Abstract
We previously reported that a single exercise session protects against fatty acid (FA)-induced insulin resistance, perhaps in part through augmented intramyocellular triacylglycerol (IMTG) synthesis.
Objective
The aim of this study was to examine the effect of elevated FA availability after exercise on factors regulating IMTG metabolism.
Materials/Methods
After exercise (90 min, 65% VO2peak), 7 healthy women (body mass index: 23 ± 1 kg/m2) were infused overnight (16h) with either a lipid and heparin solution (LIPID; 0.11 g fat/kg/h) or saline (SALINE). We measured resting FA oxidation (indirect calorimetry) and obtained a skeletal muscle biopsy sample the next morning.
Results
The 4-fold increase in overnight plasma FA concentration during LIPID increased IMTG by ~30% during LIPID vs. SALINE. This was accompanied by ~25% greater membrane-associated abundance of the FA transporter FAT/CD36 (P < 0.01), and ~8% increase in the activity of the IMTG synthesis enzyme glycerol-3-phosphate acyltransferase (GPAT; P < 0.01). In contrast, resting FA oxidation was not affected. We also found no difference in the protein abundance of GPAT1 and diacylglycerol acyltransferase-1 (DGAT1), DGAT activity, or the abundance of the lipid droplet coat proteins (perilipins 2, 3, 4, 5) between treatments.
Conclusions
Our findings suggest augmented capacity for FA flux into muscle (i.e., via membrane-associated FAT/CD36), perhaps together with a slight, yet significant increase in activity of a key IMTG synthesis enzyme (GPAT) may enhance IMTG storage when FA availability is high after exercise. The importance of the absence of a change in perilipin protein abundance despite increased muscle lipid storage remains to be determined.
Keywords: FAT/CD36, glycerol-3-phosphate acyltransferase, diacylglycerol acyltransferase, diacylglycerol, ceramide
INTRODUCTION
Excessive fatty acid availability is a primary contributor to the insulin resistance found in obesity [1, 2], and we have demonstrated a single session of exercise can protect against fatty acid-induced insulin resistance [3, 4]. We attributed at least part of this protective effect of exercise to an increase in intramyocellular triacylglycerol (IMTG) synthesis for several hours after the exercise session [3, 4]. While it is clear that high plasma fatty acid availability after exercise (as in obesity) provides more substrate necessary for IMTG synthesis, how elevated fatty acid availability alters the regulation of intramyocellular fatty acid metabolism after exercise is not completely understood.
The synthesis of IMTG occurs through the succession of four reactions. The first committed step of this process is regulated by the enzyme glycerol-3-phosphate acyltransferase (GPAT), which catalyzes the production of lysophosphatidic acid (LPA) from fatty acyl-CoA and glycerol-3-phosphate. The final key step in the triacylglycerol synthesis pathway is regulated by the enzyme diacylglycerol acyltransferase (DGAT), which catalyzes the esterification of a third fatty acyl-CoA to DAG to create a triacylglycerol. We found that a single session of exercise was sufficient to increase the protein abundance of both GPAT and DGAT [3]. However, whether elevated fatty acid availability after exercise is associated with increased GPAT and/or DGAT enzyme activity remains to be determined.
In addition to changes in activity of the triacylglycerol synthesis pathway enzymes, increased fatty acid availability may also influence other factors that can regulate IMTG metabolism after exercise. Perhaps most important is the regulation of fatty acid flux into the myocyte, to provide the necessary substrate for IMTG synthesis. Fatty acid translocase (FAT/CD36) is a principal skeletal muscle fatty acid transporter [5, 6], and the rate of fatty acid uptake is proportional to the abundance of FAT/CD36 on the plasma membrane [7, 8]. Furthermore, because IMTG accumulate largely in hydrophobic lipid droplets within the cytosol, regulation of IMTG metabolism may also be affected by a family of proteins that are known to be associated with intracellular lipid droplets (now collectively referred to as “perilipins” [9]). Although the role of this family of five perilipin proteins on lipid metabolism has predominantly been studied in adipocytes (for reviews, see [10, 11]), most of the perilipin proteins have now also been identified in skeletal muscle, and have been the subject of several recent studies. However, whether high availability of fatty acid after exercise augments muscle fatty acid transport capacity (i.e., increased fatty acid transporter abundance at the muscle membrane) or the abundance of the perilipin proteins changes in parallel with IMTG accumulation is not clear. The primary objective of this study was to determine if the accumulation of IMTG that we observed in response to a high availability of fatty acids after exercise [4], was accompanied by: 1) increased activity of key enzymes of the muscle triacylglycerol esterification pathway (i.e., GPAT and DGAT), 2) increased fatty acid transport capacity in muscle (i.e., fatty acid transporter abundance at the muscle membrane), and 3) changes in the abundance of perilipin proteins within skeletal muscle.
METHODS
Subjects
Seven sedentary but otherwise healthy women (age 27 ± 4 years, body mass 62.6 ± 3.7 kg, body mass index 22.9 ± 1.0 kg/m2) volunteered to participate in this study. Subjects were not taking any medications (except oral contraceptives), and all subjects underwent a comprehensive medical examination, including a history and physical examination, a 12-lead electrocardiogram, and standard blood and urine tests. All subjects were non-smokers, weight stable (i.e., ± 2 kg), and had been sedentary (regular exercise < 2 h/wk) for at least 6 months before the study. Any history of metabolic or cardiovascular disease resulted in exclusion from participation. All of these subjects also participated in a previous study in our laboratory [4]. Written, informed consent was obtained from all subjects before initiating participation. All procedures of this study were approved by the University of Michigan Institutional Review Board.
Preliminary testing
Prior to initiating the experimental protocol, subjects underwent an incremental peak oxygen uptake test (VO2peak; 40.9 ± 2.3 ml/kg/min) on a stationary bicycle ergometer to assess aerobic fitness, and hydrostatic weighing was used to assess body composition (body fat 28.7 ± 1.5 %). This preliminary exercise test was performed at least one week before the subjects’ first experimental trial.
Experimental protocol
All subjects performed two experimental trials, separated by ≥ 7 days, and all trials were completed during the follicular phase of the subjects’ menstrual cycle (i.e., within the first 2 weeks after the onset of menses). The order of the trials was randomized, and the two trials differed only by the contents of the overnight infusion (see below). The day before each trial, subjects received a standardized evening meal (2.25 g carbohydrate/kg, 0.5 g fat/kg, 0.375 g protein/kg) prepared by the Michigan Clinical Research Unit that was eaten at home and completed at 2130 h. The next morning (Day 1) subjects were admitted to the Michigan Clinical Research Unit at 0830 h, after an overnight fast. Beginning at 1000 h subjects began 90 min of exercise at ~65% VO2peak. Exercise consisted of 45 min of treadmill exercise, immediately followed by 45 min of exercise on a cycle ergometer. Low fat meals were provided after the exercise session at 1200, 1400, and 2030 h (total content of the three meals: 8 g carbohydrate/kg, 0.3 g fat/kg, 1.1 g protein/kg). Meal energy intake was calculated to match the estimated energy expenditure during Day 1 of each trial. At ~1415 h two intravenous catheters were placed, one in an antecubital vein for use during the overnight infusion and the other in a hand vein in the contra-lateral arm for blood sampling. The overnight infusion began at 1500 h and continued until 0700 h the next morning. The content of this infusion was the only difference between the two experimental trials. On one occasion (LIPID), subjects were infused overnight with a 20% lipid emulsion (Abbott Laboratories, North Chicago, IL; [0.55 ml/kg/h]) and heparin (Elkins-Sinn, Inc., Cherry Hill, NJ; [5 U/kg/h]) with the goal of increasing overnight plasma fatty acid concentration to a high physiologic level (~1.0 mmol/l). Because our subjects were eating a weight maintaining diet, this lipid infusion resulted in a total positive energy balance. However, even when consuming a weight-maintain diet, systemic fatty acid availability is very high in obesity. Therefore, while obese individuals may be in a state of neutral energy balance (i.e., energy intake = energy expenditure) their systemic energy availability is very high compared with a lean person when expressed as either absolute availability or relative to fat-free mass, at least in part because of their high lipolytic rates [12]. It was the objective of our study design to mimic this condition. During the other trial (SALINE), subjects were infused overnight with normal saline (0.55 ml/kg/h). Indeed, plasma fatty acid concentration was elevated to an overnight average of 0.84 ± 0.14 mmol/l during the LIPID compared with 0.22 ± 0.04 mmol/l during SALINE [4]. The next morning, resting oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured (DeltaTrac, SensorMedics Inc., Yorba Linda, CA) at 0630 h to assess rates of substrate oxidation. At 0900 h, a muscle biopsy was obtained from the vastus lateralis muscle of the thigh using the percutaneous biopsy technique. Muscle biopsy samples were dissected free of adipose and connective tissue, rinsed in saline, dried and then frozen in liquid nitrogen. Muscle samples were stored at −80°C until biochemical analysis.
