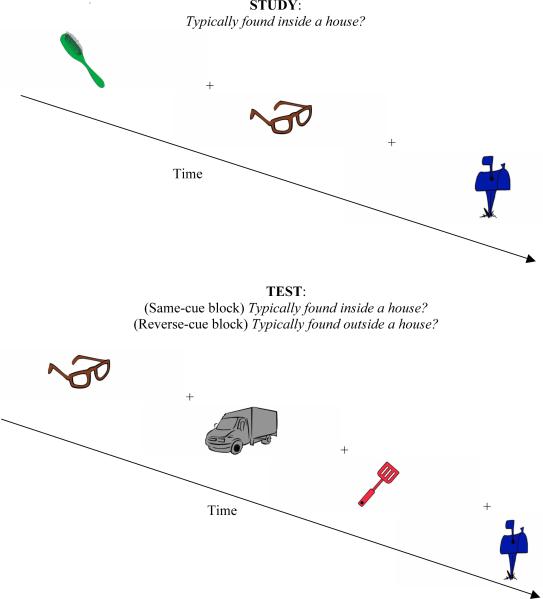
After obtaining informed consent, participants completed the experiment individually in a quiet, enclosed testing room. The experiment was introduced as a series of problem solving activities. Subjects completed two study-test blocks. Half the subjects were randomly assigned to complete the same-cue block first and the other half completed the reverse-cue block first. The same-cue test block began with encoding, in which subjects were instructed to view the trials and press the keys labeled as “yes” or “no” (the z and x keys) to indicate whether each object is typically found inside a house. Participants were told that they should respond “no” for objects that could possibly be found inside a house but are typically found elsewhere (e.g., a basketball). The same-cue version of the implicit classification test instructed subjects to make the same decision as earlier, as quickly as possible, without sacrificing the accuracy of the response. The reverse-cue test block began with encoding, which was identical to encoding in the same-cue block with the exception that a different set of objects was presented. The reverse-cue classification test instructed subjects to decide whether each object is typically found outside a house. Between each encoding and implicit test, subjects completed distractor tasks consisting of anagram word puzzles and arithmetic problems. A schematic of the stimuli and experimental design is depicted in Figure 1.
Figure 1.
Experiment 1 stimuli and design. Subjects completed two study-test blocks. Half the subjects completed the same-cue study- test block first and the remaining half began with the reverse-cue study-test block. Each block begins with the encoding task, in which subjects decide whether the presented object is typically found inside a house. An implicit test follows encoding. During the same-cue version of the test, subjects decide as quickly as possible without sacrificing accuracy whether the object is typically found inside a house. The test includes new trials and old trials from the encoding task that just preceded it. The reverse-cue study-test block begins again with encoding, which is identical to the same-cue encoding task except that a different set of trials is presented. Encoding is followed by implicit testing. During the reverse-cue version of the test, subjects decide whether the object is typically found outside a house. The test includes new trials and old trials from the encoding task that just preceded it. No objects are repeated between blocks.
Results and Discussion
Mean reaction times and standard errors for each condition are displayed in Table 2. Outliers of more than two standard deviations from the mean were removed from each condition for every subject. Similar to other studies of response learning (e.g. Horner & Henson, 2009; Schnyer et al., 2007), only correct (i.e., consistent) responses were included in analyses. The removal of outlying and inconsistent responses resulted in the exclusion of 3.6% of the total trials in young adults and 5.2% of total trials in old adults. This numerical difference was not significant (p = .42). Collapsing both retrieval tasks, older adults had slower baseline reaction times than younger adults (t(46)=−3.65, p = .001), consistent with general cognitive slowing (Salthouse, 1996). Using a mixed ANOVA with same-cue and inverted-cue baseline reaction times as a within-subjects factor and age as a between-subjects factor, there was a significant overall effect of cue reversal on baseline reaction times (F (1, 46) = 14.74, p <.001), demonstrating a cost associated with task switching, consistent with trends in some previous studies (e.g., Schnyer et al., 2007). There was no interaction between age and switch costs, F <1. Follow-up t –tests showed more specifically that both young (t(23)=−3.59, p<.01) and old (t(23)=−2.00, p=.057) had slower responses to baseline trials in the reverse-cue condition relative to the same-cue condition.
Table 2.
Mean reaction times in milliseconds (and standard errors) for each retrieval condition in Experiment 1.
| Same-Cue Condition | Reverse-Cue Condition | |||
|---|---|---|---|---|
| Studied | New | Studied | New | |
| Young | 784 (54) | 918 (67) | 1018 (58) | 1087 (63) |
| Old | 1060 (55) | 1251 (57) | 1281 (73) | 1364 (71) |
Priming was measured as a decrease in reaction time to studied trials relative to new trials. Rapid response learning was operationalized as a decrease in priming following cue reversal relative to the same-cue condition. Mean priming effects (i.e., RT differences) are presented in Table 3. We conducted a 3-way ANOVA with factors of age, priming (i.e., old vs. new item status), and cue condition (i.e., same vs. reversed). There was a significant effect of priming, (F (1, 46) = 67.84, p <.001), with reaction times to studied objects faster than to new objects. There was also a significant effect of cue condition (F (1, 46) = 31.20, p < .001), with overall slower reaction times for cue-reversed relative to same-cue trials. There was a significant interaction between priming and cue condition, (F (1, 46) = 11.40, p < .01), with larger priming effects for the same-cue relative to reversed-cue condition. There was no interaction between priming and age (F < 1), nor was there an interaction between cue condition and age (F < 1). Importantly, there was no three-way interaction between priming, cue condition, and age (F < 1). Lastly, we conducted a 3-way ANOVA with factors of condition, priming, block order (i.e., whether subjects started with the same-cue or reverse-cue study-test block). Block order did not participate in any significant interactions (all Fs <1). Thus, the results did not vary according to whether the same-cue or reverse-cue block was completed first.
Table 3.
