Abstract
Advanced age is associated with decrements in episodic memory, which are more pronounced in memory for associations than in memory for individual items. The Associative Deficit Hypothesis (ADH) states that age differences in recognition memory reflect difficulty in binding components of a memory episode and retrieving bound units. To date, ADH has received support only in studies of extreme age groups, and the influence of sex, education, and health on age-related associative deficit is unknown. We address those issues using a verbal paired-associate yes/no recognition paradigm on a lifespan sample of 278 healthy, well-educated adults. In accord with the ADH, greater age was associated with lower hit and greater false alarm rates and more liberal response bias on associative recognition tests. Women outperformed men on recognition of items and associations, but among normotensive participants, women outperformed men only on memory for associations and not on item recognition. Thus, while supporting ADH in a large lifespan sample of healthy adults, the findings indicate that the effect may be partially driven by age-related increase in liberal bias in recognition of associations. Sex differences and health factors may modify the associative deficit regardless of age.
Keywords: aging, memory, cognition, hypertension, sex differences
Many studies of cognitive aging have reported moderate age-related decrements in episodic memory (Verhaeghen, Marcoen, & Goossens, 1993). Declines in memory for complex information have been ascribed to reduced ability to bind target information and the accompanying context into a cohesive and retrievable memory episode (Chalfonte & Johnson, 1996; Light, 1991; Mitchell, Johnson, Raye, & D’Esposito, 2000). The associative deficit hypothesis (ADH) of age-related declines in episodic memory posits that the binding deficit at encoding and the additional deficit in the retrieval of bound units are responsible for age-related memory declines (Naveh-Benjamin, 2000). The ADH predicts greater age-related differences in the recognition of associations between items than in recognition of individual stimuli. Originally demonstrated on verbal paired-associate tasks (e.g., Castel & Craik, 2003; Light, Patterson, Chung, & Healy, 2004; Naveh-Benjamin, 2000), the ADH has been supported by studies that employed other stimuli (see a meta-analysis by Old & Naveh-Benjamin, 2008).
The extant studies on ADH have several limitations in design, sample characteristics, and analyses of performance that hinder interpretation of the results. First, support for ADH comes exclusively from experiments with extreme age groups, which entails overestimation of age differences (Kraemer, Yesavage, Taylor, & Kupfer, 2000; Schaie, 2005), and may distort the shape of age trajectory of cognitive performance. Second, most studies of ADH paid little attention to health indicators that decline with age and may confound its effects (Brady, Spiro, & Gaziano, 2005; Fontbonne, Berr, Ducimetiere, & Alperovitch, 2001; Joffe & Levitt, 1993; Lichtenberg, Ross, Millis, & Manning, 1995; Raz, Rodrigue, & Acker, 2003). Even in healthy participants, vascular risk factors predict reductions in episodic memory, working memory, and speed of processing (Dahle, Jacobs, & Raz, 2009). Moreover, elevated vascular risk in middle age augurs cognitive decline in the late years (Freitag et al., 2006; Piela et al., 2001). Third, it is unclear whether associative deficits reported in some studies stem from the relatively modest educational attainment of the typical participants (e.g., high school level in Naveh-Benjamin, 2000). Age differences in verbal recall are exacerbated by low education (Verhaeghen et al., 1993) suggesting a possible impact of general cognitive ability and use of strategies on performance. Furthermore, although women frequently show better memory for lexical stimuli than men do (see Herlitz & Rehnman, 2008 for review), sex differences have not been investigated in most studies of the ADH. Finally, the measurements of associative memory performance need further refinement. Because previous tests of ADH (e.g., Naveh-Benjamin, 2000) reported overall memory accuracy (proportion hits minus proportion false alarms), it is unclear whether the observed deficit reflects differential age-related decline in hits, increase in false alarms, or both. The goal of this study was to address the described limitations. We evaluated age-related differences in memory for items and associations in a lifespan sample of healthy, well-educated adults, while taking into account the potential confounding influence of vascular risk factors and refining the measures of performance.
Method
Participants
The sample consisted of 278 adult native English speakers (age 18 to 85 years, 183 women) recruited through advertisements in local media, flyers, and word of mouth from a major metropolitan area in the United States. The participants signed an informed consent form approved by the University Human Investigation Committee and were screened for history of health problems including cardiovascular, neurological or psychiatric disease, cancer, head trauma with loss of consciousness for more than five minutes, thyroid disorder, diabetes, treatment for drug and alcohol abuse, taking more than three alcoholic drinks per day, or taking anticonvulsive, anxiolytic, antipsychotic, and antidepressant medications.
The participants were screened for near, far, and color vision problems (Optec 2000 Vision Tester, Stereo Optical Co., Inc., Chicago, IL), speech-range auditory deficits (MA27 Audiometer, Maico Diagnostics, Eden Prairie, MN), depressed mood (Center for Epidemiological Studies on Depression, CES-D, Radloff, 1977, cut-off score of 15), and cognitive impairment (Mini Mental State Examination, MMSE; Folstein, Folstein, & McHugh, 1975; cut-off score 26). Participants who did not meet the eligibility requirements were not included in the study. All participants had at least a high school education, and mean education in the sample neared four years of college (15.8 ± 2.5 years). Men and women did not differ in age, years of education, prevalence of hypertension, systolic or diastolic blood pressure, or MMSE (see Table 1).