Analytical Procedures
Muscle DAG and ceramide concentration
Muscle DAG and ceramide content were assessed using the DAG-kinase assay as previously described [13]. In brief, lipid was extracted from ~5 mg (dry weight) of lyophilized muscle for each sample using a chloroform-methanol-water (1:2:0.8) homogenization buffer. The reaction was carried out for 2 h at room temperature by adding DAG-kinase and 32P-ATP to the lipid extracts. The reaction was stopped with chloroform-methanol (2:1). The organic phase was dried, re-dissolved in 65 µl chloroform-methanol (2:1), and spotted for thin layer chromatography (TLC; Whatman Inc.). Lipids were separated in chloroform-acetone-methanol-acetic acid-water (100:40:20:20:10) and 32P-labelled phosphatidic acid and ceramide-1-phosphate spots were visualized via radiography, scraped, and counted in scintillation fluid (Tri-Carb 2800TR, Perkin Elmer, Waltham, MA).
Muscle GPAT and DGAT enzyme activity
The enzyme activity of GPAT and DGAT were assessed in partially purified membrane fractions similar to as previously described [14, 15]. Briefly, ~20 mg of each muscle sample was homogenized in buffer solution (10 mM Tris pH 7.4, 1 mM EDTA, 1 mM DTT, 250 mM sucrose for GPAT and 20 mM HEPES [pH 7.4], 1 mM CaCl2, 1 mM DTT, 250 mM sucrose for DGAT). A mixture of protease inhibitors was also added to each of the homogenization buffers. Following a 30 min incubation, homogenates were centrifuged at 1500 g for 10 min at 4°C. Pellets were discarded and the supernatants were centrifuged for 2 h at 38,000 rpm (> 150,000 g) at 4°C. Supernatant was saved from the DGAT preparations for immunoblot analysis of cytosolic proteins (see below). Pellets were manually homogenized and re-dissolved in the homogenization buffer. Protein content of the resultant solution was measured (Pierce BCA Protein Assay, Thermo Scientific). For total GPAT activity, the reaction was carried out using 10 µg protein in a 200 µl reaction mixture containing 75 mM Tris pH 7.5, 1 mg/ml BSA (fatty acid-free), 4 mM MgCl2, 1 mM DTT, 8 mM NaF, 80 µM palmitol-CoA and 414 mM 14C glycerol 3-phosphate (SA >20,000 dpm/nmol) for 20min at 37°C H2O bath with agitation. The organic phase containing 14C-labelled LPA was dried, reconstituted in scintillation fluid, and measured for radioactivity. For total DGAT activity, the reaction was carried out using 10 µg protein in a 200µl reaction mixture containing 100 mM Tris pH 7.5, 250 mM sucrose, 1 mg/ml BSA (fatty acid-free), 150 mM MgCl2, 0.8 mM EDTA, 0.25 mM DAG, and 25 µM palmitoyl-CoA with 0.1 µCi 14C palmitoyl-CoA (SA >30,000 dpm/nmol) at 37°C for 20 min in a water bath with agitation. The reaction was stopped with 0.75 ml chloroform-methanol (2:1). After a 2 h room temperature lipid extraction, 0.375ml of 1 mM H2SO4/17 mM NaCl was added to facilitate lipid-aqueous phase separation. The organic phase was dried, re-dissolved in 30 µl chloroform, and spotted for TLC. Lipids were separated in chloroform-acetic acid (96:4) and triacylglycerol spots were visualized with iodine vapor, scraped, and counted in scintillation fluid.
Western blotting
Cytosolic and crude membrane fractions of muscle from DGAT activity preparations (see above) were used for immunoblot analysis of protein contents in muscle. Proteins from the centrifugation supernatant of the DGAT activity preparation were concentrated using Amicon Ultra Centrifugal Filters (MWCO 3KD, Millipore) and used for electrophoresis analysis of cytosolic proteins. 35 µg of cytosolic proteins or 25 µg of membrane proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. Crude membrane protein fractions were used to assess GPAT1 and DGAT1 protein abundance. For all other immunoblot analyses, the use of cytosolic and/or membrane protein fractions is indicated throughout the manuscript. Blots were probed with the following antibodies: α-NADH-ubiquinol oxidoreductase (COX-I; Molecular Probes, A21344), α-FAT/CD36 (Santa Cruz Biotechnology, sc-9154), α- perilipin 1, 2, 3, and 4 (all gifts from P.E. Bickel and N.E. Wolins), α-perilipin 5 (American Research Products, 03-GP31), α-GPAT1 (a gift from R.A. Coleman), and α-DGAT1 (Novus Biologicals, NB110-41487). Membranes were incubated with appropriate secondary antibodies and developed using enhanced chemiluminescence (Amersham Biosciences). Bands were imaged and then quantified via densitometry (AlphaEaseFC, Alpha Innotech Corp.). All within-subject comparisons were made using the same blot.
Calculations
Respiratory exchange ratio and fat oxidation
Respiratory exchange ratio (RER) was calculated as the ratio of VCO2 to VO2. Whole body fat/triacylglycerol oxidation (g/min) was calculated from VO2 and VCO2 measurements using the equations of Frayn [16]. Whole body fatty acid oxidation was calculated by dividing triacylglycerol oxidation by an estimated molecular weight of triacylglycerol (860 g/mol) and multiplying by 3.
Muscle GPAT enzyme activity
was calculated as:
The conversion factor of 14C glycerol 3-phosphate (G3P) was calculated by dividing the counts of 1µl 14C-G3P (dpm), by 14C-G3P concentration (pmol/µl). The fraction of 14C-G3P refers to the ratio of 14C-G3P to total G3P (mol) in the reaction mixture. GPAT activity during LIPID was calculated relative to GPAT activity during SALINE, in a within-subject manner, with the mean of these calculations expressed relative to one.
Muscle DGAT enzyme activity
was calculated as:
The conversion factor was calculated by dividing counts of 1µl 14C palmitoyl-CoA (dpm), by 14C palmitoyl-CoA concentration (pmol/µl). The fraction of 14C palmitoyl-CoA is the percentage of 14C palmitoyl-CoA in total palmitoyl-CoA (mol) in the reaction mixture. DGAT activity during LIPID was calculated relative to DGAT activity during SALINE, in a within-subject manner, with the mean of these calculations be presented relative to one.
Statistical analysis
A paired, two-tailed Student’s t-test was used to test for significant differences in all outcome variables between trials. Pearson Product Moment Correlation analysis was used to examine the relationship between outcome variables. Due to limited muscle sample acquisition during some trials, analysis of DAG and ceramide (n = 4) and GPAT1 protein content (n = 5) could not be performed using tissue from all subjects (n = 7). Statistical significance was defined as P < 0.05. All results are presented as means ± standard error.