Mean reaction time in milliseconds (and standard errors) for each retrieval condition in Experiment 2.
| Same-Cue Condition | Reverse-Cue Condition | |||||
|---|---|---|---|---|---|---|
| Intact | Recombined | New | Intact | Recombined | New | |
| Young | 853 (32) | 906 (48) | 1048 (50) | 999 (35) | 1002 (46) | 1116 (56) |
| Old | 1135 (36) | 1200 (47) | 1486 (72) | 1356 (51) | 1373 (50) | 1554 (55) |
In summary, both young and older adults demonstrated significant priming in same- and reversed-cue conditions, and the priming reduction following cue reversal did not differentially impact the age groups. Thus, Experiment 1 showed preserved rapid response learning in aging. Interestingly, despite the nonsignificant 3-way interaction between age, priming, and cue condition, the results actually show a direction of a larger impact of cue reversal on priming in the older adults, as seen in Table 3. This pattern will be discussed in more detail in the General Discussion. Irrespective of rapid response learning, Experiment 1 also showed that priming on the speeded classification task in the same-cue condition is not impaired in aging; indeed, the absolute magnitude of RT differences was actually in the direction of more priming in the older adults. This pattern is interesting given that general slowing accounts (e.g., Salthouse, 1996) might suggest that speeded access to semantic information may be impaired in aging, despite preservations in general semantic knowledge (e.g., Burke & MacKay, 1997). The finding of age-related preservations in same-cue priming is important for present purposes because reductions in priming following cue reversal can be attributed to S-R learning rather than a more general impairment on the classification task.
Of note, the pattern of age-invariant rapid response learning might be seen as inconsistent with a trend reported by Schnyer et al. (2006). In that study, the authors found that healthy age-matched controls to the MTL-amnesic patients (n=12, mean age = 55.1 years) showed some evidence of rapid response learning, with cue reversal reducing priming for high-primed items. However, unlike the healthy young adults, the age-matched controls did not also show a significant priming reduction for low-primed items. That finding suggests that the controls required multiple presentations in order to bind together the items with their response, an indication of at least partial decline in the ability to form incidental stimulus-response associations. However, there was a directional effect of reduced priming for low-primed items following cue-inversion in the controls (Experiment 2, block 2). As such, it is uncertain whether a significant effect might have emerged with a larger sample size. In the present study, only one stimulus presentation was necessary for both age groups to form an episodic association between the object and the response required for that objects. These findings present a new and important example of a form of associative processing that appears to be preserved in aging. These data fit with other evidence in the literature that despite marked age-related impairments in many measures of associative memory (Naveh-Benjamin, 2000), not all forms of associative processing are equally affected by aging (Old & Naveh-Benjamin, 2008; Naveh-Benjamin et al., 2009).
However, an alternative possibility for the age-equivalence found in the present experiment is that the formation of association between a single stimulus and its required response may have been too simple to detect reliable differences. There is evidence from the working memory literature that age-related impairments in feature binding are compounded by cognitive load (Mitchell, Raye, Johnson & D'Esposito, 2000a), with memory performance declining even when the number of features increased from one to two. Furthermore, Gagnon, Soulard, Brasgold, and Kreller (2007) found a direct correlation, with older adults' memory for contextual details (e.g., color or size) decreasing as the number of features increased, a finding that was attributed to decreased attentional resources. This interpretation fits with complementary studies (Braver, Satpute, Keys, Racine & Barch, 2005; Glisky, Rubin & Davidson, 2001) that highlight the importance of frontal lobe functioning in the encoding and maintenance of context information. The negative correlation between relational complexity and performance (Gagnon et al., 2007; Mitchell et al., 2000a) suggests that in a more complex classification paradigm, older adults may have fewer processing resources available than young adults to form a stimulus-response association following a single stimulus presentation.
Experiment 2
The primary goal of Experiment 2 was to test whether age differences in rapid response learning would emerge in a more complex speeded classification paradigm, where more than one association must be formed. In this Experiment, we take an approach based on McKoon and Ratcliff (1979). In their study, subjects first encoded a series of unrelated word pairs. Later they complete a speeded lexical decision task for words that appeared either alongside their studied associate (i.e., an intact pair), or next to a different studied word (i.e., a recombined pair). Associative priming was demonstrated by faster performance for words presented within their studied context. In the present experiment, we use an associative version of the inside/outside speeded classification task used in Experiment 1. At study, subjects associate otherwise unrelated objects by deciding which is more likely to be found inside a house. At test, subjects are presented with intact, recombined, and new object pairs. Associative priming is measured as faster reaction time for intact trials relative to recombined trials, and can be interpreted as evidence of an encoded association between the object stimuli. Of note, stimulus-response learning in an associative decision task has been previously examined in young adults by Dennis and Schmidt (2003). In their study, subjects viewed pairs of unrelated words (e.g., elephant-jeep, desk-flowerpot, thimble-squirrel) and decided which was larger. Later, reaction times were compared for trials in a re-pair match condition (recombined items that required the same item-specific response, e.g., desk-squirrel) with a re-pair mismatch condition (recombined items that required the opposite item-specific response, e.g., desk-jeep). Reaction times were faster for the re-pair match condition relative to the re-pair mismatch condition. This finding coincides with the recent studies of rapid response learning (e.g., Schnyer et al., 2007; Horner & Henson, 2008, 2009), in that performance was facilitated for trials in which subjects could rely on prior item-specific responses. Based on the findings from Dennis and Schmidt (2003), we expected that cue inversion would reduce associative priming in young adults. The critical question for present purposes was whether cue inversion would equivalently reduce associative priming in older adults. Prior evidence that older adults' relational memory performance declines with the number of features to be associated (Gagnon et al., 2007; Mitchell et al., 2000a) suggests that both age groups will show associative priming when the cue remains the same, but that only young adults' priming will be affected by cue inversion.
