Abstract
Glucose is important to the maturation of the oocyte and development of the embryo, while hyperglycemia results in profound reproductive and developmental consequences. However, the normal physiology of glucose in the ovary remains poorly understood. The goal of this study was to determine intra-follicular glucose dynamics during the periovulatory interval in non-human primates undergoing controlled ovarian stimulation protocols. Follicular fluid and mural granulosa cells were isolated before or up to 24 hr after an ovulatory hCG bolus, and the human granulosa-lutein cell line hGL5 was used. Intra-follicular glucose increased 3 hr after hCG, and remained at that level until 12 hr when levels decline back to pre-hCG concentrations. Pyruvate and lactate concentrations in the follicle were not strongly altered by hCG. Mural granulosa cell expression of hexokinase 1 and 2, and glucose-6-phosphate dehydrogenase mRNA decreased following hCG, while glycogen phosphorylase (liver form) increased following hCG. Glucose uptake by hGL5 cells was delayed until 24 hr following stimulation. In summary, intra-follicular glucose increases following an ovulatory stimulus and mural granulosa cells do not appear able to utilize it, sparing the glucose for the cumulus-oocyte complex.
Keywords: macaque, (granulosa cells), glucose, glycolysis, luteinization
Introduction
The development of the primate corpus luteum, including events leading to the extrusion of a fertilizable oocyte and luteinization of granulosa cells into luteal cells, is considered to be an energetically demanding series of events (Harris et al. 2007). Early studies using rats indicated that LH increased glucose uptake and lactate output, along with increased hexokinase activity (Armstrong et al. 1962; Flint et al. 1969; Hamberger et al. 1967), although relatively little subsequent work has been done on the metabolic fate of ovarian glucose. The exception to this is the cumulus-oocyte complex, where glucose metabolism by the cumulus cells has been shown to provide essential metabolites to the oocyte (Downs 2002; Ratchford et al. 2008; Su et al. 2009; Sutton-McDowall et al.; Wang et al. 2009). The lack of research into the basic understanding of ovarian glucose metabolism probably stems from the fact that metabolic pathologies and hyperglycemia have a profound negative influence on reproduction and development (Doblado et al. 2007; Jungheim et al. 2008), subsuming much of the attention on this topic. Hyperglycemia and hyperinsulinemia in mice can disrupt ovarian follicle development, oocyte, and the early embryo (Doblado and Moley 2007). Chabrolle et al (Chabrolle et al. 2008) recently demonstrated that presumptively high levels of glucose (500-1000 mg/dL) inhibited gonadotropin-induced steroidogenesis in isolated non-luteinized rat granulosa cells. Whether hyperglycemia associated with metabolic disorders translates to elevated intra-follicular glucose remains incompletely understood (Foong et al. 2006). Diabetes and hyperglycemia notwithstanding, physiologic levels of glucose are essential for normal ovarian function, although the intra-follicular metabolic flux of glucose, lactate, and pyruvate remain unknown.
Glucose uptake is mediated primarily through the actions of glucose transporters (GLUT) or sodium-dependent glucose transporters (SGLT) (Wood et al. 2003). There are at least 13 members of the solute carrier (SLC)-2 family, 12 of which are GLUTs that facilitate the diffusion of hexoses across the plasma membrane (Uldry et al. 2004). GLUT1-4 have received most of the attention, with GLUT3 having a higher affinity and capacity for glucose than GLUT1, 2, or 4 in Xenopus oocytes (reviewed in (Simpson et al. 2008)). Importantly, glucose movement can be inward or outward (Banhegyi et al. 1998). GLUT3 mRNA is increased in rat ovaries following an ovulatory hCG stimulus, while GLUT1, 3, and 4 mRNA are expressed in bovine follicles and corpora lutea (Nishimoto et al. 2006).
Glucose can be metabolized multiple pathways, including glycolysis, glycogen synthesis, hexosamine biosynthesis, polyol synthesis, or the pentose phosphate pathway (PPP). Regardless, the metabolism of glucose to glucose-6-phosphate is the initial step and mediated by hexokinase or glucose-6-phosphatase. Glucose-6-phosphate can enter glycolysis through the action of phosphohexose isomerase or PPP via glucose-6-phosphate dehydrogenase. The outcome of these pathways is ATP, and NADPH and ribose sugars, respectively. There are currently no data indicating changes in expression of key glucose metabolizing genes during luteinization of granulosa cells.
The goal of this study was to determine intra-follicular glucose, lactate, and pyruvate concentrations before and after an ovulatory stimulus given to rhesus monkeys undergoing a controlled ovarian stimulation protocol, and to test the hypothesis that hCG induced changes to gene expression in mural granuosa cells are consistent with glucose uptake and metabolism.