Table 1.
| A. Sample Demographics Comparing Men and Women | ||||
|---|---|---|---|---|
| Variable | Men | Women | t or χ2a | p |
| Mean ± SD | Mean ± SD | |||
| Age | 51.8 ± 16.2 | 52.3 ± 15.3 | 0.27 | .79 |
| Education | 16.0 ± 2.5 | 15.6 ± 2.5 | 0.82 | .41 |
| MMSE | 28.8 ± 1.1 | 28.9 ± 1.0 | 0.95 | .34 |
| Systolic BP (mm Hg) | 123.3 ± 11.7 | 121.2 ± 13.6 | 1.26 | .21 |
| Diastolic BP (mm Hg) | 76.4 ± 8.1 | 74.7 ± 7.5 | 1.78 | .08 |
| % Dx HTNb | 18% | 18% | 0.00a | .98 |
| B. Age Distributions for Men and Women | ||||
|---|---|---|---|---|
| Decade | Men N (%) | Women N (%) | Total N (%) | |
| 18–30 | 13 (13.7%) | 23 (12.6%) | 36 (12.9%) | |
| 31–40 | 12 (12.6%) | 15 (8.2%) | 27 (9.7%) | |
| 41–50 | 9 (9.5%) | 27 (14.8%) | 36 (12.9%) | |
| 51–60 | 28 (29.5%) | 52 (28.4%) | 80 (28.8%) | |
| 61–70 | 18 (18.9%) | 42 (23.0%) | 60 (21.6%) | |
| > 71 | 15 (15.8%) | 24 (13.1%) | 39 (14.0%) | |
Notes: Mean values ± standard deviations are presented. MMSE = Mini Mental State Examination.
A single degree of freedom chi-square test.
% Dx HTN = Proportion of sample with physician diagnosed hypertension.
Following informed consent and prior to cognitive testing, an experimenter measured blood pressure with an analog mercury sphygmomanometer (Model 12–525; Country Technology, Gays Mills, WI) using a left arm brachial cuff. The measurements were taken from seated participants in a quiet room, on three separate days. For all but 14 participants, blood pressure was averaged across at least two occasions. Hypertension was operationally defined as either a diagnosis with prescription of anti-hypertensive medication, or measured blood pressure above threshold (systolic BP > 140 mm Hg or diastolic BP > 90 mm Hg; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, 2004).
Design and Procedure
The recognition memory task was modeled after that of Experiment 2 from Naveh-Benjamin’s (2000) study. The participants were tested individually and the task consisted of two study lists of 26 word pairs each, followed by separate recognition memory tests for items and associations between them. The word-pairs were rendered in a white, sans-serif font on a black background, and were presented on a 17-inch color computer monitor (resolution 800 × 600) at a rate of 5.5 s per pair with a 200 ms inter-stimulus interval. Each participant viewed two word-pair lists randomly chosen from a subset of six lists (from a total pool of 12). The relative position of the two words (left or right) in each pair was randomized across participants. Participants responded by pressing one of two keys on a 104-key Dell computer keyboard (Dell Inc., Round Rock, Tx). A “YES” label was placed on the V key and a “NO” label on the N key.
Study instructions were intentional: participants were told to remember both the single words and the word pairs, and were informed that they would have separate memory tests for the words and the pairings. To minimize rehearsal after encoding, participants counted backward by threes from a randomly generated number over 900 for 60 s. Separate recognition memory tests for items and associations were administered, and the process was repeated for the second list of word pair stimuli. All tests were self-paced and response to one item was required before presentation of the next one. Items remained on the screen for up to 3 s, unless the participant responded sooner. Between the response and the next item there was a 200 ms blank-screen interval. Prior to the study session, participants heard instructions for both tasks and practiced on both tests. Order of presentation for the item and associative recognition tests was counterbalanced across the sample. The second word pair list was presented and the procedure was repeated following study and test phases for the first list.
During the item recognition test, participants viewed 16 words including eight words seen at study and eight new words. The experimenter instructed participants to respond ‘YES’ if the word was seen at study, or ‘NO’ if the word was new. On the associative recognition test, participants saw 16 word pairs consisting entirely of words from the study list. However, eight pairs were the same as shown at study, and eight were composed of recombined word (words that appeared during study but not together). Participants were instructed to respond ‘YES’ if they saw the pair as it was at study, or to respond ‘NO’ if they did not see that pair of words at study. A given word from the study phase appeared in only one of the tests.
Data Conditioning
To assess recognition performance, we used A′, a signal detection index based on proportion correct and proportion incorrect from targets and foils, respectively (Pollack & Norman, 1964). This nonparametric signal detection index is among the most commonly used measures of discriminability for yes/no tests of recognition memory, and is valid regardless of the distributions of hit rate and false alarm rate (Donaldson, 1992; Stanislaw & Todorov, 1999). Retrieval trials with response times shorter than 200 ms or longer than 10 s were excluded from analyses as such responses were believed to stem from errors or memory processes other than recognition. Hit rate (HR) was calculated by dividing the number of correct target responses (responding “YES” to a target), by the total number of targets presented. False alarm rate (FAR) was calculated by dividing the number of incorrect distracter responses (responding “YES” to a distracter) by the total number of distracter stimuli. These scores were then used to derive the A′ statistic and corresponding response bias measure, B″D (Donaldson, 1992). This bias index is better than Grier’s (1971) B″ because of its ability to measure bias at lower recognition performance (Donaldson, 1992).
Statistical Analyses
For data analysis, we used the general linear model (GLM) framework. In separate models, A′, B″D, HR, FAR, and hit response time (RT) served as the dependent variables for analyses of performance. In all models, Age, centered at the sample mean, was a continuous predictor, and Sex was a categorical predictor. The two Lists and two Tests (recognition of items and of associations) were treated as repeated measures. The second-order interaction of the two predictors was also included in the model, but when the interaction terms were found to be non-significant, the reduced model without those terms was re-evaluated. This approach was repeated in additional models that included the effects of hypertension. Huynh-Feldt correction was applied to significant interactions as control for violations of the sphericity assumption. To correct for significant skew, we applied a log transformation to mean RT data from hit responses, and an arcsine transformation to A′, HR, and FAR scores.