Method
Participants
Thirty young adults (ages 18–22 years, M = 19.3, SD = 1.1; mean yrs education = 12.7, SD = 1.2) and 30 community-dwelling older adults (ages 65–83 years, M = 72.6, SD = 6.2; mean yrs education = 16.3, SD = 2.4) participated in Experiment 2. None of the subjects in Experiment 2 had participated in Experiment 1. The young adults participated in partial fulfillment of an Introduction to Psychology course requirement at UNC- Chapel Hill. Older adults were compensated $10 per hour. Eligibility criteria were the same as in Experiment 1.
Design and Materials
The stimuli from Experiment 1 were paired together to create trials of two objects presented side-by-side, with each object framed by a line box. Pairings were created from pre-experimental ratings that judged the likelihood that each object would be found inside a house. Pairs were constructed such that a consistent but not necessarily obvious response would be made on each trial. This procedure was selected to ensure that subjects would need to consider both objects in every trial in order to decide correctly. For half the trials, the object more likely to be found inside a house appeared on the left side and the remaining half appeared on the right. Each object's relative left/right location remained fixed from study to test. In total there were 60 critical pairs that were counterbalanced across two study-test blocks. This counterbalance produced 6 list versions for each task. Each encoding list included 4 practice pairs followed by 20 critical pairs, and ended with 2 recency buffer pairs. Each corresponding implicit test list included 10 counterbalanced critical new pairs plus the 20 studied pairs, 10 of which were presented as intact pairs (i.e., presented together previously), and 10 of which were rearranged with each other to form recombined pairs (i.e., each object presented but not together). The recombined trials were constructed such that an object's relative classification status did not change from study to test. Thus, although the objects were presented in a new context, the response mapping for any given object remained constant. Twenty additional filler pairs appeared on each implicit test, which, like in Experiment 1, were not included in analyses but served to increase test length as well as the ratio of new to old trials. The order of trials on the implicit tests was randomized with the constraint that it began and ended with two filler pairs. For the study task, objects remained on the screen for 6000ms regardless of the timing of response, after which the next trial appeared automatically. To encourage speed during the implicit task, objects were cleared from the screen after the subject's response and were replaced with a fixation cross, which remained on the screen until the onset of the subsequent trial, with a consistent ISI of 4000ms.
Procedure
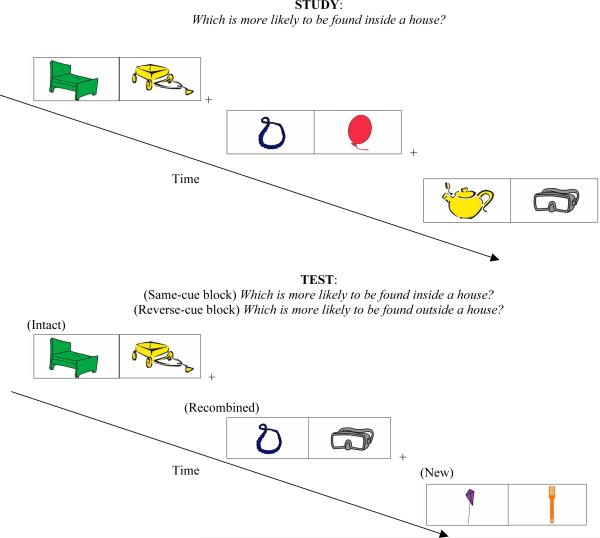
Like Experiment 1, subjects completed two study-test blocks. Half the subjects were randomly assigned to complete the same-cue block first and the other half completed the reverse-cue block first. The same-cue test block began with encoding, in which subjects were instructed to view the trials and press the keys labeled as “left” or “right” (the j and k keys) which of the two presented objects is more likely to be found inside a house. During the same-cue implicit test, subjects decided which object was more likely to be found inside a house, as quickly as possible without sacrificing accuracy. Subjects were told that a fixation cross would appear between each trial, and were instructed to keep their eyes focused on the cross. Encoding in the same-cue and reverse-cue blocks was identical with the exception that a different set of objects was presented. The reverse-cue test asked subjects to decide which of the two objects was more likely to be found outside a house. Between each encoding and implicit test, subjects completed distractor tasks consisting of anagram word puzzles and arithmetic problems. A schematic of the stimuli and experimental design is depicted in Figure 2.
Figure 2.
Experiment 2 stimuli and design. Subjects completed two study-test blocks. Half the subjects completed the same-cue study- test block first and the remaining half began with the reverse-cue study-test block. Each block begins with the encoding task, in which subjects decide which of two presented objects is more likely to be found inside a house. An implicit test follows encoding. During the same-cue version of the test, subjects decide as quickly as possible, without sacrificing accuracy, which of two presented objects is more likely to be found inside a house. The test includes pairs that are new (i.e., neither object studied), intact (i.e., objects studied together during encoding), and recombined (i.e., each object studied but not together). Intact, recombined, and new status is noted above for schematic purposes but was not identified as such during the actual experiment. The reverse-cue study-test block begins with encoding, which is identical to the encoding task from the same-cue block except that a different set of trials is presented. Encoding is followed by implicit testing. During the reverse-cue version of the test, subjects are asked to decide as quickly as possible without sacrificing accuracy which of two presented objects is more likely to be found outside a house. No objects are repeated between blocks.
Results and Discussion
Mean reaction times and standard errors for each retrieval condition are displayed in Table 3. Outliers of more than two standard deviations from the mean were removed from each condition in each subject, and only correct responses were included in analyses. These procedures yielded 4.8% of trials excluded in young adults and 6.2% excluded in old adults. This difference was not significant (p = .54). Like Experiment 1, older adults had slower baseline RTs than younger adults when collapsing across cue conditions, (t(58)= −6.55, p<.001). Using a mixed ANOVA with same-cue and inverted-cue baseline (i.e., new trial) reaction times as a within-subjects factor and age as a between-subjects factor, there was not a significant overall effect of cue reversal on baseline reaction times (F (1, 58) = 1.87, p = .18), nor was there a significant interaction between switch costs and age (F <1). Thus, unlike Experiment 1, there was no evidence of task switch costs in either age group. Although this presents a different pattern from Experiment 1, such mixed findings are not inconsistent with the prior literature: task switch costs are sometimes found (Schnyer et al., 2007, Exp. 1 block 2), and sometimes there is no baseline difference between same-cue and inverted-cue trials (Schnyer et al., 2007, Exp. 1 block 1). Baseline trials in both the same and inverted trials were slower for both age groups in Experiment 2 relative to identical conditions in Experiment 1, possibly suggesting that the associative classification is more difficult than the item-level version.