Materials and Methods
Animals
Adult female rhesus macaques (Macaca mulatta) were housed at the California National Primate Research Center as described previously (VandeVoort et al. 1991). Beginning on menstrual cycle day 1 to 4 (onset of menstruation =day 1), monkeys were treated with recombinant human FSH (r-hFSH; Ares-Serono, Randolph, MA or Organon, West Orange, NJ; 37.5 IU, im, twice daily) for 7 days. Antide (Ares-Serono; 5 mg/kg body weight, sc, single injection daily) was administered daily to prevent endogenous gonadotropin secretion. Follicles were aspirated the morning after the last dose of r-hFSH by an ultrasound-guided procedure as described previously (VandeVoort and Tarantal 1991), and the characteristics of the follicular cohort in this model have been described (VandeVoort et al. 2003). The resulting cells are referred to as non-luteinized granulosa cells (NLGC). A subset of animals received an ovulatory bolus of r-hCG (1000 IU, sc, Ares-Serono) on the morning of day 8 and follicles were aspirated before (0 h) or 3, 6, 12 and 24 h after hCG (n≥3 / time point). Aspirates representing the pooled contents of multiple follicles from each animal were maintained at approximately 35 C within a temperature-controlled isolette at all times. Oocytes were removed by transferring the aspirate to a 24-mm diameter, 70-μm pore size filter (Netwell Inserts 3479, Corning, Inc., Acton, MA), and the tube was rinsed with fresh Tyrode's lactate (TL-HEPES/0.1 mg/ml PVA) that was also poured onto the filter. This rinse was repeated until blood cells were removed from the filter. Granulosa cells were recovered by centrifugation of the cell suspension for 5 min at 300 × g to pellet the red cells and then increased to 500 × g for an additional 5 min, resulting in a thin layer of granulosa cells over the red cell pellet. The layer of granulosa cells was transferred to a 40% Percoll gradient in medium 199 (Sigma-Aldrich Corp., St. Louis, MO) and centrifuged for 30 min at 500 × g. The granulosa cells were recovered from the surface of the Percoll with a Pasteur pipette and washed twice with TL-HEPES-PVA and centrifuged at 500 × g for 10 min. The cell pellet was resuspended in 1 ml TL-HEPES-PVA in a 15 mL centrifuge tube and total cell number was determined on a hemocytometer. An additional 14 ml TL-HEPES-PVA was added to the cell suspension and tubes were capped and sealed with parafilm. The cells were shipped in a biohazard shipping container by overnight delivery at ambient temperature from September to June and the viability reassessed upon arrival (Chaffin et al. 2003). All animal procedures were performed in accordance with the NIH Guide for the Care and Use of laboratory Animals and were approved by the University of California Davis and the University of Maryland Baltimore animal care and use committees.
Glucose, lactate, pyruvate assays
Serum and follicular fluid concentrations of glucose were measured using a glucose oxidase-peroxide reaction kit (Cayman Chemical Company, Ann Arbor MI). The assay range was from 0-250 mg/dl. Pyruvate and Lactate from follicular fluid were measured using commercially available kits (BioVision Research products, Mountain View CA). All samples were run in a single assay with intra-assay variability <5%.
Real-time RT-PCR
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) and reverse transcribed with Moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA). Real-time RT-PCR (Applied Biosystems, Inc., Foster City, CA) was performed; Primers and 6-carboxy fluorescein-labeled probes for the target gene of interest and carboxy-(VIC)-labelled probe for the endogenous control ribosomal protein L19 (RPL19) was synthesized by Applied Biosystems and used in the same reaction. Probe and primer information is provided in table I. For relative quantification of mRNA levels, a standard curve was generated using a pool of human luteinized granulosa cell cDNA. The target gene was normalized to RPL19 as described (Brogan et al. 2009).
Table 1. Gene detection probe / primer sequences.
| Gene Name | Sequence | |
|---|---|---|
| RPL19 | Forward: CCCCAATGAGACCAATGAAATC Reverse: CAGCCCATCTTTGATGAGCTT Probe: VIC-ATGCCAACTCCCGTCAGCAGATC |
BC095445 |
| GLUT 1 | Assay on Demand | Hs00892682_g1 |
| GLUT 2 | Forward: AAT TGC TCC AAC CGC TCT CA Reverse: CTA ATA AGA ATG CCC CTG ACG AT Probe: FAM-AGC ACT TGG CAC TTT TCA TCA GCT GGC |
|
| GLUT 3 | Forward: GGT TTT GTG CCC ATG TAC ATT G Reverse: TGG TTG AGA GTG CCA AAG GC Probe: FAM-TCG CCT ACT GCC CTG C |
|
| GLUT 4 | Forward: GCT TCG TGG CAT TTT TGA GA Reverse: AGC TCG GCC ACG ATG AAC Probe: FAM-TGG CCC TGG CCC CAT TCC TT |
|
| G6PC3 | Assay on Demand | Hs00609178_m1 |
| PYGL | Assay on Demand | Hs00161132_m1 |
| HK1 | Assay on Demand | Hs00175976_m1 |
| HK2 | Assay on Demand | Hs00606086_m1 |
| G6PD | Assay on Demand | Hs00166169_m1 |
| PGI | Assay on Demand | Hs00976711_m1 |
hGL5 cultures, glucose uptake, steroidogenesis
Human (h) GL5 cells were provided by Dr. Bruce Carr, University of Texas Southwestern. Cells were cultured in DMEM/F12 medium (1:1, Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Invitrogen), 1× ITS+ (Sigma), 100 U/ml penicillin, 100 μg/ml streptomycin and 1 μg/ml amphotericin B (Gibco). Cells were plated in a black-walled 96-well format at an initial seeding density of 1×105 cells / well overnight in the presence of serum. Cell cultures were changed to glucose-free DMEM in the presence of ITS and with or without forskolin (10 μM) or insulin (1000 ng/ml) for up to 24 hr. A fluorescent non-metabolizable glucose analog (6-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-6-deoxyglucose; 6-NBDG; 0.3 mM; Invitrogen) was added to wells for the final 10 minutes of incubation. Media were removed and cells washed three times with warmed Hank's Balanced Salt Solution and the fluorescence measured (465/540 nm). Background fluorescence was determined in control wells receiving no 6-NBDG and empty wells in the presence of 6-NBDG.
To determine the effects of glucose on forskolin-induced progesterone synthesis, cells were grown as above and the media changed to glucose-free. In some cultures, 10% FCS was added with or without forskolin for 24 hr. In different cultures, D-glucose was kept at 0, 50, or 500 mg/dL, representing hypo-, normo-, and hyperglycemic conditions, respectively. L-glucose was used at the same concentrations as an osmotic control. Media were harvested and progesterone assayed using a commercially available kit (Siemens, Los Angeles, CA) (Cherian-Shaw et al. 2009).
Statistics
Normal distributions of data were verified using a Bartlett's chi-square test; data were log transformed if necessary. All data are presented as mean ± SEM. In vivo data were analyzed using one-way ANOVA and in vitro data by two-way ANOVA. Differences were considered significant if p<0.05. Data are presented as the mean ± SEM.