Results
Signal Detection Analyses (A′)
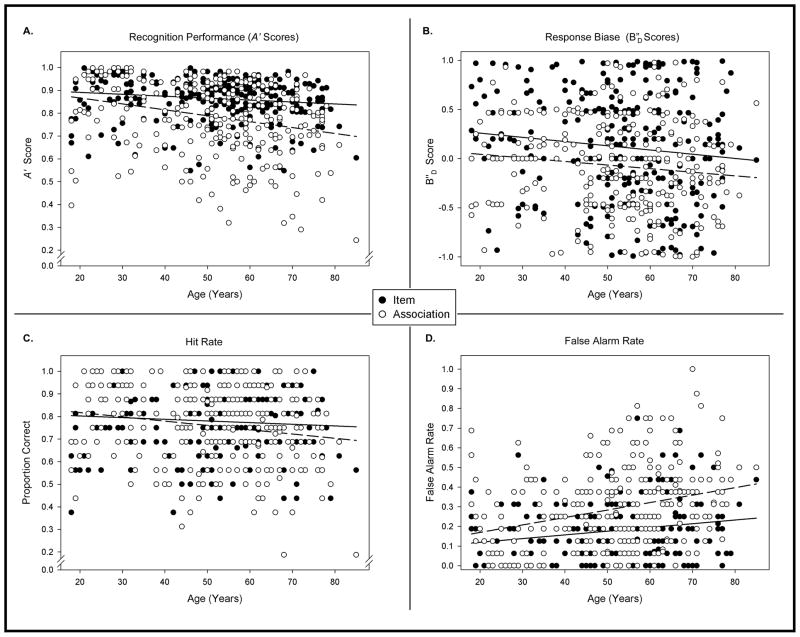
A significant main effect of Age (F[1, 274] = 17.00, p < .001) indicated that advanced age was associated with poorer recognition performance. A significant main effect of Sex (F[1, 274] = 9.00, p < .01) was due to women performing better than men. A significant main effect of Test (F[1, 274] = 95.91, p < .001), reflected better recognition of individual items than the paired associates. Notably, a significant Test × Age interaction (F[1, 274] = 13.22, p < .001) was due to difference in the correlation between age and item recognition (r = −0.18, p < .05) versus age and recognition of associations (r = −0.33, p < .001; Steiger’s Z* = 2.22, p < .05; Fig. 1A). There were no significant effects involving the List factor (p > .3 for all).
Figure 1.
Performance on the word pair task by a continuous lifespan sample of healthy adults. In all figures, solid black circles and the solid regression line depict items, and open circles and the broken regression line represent association scores. A. Scatterplot of the relationship between signal detection measure A′ reflecting recognition performance for items and associations between words and participant age in years. B. Scatterplot representing participants’ ages and response bias measure B″D from recognition performance of items and associations. Scores less than 0 reflect more liberal bias, and scores greater than zero indicate more conservative bias. C. Scatterplot of participants’ ages and hit rate scores for items and associations. D. False alarm rate scores for items and associations by age in years.
Response Bias
There was a main effect of Age on the bias measure B″D (F[1, 274] = 5.77, p < .05), due to a small but significant correlation between Age and B″D values (r = −0.15, p < .05) that reflected an age-related liberal response bias. As indicated by a significant main effect of Test (F[1, 274] = 14.47, p < .001), recognition of paired associates elicited more liberal responses than recognition of individual items (Fig. 1B). A significant main effect of List (F[1, 274] = 25.12, p < .001) showed that response criterion became more liberal from the first to second administration. There were no significant effects or interactions between response bias and sex.
Hit Rate and False Alarm Rate
Because A′ is calculated from HR and FAR values, and because age may be associated with higher rates of false recognition (for reviews, see Schacter, Koustaal, & Norman, 1997; Schacter, Norman, & Koustaal, 1998), we conducted separate analyses on hit rate (HR) and false alarm rate (FAR). Distributions of FAR were highly positively skewed due to a floor effect, whereas HR distributions were negatively skewed because of a ceiling effect. Therefore, arcsine transformations were applied to both. In the HR analyses, the nonsignificant Sex × Age interaction was removed and the model was re-evaluated. The reduced model revealed a significant main effect of Age (F[1, 276] = 7.74, p < .01): greater age was associated with lower hit rate (r = −.16, p < .01). There was a significant main effect of Sex (F[1, 276] = 10.69, p < .01) as women outperformed men in response to target stimuli. A significant main effect of List (F[1, 276] = 13.40, p < .001) was observed as performance improved from administration of the first to the second list. Importantly, a significant Test × Age interaction (F[1, 276] = 6.58, p < .05, see Fig. 1C) reflected the negative correlation between age and associative recognition hit rate (r = −.19, p < .01) but not with item recognition hit rate (r = −.08, ns).
Analysis of FAR showed a significant main effect of Age (F[1, 274] = 22.76, p < .001). Greater age was associated with more false positive responses to foil stimuli. A significant main effect of Sex (F[1, 274] = 5.60, p < .001) was due to men showing more false positive responses than women. The model also revealed a significant main effect of Test (F[1, 274] = 89.93, p < .001): recognition of associations produced more false alarms than recognition of items. Notably, a significant interaction of Test × Age was also observed (F[1, 274] = 5.03, p < .05). Figure 1D illustrates the significant differences in relationships between age and false alarms to items (r = −0.21, p < .01) and associations (r = −0.32, p < .001; Steiger’s Z* = 2.01, p < .05). As in the HR analyses, there was also a main effect of List: F(1, 274) = 5.32, p < .05); false alarm responses increased from the first administration of the task to the last.
