Abstract
De novo lipogenesis, the production of fats from simple precursors, is often dismissed as irrelevant to the pathobiology of obesity caused by positive energy balance due to typical high fat diets. However, emerging data implicate de novo lipogenesis in the generation of metabolic signals that alter disease risk. Exploiting this signaling pathway represents lipoexpediency. Lipoexpediency is the concept of directing fats toward benefit even in the setting of lipid overload, and represents a strategy to complement efforts aimed at improving energy balance. Optimizing lipid signals initiated by key lipogenic enzymes such as fatty acid synthase might limit morbidity in people unlikely to abandon the lifestyle of the sedentary gourmand.
The reality of lipid excess
An epidemic with limited novel treatment approaches
Over 40% of Americans have diabetes or prediabetes [1] and as many as a quarter of American adults have the metabolic syndrome, which predisposes to diabetes and cardiovascular disease [2]. Tobacco use increases health risks that have been decreased by public health measures targeted at smoking, but the adverse effects of obesity will probably negate the beneficial effects of declining tobacco use in the United States by 2020 [3]. The metabolic mayhem promoted by a Western lifestyle has been exported worldwide, and diabetes is now a major and accelerating public health problem in China [4]. Diabetes, obesity, and the metabolic syndrome also increase the risk of nonalcoholic fatty liver disease [5]. Lifestyle changes are difficult to institute given the pressures of Western culture, and other therapies are limited. For example, insulin therapy lowers hemoglobin A1c but may increase adiposity. Statins and reninangiotensin system (RAS) inhibitors decrease but do not eliminate cardiovascular risk, i.e. individuals with dyslipidemia, hypertension, and diabetes are still at considerable risk for heart attacks even when treated with statins and RAS inhibitors. PPAR agonists have a mixed record that is still evolving, and it is unknown if GLP-1 modulators will decrease complications of diabetes and obesity.
Not all lipid is bad—the case for lipoexpediency
Complications from diabetes and obesity may be related to excess fat in non-adipose tissues, leading to lipid-mediated cellular damage or death. This idea is encapsulated in the notion of lipotoxicity [6,7]. While there are many examples of homeostatic disruption induced by lipid overload, mere lipid excess is not always sufficient to cause disease. Humans can be obese but free of insulin resistance and apparent vascular disease [8]. Leptin-deficient mice with adiponectin overexpression are morbidly obese yet metabolically healthy [9]. Overloading macrophages with fat by increasing expression of acyl CoA:diacylglycerol acyltransferase 1 (DGAT1) (an enzyme that synthesizes triglycerides) decreases diet-induced inflammation and insulin resistance in mice [10]. Endurance-trained athletes are exquisitely insulin sensitive in the setting of increased skeletal muscle lipid content [11]. These findings and others suggest that fat has spatial and temporal relationships with individual cells that impact signaling relevant to disease. Discrete lipid molecules, when presented at the proper location and time, trigger signals that modulate adaptation to stress. This raises the possibility of lipoexpediency (Box 1) – channeling calories toward beneficial forms of lipid even in the setting of lipid overload that would ideally be resolved through altered energy balance (eating less and exercising more).
Growing evidence points to a variety of mechanisms through which lipids might impact cellular signaling [12]. These include serving as ligands for nuclear receptors, interacting with cell surface proteins such as G-protein coupled receptors (GPCRs), modulating kinase activities, altering inflammatory cascades, controlling localization and function of proteins through the post-translational modifications of palmitoylation and myristoylation, and others. Compelling data indicate that lipids can be anti-inflammatory or inflammatory depending on how polyunsaturated fatty acids (from exogenous dietary sources) are metabolized [13]. The focus of this review is to describe some of the key, mechanistically defined examples of how de novo lipogenesis (the endogenous generation of fats) participates in signaling. In particular, fatty acid synthase and other enzymes appear to play important roles in transmitting signals that regulate metabolic homeostasis.
De novo lipogenesis
Regulated, tissue-specific flow of carbons to fat
Stored energy is critical for survival during starvation. Mammals have a limited ability to store energy as carbohydrates but a seemingly unlimited capacity to store calories as fat, which is maladaptive in the industrialized world. In the setting of positive energy balance, carbohydrates can be converted to fatty acids through the process of de novo lipogenesis [14]. Fatty acid biosynthesis is thought to occur to a greater extent in rodents as compared to humans and is probably a minor contributor to whole body lipid stores in present-day humans eating a typical high fat diet [15-17]. The degree to which de novo lipogenesis can be influenced by diet is limited, but this issue is complicated by energy balance and the effects of different carbohydrates [18,19]. However, pharmacologic or genetic manipulation of enzymes in the lipogenic pathway can have profound metabolic consequences [20], consistent with a signaling role for lipogenesis.