Results
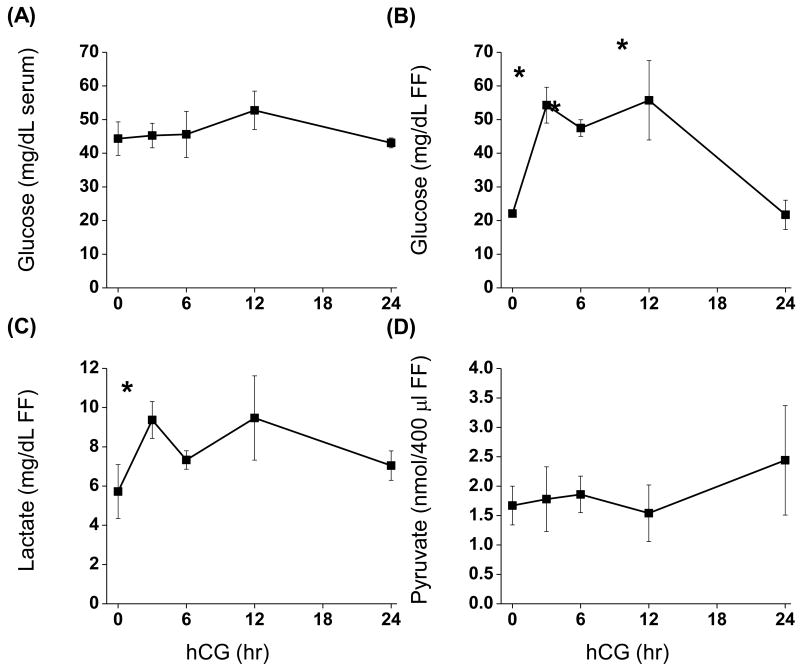
Luteinization of the macaque follicle using controlled ovarian stimulation protocols has been described in detail (Chaffin et al. 1999; Fru et al. 2006). Fasting serum glucose concentrations were approximately 46 mg/dL and did not change following administration of an ovulatory hCG bolus. The historical colony range of fasting glucose levels at the California National Primate Research Center is 43 to 71 mg/dL (unpublished observations) (Wahab et al. 2008). The concentration of glucose in follicular fluid prior to hCG was 22.0 mg/dL ± 1.1 and increased 2.5-fold (p<0.05; 54.3 ± 5.3) 3 hr after hCG. Glucose concentrations remained at this level until 24 hr post-hCG, at which levels returned to pre-hCG (21.7 ± 4.4) (Fig. 1A, B). Intra-follicular levels of lactate increased significantly 3 hr following hCG (1.7-fold; p<0.05). However, lactate levels 6, 12, and 24 hr post-hCG were not different than 0 or 3 hr (Fig. 1C). Levels of pyruvate in follicular fluid did not change as a result of hCG (Fig. 1D).
Figure 1. Follicular fluid concentrations of glucose, lactate, and pyruvate before and after an ovulatory stimulus.
Follicular fluid was aspirated from rhesus monkeys undergoing controlled ovarian stimulation protocols before (0 hr), 3, 6, 12 and 24 hr after an ovulatory hCG bolus (n=3, 3, 3, 3, 5). Glucose (A, B), lactate (C), and pyruvate (D) assays are described in Materials and Methods. *, significantly different than pre-hCG (0 hr).
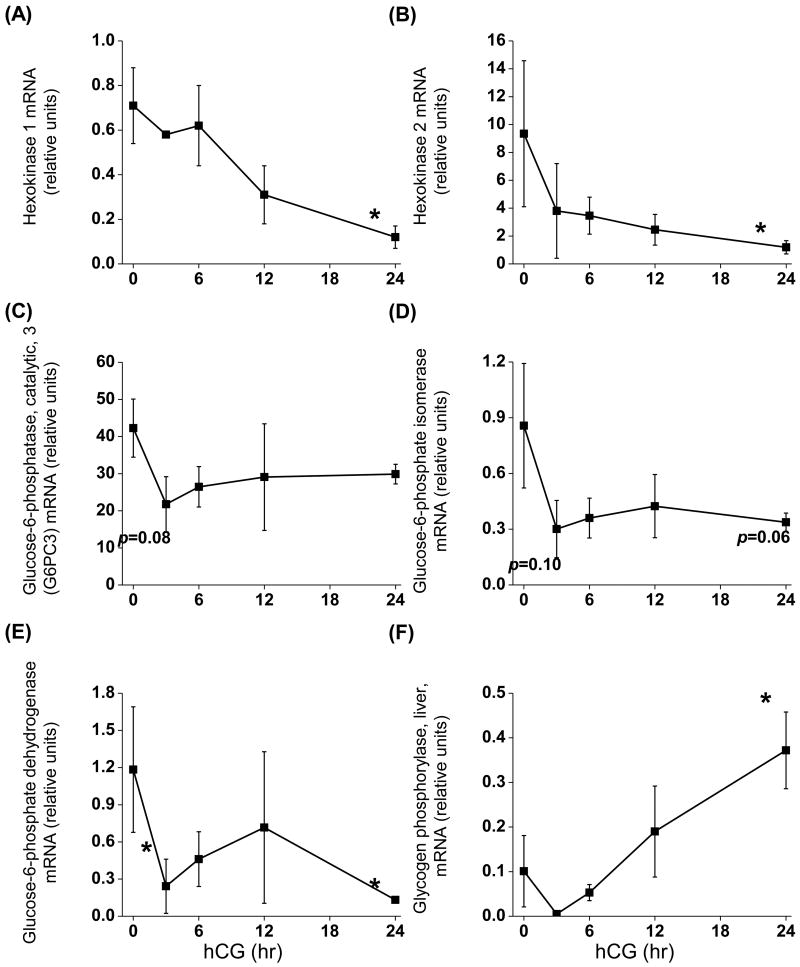
Many of the glucose and glycogen metabolizing genes exist as multiple isoforms, including hexokinase, glucose-6-phosphatase, and phophorylase. Data from a rhesus macaque granulosa cell microarray were screened in order to determine which isoforms are expressed by mural granulosa cells (K. Latham, Temple University, personal communication). Both hexokinase 1 and 2 (glucose → glucose-6-phosphate) were expressed by mural granulosa cells before and after hCG; however, the expression of both genes was reduced 24 hr post hCG (p<0.05; 6- and 8-fold, respectively) (Fig. 2A, B). The only detectable form of glucose-6-phosphatase (glucose-6-phosphate → glucose) was the ubiquitously expressed catalytic subunit (G6PC3; (Martin et al. 2002). The expression of G6PC3 mRNA did not change after hCG, although there was a tendency for the expression to decline 3 hr post-hCG (p=0.08) (Fig. 2C). Similarly, glucose phosphate isomerase mRNA (glucose-6-phosphate → fructose-6-phosphate) tended to be reduced following hCG (p=0.10 and 0.06 at 3 and 24 hr, respectively) (Fig. 2D). The mRNA expression of glucose-6-phosphate dehydrogenase (glucose-6-phosphate → 6-phosphogluconate; the first step in the pentose phosphate pathway) was reduced 3 hr (p<0.05; 5-fold) and 24 hr (p<0.05; 9-fold) post-hCG (Fig. 2E). The liver isoform of glycogen phosphorylase (glycogen →→→glucose-6-phosphate) was the only form consistently detectable in primate granulosa cells. The brain isoform was present in approximately 50% of samples, but at very low levels. The expression of glycogen phosphorylase (liver) mRNA increased nearly 4-fold (p<0.05) 24 hr after hCG (Fig. 2F).