RESULTS
Muscle lipids
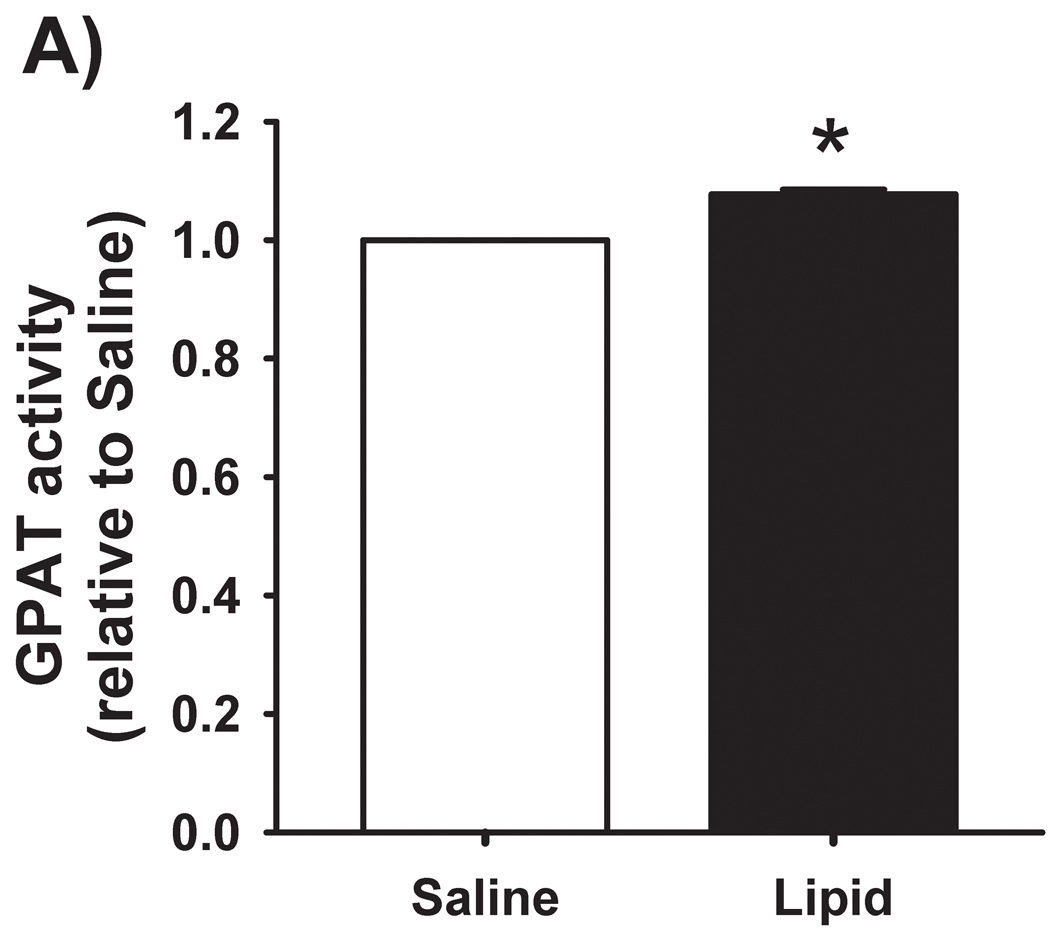
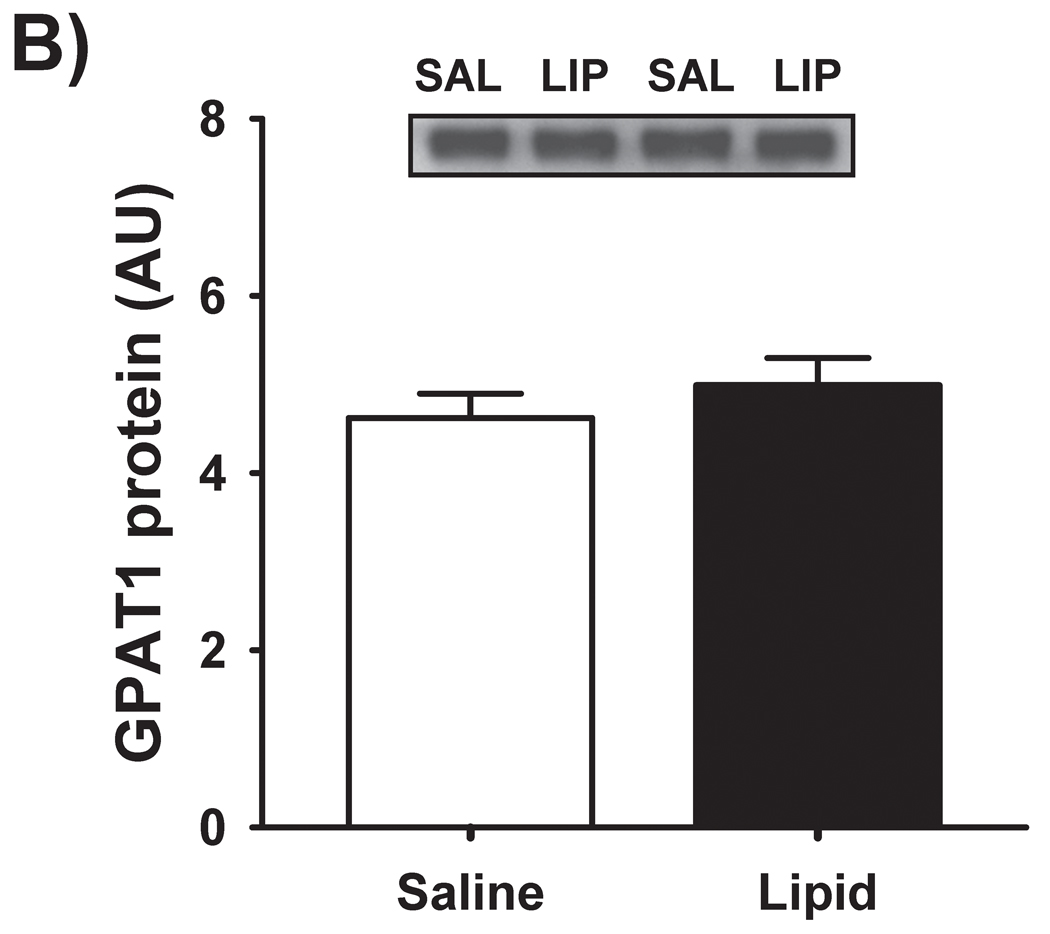
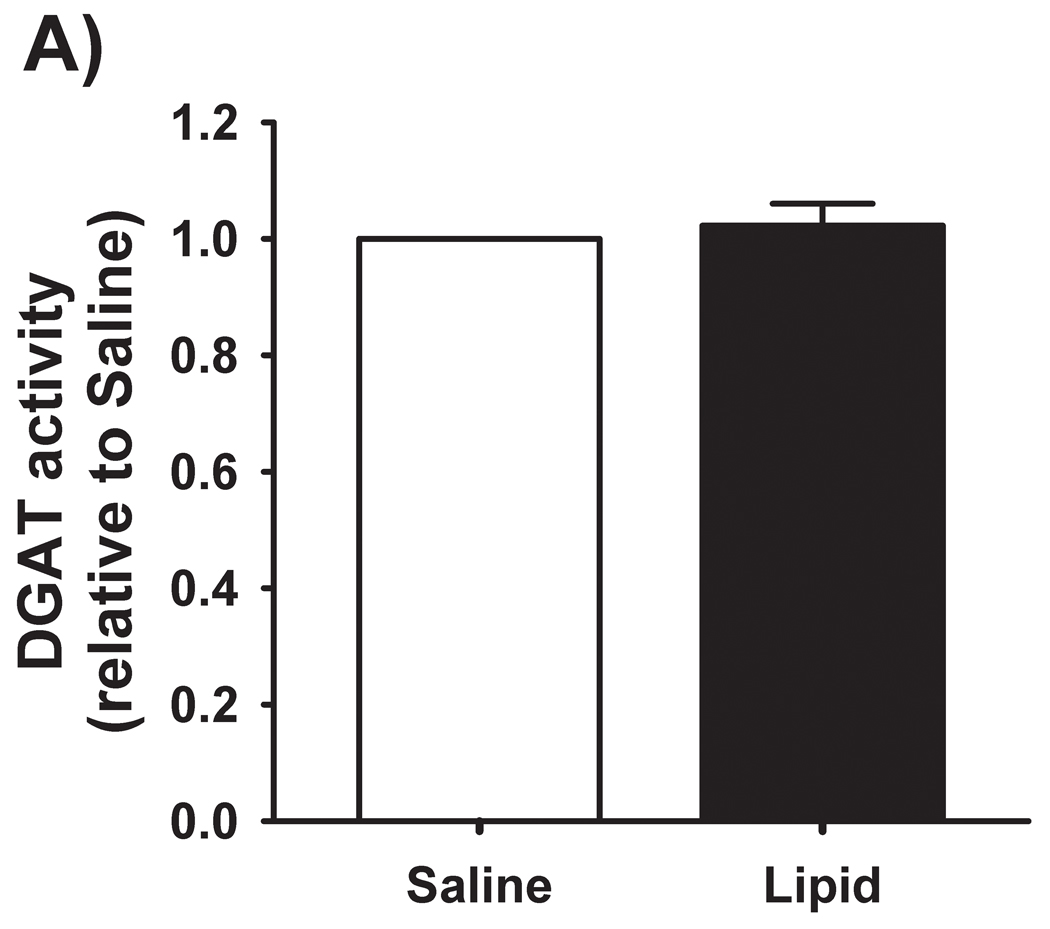
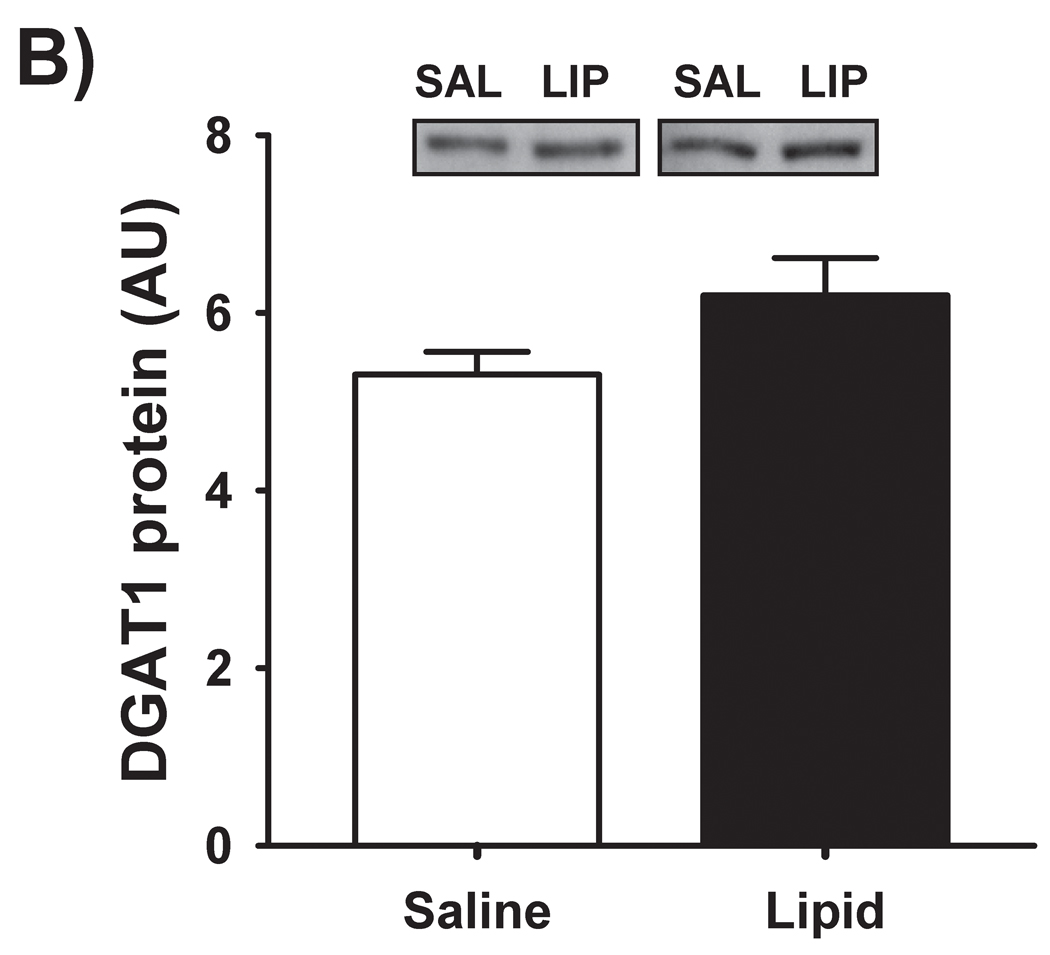
We have previously reported [4] that IMTG concentration was significantly increased in response to elevated fatty acid availability during LIPID compared with SALINE. In parallel with the marked increase in IMTG concentration, presently we found GPAT enzyme activity in skeletal muscle to be slightly, yet significantly greater during LIPID compared with SALINE (P = 0.01; Figure 1A). However, the small increase in muscle GPAT1 protein abundance between trials did not reach statistical significance (Figure 1B). We found no differences in muscle DGAT activity or DGAT1 protein abundance between trials (Figure 2). In contrast to the elevated IMTG concentration with the lipid infusion, muscle concentrations of the lipid intermediates, DAG (1839 ± 251 vs. 1494 ± 229 pmol/mg protein for LIPID and SALINE, respectively; P = 0.35) and ceramide (665 ± 37 vs. 636 ± 72 pmol/mg protein for LIPID and SALINE, respectively; P = 0.52) were not different between trials.
Figure 1. Skeletal muscle GPAT activity and abundance.
(A) Total muscle GPAT activity and (B) muscle GPAT-1 protein abundance the morning after the overnight infusion. The inset figure is a representative western blot for two subjects. * P ≤ 0.01 for LIPID compared with SALINE. GPAT, glycerol-3-phosphate acyltransferase; SAL, SALINE; LIP, LIPID; AU, arbitrary units.
Figure 2. Skeletal muscle DGAT activity and abundance.
(A) Muscle DGAT activity and (B) muscle DGAT-1 protein abundance the morning after the overnight infusion. The inset figures are representative western blots for two subjects. DGAT, diacylglycerol acyltransferase; SAL, SALINE; LIP, LIPID; AU, arbitrary units.