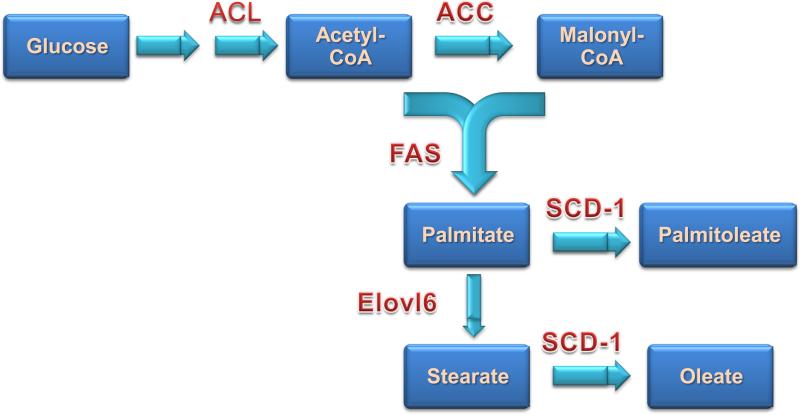
De novo fatty acid biosynthesis occurs in essentially all cells, but adipose tissue and liver are the major sites with liver thought to be quantitatively more important than fat. The flow of carbons from glucose to fatty acids requires a coordinated series of enzymatic reactions shown in Figure 1. Citrate produced by the tricarboxylic acid cycle in mitochondria is converted by ATP-citrate lyase (ACL) to acetyl-CoA, which is converted to malonyl-CoA by acetyl CoA carboxylase (ACC). ACC exists as ACC1, a cytosolic isoform important in liver and fat for de novo lipogenesis, and ACC2, a mitochondria-associated isoform with highest expression in muscle tissues. Antisense knockdown of ACC isoforms improves insulin sensitivity and fatty liver [21].
Figure 1.
Schematic of pathways involved in de novo lipogenesis. ACL-ATP citrate lyase; ACC-acetyl-CoA carboxylase; FAS-fatty acid synthase; SCD-1-stearyl-CoA desaturase; Elovl6-elongation of very long-chain fatty acids protein 6.
Fatty acid biosynthesis
The first committed step in fatty acid synthesis is catalyzed by fatty acid synthase (FAS), a multifunctional cytosolic protein that primarily synthesizes palmitate, a 16 carbon saturated fatty acid. Mammalian FAS is considered a type I fatty acid synthase complex, with multiple domains in a single large peptide [22]. Many lower organisms have a type II FAS with different functions carried out by separate proteins, although there is a type II FAS in mammalian mitochondria [23], raising the specter of lipid signaling at a site implicated in insulin resistance and diabetes. After priming with acetyl CoA, FAS uses malonyl-CoA as a carbon donor and NADPH as a cofactor. Active FAS exists as a homodimer of two 260 kD subunits each with multiple functional domains. These domains contain six distinct enzymatic activities required for the initiation of synthesis and elongation of the fatty acid by two carbon increments: acetyl transferase, β-ketoacyl synthase, malonyl transferase, β-ketoacyl reductase, β-hydroxyacyl dehydratase, and enoyl reductase. The growing fatty acid chain is tethered to an acyl carrier protein (ACP) domain, and a final domain contains a thioesterase that releases the fatty acid.
FAS is expressed to widely varying degrees in nearly all human and mouse tissues. It is regulated predominantly by the anabolic effects of insulin mostly through transcriptional effects [24,25], although other mechanisms are described and many other nutrients and hormones affect FAS expression [14]. Variations in FAS expression and enzyme activity have now been implicated in insulin resistance and obesity in humans [26-28]. These observations may be related to the observation that in liver, insulin resistance is selective, with elevated insulin levels failing to suppress gluconeogenesis yet continuing to stimulate lipogenesis [29]. A circulating form of FAS has been reported as a biomarker of metabolic stress and insulin sensitivity, changing with weight loss and reflecting insulin sensitivity in humans [30].
Fatty acid elongation
Fatty acids derived from the FAS reaction can be further elongated and/or desaturated by membrane-bound enzymes located in the endoplasmic reticulum. Fatty acid elongation is catalyzed by Elovl (elongation of very long-chain fatty acid) proteins, of which there are seven distinct members (Elovl1-7). Elovl6 is thought to be involved in de novo lipogenesis and is regulated by dietary, hormonal and developmental factors [31]. As with FAS, Elovl6 uses malonyl-CoA as a substrate and NADPH as a reducing agent. Elovl6 primarily catalyzes elongation of fatty acids consisting of 12, 14 or 16 carbons. Saturated fatty acids with 12, 14 or 16 carbons can be synthesized by FAS (C16:0 is the primary product) or derived from dietary coconut oil (C12:0, C14:0), butter (C14:0, C16:0), and meat or cheese (C16:0). Mice with Elov6 deficiency are obese but protected from insulin resistance [31], comprising another example of lipoexpedient flow of calories resulting in beneficial signaling despite obesity.