Figure 2. Expression of genes associated with glucose metabolism before and after an ovulatory stimulus.
Granulosa cells were aspirated before (0 hr), 3, 6, 12 and 24 hr after an ovulatory hCG bolus given to rhesus monkeys undergoing controlled ovarian stimulation (n=3, 3, 4, 3, 4). Levels of mRNA were determined using real-time RT-PCR and data normalized to the internal standard ribosomal protein L19. *, significantly different than pre-hCG (0 hr).
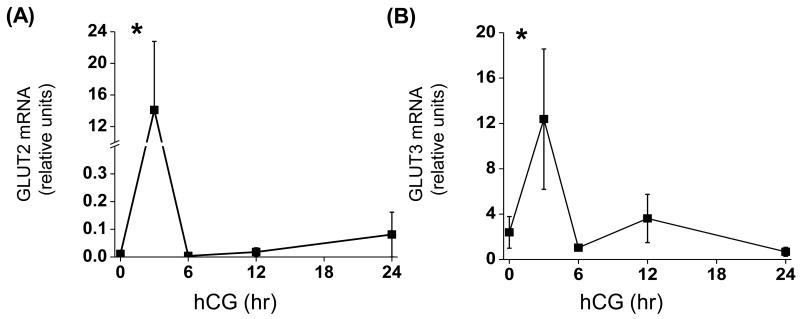
The mRNA expression of GLUT 1-4 by luteinizing mural granulosa cells was determined. GLUT1 and GLUT4 mRNA were not detectable at any time point in isolated granulosa cells (the adrenocortical cell line H295R (Cherian-Shaw et al. 2009) was used as a positive control). GLUT2 and GLUT3 mRNA increased transiently (p<0.05; 1,280- and 5-fold) 3 hr post-hCG; thereafter, levels returned to baseline (0 hr).
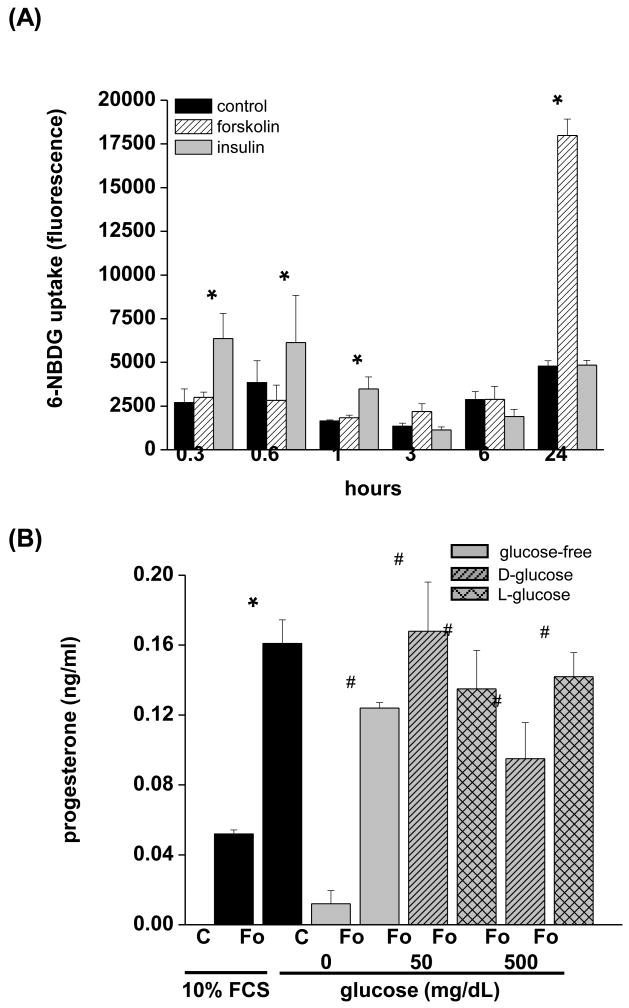
Primary macaque granulosa cells are susceptible to apoptosis in the absence of trophic stimuli, including insulin, glucose, and gonadotropins. In order to determine the time-course of glucose uptake following an acute trophic stimulus, the human granulosa-lutein cell line hGL5 was used. Insulin, IGF-I, and IGF-II levels in macaque follicular fluid do not change following hCG (Brogan et al. 2009); Thus, ITS was maintained in the culture media. Additional insulin was added to determine if rapid, insulin-mediated glucose uptake was functional in forskolin-stimulated hGL5 cells. The uptake of the non-metabolizable fluorescent glucose analog 6-NBDG was modestly stimulated by insulin 0.3, 0.6, and 1 hr after exposure (2.4-, 1.6- and 2.1-fold, respectively; p<0.05) (Fig. 4A). Thereafter, insulin had no effect on glucose uptake. In contrast, glucose uptake by forskolin was stimulated only 24 hr after the onset of treatment (3.8-fold; p<0.05). Chabrolle et al (Chabrolle et al. 2008) showed that high levels of glocuse decrease ovarian steroidogenesis by rat granulosa cells. In order to determine if this occurs also in hGL5 cells, forskolin was added in the presence of 10% FCS, resulting in a 3-fold increase (p<0.05) in media progesterone levels (Fig. 4B). A similar forskolin-induced increase in progesterone was seen in serum-free conditions regardless of glycemic levels of the culture.