Associative priming was measured as response times to recombined trials minus response times to intact trials. We conducted a 3-way ANOVA with factors of cue condition (same-cue versus reverse-cue trials), associative priming (intact versus recombined trial status), and age. There was a significant effect of condition, (F (1, 62) = 21.32, p <.001), with overall RTs slower in the reverse-cue condition relative to the same-cue condition. There was no interaction between age and condition, (F (1, 58) = 1.59, p =.22), indicating that task switch costs for the studied objects (i.e., collapsed across intact and recombined trials) were equivalent in older and younger adults. The overall main effect of associative priming was only marginally significant (F (1, 58) = 3.4, p =.07), a result that was likely a function of the interaction between priming and condition. Indeed, there was a significant interaction between condition and associative priming, (F (1, 58) = 4.00, p = .05), showing that cue reversal significantly reduced associative priming. Follow-up t-tests showed that cue reversal in fact eliminated associative priming in both age groups: priming was significantly greater than zero in the same-cue condition in young (t (29) = 2.74, p =.01) and older (t (29) = 2.19, p <.05) adults, but was not significantly greater than zero in the reverse-cue condition in either young (p =.95) or old (.65) adults. There was no interaction between age and associative priming (F <1), showing that the context change between intact and recombined trials produced an equivalent effect on both age groups. Importantly, in the omnibus test, there was no 3-way interaction between condition, associative priming, and age (F <1), showing that the effect of cue reversal on associative priming was not modulated by age. Lastly, like Experiment 1, block order did not participate in any significant effects (all Fs <1).
Additionally, because the individual objects within recombined pairs were previously studied but not together, the comparison between recombined and new trials offers a measure of item priming in the absence of an association. Subsequently, rapid response learning in item priming can be measured as an item priming reduction for the reverse-cue condition relative to the same-cue condition. We conducted a 3-way ANOVA with factors of age, item priming (recombined versus new trial status) and cue condition (same versus reversed). There was a significant effect of item priming (F (1, 58) = 72.91, p < .001), with reaction times faster for recombined trials than new trials. There was also a significant effect of cue condition (F (1, 62) = 6.55, p =.01), with overall reaction times in the reverse-cue condition slower than in the same-cue condition. There was a significant interaction between item priming and age (F (1, 62) = 6.08, p <.05), with in fact more item priming in the older than young adults. This age difference is likely a function, at least in part, of the slower baseline reaction times in older adults; when we re-calculated priming as a function of percent change from baseline (i.e., rather than RT difference), there was not a significant age difference (p = .16), with 10.0% change in young adults and 14.2% change in older adults. Unlike Experiment 1, there was not a significant interaction between priming and cue condition (F (1, 58) = 2.60, p =.11), indicating that cue reversal did not significantly reduce item priming. Thus, unlike associative priming or the priming of single objects in Experiment 1, rapid response learning in the current measure of item priming was not clearly evident. Still, the numerical direction of the difference indicated less item priming under conditions of cue reversal in both age groups, especially in the older adults. This interesting pattern will be discussed in more detail in the General Discussion. There was not a significant interaction between condition and age group (F <1), demonstrating that the effect of cue reversal on priming was not moderated by age. Importantly, there was not a significant 3-way interaction between priming, cue condition, and age (F <1). Like with associative priming, block order did not participate in any significant effects (all Fs<1).
In summary, the pattern of equivalent associative priming in the same-cue condition indicates that the older adults were able to form an associative link between unrelated objects as well as young adults under an incidental encoding condition. Moreover, inverting the decision cue disrupted associative priming in both age groups. It is therefore unlikely that the pattern in Experiment 1 emerged only because of the simplicity of the paradigm. In contrast with prior findings in the literature in which age-related memory performance was disrupted by an increased number of features (Gagnon et al., 2007; Mitchell et al., 2000a), older adults formed a stimulus-response link even when required to form an additional association between the object stimuli. Although one could argue that the lack of associative priming was not due to stimulus-response learning but rather reflected insufficient encoding of the association between the object stimuli, this possibility is unlikely. The encoding conditions were identical under both the same-cue and reversed-cue conditions, with the order of the blocks counterbalanced across subjects. It was only when the retrieval instructions were manipulated that priming was affected; therefore an encoding explanation is not tenable. Rather, the retrieval manipulation in Experiment 2 highlights stimulus-response learning as a mechanism that underlies priming in this associative priming paradigm, consistent with recent interpretations of repetition priming on speeded classification tasks (Dobbins et al., 2004; Horner & Henson, 2008, 2009; Schnyer et al., 2006, 2007).
General Discussion
The present experiments provide an example of a form of associative processing that appears to be unimpaired by healthy aging. As described previously, aging has been linked with a disproportionate decline in associative memory relative to item memory, particularly under intentional encoding and retrieval conditions (Naveh-Benjamin, 2000; Old & Naveh-Benjamin, 2008; Spencer & Raz, 1995). However, the status of incidental associative processing has been controversial, both within the explicit (see Old & Naveh-Benjamin, 2008) and implicit (see Dew et al., 2007) memory literatures. The present paper offers two measures of incidental associative processing in aging: First, an RT benefit for intact relative to recombined pairs in the same-cue condition in Experiment 2 indicates facilitated task performance for objects presented in their studied context. In this measure, older and younger adults produced equivalent levels of associative priming, providing evidence that the association between studied objects was encoded. This finding replicates results from our laboratory using a similar paradigm (Dew & Giovanello, submitted). Second, a reduction in priming for trials in the reverse-cue relative to same-cue conditions indicates an increased reliance on previously learned responses, an effect that is dependent on a stimulus-response associative learning mechanism. Critically, we found no evidence of an age difference in rapid response learning, either in repetition priming (Exp. 1) or in the more complex associative priming paradigm (Exp. 2). As such, rapid response learning appears to be preserved in healthy aging.