Fatty acid desaturation
Introduction of double bonds at specific positions in fatty acids has profound effects on their metabolic fate. Stearoyl-CoA desaturases (SCDs), existing as four isoforms, introduce a single double bond at the Δ9 position of saturated fatty acids such as palmitate and stearate. SCD-1 is the predominant isoform in adipose tissue and liver and is upregulated in animals fed a diet rich in carbohydrates. Inactivation of SCD-1 in liver prevents fatty liver and obesity induced by high carbohydrate feeding [32].
Thus, synthesizing fats from simple precursors involves the generation of saturated fatty acids and the processes of elongating as well as desaturating these molecules. Enzymes implicated in obesity and insulin resistance carry out these steps.
De novo lipogenesis and activation of PPARs
Lipogenesis and PPARα
PPARs are attractive potential molecular mediators of signaling triggered by de novo lipogenesis since this subfamily of nuclear receptors regulates lipid metabolism and inflammation [33]. The three members of this family, PPARα, PPARγ and PPARδ, function as ligand-activated transcription factors that form obligate heterodimers with retinoid X receptors (RXRs) and bind to specific DNA sites known as PPAR response elements (PPREs) located in target gene promoters. Ligand binding induces a conformational change in PPARs, resulting in dissociation of repressors and recruitment of co-activators with subsequent activation of target gene expression. Owing to their unusually large ligand binding pocket, these receptors can interact with a wide variety of lipids, including unsaturated and polyunsaturated fatty acids, fatty acyl-CoA species, eicosanoids (including prostaglandins, leukotrienes and HETEs), oxidized fatty acids and oxidized phospholipids. However, these lipids generally have low affinity for the receptor and/or are present at low concentrations, raising doubts that they are true endogenous ligands. The concept that lipogenic pathways are involved in the activation of PPARs was introduced more than a decade ago [34]. However, identification of specific de novo synthesized lipids that activate these receptors has been difficult. Recent studies suggest that lipogenesis mediated by fatty acid synthase plays a critical role in generating an endogenous ligand for PPARα in liver [35,36].
PPARα is the target of fibrate drugs used to treat disorders of lipid metabolism. It is expressed in various tissues, but is enriched in liver, where it promotes fatty acid oxidation, ketogenesis, lipid transport, and gluconeogenesis [37,38]. PPARα, or the signaling network for which it is an effector, appears to sense changes in nutritional status and respond by promoting fatty acid metabolism. However, it seems unlikely that circulating levels of fatty acids serve as a direct signal for PPARα activation because fatty acids cannot enter the nucleus where PPARα is localized, without first undergoing addition of an acyl-CoA group, which occurs upon crossing the plasma membrane. These CoA derivatives have numerous potential fates, including incorporation into the external plasma membrane or various endomembranes, synthesis of phospholipids, storage in lipid droplets, transport to the mitochondria for β-oxidation, and others. It is possible then that FAS, a nutritionally responsive enzyme, may be involved in generating an endogenous ligand for PPARα.
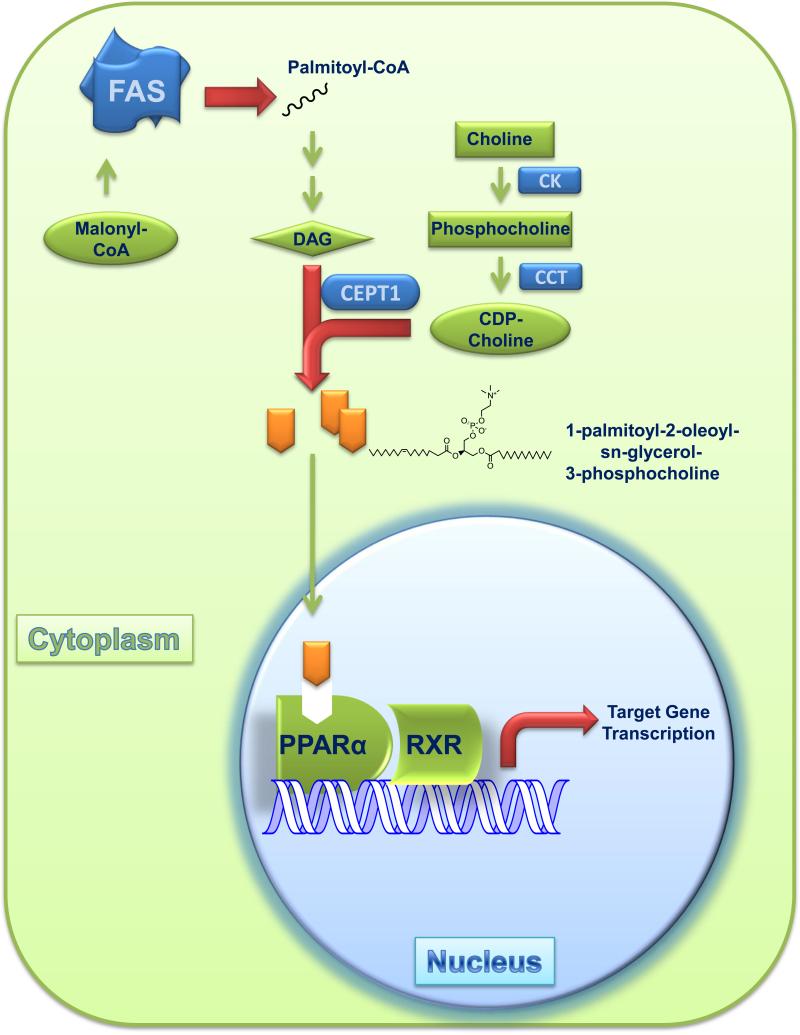