Because significant Age × Test interactions were independently observed in analyses of hit rate and false alarm rate, we also compared the correlations between Age and associative HR (r = −.19, p < .01) and Age with associative FAR (r = .32, p < .001). The relationship between Age and FAR was larger than between Age and HR (Steiger’s Z* = 2.06, p < .05), indicating that the magnitude of age-related increase in false alarms was greater than age-related reduction in hits.
Response Times
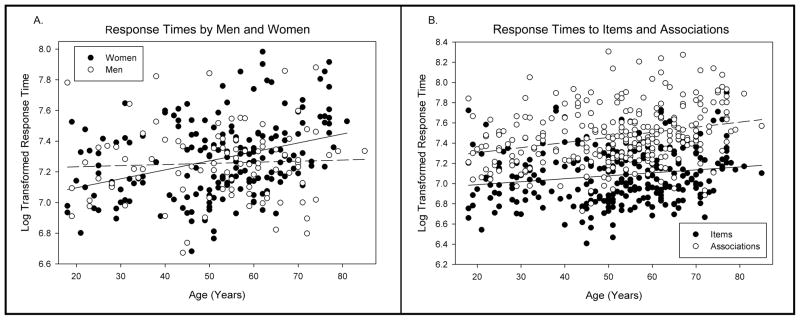
Absence of false alarm responses by some participants precluded analysis of response times to both hits and false alarms. Therefore, only log-transformed hit RTs were subjected to the same GLM analysis as for the accuracy measures above. Analysis of hit RTs showed a significant main effect of Age (F[1, 274] = 12.12, p < .001), as advanced age was associated with longer response times (r = .25, p < .001). Although there was no main effect of Sex, there was a significant interaction of Sex × Age (F[1, 274] = 7.32, p < .01). The relationship between RT and Age was significant for women (r = .35, p < .001) but not for men (r = .05, ns; Fig. 2a). A significant main effect of Test (F[1, 274] = 692.15, p < .001) was the result of longer RTs to recognition of associations (M = 7.47, SD = .28) than of items (M = 7.08, SD = .26). There was also a significant interaction of Test × Age (F[1, 274] = 5.03, p < .05). Greater age was more strongly associated with longer RTs for associations (r = .28, p < .001) than for items (r = .18, p < .01; Steiger’s Z* = 2.02, p < .05; Fig. 2b). A significant main effect of List was also observed (F[1, 274] = 15.73, p < .001) as participants responded faster in the second administration than in the first. Despite a significant List × Age interaction (F[1, 274] = 4.30, p < .05), the difference between the relationships of Age and RTs for List 1 (r = .27, p < .001) and List 2 (r = .20, p < .01) were not significant (Steiger’s Z* = 1.66, p > .05).
Figure 2.
Response times in the word pair task by a continuous sample of healthy adults. A. Scatterplot depicting the relationship between log transformed response times and participant age in years for women (solid black circles, dashed regression line) and men (open circles, solid regression line). B. Scatterplot of the relationships between log transformed response times to items (solid black circles, solid regression line) and associations (open circles, dashed regression line) and participant age in years.
Even within a healthy sample as the one employed in the present study, medically controlled hypertension (Raz et al., 2003) has a negative effect on cognition. Furthermore, our sample did not contain individuals with health conditions that are rarely excluded from cognitive aging studies but which may negatively impact cognition such as thyroid disorder (Van Boxtel, Menheere, Bekers, Hogervorst, & Jolles, 2003), or history of cancer (Ahles et al., 2002; see Anderson-Hanley, Sherman, Riggs, Agocha, & Compas, 2003 for review). Thus, these sample characteristics afforded the opportunity to investigate the effects of medicated hypertension uncontaminated by co-morbidities. Because hypertension is rarely found in younger age, additional models were tested to investigate the effects of hypertension on recognition indices and response times in participants aged 40 years and older (N = 219; 147 women). Among the women, there were 103 normotensive and 44 hypertensive participants, and among men, there were 51 normotensive and 21 hypertensive participants. After testing and removal of nonsignificant interactions (p > .1 for all), the reduced model testing recognition of items and associations (arcsine transformed A′ scores) included the predictors Age, Sex, Hypertension, and the interaction of Hypertension and Sex; the dependent variables and repeated measures were the same as specified in the prior models with the larger sample. As in the full sample, we observed significant main effects of Age, Sex, and Test (F[1, 214] = 7.65, p < .01, F[1, 214] = 4.09, p < .05, and F[1, 214] = 86.86, p < .001, respectively). Older participants and men performed worse than young participants and women, and items were better recognized than associations. Although there was no main effect of Hypertension (F < 0.15), there were significant interactions of Test × Hypertension × Sex and List × Test × Hypertension × Sex (F[1, 214] = 5.04, p < .05, and F[1, 214] = 4.58, p < .05, respectively). Post hoc analyses on the Test × Hypertension × Sex interaction (Newman-Keuls tests) indicated that among normotensive participants, men performed worse than women on both associative tasks but not on the item tasks. In the analysis of B″D, none of the higher order interactions was significant (p > .15 for all). The final model evaluating B″D on the two lists’ item and association tasks included Age and the dichotomous predictors of Sex and Hypertension. As in the full sample, main effects of Test (F[1, 215] = 14.54, p < .001) and List (F[1, 215] = 7.14, p < .01) were observed; no other effects or interactions were evident.