Interestingly, both experiments show a direction of more priming in the older than younger adult group, as well as a relatively larger impact of cue reversal on priming in the older adults. One possible explanation for this trend is that it might be consistent with an inhibitory deficit in aging. Older adults often show impairments in the process of inhibiting irrelevant information, both at encoding and retrieval (see Hasher & Zacks, 1988). As such, it would follow that a test manipulation that renders a learned response inappropriate might have a relatively larger impact on older than younger adults, to the extent that responding correctly under conditions of cue inversion requires inhibition of the learned response. An inhibitory deficit in aging might maximize age differences under conditions of a full reversal of the decision cue, as employed in the current paradigm, relative to a manipulation in which the decision cues at study and test are simply orthogonal. A future study that examines age differences in the effect of cue reversal versus an orthogonal change in decision cue might help inform the role of an age-related inhibitory deficit in rapid response learning. More generally, the current findings complement other paradigms in the literature that have examined the role of inhibition in repetition priming. For instance, Hasher, Quig and May (1997) also showed that when older adults process irrelevant information at encoding (i.e., due to an inhibitory deficit), they produce a priming advantage during implicit testing. Likewise, the present results are also consistent with those found by Marczinski, Milliken and Nelson (2003), who examined the relative contributions of specific and nonspecific influences to repetition effects. Specific influences referred to the mapping between a specific stimulus and its response, and nonspecific influences referred to a more general response repetition independent of the stimulus. Reaction time in older adults was disproportionately slowed when the same response was required for a different stimulus versus when the same response was required for the same stimulus. Consistent with the present studies, this pattern suggests a perseveration of the initial specific stimulus-response mapping.
Importantly, a perseveration of a stimulus-response mapping in the present experiments indicates age-invariance in the initial binding of the stimulus and its associated response. Thus, despite the tendency to observe age-related decline across various associative paradigms (Old & Naveh-Benjamin, 2008; Spencer & Raz, 1995), the results in the present experiments fit with a growing body of research showing that when associative tasks do not require elaborative encoding or strategic retrieval processes, older adults perform at the level of young adults (e.g., Laver, 2009; Wegesin, et al., 2004). As such, the present studies are consistent with the position that not all forms of associative processing are impaired by aging (Old & Naveh-Benjamin, 2008). Interestingly, not all the literature is suggestive of an age-related deficit in intentional associative encoding, either. For instance, while older adults typically show poorer recall performance, they actually chunk information similarly when to-be-learned sequences are not longer than 6 items (e.g., Allen & Coyne, 1988, Allen & Coyne, 1989; Allen & Crozier, 1991). When sequences are longer (i.e., to the extent that processing resources can become exceeded), older adults do show deficits in chunking (e.g., Naveh-Benjamin, Cowan, Kilb, & Chen, 2007). These findings highlight a joint contribution of declines in executive control processes and declines in more general processing resources to the typically observed age-related associative memory declines.
In addition to the aging findings, the present experiments also identified several noteworthy patterns in the young adults. First, the present experiments provide further evidence of rapid response learning as a mechanism that underlies priming on speeded classification tasks (Dobbins et al., 2004; Horner & Henson, 2008, 2009; Schnyer et al., 2007), and generalized this pattern to a new task. Experiment 1 showed that cue inversion reduced priming using a speeded inside/outside judgment, and in Experiment 2 cue inversion eliminated priming on an associative version of this task. Although previous theoretical accounts, such as a neural sharpening mechanism (Wiggs & Martin, 1998), have been successful in explaining certain instances of priming, especially tasks in which the stimulus is changed from study to test (Schacter, Wig & Stevens, 2007), rapid response learning may be more successful in accounting for priming on speeded classification tasks in which the stimulus remains constant (Dobbins et al., 2004; Horner & Henson, 2008, 2009; Schnyer et al., 2007; see also Logan, 1988, 1990). The present experiments thus fit well within this literature.
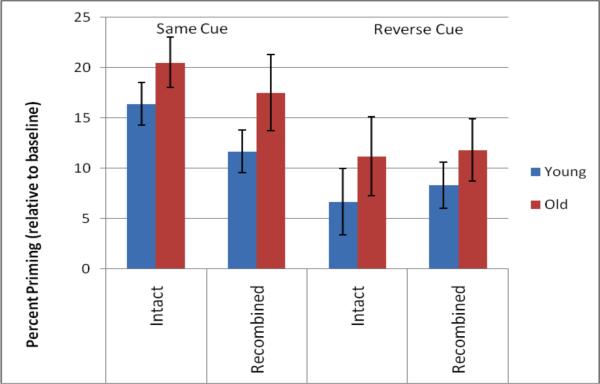
Additionally, Experiment 2 assessed the role of context re-instatement in rapid response learning. Cue reversal produced a small and nonsignificant effect on item priming (in which RTs to recombined pairs were compared with RTs to new pairs). In contrast, cue reversal produced a significant effect on associative priming, such that cue reversal wholly eliminated the benefit that the intact pairs previously held over the recombined pairs, as reflected in Figure 3. This difference between item and associative priming is intriguing, given that the object-level decision was always preserved, regardless of whether any given object was later presented in an intact or a recombined pair. Because the prior object-level response always remained appropriate, one might have reasonably assumed that this preserved correspondence would contribute to facilitation of performance in both item and associative priming. In contrast to this assumption, the preserved priming of recombined pairs relative to novel pairs was relatively unaffected by whether or not the prior response remained appropriate. There are several interesting possibilities as to the mechanism of this effect. Perhaps the most likely explanation is that the paradigm emphasized the relational judgment rather than the item-level judgment. If subjects were truly engaging in a relational decision, then the context manipulation would be essential to the classification decision and thus to response mapping. In this case, the individual-object-level consistency should be relatively unimportant. Two findings – 1) the RT benefit in intact relative to recombined trials in the same-cue condition, and 2) the larger effect on associative relative to item priming in the reverse-cue condition – provide evidence that in subjects are indeed engaging in a relational, rather than item-level, judgment. This has important implications also for aging, as it argues against the possibility that the unimpaired associative priming in the older adults (i.e., in the same-cue condition) was not due to preservations in associative binding but rather to the preserved encoding of individual objects.