Figure 4. Glucose uptake and effects of glucose on steroidogenesis in hGL5 cells.
(A) The human granulosa-lutein cell line hGL5 was cultured in glucose-free medium for up to 24 hr in the presence or absence of forsklin (Fo, 10 μM) or insulin (1000 ng/ml) (n=3 discrete replicates). The non-metabolizable fluorescent glucose analog 6-NBDG was added for the final 10 minutes of culture and the amount of intracellular 6-NBDG fluorescently. *, significantly different than time-matched controls. (B) The effects of hypo-, normo-, and hyperglycemic culture conditions on progesterone synthesis. Cells were culture in serum-free media with 0, 50, or 500 mg/dL D- or L-glucose for 24 hr and progesterone measured by radioimmunoassay. *, significantly different than controls in 10% FCS; #, significantly different than glucose-free control cultures.
Discussion
Glucose plays an important role in oocyte maturation (reviewed in (Sutton-McDowall et al.; Sutton et al. 2003). However, because most IVF aspirations are performed well after an ovulatory stimulus with resulting materials aspirated into media containing glucose, it has not been possible to describe changes in glucose levels and metabolism in a physiologically relevant setting. In addition, the metabolism of glucose by mural granulosa cells remains largely unknown. In rhesus monkeys undergoing a controlled ovarian stimulation protocol, an ovulatory hCG stimulus rapidly and transiently increases intra-follicular concentrations of glucose, while serum levels remain unchanged. Lactate in follicular fluid modestly increases after hCG, while pyruvate does not change. Expression of genes associated with glucose metabolism examined in this report decline as a result of an ovulatory stimulus, while glycogen phosphorylase increases. The expression of glucose transporters 2 and 3 is transiently increased by hCG.
Direct measurement of intra-ovarian glucose concentrations are hampered in rodents due to the difficulty in obtaining pure follicular fluid, and in humans by the lack of preovulatory (pre-hCG) samples. Monkeys offer an ideal model with which to examine changes in levels of intra-follicular glucose and metabolites; however, fasting serum glucose levels in macaques are typically lower than seen in humans (Wahab et al. 2008) (unpublished observations). Fasting serum glucose in the present study was in the historic range of the colony maintained by the California National Primate Research Center, and did not change as a result of hCG. In contrast, intra-follicular glucose levels increased rapidly following hCG, and declined by 24 hr. Given the rapid increase in glucose (<3 hr), it is difficult to envision a mechanism whereby the follicle specifically accumulates glucose from the serum. However, serum and follicular fluid glucose are both increased by approximately 25% in PCOS patients compared to normoandrogenic women (Foong et al. 2006) and significant correlations in glucose levels also exist between bovine serum and follicular fluid (Leroy et al. 2004), raising the possibility that ovarian glucose can be derived from serum. Despite this, it seems more likely that serum-borne glucose contributes to basal levels of follicular glucose rather than acute increases. Another possible source of follicular glucose is through glycogenolysis. This hypothesis is supported by the induction of glycogen phosphorylase, although this is after the increase in glucose. Thus, the origin of follicular glucose in the primate remains uncertain.
In contrast to glucose, there are no changes in follicular fluid pyruvate and a modest (60%) increase in lacatate. The increase in lactate could be indicative of anaerobic respiration in response to increased energetic demands associated with ovulation and luteal formation. Mouse preovulatory follicles can develop in vitro in the presence of an inhibitor of oxidative phosphorylation, evidence that follicular growth can be driven by glycolysis alone (Boland et al. 1993; Boland et al. 1994). In women undergoing IVF cycles, follicular fluid lactate correlates with follicle size (Fischer et al. 1992; Gull et al. 1999), suggesting that larger follicles have progressively lower intra-follicular oxygen concentrations. Interestingly, Redding et al (Redding et al. 2008) have modeled intra-follicular oxygen levels to < 7%. It is unlikely that vascular permeability dramatically increases oxygen in the luteinizing follicle until at least 12 hr post-hCG (Hazzard et al. 1999); thus the early period of the periovulatory interval is likely marked by low oxygen tension and increased anaerobic respiration.
The role of glucose in the luteinizing primate follicle has not been fully elucidated. Glucose and glucose metabolites such as pyruvate are important mediators of events at the cumulus-oocyte complex (Downs 2002; Downs et al. 2000; Downs et al. 1998; Downs et al. 2002; Downs et al. 1996; Eppig 1976; Su et al. 2009), although glucose uptake and metabolism by mural granulosa cells has not been explored. Harris et al (Harris et al. 2007) showed that murine follicles grown in vitro increase glucose consumption following hCG, although it is difficult to determine the relative contributions of mural versus cumulus granulosa cells in intact follicles. Similarly, the increase in follicular lactate may suggest an increase in glycolysis; however, it is hypothesized that this occurs at the level of the cumulus cell rather than in mural granulosa cells (Harris et al. 2007).