Previous studies of mice lacking FAS in liver (FASKOL mice) suggested that de novo lipogenesis mediated by FAS may be required for PPARα activation [35]. When subjected to fasting or fed a diet that lacks fat, these mice exhibit decreased PPARα-dependent gene expression and a phenotype resembling PPARα deficiency, including hypoglycemia, high serum levels of non-esterified fatty acids, and fatty liver. Each is corrected by treatment with a selective PPARα agonist, providing evidence that FAS may be involved in synthesizing an endogenous ligand for PPARα [35]. To isolate this ligand, tagged PPARα was immunoprecipitated from the liver, and PPARα-associated lipids were identified by tandem mass spectrometry [36]. A distinct phosphatidylcholine species, 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (16:0/18:1-GPC), was associated with PPARα in samples from wild-type mice, but not samples from FASKOL mice. Levels of PPARα-associated GPC increased when mice were fed a fat-free, high carbohydrate diet, a condition that promotes FAS activity. In vitro binding studies showed that 16:0/18:1-GPC preferentially interacts with the ligand binding domain of PPARα, but not PPARγ, and only weakly with PPARδ. Depletion of CEPT-1, an enzyme required for phosphatidylcholine synthesis, impaired PPARα-dependent gene expression and caused fatty liver. Together, these studies suggest that FAS is required for generating an endogenous ligand for PPARα [36] in the liver (Figure 2). In the hypothalamus [39] and macrophages [40], FAS deficiency is also associated with metabolic phenotypes, decreased PPARα-dependent gene expression, and restoration of expression with a PPARα ligand. It is unknown if the FAS-dependent PPARα ligand in brain or macrophages is the same as that in liver. Whether FAS is required for activating other PPARs is also unknown.
Figure 2.
FAS generates an endogenous ligand for PPARα in liver. FAS, a homodimer, synthesizes palmitate, which is converted to a CoA species that is incorporated into diacylglycerol (DAG). CDP-choline is generated by the sequential actions of CK (choline kinase) and CCT (CTP:phosphocholine cytidylyltransferase), enzymes in the Kennedy pathway of phosphatidylcholine synthesis. DAG and CDP-choline are converted by the ER protein choline-ethanolamine phosphotransferase-1 (CEPT1) to 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine. This phosphatidylcholine species binds to PPARα, which forms a heterodimer with RXR to activate gene expression.
Lipogenesis and PPARγ
PPARγ is enriched in adipose tissue, an important metabolic organ that regulates whole body energy balance. In contrast to PPARα, which promotes fatty acid oxidation, PPARγ regulates storage of fat. In vivo and cell culture studies have established PPARγ as both necessary and sufficient for differentiation of preadipocytes (fibroblasts) into adipocytes [41]. PPARγ is the target of thiazolidinediones used to treat type 2 diabetes. Although the identity of the endogenous ligand for PPARγ remains unclear, several lipids, including polyunsaturated fatty acids and eicosanoids, have been implicated as ligands [42-44]. In particular, 15-deoxy-Δ12,14-prostaglandin J2 was proposed as an endogenous ligand for PPARγ [45], but its concentration in adipocytes is likely too low to be physiologically relevant [46]. A study using a β-galactosidase reporter under the control of the Gal4 DNA binding domain fused to the PPARγ ligand-binding domain demonstrated that endogenous ligand(s) for PPARγ may be produced during early phase adipocyte differentiation [47] but these ligands remain unidentified.
Several studies have suggested that de novo lipogenesis may be involved in synthesizing an endogenous ligand for PPARγ. For example, ectopic expression of wild-type SREBP1, a transcriptional regulator of de novo lipogenesis, stimulates adipocyte differentiation while overexpression of a dominant negative mutant impairs adipogenesis [34]. It was then found that SREBP1 regulates adipogenesis through production of an as yet unidentified endogenous ligand for PPARγ, since supernatants from cultures that overexpress SREBP1 contained one or more secreted lipids that stimulated transcriptional activity of a PPARγ reporter and bound directly to PPARγ to displace radioactive thiazolidinedione ligands [48]. As downstream targets of SREBP1, FAS and SCD-2 may be involved in synthesis of endogenous ligands for PPARγ since siRNA-mediated knockdown and/or pharmacological inhibition of FAS and SCD-2 affect adipogenesis [49-51].