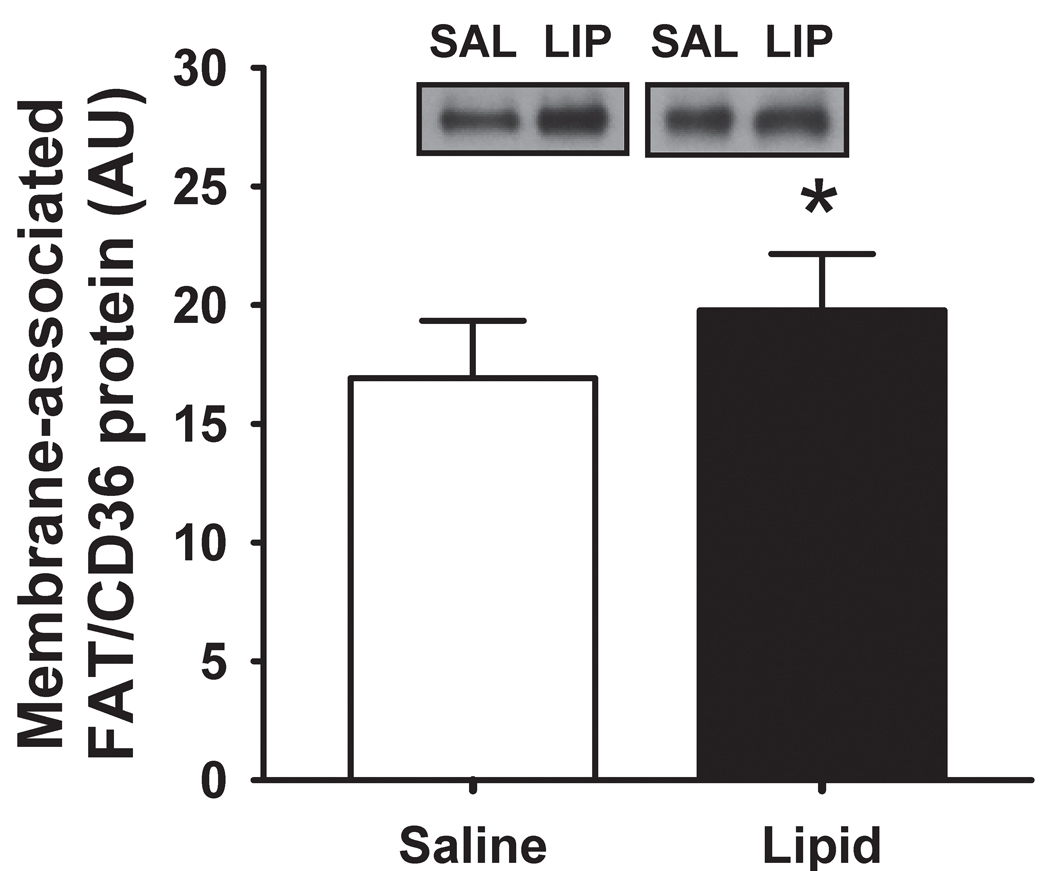
FAT/CD36 protein abundance
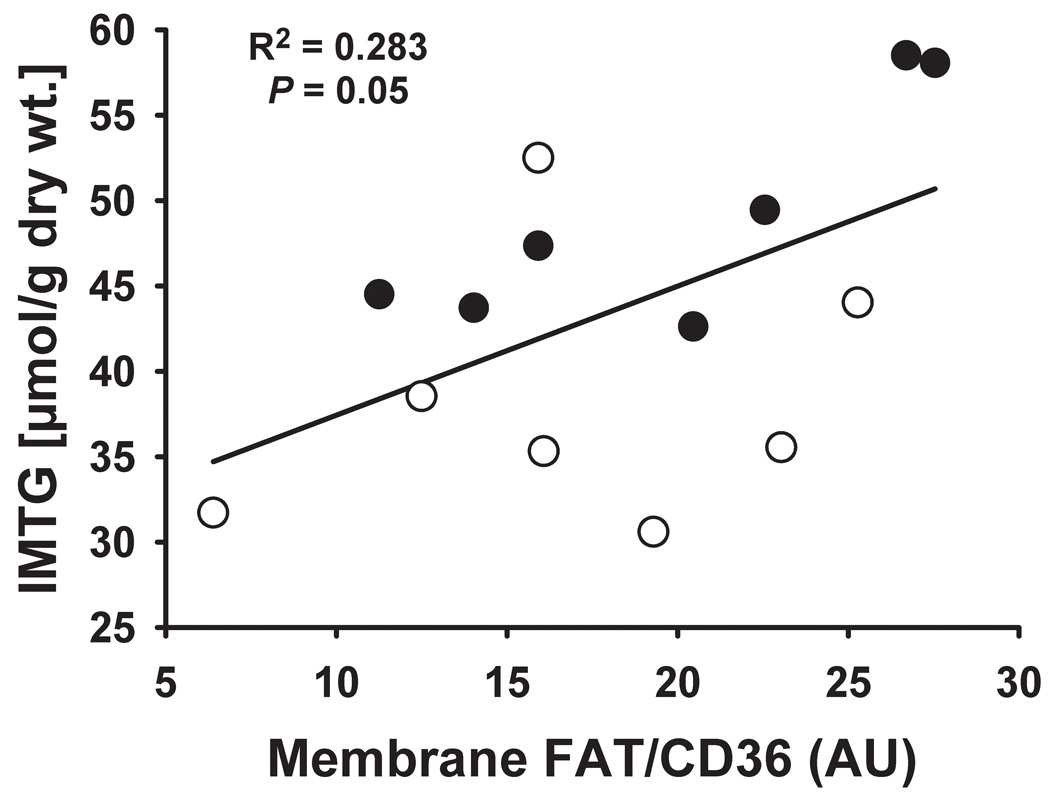
The lipid infusion significantly increased membrane-associated FAT/CD36 (P = 0.005; Figure 3), while cytosolic FAT/CD36 protein content was not affected (3.7 ± 0.6 vs. 3.8 ± 0.5 arbitrary units (AU) for LIPID and SALINE, respectively; P = 0.59). Interestingly, we found membrane-associated FAT/CD36 protein content to be positively correlated with IMTG concentration (Figure 4).
Figure 3. Skeletal muscle FAT/CD36 abundance.
Muscle membrane-associated FAT/CD36 protein abundance the morning after the overnight infusion. Inset figures are representative western blots for two subjects. * P ≤ 0.01 for LIPID compared with SALINE. SAL, SALINE; LIP, LIPID; AU, arbitrary units.
Figure 4. Relationship between membrane-associated fatty acid transporter abundance skeletal muscle lipid accumulation.
Correlation between membrane-associated FAT/CD36 protein abundance and IMTG concentration the morning after the overnight infusion. Open circles (○) represent SALINE, whereas closed circles (●) represent LIPID. IMTG, intramyocellular triacylglycerol; AU, arbitrary units.
Fatty acid oxidation
Despite the increase in muscle membrane-associated FAT/CD36 abundance and a near three-fold increase in overnight plasma fatty acid concentration during LIPID compared with SALINE, neither RER (0.83 ± 0.02 vs. 0.81 ± 0.02, respectively, P = 0.38) nor whole body fatty acid oxidation (3.0 ± 0.6 vs. 3.5 ± 0.4 µmol/kg/min, respectively, P = 0.35) were different between trials the next morning. COX-I protein content in the muscle membrane fraction, an indicator of oxidative capacity, was also identical between trials (1.2 ± 0.2 vs. 1.2 ± 0.2 AU for LIPID and SALINE, respectively; P = 0.49).