Figure 3.
Priming effects as a function of percent change from baseline in Experiment 2. While cue reversal produced a small, nonsignificant reduction in the magnitude of item priming, it reversed the direction of associative priming, such that priming in the intact condition was no longer facilitated relative to the recombined condition. This pattern was observed in both young and older adults.
Alternatively, it is possible that the larger effect of cue reversal on associative relative to item priming speaks to the importance of stimulus-level specificity required for priming to be affected by a change in decision cue. In the current literature, there have been mixed findings as to whether rapid response learning is specific to studied stimuli or occurs at a more abstract level of analysis. For instance, Schnyer et al. (2007) found that cue reversal did not have a larger impact on high- relative to low-primed items when a different object exemplar was presented at test, despite the fact that presentation of a different exemplar should not affect the classification decision. These results imply that rapid response learning may be highly perceptually specific. In contrast, Denkinger and Koutstaal (2009) recently showed that both a reversal and an orthogonal change in decision cue reduced priming for objects that were perceptually or conceptually similar but not identical to the original stimulus (see also Waszak, Hommel, & Alpert, 2004 for a similar result). If rapid response learning is highly stimulus-specific (Schnyer et al., 2007), this would imply that a form of item priming in which the context of a studied object is changed might not be impacted by cue reversal. As such, the present study might be seen as consistent with the results found by Schnyer et al. (2007), converging on the suggestion of a high degree of perceptual specificity in rapid response learning. The current data merely speculate on this possibility, because context in the present study was relevant for the classification decision. Further data are needed to determine whether rapid response learning reduces priming when a context manipulation is irrelevant for the task.
Lastly, it is worth noting that the lack of age differences presents an important difference between healthy aging and MTL-amnesia. While Schnyer et al. (2006) found that repetition priming in patients with MTL damage was unaffected by cue inversion, priming in the healthy older adults in the current studies was affected to the same extent as young adults. Although both populations have been described as having a deficit in associative processing, the impairments may have different sources. The prefrontal cortex (PFC) is known to be involved in the strategic organization of associative features (Buckner, 2003; Dobbins, Foley, Schacter & Wagner, 2002), and there is evidence that this region is particularly susceptible to age-related decline (e.g., Head, Snyder, Girton, Morris & Buckner, 2005). Age differences may also stem from impairments in the circuitry between the PFC and the hippocampus (Li, Naveh-Benjamin & Lindenberger, 2005; Mitchell, et al., 2000b). As described earlier, Dobbins et al. (2004) found that cue inversion disrupted priming in left PFC, a finding that supports rapid response learning as a reflection of increased automatized processing in the context of decreased executive processes. A population with impaired PFC function should thus benefit by relying on prior responses, and the lack of age differences in the present experiments fits with this hypothesis. A functional neuroimaging approach would be an important future direction for the present studies, to determine whether, under conditions of cue reversal, neural priming operates similarly in both age groups.
Table 4.
Priming effects in msec (and standard errors) in Experiments 1 and 2.
| Same Cue | Reverse Cue | ||
|---|---|---|---|
| Exp. 1 | YA | 134 (20) | 69 (24) |
| OA | 191 (19) | 83 (41) | |
| Exp. 2: Item Priming | YA | 143 (47) | 115 (38) |
| OA | 286 (41) | 181 (41) | |
| Exp. 2: Assoc. Priming | YA | 53 (36) | 2.4 (39) |
| OA | 65 (30) | 17 (38) | |
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/PAG
References
- Allen PA, Coyne AC. Age differences in primary organization or processing variability Part I: An examination of age and primary organization. Experimental Aging Research. 1988;14(2-3):143–9. doi: 10.1080/03610738808259739. [DOI] [PubMed] [Google Scholar]
- Allen PA, Coyne AC. Are there age differences in chunking? Journal of Gerontology. 1989;44(6):181–3. doi: 10.1093/geronj/44.6.p181. [DOI] [PubMed] [Google Scholar]
- Allen PA, Crozier AC. Age and ideal chunk size. Journal of Gerontology. 1992;47(1):47–51. doi: 10.1093/geronj/47.1.p47. [DOI] [PubMed] [Google Scholar]
- Bastin C, Van der Linden M. The contribution of recollection and familiarity to recognition memory: A study of the effects of test format and aging. Neuropsychology. 2003;17:14–24. [PubMed] [Google Scholar]
- Bowers JS, Schacter DL. Implicit memory and test awareness. Journal of Experimental Psychology: Learning, Memory and Cognition. 1990;3(16):404–416. doi: 10.1037//0278-7393.16.3.404. [DOI] [PubMed] [Google Scholar]
- Braver TS, Satpute AB, Rush BK, Racine CA, Barch DM. Context processing and context maintenance in healthy aging and early stage dementia of the Alzheimer's type. Psychology and Aging. 2005;20(1):33–46. doi: 10.1037/0882-7974.20.1.33. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Functional-anatomic correlates of control processes in memory. The Journal of Neuroscience. 2003;23(10):3999–4004. doi: 10.1523/JNEUROSCI.23-10-03999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, MacKay DG. Memory, language, and ageing. Philosophical Transactions of the Royal Society of London B. 1997;352:1845–1856. doi: 10.1098/rstb.1997.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Memory & Cognition. 1996;24:403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Poldrack RA, Eichenbaum H. Memory for items and memory for relations in the procedural/declarative memory framework. Memory. 1997;5(1-2):131–78. doi: 10.1080/741941149. [DOI] [PubMed] [Google Scholar]