Lipogenic pathways and insulin resistance
Although its clinical hallmark is hyperglycemia, type 2 diabetes is also a disease of impaired lipid metabolism. Fatty acid metabolism is an important regulator of insulin resistance, a central component in the pathogenesis of type 2 diabetes [52]. Insulin, the most potent anabolic hormone, promotes the synthesis and storage of carbohydrates, lipids and proteins and inhibits their degradation [53]. In the setting of insulin resistance, normal circulating concentrations of insulin are insufficient to properly regulate metabolism, and the resultant lack of suppression of lipolysis in adipose tissue releases more fatty acids into the systemic circulation. Elevated levels of free fatty acids perpetuate insulin resistance by inhibiting glucose uptake, storage and oxidation and by increasing hepatic glucose production [54]. But not all fatty acids are detrimental and some, such as n-3 polyunsaturated fatty acids, may diminish end organ effects related to insulin resistance [55]. Whether endogenously synthesized lipids affect insulin sensitivity has been an unanswered question.
Lipokine regulation of insulin sensitivity
Recent work addressed this question by uncovering a novel link between de novo lipogenesis and insulin resistance [56]. This discovery was prompted by the observation that mice with combined loss of the adipocyte fatty acid binding proteins aP2 (FABP4) and mal1 (FABP5) have altered lipid profiles, are protected from high fat diet-induced obesity and fatty liver, and display profound insulin sensitivity [57]. To determine if a specific lipid correlates with the insulin sensitivity in these mice, Cao and colleagues used an unbiased lipidomics approach. This strategy led to the identification of C16:1n7-palmitoleate, an adipose tissue-derived monounsaturated fatty acid, as a circulating factor that regulates insulin sensitivity in liver and muscle. Because dietary levels of this fatty acid are generally low, de novo synthesis in adipose tissue is required for its production, presumably by FAS-mediated synthesis of palmitate followed by SCD-1-catalyzed desaturation. Not only was palmitoleate enriched in adipose tissue of mice lacking the two FABPs, but its levels were also considerably elevated in the serum of these mice. Since SCD-1 is transcriptionally regulated by various hormones and nutritional factors, Cao et al. tested the possibility that circulating palmitoleate regulates hepatic SCD-1 expression. In cultured cells and in vivo, palmitoleate treatment blocked hepatic SCD-1 promoter activity, illustrating a novel negative feedback mechanism in which adipose tissue-derived palmitoleate blocks the expression of hepatic SCD-1. The authors speculated that this mechanism might explain the protection from fatty liver in FABP4/5 knockout mice. They also showed that palmitoleate treatment of muscle improved insulin sensitivity reflected as increased Akt phosphorylation and insulin-stimulated glucose uptake. These findings are consistent with the notion of lipoexpediency by directing lipid flow toward the production of palmitoleate, a potentially beneficial lipokine.
Prostanoids and insulin sensitivity
Another class of endogenously synthesized lipids, prostanoids, may also affect insulin sensitivity, albeit through indirect effects. There are at least two clear examples of the potential for directing lipid flow in a lipoexpedient manner, i.e. toward physiological benefit despite positive energy balance, involving prostaglandins. First, adipose tissue is enriched in AdPLA, a type of phospholipase A2, the class of calcium-dependent enzymes critical for the production of eicosanoids such as prostaglandins. Inactivation of AdPLA results in mice that are lean with accelerated lipolysis, decreased levels of prostaglandin E2 (PGE2 is known to suppress lipolysis), and decreased insulin sensitivity [58]. These findings suggest that increasing PGE2 levels in adipose tissue could improve insulin sensitivity by safely storing lipids in adipocytes. Second, overexpression of cyclooxygenase-2, a rate-limiting enzyme for prostaglandin production, in mice promotes the differentiation of mesenchymal precursors toward brown fat, resulting in resistance to diet-induced obesity and improved insulin sensitivity [59]. Identifying the lipid mediators of this effect could allow the development of strategies to increase brown fat in humans, thought to be metabolically beneficial through adaptive thermogenesis [60].
Feeding behavior and lipogenesis
Central nervous system lipogenesis and food intake
Eating less, a more effective approach for weight loss than increasing energy expenditure, is an attractive goal for treating obesity-related diseases. De novo lipogenesis in the brain is involved in regulating food intake but the specific mediators are unknown. FAS inhibitors cause weight loss in mice [61], an effect that may be due to interactions between FAS and the mammalian target of rapamycin complex 1 [62]. Elevated levels of malonyl-CoA, one consequence of FAS inhibition, are correlated with inhibition of food intake, and lowering these levels through the action of the enzyme malonyl-CoA decarboxylase increases food intake. The inhibition of carnitine palmitoyltransferase-1 by malonyl-CoA leading to decreased mitochondrial β-oxidation and the potential accumulation of long chain acyl CoA molecules may activate neurons that control food intake [63].