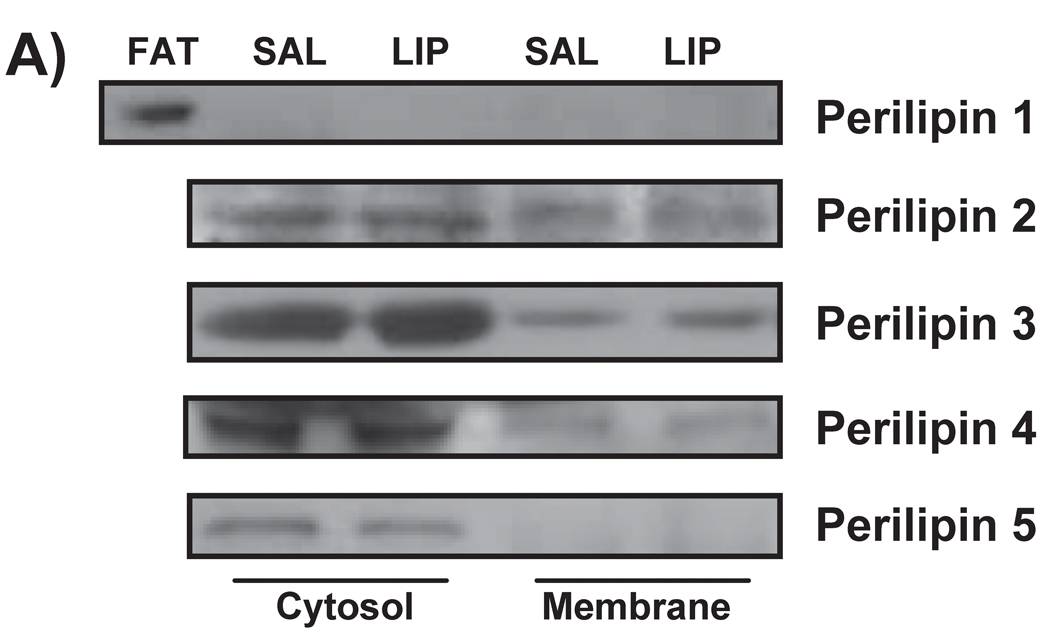
Perilipin proteins
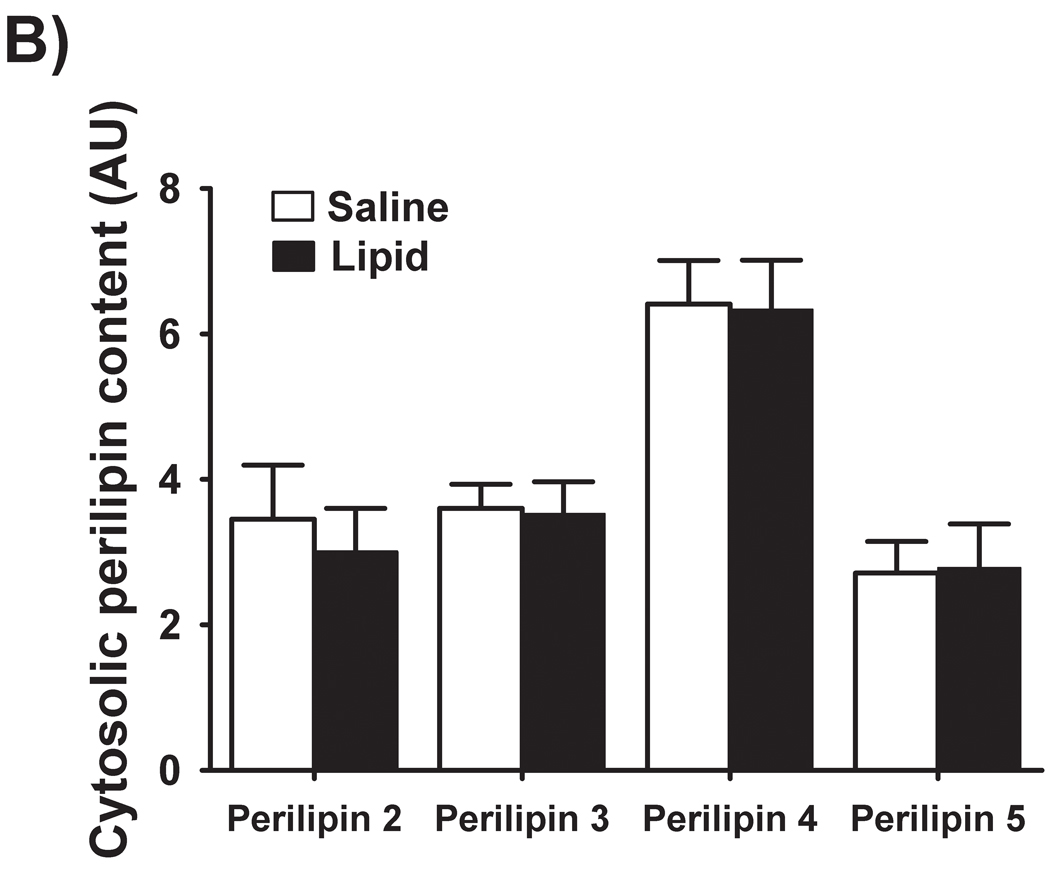
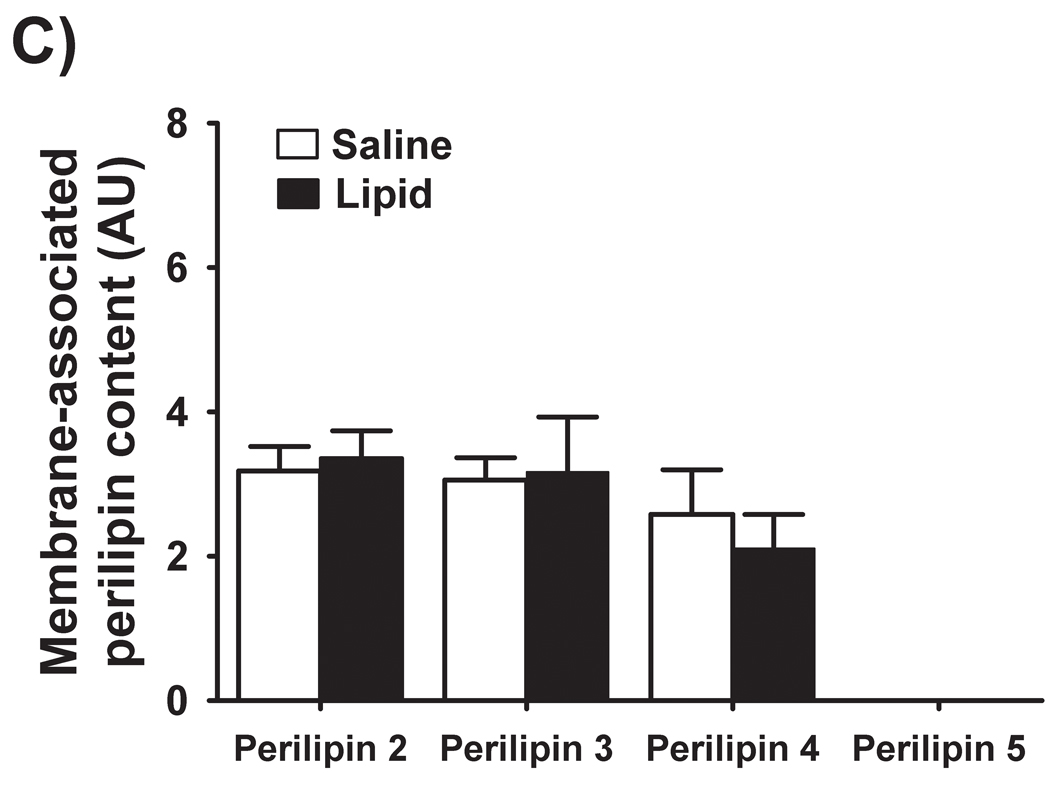
Perilipin 1 was not detected in muscle homogenates, indicating that our muscle samples were free of adipose tissue contamination (Figure 5A). We did detect perilipins 2, 3, 4, and 5 in the skeletal muscle samples from both trials. Interestingly, the augmented IMTG concentration during LIPID vs. SALINE was not accompanied by elevated concentrations of any of these perilipin proteins within either the cytosolic or membrane fractions (Figure 5B and 5C). We did not detect any perilipin 5 protein in the membrane fractions of any of our muscle samples (Figure 5C).
Figure 5. Perilipin protein abundance in skeletal muscle.
(A) Representative western blots from two subjects for perilipins 1, 2, 3, 4, and 5. (B) Muscle protein abundance of cytosolic and (C) membrane-associated perilipins 2, 3, 4 and 5. Perilipin 5 was not detected in muscle membrane preparations. FAT, a control adipose tissue homogenate; SAL, SALINE; LIP, LIPID; AU, arbitrary units.
DISCUSSION
We have previously demonstrated that alterations in muscle lipid metabolism in the several hours after exercise can help offset insulin resistance stemming from the excessive fatty acid availability commonly found in obesity [3, 4]. However, the influence of elevated fatty acid availability on specific changes in intramyocellular lipid metabolism after exercise is not completely understood. Here we found that the accumulation of IMTG resulting from high systemic fatty acid availability after exercise was accompanied by a small but significant increase in the activity of GPAT, which is a key regulating step in the triacylglycerol esterification pathway. Perhaps more importantly, we found the lipid infusion increased the abundance of FAT/CD36 in the membrane fraction from skeletal muscle, suggesting that the capacity to transport fatty acids into the cell was enhanced. In fact, the abundance of membrane-associated FAT/CD36 was significantly correlated with IMTG concentration. Additionally, we found that the abundance of lipid droplet coating proteins (i.e., perilipins) was not increased despite the marked elevation in lipid storage within muscle.