Analyses of HR revealed a significant main effect of Sex (F[1, 215] = 5.28, p < .05), with women outperforming men. The main effect of Test was also significant (F[1, 215] = 6.57, p < .05); the HR for items was greater than for associations. A significant main effect of List was also observed with hit rate increasing between administration of the first and second lists (F[1, 215] = 7.14, p < .05). There were no effects of or interactions with hypertension. Analyses of FAR revealed a significant main effect of Age (F[1, 214] = 8.55, p < .01) as greater age was associated with higher FAR (r = .21, p < .01). The main effect of Test was also significant (F[1, 214] = 71.39, p < .001); there were substantially more false alarms produced in response to the association recognition task than the item task. A significant main effect of List was also observed; FAR increased from the first administration to the second (F[1, 214] = 4.41, p < .05). There were no effects of hypertension (main or interactive) on false alarm rate.
Analysis of the effects of Hypertension on hit RT revealed neither significant main effect nor interactions involving that factor. The main effects of Age (F[1, 214] = 16.78, p < .001), Sex (F[1, 214] = 3.92, p < .05), Test (F[1, 215] = 317.39, p < .001) and List (F[1, 214] = 9.29, p < .01) were observed, as in the full sample. Younger age and female sex were associated with shorter RT. Items produced faster RTs than associations did, and faster RTs were observed in the second administration, relative to the first.
Discussion
The main result of this study is a generalization of the ADH to a continuous lifespan sample of healthy adults with high educational attainment. Several factors predicted performance on recognition memory tests. Advanced age and male sex were associated with slower and less accurate recognition performance. In accord with prior findings (e.g., Naveh-Benjamin, 2000), recognition of individual items was better than recognition of paired associates, and disproportionally so for older adults. The male associative memory disadvantage was evident only in normotensive participants, although it is unclear whether the sex differences among persons with hypertension were not revealed due to low statistical power or indeed were overshadowed by the influence of vascular risk.
Older adults evidenced a small but significant liberal bias in response criterion, and across all ages, response criterion became more liberal from the first to second administration of the task. Analyses of hit and false alarm rates reveal that advanced age and male sex were linked to lower hit rate and higher false alarm rate, and that relationship was stronger for the associations compared to the items. Notably, age-related differences in FAR were significantly greater than those in HR. These findings add to prior work that focused on the indices of recognition memory that combine FAR and HR. The results of separate HR and FAR analyses reported here are consistent with the findings from a similar study using extreme age groups, in which older adults had more false alarms than younger adults for associations but not items (Castel & Craik, 2003). Thus, as evident both from the regression plots in Figures 1C and 1D, and from a greater association between age and false alarm rates in comparison to hit rates, age-related associative deficits appear to be driven in particular by higher false alarm rates in recognition of associations. This finding corresponds to a similar result obtained for word pairs (Shing, Werkle-Bergner, Li, & Lindenberger, 2009) and face-name associations (Naveh-Benjamin et al. 2009).
One explanation for such a pattern of results is based on the dual process accounts of recognition memory, which suggests that recognition of previously studied information depends on separate, dissociable processes of recollection and familiarity (Jacoby, 1991). Recollection requires memory for contextual details as well as for the target items, with which the context is associated. Familiarity, on the other hand, involves a unitary sense of knowing. One widely held view suggests that older adults rely more on familiarity than on recollective processing due to relative sparing of the former and age-related declines in the latter (see Yonelinas, 2002, for a review). Whereas the tests of item memory in the present study involved previously studied target words and lures, the recognition test for associations used recombined pairs as mnemonic foils. Thus, the age-related increase in liberal response bias observed in the present study may reflect an increased reliance on familiarity processes over recollective processes in recognition. Moreover, because all words composing target and lure pairs had been shown during study, greater reliance on item familiarity, combined with poorer recollection in older adults, due to inability to use recall-to-reject strategy (Gallo, Bell, Beier, & Schacter, 2006), may have biased older observers towards erroneous “yes” responses to the recombined distracter pairs. Such bias could lead to the high false alarm rates in the associative memory test. Alternately, age-related reduction in the ability to inhibit distracting thoughts at encoding (see Lustig, Hasher, & Zacks, 2007 for a review), or failure to inhibit lexical representations semantically linked to a target word at retrieval (see Burke & Light, 1981 for a review) have also been implicated in age-related associative memory deficits. Other findings from similar tasks that compared older and young adults’ memory for related and non-related word pairs showed the semantic relatedness of the two words also mitigates the ADH (Naveh-Benjamin, Hussain, Guez, & Bar-On, 2003). Similarly, Castel (2005) showed that older adults can create associations between a grocery item and its price as well as younger ones when the price is at market value of the item. Thus, modulation of mnestic noise that may contribute to poorer discriminability may only be necessary when representations are not already cognitively linked. Notably, prior research indicates that older adults are not only prone to greater false alarm rates, but they commit those errors with high confidence (Shing et al., 2009). Thus, the liberal shift in response bias may indicate that increased noise may be compounded and strengthened by strategic influence.
Orienting task and use of strategy can mitigate associative memory decrements in older adults (see Hertzog & Dunlosky, 2004, Verhaegen et al, 1993 for reviews; Naveh-Benjamin, Brav, & Levi, 2007). In associative memory tests, effective encoding strategies such as sentence formation and interactive imagery enhance older adults’ associative recognition (Dunlosky, Hertzog, & Powell-Mann, 2005; Naveh-Benjamin et al., 2007). However, older adults rarely engage such strategies spontaneously during encoding (Kausler, 1994), although they do benefit substantially when instructed to use a particular strategy (see Hertzog & Dunlosky, 2004 for a review; Naveh-Benjamin et al., 2007). In the present study encoding was intentional, but participants were not instructed on strategy use. Thus, although individual differences in strategy use may explain some additional variance in memory performance beyond that of age alone, the role of such metacognitive factors in the present study is unclear.