Gastrointestinal lipogenesis and food intake
The gut is another site of lipid assembly that determines feeding behavior to impact systemic metabolism. Fatty acid ethanolamides appear to be particularly important satiety signals. Feeding stimulates oleoylethanolamide (OEA) biosynthesis in the small intestine of rodents, and systemic administration of OEA suppresses food intake through mechanisms that may involve PPARα [64] and the G protein coupled receptor GPR119 [65]. C16:0 N-acylphosphatidylethanolamine (NAPE), another lipid secreted from the gut, also decreases food intake, probably by interacting with as yet unidentified targets in the hypothalamus [66]. For both NAPE and OEA, lipid biogenesis is stimulated from intestine by the ingestion of fat but not glucose or protein. Ghrelin O-acetyltransferase (GOAT) [67], which acylates and thus activates the orexigenic hormone ghrelin, is also triggered by dietary fat [68]. Since ghrelin promotes anabolism through complex effects that involve CNS effects on FAS [69], signals from de novo lipogenesis may also participate in modulation of ghrelin-driven physiology induced by fat intake.
Collectively, studies in both the brain and the gastrointestinal tract lend credence to the concept that feeding behavior can be modified by altering the synthesis and availability of discrete lipid molecules with potential signaling functions.
Future directions
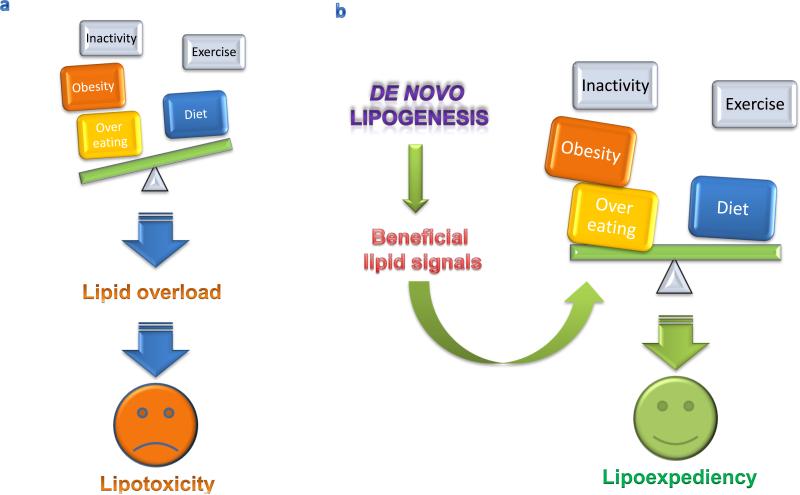
It is unrealistic to assume that a culture with ready access to food and social structures that encourage interactions with video monitors will suddenly choose to adopt prudent life style interventions for obesity and its consequences. Since dealing with people in positive energy balance may be inevitable, lipoexpediency, the notion of directing excess calories toward potentially beneficial fats, provides a practical conceptual framework for developing novel treatment paradigms (Figure 3).
Figure 3.
De novo lipogenesis as a potential mediator of lipoexpediency. (a) Inactivity, obesity, and overeating tip energy balance away from the benefits of proper diet and exercise, leading to lipid overload and tissue damage induced by lipotoxicity. (b) The appropriate manipulation of de novo lipogenesis pathways has the potential to generate beneficial lipid transmitters with the capacity to tip metabolic scales in favor of greater benefit from diet and exercise and leading to the integrative physiology of lipoexpediency.
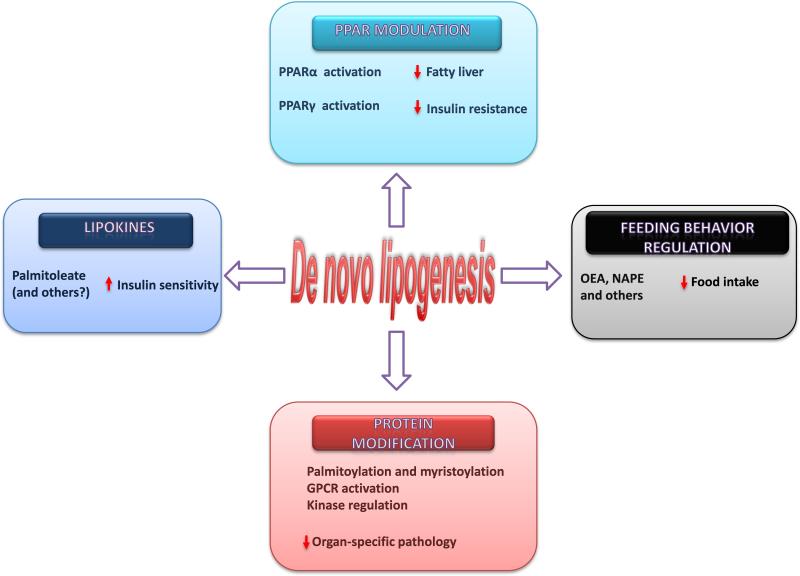
De novo lipogenesis has the potential to trigger several effector mechanisms leading to lipoexpediency (Figure 4). However, key questions must be addressed before modulation of de novo lipogenesis can be considered a therapeutic option. How do target tissues distinguish between endogenously produced lipids serving signaling functions and apparently identical lipids from exogenous sources that are directed to structural or other functions? Do unrecognized lipid chaperones, potential targets of drug or nutraceutical development, participate in this process? Do FAS and other enzymes involved in de novo lipogenesis serve signaling functions beyond the activation of PPARs? Do these enzymes signal in tissues relevant to the complications of obesity and diabetes such as peripheral nerves, the vasculature, and the heart? While living with excess fat may be inevitable for many in contemporary cultures, lipoexpediency might make it possible to steer that fat toward pathways that restore instead of ravage normal tissue.