Oocyte meiosis in macaques resumes between 18-24 hr post-hCG (Borman et al. 2004; Nyholt de Prada et al. 2009), thus the increased glucose in the follicle likely serves to support the cumulus-oocyte complex through the initiation of meiosis and cumulus expansion (Gutnisky et al. 2007; Herrick et al. 2006; Thompson et al. 2007). Oocyte-secreted factors in the mouse increase the expression of gycolytic genes in cumulus cells, which in turn provide the GV-intact oocyte with pyruvate (Sugiura et al. 2005), while oocytes do not appear to have glycolytic capacity (Biggers et al. 1967). Whether this relationship holds true for primate cumulus-oocyte complexes following an ovulatory signal remains to be established, although it is interesting to note that the expression of GLUT4 in macaque oocytes increases as the oocyte resumes meiosis (Zheng et al. 2007). It is tempting to hypothesize that the decline in intra-follicular glucose 12-24 hr post-hCG is due to glucose metabolism by cumulus cells (cf. (Su et al. 2009)); certainly mouse cumulus-oocyte complexes can metabolize substantial quantities of glucose (Harris et al. 2007). Alternatively, the basement membrane begins to breakdown around this same time point, along with increased vascular permeability (Hazzard et al. 1999), and thus the reduction in intra-follicular glucose may simply be a result of follicular fluid dilution by serum.
Non-luteinized (pre-hCG) mural granulosa cells express several key components of glucose metabolism with the exception of GLUT1 and GLUT4. Contrary to expectations, hCG reduces hexokinase and glucose-6-phosphate isomerase in mural granulosa cells, suggesting that mural cells do not actively metabolize glucose during the first 24 hr after an ovulatory stimulus. In addition, the granulosa cell line hGL5 does not increase glucose uptake in response to forskolin until 24 hr, while there is a rapid (<1 hr) and transient effect of insulin Thus two discrete mechanisms of glucose uptake may be present in granulosa cells; one driven by insulin and a second by a slower, gonadotropin mediated pathway. The hGL5 cell line used herein has marked differences from primary mural granulosa cells (Rainey et al. 1994), including a lack of gonadotropin receptors; thus, whether these results are directly applicable to the intact primate follicle remains to be tested, although they do suggest that mural granulosa cells defer glucose uptake long enough for the oocyte to resume meiosis and the ovulatory cascade to be well underway. Given that it can be reasonably assumed that the high rate of steroid synthesis carries a high energetic cost, the source of energy for mural cells during the first 24 hr after an ovulatory stimulus needs to be experimentally determined.
Out of necessity, we examined mRNA expression in the current study. It is important to note that mRNA expression and enzyme activity are not always equivalent. Hexokinase activity is traditionally thought of as the rate limiting step in the conversion of glucose to G-6-P and is an irreversible reaction. On the other hand, glucose-6-phosphate isomerase (G-6-P to fructose-6-P) is freely reversible. The rate of enzymatic activity in reversible reactions depends on and is regulated by the substrate concentration. Our study did not examine the concentrations of G-6-P or F-6-P, or enzymatic activity, although the mRNA data presented herein support the notion that that glucose is utilization is decreased after treatment with hCG in the non-human primate.
Based on the data presented herein, it is hypothesized that mural granulosa cells “spare” glucose for the cumulus-oocyte complex for 24 hr, and then begin to take up glucose thereafter. These data indicate a novel mechanism whereby mural granulosa cells interact with the cumulus-oocyte complex in primates.
Figure 3. Expression of glucose transporter 1-4 mRNA before and after an ovulatory stimulus.
Granulosa cells were aspirated before (0 hr), 3, 6, 12 and 24 hr after an ovulatory hCG bolus given to rhesus monkeys undergoing controlled ovarian stimulation (n=3, 3, 4, 3, 4). Levels of mRNA were determined using real-time RT-PCR and data normalized to the internal standard ribosomal protein L19. GLUT 1 and GLUT 4 mRNA were not detectable in granulosa cells before or after hCG. *, significantly different than pre-hCG (0 hr).
Acknowledgments
The authors are grateful to Organon Inc., West Orange, NJ, USA, for the generous supply of recombinant human FSH and Serono laboratories (Ares Advanced Technology), Randolph, MA, USA, for the gift of recombinant hCG. The hGL5 cells were generously provided by Dr. Bruce Carr, University of Texas Southwestern Medical School (Dallas, TX). This research was supported in part by NIH HD043358 (CLC), RR13439 (CAV), RR00169 (CNPRC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Armstrong DT, Greep RO. Effect of gonadotrophic hormones on glucose metabolism by luteinized rat ovaries. Endocrinology. 1962;70:701–10. doi: 10.1210/endo-70-5-701. [DOI] [PubMed] [Google Scholar]
- Banhegyi G, Marcolongo P, Burchell A, Benedetti A. Heterogeneity of glucose transport in rat liver microsomal vesicles. Arch Biochem Biophys. 1998;359:133–8. doi: 10.1006/abbi.1998.0888. [DOI] [PubMed] [Google Scholar]
- Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci U S A. 1967;58:560–7. doi: 10.1073/pnas.58.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland NI, Humpherson PG, Leese HJ, Gosden RG. Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol Reprod. 1993;48:798–806. doi: 10.1095/biolreprod48.4.798. [DOI] [PubMed] [Google Scholar]