- Cohn M, Emrich SM, Moscovitch M. Age-related deficits in associative memory: The influence of impaired strategic retrieval. Psychology and Aging. 2008;23(1):93–103. doi: 10.1037/0882-7974.23.1.93. [DOI] [PubMed] [Google Scholar]
- Dennis I, Schmidt L. Associative processes in repetition priming. Journal of Experimental Psychology: Learning, Memory and Cognition. 2003;29:532–538. doi: 10.1037/0278-7393.29.4.532. [DOI] [PubMed] [Google Scholar]
- Denkinger B, Koutstaal W. Perceive-decide-act, perceive-decide-act: How abstract is repetition-related decision learning? Journal of Experimental Psychology: Learning, Memory and Cognition. 2009;35(3):742–756. doi: 10.1037/a0015263. [DOI] [PubMed] [Google Scholar]
- Dew ITZ, Bayen UJ, Giovanello KS. Implicit relational memory in young and older adults. Zeitschrift für Psychologie [Journal of Psychology]: Special Issue on Human Memory. 2007;215(1):25–34. [Google Scholar]
- Dew ITZ, Giovanello KS. Differential age effects for implicit and explicit conceptual associative memory. doi: 10.1037/a0019940. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428(6980):316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- Ergis A, Van der Linden M, Deweer B. Priming for new associations in normal aging and in mild dementia of the Alzheimer type. Cortex. 1998;34:357–373. doi: 10.1016/s0010-9452(08)70760-3. [DOI] [PubMed] [Google Scholar]
- Gagnon S, Soulard K, Brasgold M, Kreller J. Effects of normal aging on memory for multiple contextual features. Brain and Cognition. 2007;64(3):208–216. doi: 10.1016/j.bandc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gibson JM, Brooks JO, Friedman L, Yesavage JA. Typography manipulations can affect priming of word stem completion in older and younger adults. Psychology and Aging. 1993;8(4):481–489. doi: 10.1037//0882-7974.8.4.481. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Rubin SR, Davidson PSR. Source memory in older adults: An encoding or retrieval problem? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: Neural models of stimulus-specific effects. Trends in Cognitive Science. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hasher L, Quig MB, May CP. Inhibitory control over no-longer-relevant information: Adult age differences. Memory & Cognition. 1997;25:286–295. doi: 10.3758/bf03211284. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new vies. In: Bower G, editor. The psychology of learning and motivation. Academic Press; New York: 1988. pp. 193–225. [Google Scholar]
- Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Frontal-hippocampal double dissociation between normal aging and Alzheimer's disease. 2005 doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- Horner AJ, Henson RN. Priming, response learning and repetition suppression. Neuropsychologia. 2008;46(7):1979–1991. doi: 10.1016/j.neuropsychologia.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner AJ, Henson RN. Bindings between stimuli and multiple response codes dominate long-lad repetition priming in speeded classification tasks. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35(3):757–779. doi: 10.1037/a0015262. [DOI] [PubMed] [Google Scholar]
- Howard DV, Fry AF, Brune CM. Aging and memory for new associations: Direct versus indirect measures. Journal of Experimental Psychology: Learning, Memory and Cognition. 1991;17:779–792. doi: 10.1037//0278-7393.17.4.779. [DOI] [PubMed] [Google Scholar]
- Howard DV, Heisey JG, Shaw R. Aging and the priming of newly learned associations. Developmental Psychology. 1986;22:78–85. [Google Scholar]
- Jacoby LL. Remembering the data: analyzing interactive processes in reading. Journal of Verbal Learning and Verbal Behavior. 1983;22:485–508. [Google Scholar]
- Jacoby LL. Ironic effects of repetition: Measuring age-related differences in memory. Journal of Experimental Psychology: Learning, Memory and Cognition. 1999;25(1):3–22. doi: 10.1037//0278-7393.25.1.3. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: Aging, attention, and control. Psychology and Aging. 1993;8:283–293. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Kinoshita S. Priming for novel associations: Evidence for an attentional component. Memory. 1999;7:385–404. [Google Scholar]
- Laver GD. Adult aging effects on semantic and episodic priming in word recognition. Psychology and Aging. 2009;24(1):28–39. doi: 10.1037/a0014642. [DOI] [PubMed] [Google Scholar]
- Li SC, Naveh-Benjamin M, Lindenberger U. Aging neuromodulation impairs associative binding: a neurocomputational account. Psychological Science. 2005;16(6):445–50. doi: 10.1111/j.0956-7976.2005.01555.x. [DOI] [PubMed] [Google Scholar]
- Light LL. Memory and aging: Four hypotheses in search of data. Annual Review of Psychology. 1991;42:333–377. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Light LL, La Voie D, Valencia-Laver D, Albertson Owens SA, Mead G. Direct and indirect measures of memory for modality in young and older adults. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1992;18:1284–1297. doi: 10.1037//0278-7393.18.6.1284. [DOI] [PubMed] [Google Scholar]
- Light LL, Prull MW, LaVoie DJ, Healy MR. Dual-process theories of memory in old age. In: Perfect TJ, Maylor EA, editors. Models of cognitive aging. Oxford University Press; New York: 2000. pp. 238–300. [Google Scholar]
- Lloyd-Jones T. The role of color in the implicit memory performance of healthy older adults and individuals with Alzheimer's disease. Neuropsychology. 2005;19(1):44–53. doi: 10.1037/0894-4105.19.1.44. [DOI] [PubMed] [Google Scholar]
- Logan GD. Toward an instance theory of automatization. Psychological Review. 1988;95:492–527. [Google Scholar]
- Logan GD. Repetition priming and automaticity: Common underlying mechanisms? Cognitive Psychology. 1990;22:1–35. [Google Scholar]
- Macstim [computer software] WhiteAnt Occasional Publishing; West Melbourne: [Google Scholar]
- Marczinski CA, Milliken B, Nelson S. Aging and repetition effects: Separate specific and nonspecific influences. Psychology and Aging. 2003;18(4):780–790. doi: 10.1037/0882-7974.18.4.780. [DOI] [PubMed] [Google Scholar]