High systemic fatty acid availability after exercise is known to augment triacylglycerol resynthesis in muscle and elevate IMTG concentration [3, 4, 17]. Because fatty acids largely enter skeletal muscle via protein-mediated transport [18], the abundance of fatty acid transporters at the myocyte membrane largely dictates the capacity for fatty acid transport into the cell [7, 8]. Our finding that FAT/CD36 abundance in muscle membrane fractions was greater after the lipid infusion compared with saline despite no difference in the cytosolic fraction between trials suggests that augmented fatty acid availability likely increased the total abundance of FAT/CD36 protein, but that the additional FAT/CD36 was exclusively localized at the muscle membrane. This expands on previous studies that have reported augmented muscle membrane FAT/CD36 in obesity [7, 8], by suggesting the chronic elevation in fatty acid availability found in obesity may be responsible for the increased abundance and altered basal localization of muscle FAT/CD36. How fatty acid may be inducing this effect has yet to be determined, as pharmacological activation of the fatty acid ligand inducible transcription factors peroxisome proliferator-activated receptors (PPARs) α and γ does not augment FAT/CD36 transcription in rat skeletal muscle [19], despite the known presence of a peroxisome proliferator response element (PPRE) in the promoter region of the FAT/CD36 gene [20]. Because our measurement of FAT/CD36 in crude membrane preparations did not allow us to determine the specific localization of FAT/CD36, we do not have definitive evidence to support that this increased abundance of FAT/CD36 in our study occurred within the plasma membrane. However, based on the previous finding of increased sarcolemmal FAT/CD36 protein content in obese compared with lean individuals [7, 8], it is likely that the lipid infusion in our study increased FAT/CD36 within the plasma membrane. We surmise that elevated fatty acid availability can increase membrane-associated FAT/CD36, thereby augmenting long-chain fatty acid uptake capacity into the myocyte. The significant correlation we observed between membrane-associated FAT/CD36 and IMTG concentration suggests that the increased abundance of FAT/CD36 in the plasma membrane may be a key step in augmenting IMTG accumulation when circulating fatty acid availability is elevated after exercise, and is in agreement with other recent studies highlighting the role of FAT/CD36 in muscle lipid accumulation [7, 8].
Accompanying the greater capacity for fatty acid flux into muscle in response to the lipid infusion, we also found a slight, yet significant increase in the activity of the enzyme that catalyzes the first committed step of the triacylglycerol synthesis pathway in muscle (i.e., GPAT). Conversely, we found no effect of the lipid infusion on DGAT activity, which catalyzes the final step of the esterification pathway. The relatively small increase in total GPAT activity that we found occurred in absence of a significant increase in GPAT1 protein content, which could suggest that either the intrinsic activity of the enzyme was increased, or isoforms of GPAT other than GPAT1 were increased. Alternatively, it is possible that a small increase in GPAT1 protein abundance was simply not detected with our immunoblotting technique, as GPAT activity was identical between trials when expressed relative to GPAT1 protein abundance (8.3 ± 0.4 vs. 8.3 ± 0.5 AU, respectively). We previously reported that a prior session of exercise increased the protein abundance of both GPAT1 and DGAT1 in skeletal muscle in a similar time frame as in this study [3]. Because our subjects performed a session of exercise the day before the muscle biopsy in both the LIPID and SALINE trials of this study, it is important to acknowledge that this session of exercise may have increased GPAT and DGAT protein abundance (and activity) during both trials compared with if no exercise had been performed.
It has been proposed that an impaired ability to oxidize fatty acids may underlie an accumulation of lipid intermediates, and the resultant suppression in insulin action in obesity [21, 22]. Accordingly, several studies have suggested that increasing oxidative disposal of fatty acids provides important protection against lipid-induced insulin resistance [22, 23]. However, whether or not fat oxidation plays a key role in the accumulation of intramyocellular lipids and insulin resistance in obesity is controversial [24–27]. We recently reported that in these same subjects a single session of exercise protected against the lipid-induced insulin resistance [4], and here we confirm that this protection occurred in absence of an increase in fat oxidation. Moreover, our present finding that muscle DAG and ceramide concentrations were no greater after LIPID compared with SALINE, indicates that an increase in fatty acid oxidation is not required to prevent the accumulation of these lipid intermediates. Together, these data suggest that exercise-induced protection against lipid-induced insulin resistance is not dependent on an increase in fat oxidation.
Intramyocellular lipids are mainly stored in lipid droplets that are coated by specialized proteins. The largest family of these lipid droplet-associated proteins are now collectively referred to as perilipins [9]. Perilipins help establish and maintain the partition between insoluble triacylglycerols and the aqueous cytosol, while retaining a physical connection between the phases. Perilipin proteins have been suggested to be involved in the metabolic regulation of the triacylglycerols within the lipid droplet (e.g., storage [28, 29], lipolysis [30, 31], oxidation [32, 33]), as well as involved in trafficking the lipid droplet toward specific sites and/or signaling pathways within the cell (for review, see [11]). Still, the specific roles of each of the perilipin proteins in muscle have yet to be completely elucidated. Our findings indicate that the ~30% increase in IMTG concentration during LIPID was not paralleled by an increased protein abundance of perilipin 2, 3, 4 or 5. This suggests that the perilipin coat surrounding the intramyocellular lipid droplets was less dense during LIPID compared with SALINE. This finding was somewhat unexpected given that the expression of perilipins 2, 4 and 5 is known to be upregulated by PPARs α and γ [34, 35]. One potential functional outcome associated with a lower perilipin density is that with less of this protein coat, the lipid droplet may be more susceptible to lipase activity. However, we did not observe an increase in DAG concentration as one might expect if IMTG lipolytic rate was accelerated. The effect of changes in perilipin density on insulin sensitivity is also equivocal. The improvement in insulin sensitivity during weight loss has been associated with increased muscle perilipin 2 relative to IMTG content [36]. In contrast, it has recently been reported that the improvement in insulin sensitivity after thiazoladinedione treatment was accompanied by a reduction in the abundance of perilipin proteins relative to the IMTG content [37]. Therefore, whether a change in perilipin density relative to IMTG content is important for the metabolic regulation of lipid storage and downstream effects on insulin sensitivity remains to be determined.
In summary, our findings suggest that an increased fatty acid transport capacity (as indicated by greater membrane-associated FAT/CD36 transporter protein abundance), together with a slight, yet significant increase in muscle GPAT activity underlie the increased accumulation of IMTG when fatty acid availability is high after exercise. Because partitioning of fatty acids toward neutral lipid (i.e., IMTG) has been proposed to protect against insulin resistance [2–4], these adaptations may be key contributors to the improvement in insulin sensitivity found after a single session of exercise in obesity. However, the relatively small increase in muscle GPAT activity with no rise in DGAT activity during the lipid infusion, suggests that direct adaptations within the triacylglycerol esterification pathway may be secondary to the increase fatty acid transport capacity. The significant correlation we observed between membrane-associated FAT/CD36 and IMTG concentration does not prove causality, but helps support the potential impact of augmented transport capacity on IMTG synthesis. Additionally, our novel finding that the protein abundance of perilipins 2, 3, 4 and 5 did not increase in parallel with IMTG accumulation suggests that elevating fatty acid availability after exercise reduced the IMTG perilipin coating density. While changes in perilipin content of a lipid droplet can impact the metabolic fate of cellular lipids, the functional significance of the lower perilipin density we observed here has yet to be determined.
Acknowledgements
We are very grateful to Christopher W. Paran for his outstanding assistance in the laboratory, the staff of the Michigan Clinical Research Unit and the Michigan Metabolomics Obesity Center for help conducting the experimental protocols, the staff in the Michigan Diabetes Research and Training Center chemistry core laboratory for assistance with the insulin assay, and the subjects for their enthusiastic participation.
List of Abbreviations
- ATP
adenosine triphosphate
- AU
arbitrary units
- BSA
bovine serum albumin
- COX-I
NADH-ubiquinone oxidoreductase
- DAG
diacylglycerol
- DGAT
diacylglycerol acyltransferase
- dw
dry weight
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- FA
fatty acid
- FAT/CD36
fatty acid translocase
- G3P
glycerol-3-phosphate
- GPAT
glycerol-3-phosphate acyltransferase
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IMTG
intramyocellular triacylglycerol
- LIPID or LIP
lipid infusion trial
- LPA
lysophosphatidic acid
- NADH
reduced nicotinamide adenine dinucleotide
- PPAR
peroxisome proliferator-activated receptor
- PPRE
peroxisome proliferator response element
- RER
respiratory exchange ratio
- SA
specific activity
- SALINE or SAL
saline infusion trial
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TLC
thin layer chromatography
- VCO2
carbon dioxide production
- VO2
oxygen consumption
- VO2peak
peak oxygen uptake
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None of the authors had any conflict of interest pertaining to the topic or contents of this article.