- Boland NI, Humpherson PG, Leese HJ, Gosden RG. Characterization of follicular energy metabolism. Hum Reprod. 1994;9:604–9. doi: 10.1093/oxfordjournals.humrep.a138557. [DOI] [PubMed] [Google Scholar]
- Borman SM, Chaffin CL, Schwinof KM, Stouffer RL, Zelinski-Wooten MB. Progesterone promotes oocyte maturation, but not ovulation, in nonhuman primate follicles without a gonadotropin surge. Biol Reprod. 2004;71:366–73. doi: 10.1095/biolreprod.103.023390. [DOI] [PubMed] [Google Scholar]
- Brogan RS, Mix S, Puttabyatappa M, Vandevoort CA, Chaffin CL. Expression of the insulin-like growth factor and insulin systems in the luteinizing macaque ovarian follicle. Fertil Steril. 2009 doi: 10.1016/j.fertnstert.2008.12.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushia RJ, Walsh DA. Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure. Front Biosci. 1999;4:D618–41. doi: 10.2741/brushia. [DOI] [PubMed] [Google Scholar]
- Chabrolle C, Jeanpierre E, Tosca L, Rame C, Dupont J. Effects of high levels of glucose on the steroidogenesis and the expression of adiponectin receptors in rat ovarian cells. Reprod Biol Endocrinol. 2008:6–11. doi: 10.1186/1477-7827-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin CL, Brogan RS, Stouffer RL, VandeVoort CA. Dynamics of Myc/Max/Mad expression during luteinization of primate granulosa cells in vitro: association with periovulatory proliferation. Endocrinology. 2003;144:1249–56. doi: 10.1210/en.2002-220664. [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Hess DL, Stouffer RL. Dynamics of periovulatory steroidogenesis in the rhesus monkey follicle after ovarian stimulation. Hum Reprod. 1999;14:642–9. doi: 10.1093/humrep/14.3.642. [DOI] [PubMed] [Google Scholar]
- Cherian-Shaw M, Puttabyatappa M, Greason E, Rodriguez A, VandeVoort CA, Chaffin CL. Expression of scavenger receptor-BI and low-density lipoprotein receptor and differential use of lipoproteins to support early steroidogenesis in luteinizing macaque granulosa cells. Endocrinology. 2009;150:957–65. doi: 10.1210/en.2008-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Prada JK, Hill DL, Chaffin CL, VandeVoort CA. Nuclear maturation and structural components of nonhuman primate cumulus-oocyte complexes during in vivo and in vitro maturation. Fertil Steril. 2009;91:2043–50. doi: 10.1016/j.fertnstert.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblado M, Moley KH. Glucose metabolism in pregnancy and embryogenesis. Curr Opin Endocrinol Diabetes Obes. 2007;14:488–93. doi: 10.1097/MED.0b013e3282f1cb92. [DOI] [PubMed] [Google Scholar]
- Downs SM. The biochemistry of oocyte maturation. Ernst Schering Res Found Workshop. 2002:81–99. doi: 10.1007/978-3-662-04960-0_6. [DOI] [PubMed] [Google Scholar]
- Downs SM, Hudson ED. Energy substrates and the completion of spontaneous meiotic maturation. Zygote. 2000;8:339–51. doi: 10.1017/s0967199400001131. [DOI] [PubMed] [Google Scholar]
- Downs SM, Humpherson PG, Leese HJ. Meiotic induction in cumulus cell-enclosed mouse oocytes: involvement of the pentose phosphate pathway. Biol Reprod. 1998;58:1084–94. doi: 10.1095/biolreprod58.4.1084. [DOI] [PubMed] [Google Scholar]
- Downs SM, Humpherson PG, Leese HJ. Pyruvate utilization by mouse oocytes is influenced by meiotic status and the cumulus oophorus. Mol Reprod Dev. 2002;62:113–23. doi: 10.1002/mrd.10067. [DOI] [PubMed] [Google Scholar]
- Downs SM, Humpherson PG, Martin KL, Leese HJ. Glucose utilization during gonadotropin-induced meiotic maturation in cumulus cell-enclosed mouse oocytes. Mol Reprod Dev. 1996;44:121–31. doi: 10.1002/(SICI)1098-2795(199605)44:1<121::AID-MRD14>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Analysis of mouse oogenesis in vitro. Oocyte isolation and the utilization of exogenous energy sources by growing oocytes. J Exp Zool. 1976;198:375–82. doi: 10.1002/jez.1401980311. [DOI] [PubMed] [Google Scholar]
- Fischer B, Kunzel W, Kleinstein J, Gips H. Oxygen tension in follicular fluid falls with follicle maturation. Eur J Obstet Gynecol Reprod Biol. 1992;43:39–43. doi: 10.1016/0028-2243(92)90241-p. [DOI] [PubMed] [Google Scholar]
- Flint AP, Denton RM. Glucose metabolism in the superovulated rat ovary in vitro. Effects of luteinizing hormone and the role of glucose metabolism in steroidogenesis. Biochem J. 1969;112:243–54. doi: 10.1042/bj1120243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong SC, Abbott DH, Zschunke MA, Lesnick TG, Phy JL, Dumesic DA. Follicle luteinization in hyperandrogenic follicles of polycystic ovary syndrome patients undergoing gonadotropin therapy for in vitro fertilization. J Clin Endocrinol Metab. 2006;91:2327–33. doi: 10.1210/jc.2005-2142. [DOI] [PubMed] [Google Scholar]
- Fru KN, Vandevoort CA, Chaffin CL. Mineralocorticoid synthesis during the periovulatory interval in macaques. Biol Reprod. 2006;75:568–74. doi: 10.1095/biolreprod.106.053470. [DOI] [PubMed] [Google Scholar]
- Gull I, Geva E, Lerner-Geva L, Lessing JB, Wolman I, Amit A. Anaerobic glycolysis. The metabolism of the preovulatory human oocyte. Eur J Obstet Gynecol Reprod Biol. 1999;85:225–8. doi: 10.1016/s0301-2115(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Gutnisky C, Dalvit GC, Pintos LN, Thompson JG, Beconi MT, Cetica PD. Influence of hyaluronic acid synthesis and cumulus mucification on bovine oocyte in vitro maturation, fertilisation and embryo development. Reprod Fertil Dev. 2007;19:488–97. doi: 10.1071/rd06134. [DOI] [PubMed] [Google Scholar]
- Hamberger LA, Ahren KE. Effects of gonadotrophins in vitro on glucose uptake and lactic acid production of ovaries from prepubertal and hypophysectomized rats. Endocrinology. 1967;81:93–100. doi: 10.1210/endo-81-1-93. [DOI] [PubMed] [Google Scholar]