- Marsolek CJ, Field JE. Perceptual-motor sequence learning of general regularities and specific sequences. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:815–836. doi: 10.1037//0096-1523.25.3.815. [DOI] [PubMed] [Google Scholar]
- McKoon G, Ratcliff R. Priming in episodic and semantic memory. Journal of Verbal Learning and Verbal Behavior. 1979;18:463–480. [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, Mather M, D'Esposito M. Aging and reflective processes of working memory: Binding and test load deficits. Psychology and Aging. 2000a;15:527–541. doi: 10.1037//0882-7974.15.3.527. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D'Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Brain Research Interactive -- Cognitive Brain Research. 2000b;1 doi: 10.1016/s0926-6410(00)00029-x. [DOI] [PubMed] [Google Scholar]
- Monti LA, Gabrieli JDE, Wilson RS. Sources of priming in text rereading: Intact implicit memory for new associations in older adults and in patients with Alzheimer's disease. Psychology and Aging. 1997;12(3):536–547. doi: 10.1037//0882-7974.12.3.536. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory and Cognition. 2000;26:1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, Levy O. The associative memory deficit of older adults: The role of strategy utilization. Psychology and Aging. 2007;22(1):202–208. doi: 10.1037/0882-7974.22.1.202. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Cowan N, Kilb A, Chen Z. Age-related differences in immediate serial recall: dissociating chunk formation and capacity. Memory. 2007;35(4):724–37. doi: 10.3758/bf03193310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Craik FIM, Guez J, Kreuger S. Divided attention in younger and older adults: Effects of strategy and relatedness on memory performance and secondary task costs. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:520–537. doi: 10.1037/0278-7393.31.3.520. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Kilb A, Reedy S. The associative memory deficit of older adults: Further support using face-name associations. Psychology and Aging. 2004;19:541–546. doi: 10.1037/0882-7974.19.3.541. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Hussain Z, Guez J, Bar-On M. Adult age differences in episodic memory: Further support for an associative-deficit hypothesis. Journal of Experimental Psychology: Learning, Memory and Cognition. 2003;29(5):826–837. doi: 10.1037/0278-7393.29.5.826. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Shing YL, Kilb A, Werkle-Bergner M, Lindenberger U. Adult age differences in memory for name-face associations: The effects of intentional and incidental learning. Memory. 2009;17(2):220–232. doi: 10.1080/09658210802222183. [DOI] [PubMed] [Google Scholar]
- O'Hanlon L, Wilcox KA, Kemper S. Age differences in implicit and explicit associative memory: Exploring elaborative processing effects. Experimental Aging Research. 2001;27:341–359. doi: 10.1080/03610730109342353. [DOI] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: A meta-analysis. Psychology and Aging. 2008;23(1):104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Cohen NJ. Priming of new associations in reading time: What is learned? Psychonomic Bulletin & Review. 1997;4(3):398–402. [Google Scholar]
- Roediger HL. Implicit memory: Retention without remembering. American Psychologist. 1990;45(9):1043–1056. doi: 10.1037//0003-066x.45.9.1043. [DOI] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. Implicit memory in normal human subjects. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Vol. 8. Elsevier; Amsterdam: 1993. pp. 63–131. [Google Scholar]
- Ryan LD, Leung G, Turk-Brown NB, Haser L. Assessment of age-related changes in inhibition and binding using eye movement monitoring. Psychology and Aging. 2007;22(2):239–250. doi: 10.1037/0882-7974.22.2.239. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Dobbins IG, Schnyer DM. Specificity of priming: A cognitive neuroscience perspective. Nature Reviews Neuroscience. 2004;5:853–862. doi: 10.1038/nrn1534. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Current Opinion in Neurobiology. 2007;17(2):171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Dobbins IG, Nicholls L, Schacter DL, Verfaellie M. Rapid response learning in amnesia: Delineating associative learning components in repetition priming. Neuropsychologia. 2006;44(1):140–149. doi: 10.1016/j.neuropsychologia.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Dobbins IG, Nicholls L, Davis S, Verfaillie M, Schacter DL. Item to decision mapping in rapid response learning. Memory and Cognition. 2007;35(6):1472–82. doi: 10.3758/bf03193617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP, Squire LR. Impaired priming of new associations in amnesia. Journal of Experimental Psychology: Learning, Memory & Cognition. 1989;15:721–728. doi: 10.1037//0278-7393.15.4.721. [DOI] [PubMed] [Google Scholar]
- Smith AD, Park DC, Earles JLK, Shaw RJ, Whiting WL., IV Age differences in context integration in memory. Psychology and Aging. 1998;13:21–28. doi: 10.1037//0882-7974.13.1.21. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: A meta-analysis. Psychology and Aging. 1995;10(4):527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Spieler DH, Balota DA. Characteristics of associative learning in younger and older adults: Evidence from an episodic priming paradigm. Psychology and Aging. 1996;11(4):607–620. doi: 10.1037//0882-7974.11.4.607. [DOI] [PubMed] [Google Scholar]
- Waszak F, Hommel B, Allport A. Semantic generalization of stimulus-task bindings. Psychonomic Bulletin and Review. 2004;11:1027–1033. doi: 10.3758/bf03196732. [DOI] [PubMed] [Google Scholar]
- Wegesin DJ, Ream JM, Stern Y. Explicit contamination contributes to aging effects in episodic priming: behavioral and ERP evidence. J Gerontol B Sci Soc Sci. 2004;59(6):317–324. doi: 10.1093/geronb/59.6.p317. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Aging and feature-specific priming of familiar and novel stimuli. Psychology and Aging. 1994;9(4):578–588. doi: 10.1037//0882-7974.9.4.578. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Current Opinion in Neurobiology. 1998;8(2):227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]