Our primary objective in the present study was to replicate the prior findings using Naveh-Benjamin’s (2000) single-item, yes/no recognition task in a lifespan sample of healthy adults. However, this experimental paradigm has limitations in comparison to n-alternative forced choice, remember/know, and remember-confidence ratings tasks. Thus, despite extending the findings through separate evaluations of the various signal detection indices in an adult lifespan sample, we are unable to evaluate other potentially informative aspects of memory performance, such as receiver operating characteristic curves (Healy, Light, & Chung, 2005). Age-related associative memory deficit has been observed in yes/no and forced choice tasks using various stimuli. It was generalized across verbal paired-associates (e.g., Castel & Craik, 2003; Light et al., 2004; Naveh-Benjamin, 2000), face-name pairs (Naveh-Benjamin, Guez, Kilb, & Reedy, 2004; Naveh-Benjamin et al., 2009), objects (Naveh-Benjamin et al., 2003), face-spatial location pairs (Bastin & Van der Linden, 2006), and person-activity pairs (Old & Naveh-Benjamin, 2008b; see Old & Naveh-Benjamin, 2008a for a meta-analysis and review), although only few such studies have used confidence ratings, or remember/know (Tulving, 1985) tasks. However, some experimental paradigms have been used in combination with the process dissociation procedure (Jacoby, 1991; Yonelinas, Regehr, & Jacoby, 1995) to investigate the differential roles of familiarity and recollection in cognitive aging (Cohn, Emrich, & Moscovitch, 2008; Healy et al., 2005). In a recent test of the ADH, Cohn and colleagues found that older adults show pronounced impairments in recall-to-reject processes (see Clark & Gronlund, 1996 for a review) that are important in the correct identification and rejection of previously unstudied lures (Rotello & Heit, 2000). Cohn and colleagues found that recall-to-accept processes associated with hit responses were less affected by age; similarly, we found smaller age-related declines in hits than in false alarms in the associative test. To replicate the original ADH report (Naveh-Benjamin et al., 2000), we exclusively used recombined word pairs as associative test lures, making it unfeasible to use the process dissociation procedure (Yonelinas et al., 1995) as Cohn and colleagues (2008) did. However, the similarity of the patterns of results reported here and by Cohn et al. (2008) is in line with the suggestion that age-related differences in the contributions of recollection and familiarity may underlie some of the observed age-related differences in associative memory.
Regarding the role of potentially influential participant characteristics on recognition performance indices, the present study used a more comprehensive health screening procedure and stricter eligibility requirements than in previous studies (e.g., Naveh-Benjamin, 2000). The use of such an exceptionally healthy sample that reflects a ‘best case scenario’ for aging research further validates the ADH. Finally, lack of effect of hypertension on performance cannot be taken as an indication of the benign role of vascular risk. In a sample of highly-educated and otherwise healthy adults, the negative findings could mean that the influence of hypertension was compensated by other factors. In addition, despite the relatively large sample size employed here, the power could have been too low to detect subtle effects of controlled hypertension.
Unlike the extant studies of ADH, whose results are based on comparisons of extreme age groups, our use of a continuous lifespan sample permits evaluation of factors contributing to age-related heteroscedasticity. Importantly, we found significant interactions of Age × Test in analyses of A′, hit rate, and false alarm rate in the overall sample, but not in the age-restricted sub-group (persons older than 40) used in analysis of hypertension effects. This suggests that the age-related associative deficit may begin in middle age. However, as with any cross-sectional investigation into aging phenomena, caution must be advised in the interpretation of such findings (Schaie, 2005, as cross-sectional results from lifespan samples cannot be considered reliable measures of change.
A notable difference between the present study and previous investigations of ADH (e.g., Naveh-Benjamin, 2000) was the evaluation of the effects of the individual differences variables sex and hypertension. We found that men had fewer hits, more false alarms, and worse overall performance than women, in accord with findings from other studies of aging and episodic memory (Herlitz & Rehnman, 2008). Furthermore, among adults 40 years and older (70% of the total sample) who were normotensive, women outperformed men on the associative but not item tests. This suggests that older men may be more vulnerable than women to age-related associative deficits. However, although few studies have reported female superiority in recognition of paired associates in young (Birenbaum, Kelly, & Levi-Keren, 1994) and older (Gerstorf, Herlitz, & Smith, 2006) adults, such effects have not been reported from studies using a paradigm employed in this study. Finally, the fact that the present results confirming the ADH were obtained in a sample with mean level of education corresponding to almost a full college degree (compared to average education at a high school level in Naveh-Benjamin, 2000) indicates that greater mean education does not mitigate associative recognition deficits in old age. However, because of the curtailed range of formal education in this sample, we were unable to test the effect of educational attainment on ADH.
The differential age-related declines in associative memory reported in this behavioral study are in line with some age-related changes in brain structures noted in the literature. Two major systems have been proposed as the essential brain foundation of memory for association and item-context binding: Prefrontal (Moscovitch & Winocur, 2000) and medial temporal (Davachi, 2006; Davachi & Wagner, 2002; Scoville & Milner, 1957; Squire, Stark, & Clark, 2004). Both of those brain systems exhibit age-related decline (Raz et al., 2005), although it is unclear which is more important in the observed associative memory deficit. As recently demonstrated, age-related deficit in binding faces and scenes is associated with decreases in hippocampal and prefrontal activation (Dennis et al., 2008).