- Harris SE, Adriaens I, Leese HJ, Gosden RG, Picton HM. Carbohydrate metabolism by murine ovarian follicles and oocytes grown in vitro. Reproduction. 2007;134:415–24. doi: 10.1530/REP-07-0061. [DOI] [PubMed] [Google Scholar]
- Hazzard TM, Molskness TA, Chaffin CL, Stouffer RL. Vascular endothelial growth factor (VEGF) and angiopoietin regulation by gonadotrophin and steroids in macaque granulosa cells during the periovulatory interval. Mol Hum Reprod. 1999;5:1115–21. doi: 10.1093/molehr/5.12.1115. [DOI] [PubMed] [Google Scholar]
- Herrick JR, Brad AM, Krisher RL. Chemical manipulation of glucose metabolism in porcine oocytes: effects on nuclear and cytoplasmic maturation in vitro. Reproduction. 2006;131:289–98. doi: 10.1530/rep.1.00835. [DOI] [PubMed] [Google Scholar]
- Jungheim ES, Moley KH. The impact of type 1 and type 2 diabetes mellitus on the oocyte and the preimplantation embryo. Semin Reprod Med. 2008;26:186–95. doi: 10.1055/s-2008-1042957. [DOI] [PubMed] [Google Scholar]
- Leroy JL, Vanholder T, Delanghe JR, Opsomer G, Van Soom A, Bols PE, de Kruif A. Metabolite and ionic composition of follicular fluid from different-sized follicles and their relationship to serum concentrations in dairy cows. Anim Reprod Sci. 2004;80:201–11. doi: 10.1016/S0378-4320(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Martin CC, Oeser JK, Svitek CA, Hunter SI, Hutton JC, O'Brien RM. Identification and characterization of a human cDNA and gene encoding a ubiquitously expressed glucose-6-phosphatase catalytic subunit-related protein. J Mol Endocrinol. 2002;29:205–22. doi: 10.1677/jme.0.0290205. [DOI] [PubMed] [Google Scholar]
- Nishimoto H, Matsutani R, Yamamoto S, Takahashi T, Hayashi KG, Miyamoto A, Hamano S, Tetsuka M. Gene expression of glucose transporter (GLUT) 1, 3 and 4 in bovine follicle and corpus luteum. J Endocrinol. 2006;188:111–9. doi: 10.1677/joe.1.06210. [DOI] [PubMed] [Google Scholar]
- Rainey WH, Sawetawan C, Shay JW, Michael MD, Mathis JM, Kutteh W, Byrd W, Carr BR. Transformation of human granulosa cells with the E6 and E7 regions of human papillomavirus. J Clin Endocrinol Metab. 1994;78:705–10. doi: 10.1210/jcem.78.3.8126145. [DOI] [PubMed] [Google Scholar]
- Ratchford AM, Esguerra CR, Moley KH. Decreased oocyte-granulosa cell gap junction communication and connexin expression in a type 1 diabetic mouse model. Mol Endocrinol. 2008;22:2643–54. doi: 10.1210/me.2007-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding GP, Bronlund JE, Hart AL. Theoretical investigation into the dissolved oxygen levels in follicular fluid of the developing human follicle using mathematical modelling. Reprod Fertil Dev. 2008;20:408–17. doi: 10.1071/rd07190. [DOI] [PubMed] [Google Scholar]
- Roy L, McDonald CA, Jiang C, Maroni D, Zeleznik AJ, Wyatt TA, Hou X, Davis JS. Convergence of 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A and glycogen synthase kinase-3beta/beta-catenin signaling in corpus luteum progesterone synthesis. Endocrinology. 2009;150:5036–45. doi: 10.1210/en.2009-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008;295:E242–53. doi: 10.1152/ajpendo.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27:32–42. doi: 10.1055/s-0028-1108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K, Pendola FL, Eppig JJ. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol. 2005;279:20–30. doi: 10.1016/j.ydbio.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Sutton-McDowall M, Gilchrist R, Thompson J. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction. doi: 10.1530/REP-09-0345. [DOI] [PubMed] [Google Scholar]
- Sutton ML, Gilchrist RB, Thompson JG. Effects of in-vivo and in-vitro environments on the metabolism of the cumulus-oocyte complex and its influence on oocyte developmental capacity. Hum Reprod Update. 2003;9:35–48. doi: 10.1093/humupd/dmg009. [DOI] [PubMed] [Google Scholar]
- Thompson JG, Lane M, Gilchrist RB. Metabolism of the bovine cumulus-oocyte complex and influence on subsequent developmental competence. Soc Reprod Fertil Suppl. 2007;64:179–90. doi: 10.5661/rdr-vi-179. [DOI] [PubMed] [Google Scholar]
- Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 2004;447:480–9. doi: 10.1007/s00424-003-1085-0. [DOI] [PubMed] [Google Scholar]
- VandeVoort CA, Leibo SP, Tarantal AF. Improved collection and developmental competence of immature macaque oocytes. Theriogenology. 2003;59:699–707. doi: 10.1016/s0093-691x(02)01129-9. [DOI] [PubMed] [Google Scholar]
- VandeVoort CA, Tarantal AF. The macaque model for in vitro fertilization: superovulation techniques and ultrasound-guided follicular aspiration. J Med Primatol. 1991;20:110–6. [PubMed] [Google Scholar]
- Wahab F, Aziz F, Irfan S, Zaman WU, Shahab M. Short-term fasting attenuates the response of the HPG axis to kisspeptin challenge in the adult male rhesus monkey (Macaca mulatta) Life Sci. 2008;83:633–7. doi: 10.1016/j.lfs.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ratchford AM, Chi MM, Schoeller E, Frolova A, Schedl T, Moley KH. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol. 2009;23:1603–12. doi: 10.1210/me.2009-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89:3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]
- Zheng P, Vassena R, Latham KE. Effects of in vitro oocyte maturation and embryo culture on the expression of glucose transporters, glucose metabolism and insulin signaling genes in rhesus monkey oocytes and preimplantation embryos. Mol Hum Reprod. 2007;13:361–71. doi: 10.1093/molehr/gam014. [DOI] [PubMed] [Google Scholar]