Box 1. Lipoexpediency.
Lipoexpediency is the notion that lipids can be directed toward benefit even in the setting of lipid abundance that predisposes to disease. The term reflects an admission that while lipid overload is ideally treated by altering energy balance, namely eating less and exercising more, this approach is not always successful. Lipoexpediency thus implies a utilitarian or makeshift mechanistic approach of attempting to direct excess lipids toward signaling pathways with the potential to ameliorate disease risk caused in part by the presence of excess lipids.
Figure 4.
Potential effector mechanisms of lipoexpediency. Tissue specific modulation of de novo lipogenesis has the potential to decrease fatty liver and insulin resistance by altering PPAR signals, affecting insulin sensitivity through lipokine production, dampening feeding behavior through the synthesis of signaling lipids (such as oleoylethanolamide [OEA] and C16:0 N-acylphosphatidylethanolamine [NAPE]), and decreasing organ pathology through several effects on protein modification.
Acknowledgements
This work was supported by grants DK076729, DK088083, and F32 DK083895 from the NIDDK, and Fellowship Awards from the American Heart Association and American Diabetes Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cowie CC, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford ES, et al. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 3.Stewart ST, et al. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361:2252–2260. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang W, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 5.Fabbrini E, et al. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brookheart RT, et al. As a matter of fat. Cell Metab. 2009;10:9–12. doi: 10.1016/j.cmet.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusminski CM, et al. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14:1484–1495. doi: 10.1007/s10495-009-0352-8. [DOI] [PubMed] [Google Scholar]
- 8.Stefan N, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 9.Kim JY, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koliwad SK, et al. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Invest. 2010;120:756–767. doi: 10.1172/JCI36066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodpaster BH, et al. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 12.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 13.Serhan CN, et al. Anti-inflammatory and proresolving lipid mediators. Annu Rev Path. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semenkovich CF. Regulation of fatty acid synthase (FAS). Prog Lipid Res. 1997;36:43–53. doi: 10.1016/s0163-7827(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 15.Aarsland A, et al. Contributions of de novo synthesis of fatty acids to total VLDL-triglyceride secretion during prolonged hyperglycemia/hyperinsulinemia in normal man. J Clin Invest. 1996;98:2008–2017. doi: 10.1172/JCI119005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Letexier D, et al. Comparison of the expression and activity of the lipogenic pathway in human and rat adipose tissue. J Lipid Res. 2003;44:2127–2134. doi: 10.1194/jlr.M300235-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.McDevitt RM, et al. De novo lipogenesis during controlled overfeeding with sucrose or glucose in lean and obese women. Am J Clin Nutr. 2001;74:737–746. doi: 10.1093/ajcn/74.6.737. [DOI] [PubMed] [Google Scholar]
- 18.Hellerstein MK. De novo lipogenesis in humans: metabolic and regulatory aspects. Eur J Clin Invest. 1999;53:S53–65. doi: 10.1038/sj.ejcn.1600744. [DOI] [PubMed] [Google Scholar]
- 19.Stanhope KL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savage DB, et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maier T, et al. The crystal structure of a mammalian fatty acid synthase. Science. 2008;321:1315–1322. doi: 10.1126/science.1161269. [DOI] [PubMed] [Google Scholar]
- 23.Witkowski A, et al. Coupling of the de novo fatty acid biosynthesis and lipoylation pathways in mammalian mitochondria. J Biol Chem. 2007;282:14178–14185. doi: 10.1074/jbc.M701486200. [DOI] [PubMed] [Google Scholar]
- 24.Goodridge AG. Regulation of the gene for fatty acid synthase. Fed Proc. 1986;45:2399–2405. [PubMed] [Google Scholar]
- 25.Wong RH, et al. A role of DNA-PK for the metabolic gene regulation in response to insulin. Cell. 2009;136:1056–1072. doi: 10.1016/j.cell.2008.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts R, et al. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52:882–890. doi: 10.1007/s00125-009-1300-4. [DOI] [PubMed] [Google Scholar]
- 27.Moreno-Navarrete JM, et al. Val1483Ile in FASN gene is linked to central obesity and insulin sensitivity in adult white men. Obesity (Silver Spring) 2009;17:1755–1761. doi: 10.1038/oby.2009.65. [DOI] [PubMed] [Google Scholar]
- 28.Schleinitz D, et al. Effect of genetic variation in the human fatty acid synthase gene (FASN) on obesity and fat depot-specific mRNA expression. Obesity (Silver Spring) 2010;18:1218–1225. doi: 10.1038/oby.2009.392. [DOI] [PubMed] [Google Scholar]