Author Contributions
SAN, SS, ML, ACE, and JFH participated in study design and/or conduct, as well as data collection and analysis. Data interpretation and manuscript writing were performed by SAN and JFH.
REFERENCES
- 1.Schenk S, Harber MP, Shrivastava CR, et al. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J Physiol. 2009;587(Pt 20):4949–4961. doi: 10.1113/jphysiol.2009.175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann OP, Dahl DB, Brechtel K, et al. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes. 2001;50(11):2579–2584. doi: 10.2337/diabetes.50.11.2579. [DOI] [PubMed] [Google Scholar]
- 3.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest. 2007;117(6):1690–1698. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schenk S, Cook JN, Kaufman AE, et al. Postexercise insulin sensitivity is not impaired after an overnight lipid infusion. Am J Physiol Endocrinol Metab. 2005;288(3):E519–E525. doi: 10.1152/ajpendo.00401.2004. [DOI] [PubMed] [Google Scholar]
- 5.Bonen A, Luiken JJ, Arumugam Y, et al. Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J Biol Chem. 2000;275(19):14501–14508. doi: 10.1074/jbc.275.19.14501. [DOI] [PubMed] [Google Scholar]
- 6.Coburn CT, Knapp FF, Jr, Febbraio M, et al. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275(42):32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 7.Aguer C, Mercier J, Man CY, et al. Intramyocellular lipid accumulation is associated with permanent relocation ex vivo and in vitro of fatty acid translocase (FAT)/CD36 in obese patients. Diabetologia. 53(6):1151–1163. doi: 10.1007/s00125-010-1708-x. [DOI] [PubMed] [Google Scholar]
- 8.Bonen A, Parolin ML, Steinberg GR, et al. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 2004;18(10):1144–1146. doi: 10.1096/fj.03-1065fje. [DOI] [PubMed] [Google Scholar]
- 9.Kimmel AR, Brasaemle DL, McAndrews-Hill M, et al. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res. 51(3):468–471. doi: 10.1194/jlr.R000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791(6):419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580(23):5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz JF, Coppack SW, Paramore D, et al. Effect of short-term fasting on lipid kinetics in lean and obese women. Am J Physiol. 1999;276(2 Pt 1):E278–E284. doi: 10.1152/ajpendo.1999.276.2.E278. [DOI] [PubMed] [Google Scholar]
- 13.Preiss J, Loomis CR, Bishop WR, et al. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986;261(19):8597–8600. [PubMed] [Google Scholar]
- 14.Yu YH, Zhang Y, Oelkers P, et al. Posttranscriptional control of the expression and function of diacylglycerol acyltransferase-1 in mouse adipocytes. J Biol Chem. 2002;277(52):50876–50884. doi: 10.1074/jbc.M207353200. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Lee DP, Gong N, et al. Cloning and functional characterization of a novel mitochondrial N-ethylmaleimide-sensitive glycerol-3-phosphate acyltransferase (GPAT2) Arch Biochem Biophys. 2007;465(2):347–358. doi: 10.1016/j.abb.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 17.Fox AK, Kaufman AE, Horowitz JF. Adding fat calories to meals after exercise does not alter glucose tolerance. J Appl Physiol. 2004;97(1):11–16. doi: 10.1152/japplphysiol.01398.2003. [DOI] [PubMed] [Google Scholar]
- 18.Schwenk RW, Holloway GP, Luiken JJ, et al. Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins Leukot Essent Fatty Acids. 82(4–6):149–154. doi: 10.1016/j.plefa.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 19.Benton CR, Koonen DP, Calles-Escandon J, et al. Differential effects of contraction and PPAR agonists on the expression of fatty acid transporters in rat skeletal muscle. J Physiol. 2006;573(Pt 1):199–210. doi: 10.1113/jphysiol.2006.106013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato O, Kuriki C, Fukui Y, et al. Dual promoter structure of mouse and human fatty acid translocase/CD36 genes and unique transcriptional activation by peroxisome proliferator-activated receptor alpha and gamma ligands. J Biol Chem. 2002;277(18):15703–15711. doi: 10.1074/jbc.M110158200. [DOI] [PubMed] [Google Scholar]
- 21.Kelley DE, Goodpaster B, Wing RR, et al. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277(6 Pt 1):E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 22.Kelley DE, He J, Menshikova EV, et al. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 23.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 24.Hancock CR, Han DH, Chen M, et al. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci U S A. 2008;105(22):7815–7820. doi: 10.1073/pnas.0802057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boushel R, Gnaiger E, Schjerling P, et al. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50(4):790–796. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holloway GP, Thrush AB, Heigenhauser GJ, et al. Skeletal muscle mitochondrial FAT/CD36 content and palmitate oxidation are not decreased in obese women. Am J Physiol Endocrinol Metab. 2007;292(6):E1782–E1789. doi: 10.1152/ajpendo.00639.2006. [DOI] [PubMed] [Google Scholar]
- 27.De Feyter HM, Lenaers E, Houten SM, et al. Increased intramyocellular lipid content but normal skeletal muscle mitochondrial oxidative capacity throughout the pathogenesis of type 2 diabetes. FASEB J. 2008;22(11):3947–3955. doi: 10.1096/fj.08-112318. [DOI] [PubMed] [Google Scholar]
- 28.Wolins NE, Quaynor BK, Skinner JR, et al. S3-12, Adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem. 2005;280(19):19146–19155. doi: 10.1074/jbc.M500978200. [DOI] [PubMed] [Google Scholar]
- 29.Wolins NE, Skinner JR, Schoenfish MJ, et al. Adipocyte protein S3-12 coats nascent lipid droplets. J Biol Chem. 2003;278(39):37713–37721. doi: 10.1074/jbc.M304025200. [DOI] [PubMed] [Google Scholar]
- 30.Tansey JT, Sztalryd C, Gruia-Gray J, et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci U S A. 2001;98(11):6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brasaemle DL, Rubin B, Harten IA, et al. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J Biol Chem. 2000;275(49):38486–38493. doi: 10.1074/jbc.M007322200. [DOI] [PubMed] [Google Scholar]
- 32.Wolins NE, Quaynor BK, Skinner JR, et al. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55(12):3418–3428. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 33.Dalen KT, Dahl T, Holter E, et al. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta. 2007;1771(2):210–227. doi: 10.1016/j.bbalip.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Dalen KT, Schoonjans K, Ulven SM, et al. Adipose tissue expression of the lipid droplet-associating proteins S3-12 and perilipin is controlled by peroxisome proliferator-activated receptor-gamma. Diabetes. 2004;53(5):1243–1252. doi: 10.2337/diabetes.53.5.1243. [DOI] [PubMed] [Google Scholar]
- 35.Dalen KT, Ulven SM, Arntsen BM, et al. PPARalpha activators and fasting induce the expression of adipose differentiation-related protein in liver. J Lipid Res. 2006;47(5):931–943. doi: 10.1194/jlr.M500459-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Phillips SA, Choe CC, Ciaraldi TP, et al. Adipocyte differentiation-related protein in human skeletal muscle: relationship to insulin sensitivity. Obes Res. 2005;13(8):1321–1329. doi: 10.1038/oby.2005.160. [DOI] [PubMed] [Google Scholar]
- 37.Minnaard R, Schrauwen P, Schaart G, et al. Adipocyte differentiation-related protein and OXPAT in rat and human skeletal muscle: involvement in lipid accumulation and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94(10):4077–4085. doi: 10.1210/jc.2009-0352. [DOI] [PubMed] [Google Scholar]