In conclusion, we demonstrate that associative deficit in verbal recognition memory represent a generalizable and replicable phenomenon. The deficit is apparently due to a decrease in recognizing target pairs (hits) and an increase in falsely recognizing non-target recombined pairs (false alarms), relative to memory for single items. These associative deficits are observed throughout the lifespan, and are not mitigated by high educational attainment or good health.
Acknowledgments
This study was supported in part by grant R37-AG011230 from the National Institute on Aging. We thank Cheryl Dahle, Kristen Kennedy, Angela Kilb, and Peng Yuan for assistance in various aspects of this study.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/PAG
Contributor Information
Andrew R. Bender, Department of Psychology & Institute of Gerontology, Wayne State University
Moshe Naveh-Benjamin, Department of Psychological Sciences, University of Missouri.
Naftali Raz, Department of Psychology & Institute of Gerontology, Wayne State University.
References
- Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, Silberfarb PM. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. Journal of Clinical Oncology. 2002;20:485–93. doi: 10.1200/JCO.2002.20.2.485. Retrieved from http://jco.ascopubs.org/cgi/content/full/20/2/485. [DOI] [PubMed]
- Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: A meta-analysis and review of the literature. Journal of the International Neuropsychological Society. 2003;9(7):967–982. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- Bastin C, Van der Linden M. The effects of aging on the recognition of different types of associations. Experimental Aging Research. 2005;32(1):61–77. doi: 10.1080/03610730500326291. [DOI] [PubMed] [Google Scholar]
- Birenbaum M, Kelly AE, Levi-Keren M. Stimulus features and sex differences in mental rotation test performance. Intelligence. 1994;19:51–64. [Google Scholar]
- Brady CB, Spiro A, Gaziano M. Effects of age and hypertension status on cognition: The Veterans Affairs Normative Aging Study. Neuropsychology. 2005;19(6):770–777. doi: 10.1037/0894-4105.19.6.770. [DOI] [PubMed] [Google Scholar]
- Burke DM, Light LL. Memory and aging: The role of retrieval processes. Psychological Bulletin. 1981;90(3):513–546. [PubMed] [Google Scholar]
- Castel A. Memory for grocery prices in younger and older adults: The role of schematic support. Psychology and Aging. 2005;20(4):718–721. doi: 10.1037/0882-7974.20.4.718. [DOI] [PubMed] [Google Scholar]
- Castel AD, Craik FIM. The effects of aging and divided attention on memory for item and associative information. Psychology and. 2003;18(4):873–885. doi: 10.1037/0882-7974.18.4.873. [DOI] [PubMed] [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Memory and Cognition. 1996;24(4):403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Clark SE, Gronlund SD. Global matching models of recognition memory: How the models match the data. Psychonomic Bulletin & Review. 1996;3(1):37–60. doi: 10.3758/BF03210740. [DOI] [PubMed] [Google Scholar]
- Cohn M, Emrich SM, Moscovitch M. Age-related deficits in associative memory: The influence of impaired strategic retrieval. Psychology and Aging. 2008;23(1):93–103. doi: 10.1037/0882-7974.23.1.93. [DOI] [PubMed] [Google Scholar]
- Dahle CL, Jacobs BS, Raz N. Aging, vascular risk, and cognition: Blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychology and Aging. 2009;24(1):154–162. doi: 10.1037/a0014283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context, and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: Insights from relational and item-based learning. Journal of Neurophysiology. 2002;88:982–990. doi: 10.1152/jn.00046.2002. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. Journal of Experimental Psychology: Learning Memory & Cognition. 2008;34(4):791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson W. Measuring recognition memory. Journal of Experimental Psychology: General. 1992;121(3):275–277. doi: 10.1037//0096-3445.121.3.275. [DOI] [PubMed] [Google Scholar]
- Dunlosky J, Hertzog C, Powell-Moman A. The contribution of mediator-based deficiencies to age differences in associative learning. Developmental Psychology. 2005;41:389–400. doi: 10.1037/0012-1649.41.2.389. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects. Diabetes Care. 2001;24(2):366–370. doi: 10.2337/diacare.24.2.366. [DOI] [PubMed] [Google Scholar]
- Freitag MH, Piela R, Masaki K, Petrovich H, Ross GW, White LR, Launer LJ. Midlife pulse pressure and incidence of dementia: The Honolulu-Asia Aging Study. Stroke. 2006;37:33–37. doi: 10.1161/01.STR.0000196941.58869.2d. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Bell DM, Beier JS, Schacter DL. Two types of recollection-based monitoring in young and older adults: Recall-to-reject and the distinctiveness heuristic. Memory. 2006;14:730–741. doi: 10.1080/09658210600648506. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Herlitz A, Smith J. Stability of sex differences in cognition in advanced old age: The role of education and attrition. Journal of Gerontology: Psychological Sciences. 2006;61B(4):245–249. doi: 10.1093/geronb/61.4.p245. Retrieved from http://psychsocgerontology.oxfordjournals.org/content/61/4/P245.full.pdf. [DOI] [PubMed]
- Grier JB. Nonparametric indexes for sensitivity and bias: Computing formulas. Psychological Bulletin. 1971;75:424–429. doi: 10.1037/h0031246. [DOI] [PubMed] [Google Scholar]
- Healy MR, Light LL, Chung C. Dual-process models of associative recognition in young and older adults: Evidence from receiver operating characteristics. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31(4):768–788. doi: 10.1037/0278-7393.31.4.768. [DOI] [PubMed] [Google Scholar]
- Herlitz A, Rehnman J. Sex differences in episodic memory. Current Directions in Psychological. 2008;17(1):52–56. doi: 10.1111/j.1467-8721.2008.00547.x. [DOI] [Google Scholar]
- Hertzog C, Dunlosky J. Aging, metacognition, and cognitive control. In: Ross BH, editor. Psychology of Learning and Motivation. San Diego: CA: Academic Press; 2004. pp. 215–251. [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory &Language. 1991;30:513–541. [Google Scholar]
- Joffe R, Levitt A. The thyroid and depression. In: Joffe R, Levitt A, editors. The thyroid axis and psychiatric illness. Washington, D. C: American Psychiatric Press, Inc; 1993. pp. 195–254. [Google Scholar]
- Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. NIH Publication No. 04-5230. 2004 Retrieved from http://www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf.
