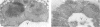Abstract
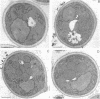
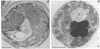
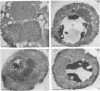
The peroxisomal flavoprotein alcohol oxidase (AO) is an octamer (600 kDa) consisting of eight identical subunits, each of which contains one flavin adenine dinucleotide molecule as a cofactor. Studies on a riboflavin (Rf) auxotrophic mutant of the yeast Hansenula polymorpha revealed that limitation of the cofactor led to drastic effects on AO import and assembly as well as peroxisome proliferation. Compared to wild-type control cells Rf-limitation led to 1) reduced levels of AO protein, 2) reduced levels of correctly assembled and activated AO inside peroxisomes, 3) a partial inhibition of peroxisomal protein import, leading to the accumulation of precursors of matrix proteins in the cytosol, and 4) a significant increase in peroxisome number. We argue that the inhibition of import may result from the saturation of a peroxisomal molecular chaperone under conditions that normal assembly of a major matrix protein inside the target organelle is prevented.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellion E., Goodman J. M. Proton ionophores prevent assembly of a peroxisomal protein. Cell. 1987 Jan 16;48(1):165–173. doi: 10.1016/0092-8674(87)90367-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Distel B., Veenhuis M., Tabak H. F. Import of alcohol oxidase into peroxisomes of Saccharomyces cerevisiae. EMBO J. 1987 Oct;6(10):3111–3116. doi: 10.1002/j.1460-2075.1987.tb02620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H., Didion T., Thiemann A., Veenhuis M., Roggenkamp R. Targeting sequences of the two major peroxisomal proteins in the methylotrophic yeast Hansenula polymorpha. Mol Gen Genet. 1992 Nov;235(2-3):269–278. doi: 10.1007/BF00279370. [DOI] [PubMed] [Google Scholar]
- Kato N., Omori Y., Tani Y., Ogata K. Alcohol oxidases of Kloeckera sp. and Hansenula polymorpha. Catalytic properties and subunit structures. Eur J Biochem. 1976 May 1;64(2):341–350. doi: 10.1111/j.1432-1033.1976.tb10307.x. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nicolay K., Veenhuis M., Douma A. C., Harder W. A 31P NMR study of the internal pH of yeast peroxisomes. Arch Microbiol. 1987 Feb;147(1):37–41. doi: 10.1007/BF00492902. [DOI] [PubMed] [Google Scholar]
- Roa M., Blobel G. Biosynthesis of peroxisomal enzymes in the methylotrophic yeast Hansenula polymorpha. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6872–6876. doi: 10.1073/pnas.80.22.6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhuis M., Sulter G., van der Klei I., Harder W. Evidence for functional heterogeneity among microbodies in yeasts. Arch Microbiol. 1989;151(2):105–110. doi: 10.1007/BF00414422. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., van Dijken J. P., Harder W. Cytochemical studies on the localization of methanol oxidase and other oxidases in peroxisomes of methanol-grown Hansenula polymorpha. Arch Microbiol. 1976 Dec 1;111(1-2):123–135. doi: 10.1007/BF00446559. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., van Dijken J. P., Pilon S. A., Harder W. Development of crystalline peroxisomes in methanol-grown cells of the yeast Hansenula polymorpha and its relation to environmental conditions. Arch Microbiol. 1978 May 30;117(2):153–163. doi: 10.1007/BF00402303. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., van der Klei I. J., Titorenko V., Harder W. Hansenula polymorpha: an attractive model organism for molecular studies of peroxisome biogenesis and function. FEMS Microbiol Lett. 1992 Dec 15;100(1-3):393–403. doi: 10.1111/j.1574-6968.1992.tb14068.x. [DOI] [PubMed] [Google Scholar]
- Waterham H. R., Keizer-Gunnink I., Goodman J. M., Harder W., Veenhuis M. Development of multipurpose peroxisomes in Candida boidinii grown in oleic acid-methanol limited continuous cultures. J Bacteriol. 1992 Jun;174(12):4057–4063. doi: 10.1128/jb.174.12.4057-4063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoop M., Asgeirsdottir S., Blaauw M., Veenhuis M., Cregg J., Gleeson M., Geert A. B. Mutations in the FAD-binding fold of alcohol oxidase from Hansenula polymorpha. Protein Eng. 1991 Oct;4(7):821–829. doi: 10.1093/protein/4.7.821. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., Otto R., Harder W. Growth of Hansenula polymorpha in a methanol-limited chemostat. Physiological responses due to the involvement of methanol oxidase as a key enzyme in methanol metabolism. Arch Microbiol. 1976 Dec 1;111(1-2):137–144. doi: 10.1007/BF00446560. [DOI] [PubMed] [Google Scholar]
- van der Klei I. J., Harder W., Veenhuis M. Biosynthesis and assembly of alcohol oxidase, a peroxisomal matrix protein in methylotrophic yeasts: a review. Yeast. 1991 Apr;7(3):195–209. doi: 10.1002/yea.320070302. [DOI] [PubMed] [Google Scholar]