- 29.Li S, et al. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Real JM, et al. Extracellular fatty acid synthase: a possible surrogate biomarker of insulin resistance. Diabetes. 2010;59:1506–1511. doi: 10.2337/db09-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuzaka T, et al. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat Med. 2007;13:1193–1202. doi: 10.1038/nm1662. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki M, et al. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6:484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 34.Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10:1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- 35.Chakravarthy MV, et al. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Chakravarthy MV, et al. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 2009;138:476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernal-Mizrachi C, et al. Dexamethasone induction of hypertension and diabetes is PPAR-alpha dependent in LDL receptor-null mice. Nat Med. 2003;9:1069–1075. doi: 10.1038/nm898. [DOI] [PubMed] [Google Scholar]
- 38.Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- 39.Chakravarthy MV, et al. Brain fatty acid synthase activates PPARalpha to maintain energy homeostasis. J Clin Invest. 2007;117:2539–2552. doi: 10.1172/JCI31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider JG, et al. Macrophage fatty acid synthase deficiency decreases diet-induced atherosclerosis. J Biol Chem. 2010;285:23398–23409. doi: 10.1074/jbc.M110.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 42.Kliewer SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krey G, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 44.Yu K, et al. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995;270:23975–23983. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 45.Forman BM, et al. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 46.Bell-Parikh LC, et al. Biosynthesis of 15-deoxy-delta12,14-PGJ2 and the ligation of PPARgamma. J Clin Invest. 2003;112:945–955. doi: 10.1172/JCI18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tzameli I, et al. Regulated production of a peroxisome proliferator-activated receptor-gamma ligand during an early phase of adipocyte differentiation in 3T3-L1 adipocytes. J Biol Chem. 2004;279:36093–36102. doi: 10.1074/jbc.M405346200. [DOI] [PubMed] [Google Scholar]
- 48.Kim JB, et al. ADD1/SREBP1 activates PPARgamma through the production of endogenous ligand. Proc Natl Acad Sci U S A. 1998;95:4333–4337. doi: 10.1073/pnas.95.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christianson JL, et al. Stearoyl-CoA desaturase 2 is required for peroxisome proliferator-activated receptor gamma expression and adipogenesis in cultured 3T3-L1 cells. J Biol Chem. 2008;283:2906–2916. doi: 10.1074/jbc.M705656200. [DOI] [PubMed] [Google Scholar]
- 50.Liu LH, et al. Effects of a fatty acid synthase inhibitor on adipocyte differentiation of mouse 3T3-L1 cells. Acta Pharmacol Sin. 2004;25:1052–1057. [PubMed] [Google Scholar]
- 51.Schmid B, et al. Inhibition of fatty acid synthase prevents preadipocyte differentiation. Biochem Biophys Res Commun. 2005;328:1073–1082. doi: 10.1016/j.bbrc.2005.01.067. [DOI] [PubMed] [Google Scholar]
- 52.McGarry JD. What is Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 53.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 54.Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351–356. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- 55.Perez-Martinez P, et al. n-3 PUFA and lipotoxicity. Biochim Biophys Acta. 2010;1801:362–366. doi: 10.1016/j.bbalip.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Cao H, et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maeda K, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1:107–119. doi: 10.1016/j.cmet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Jaworski K, et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med. 2009;15:159–168. doi: 10.1038/nm.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vegiopoulos A, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- 60.van Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 61.Loftus TM, et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 62.Proulx K, et al. Fatty acid synthase inhibitors modulate energy balance via mammalian target of rapamycin complex 1 signaling in the central nervous system. Diabetes. 2008;57:3231–3238. doi: 10.2337/db07-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolfgang MJ, Lane MD. Control of energy homeostasis: role of enzymes and intermediates of fatty acid metabolism in the central nervous system. Annu Rev Nutr. 2006;26:23–44. doi: 10.1146/annurev.nutr.25.050304.092532. [DOI] [PubMed] [Google Scholar]
- 64.Fu J, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 65.Overton HA, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Gillum MP, et al. N-acylphosphatidylethanolamine, a gut- derived circulating factor induced by fat ingestion, inhibits food intake. Cell. 2008;135:813–824. doi: 10.1016/j.cell.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang J, et al. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 68.Kirchner H, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–745. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopez M, et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008;7:389–399. doi: 10.1016/j.cmet.2008.03.006. [DOI] [PubMed] [Google Scholar]