- Kausler DH. Learning and memory in normal aging. New York: Academic Press; 1994. [Google Scholar]
- Kraemer H, Yesavage J, Taylor J, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? American Journal of Psychiatry. 2000;157(2):163. doi: 10.1176/appi.ajp.157.2.163. Retrieved from http://ajp.psychiatryonline.org/cgi/content/abstract/157/2/163. [DOI] [PubMed]
- Lichtenberg PA, Ross T, Millis SR, Manning CA. The relationship between depression and cognition in older adults: A cross-validation study. Journal of Gerontology. 1995;50B:25–32. doi: 10.1093/geronb/50B.1.P25. [DOI] [PubMed] [Google Scholar]
- Light LL. Memory and aging: Four hypotheses in search of data. Annual Review of Psychology. 1991;43:333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Light LL, Patterson MM, Chung C, Healy MR. Effects of repetition and response deadline on associative recognition in young and older adults. Memory and Cognition. 2004;32(7):1182–1193. doi: 10.3758/bf03196891. Retrieved from http://mc.psychonomic-journals.org/content/32/7/1182. [DOI] [PubMed]
- Lustig C, Hasher L, Zacks R. Inhibitory deficit theory: Recent developments in a “new view”. In: Gorfein DS, MacLeod CM, editors. The place of inhibition in cognition. Washington, DC: American Psychological Association; 2007. [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D’Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Cognitive Brain Research. 2000;10:197–206. doi: 10.1037/0882-7974.15.3.527. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The frontal cortex and working with memory. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2000. pp. 188–209. [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2000;26:1170–1188. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, Levi O. The associative memory deficit of older adults: The role of efficient strategy utilization. Psychology and Aging. 2007;22:202–208. doi: 10.1037/0882-7974.22.1.202. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Hussain Z, Guez J, Bar-On M. Adult-age differences in memory performance: Further support for an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory and Cognition. 2003;29:826–837. doi: 10.1037/0278-7393.29.5.826. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Kilb A, Reedy S. The associative deficit of older adults: Further support using face-name associations. Psychology & Aging. 2004;19(3):541–546. doi: 10.1037/0882-7974.19.3.541. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Shing YL, Kilb A, Werkle-Bergner M, Lindenberger U, Li SC. Adult age differences in memory for name-face associations: The effects of intentional and incidental learning. Memory. 2009;17(2):220–232. doi: 10.1080/09658210802190596. [DOI] [PubMed] [Google Scholar]
- Old S, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: A meta-analysis. Psychology and Aging. 2008a;23(1):104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Old S, Naveh-Benjamin M. Memory for people and their actions: Further evidence for an age-related associative deficit. Psychology and Aging. 2008b;23:467–472. doi: 10.1037/0882-7974.23.2.467. [DOI] [PubMed] [Google Scholar]
- Pollack I, Norman DA. A non-parametric analysis of recognition experiments. Psychonomic Science. 1964;1(5):125–126. [Google Scholar]
- Piela R, White LR, Petrovich H, Masaki K, Ross GW, Havlik RJ, Launer LJ. Joint effect of apoE gene and midlife systolic blood pressure on late-life cognitive impairment: The Honolulu-Asia Aging Study. Stroke. 2001;32:2882–2889. doi: 10.1161/hs1201.100392. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences, and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: Vulnerability of prefrontal and executive functions. Behavioral Neuroscience. 2003;117(6):1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Rotello CM, Heit E. Associative recognition: A case of recall-to-reject processing. Memory & Cognition. 2000;28(6):907–922. doi: 10.3758/bf03209339. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Koutstaal W, Norman KA. False memories and aging. Trends in Cognitive Sciences. 1997;1(6):229–236. doi: 10.1016/S1364-6613(97)01068-1. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Norman KA, Koutstaal W. The cognitive neuroscience of constructive memory. Annual Review of Psychology. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- Schaie K. What can we learn from longitudinal studies of adult development? Research in Human Development. 2005;2(3):133. doi: 10.1207/s15427617rhd0203_4. NIH Public Access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing YL, Werkle-Bergner M, Li SC, Lindenberger U. Committing memory errors with high confidence: Older adults do but children don’t. Memory. 2009;17(2):169–179. doi: 10.1080/09658210802190596. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers. 1999;31(1):137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- Van Boxtel MP, Menheere PP, Bekers O, Hogervorst E, Jolles J. Thyroid function, depressed mood, and cognitive performance in older individuals: The Maastricht Aging Study. Psychoneuroendocrinology. 2003;29(7):891–898. doi: 10.1016/j.psyneuen.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: A quantitative integration of research findings. Journal of Gerontology. 1993;48(4):157–171. doi: 10.1093/geronj/48.4.P157. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46(3):441–517. doi: 10.1006/jmla.2002.2864. [DOI] [Google Scholar]
- Yonelinas AP, Regehr G, Jacoby LL. Incorporating response bias into a dual-process theory of memory. Journal of Memory and Language. 1995;34(6):821–835. [Google Scholar]