INTRODUCTION
Glitazones (GZs) or thiazolidinediones are oral hypoglycemic agents used in the treatment of type II diabetes. They are acting as insulin sensitizers by regulating the transcription of insulin-responsive genes involved in the control of glucose production, transport, and utilization. The first report of their glucose-lowering effects was published in 1980s, and troglitazone was launched as an oral hypoglycemic agent in 1997, and two others, i.e., pioglitazone (PGZ) and rosiglitazone, were approved by the FDA in 1999 (1,2). Troglitazone was withdrawn because of its hepato-toxicity; the incidence of liver toxicity of PGZ and rosiglitazone are minor; however, investigations for finding more active glitazones with less side effects are still ongoing (2). PGZ is the most popular drug, and its prescription is increased by the rate of 14% during 2005–2007 (3). In addition to its antidiabetic activity, it demonstrates other activities including reduction of reactive oxygen species from adipose tissue (4) and improving cognition and regional cerebral blood flow in patients with mild Alzheimer (5). PGZ hydrochloride (PGZ-HCl) is used in pharmaceutical formulations to achieve a more soluble form of the drug; however, the aqueous solubility of PGZ-HCl is still low, and a number of investigations were reported dealing with solubilization of PGZ or PGZ-HCl (6–10).
Common cosolvents in pharmacy are ethanol, propylene glycol (PG), glycerin, polyethylene glycol 400 (PEG 400), and N-methyl-2-pyrrolidone (NMP; 11). Polyethylene glycols (PEGs) are linear or branched polyethers with the approximate molecular weight of 200–36,000. PEG 200 to PEG 800 are in liquid form, whereas PEG 1000 and higher molar masses are solids. Liquid PEGs are commonly used as cosolvents for solubilization of drugs in preclinical and clinical studies (12). Because of strong H-bonding between PEGs and water, they are freely soluble in water and in many organic solvents. PEGs have variety of applications in the pharmaceutical, chemical, cosmetic, and food industries (13). Their low toxicity and high water solubility enable their use for purification of biological materials. Among them, PEG 400 is the most commonly used cosolvent in the pharmaceutical industries for preparation of cosmetics, ointments, suppositories, ophthalmic solutions, and sustained-released oral pharmaceutical formulations (14). Propylene glycol is a stable and low toxic pharmaceutical cosolvent which is used in many commercially available oral and parenteral formulations of poorly soluble drugs (15,16). The well-known parenteral formulations containing PG are diazepam, fenoldopam mesylate, melphalan HCl, oxytetracycline, paricalcitol, pentobarbital Na, phenytoin Na, chlordiazepoxide HCl, lorazepam, and phenobarbital. PG is also used in many oral formulations of drugs including amprenavir, clofazimine, cyclosporine A, digoxin, lopinavir, ritonavir, sirolimus, loratadin, and itraconazole (15).
Our intent was to measure the solubilities of PGZ-HCl in a series of aqueous and non-aqueous solvent systems containing ethanol, PG, NMP, and PEGs at 298.2 K which extends the available database of drugs solubilities in mixed solvents (17), fitting the data to the Jouyban-Acree model that relates the solubilities in solvent mixtures to the fractions of the solvent components and constants computed by a regression analysis (18). In previous reports, the solubility of PGZ-HCl in aqueous solutions of ethanol, PG, and NMP (8), in binary mixtures of PEG 600 with water and ethanol, ternary mixtures of PEG 600-ethanol-water (9), and also in binary mixtures of PEG 400 with ethanol, PG, NMP, and water (10) were discussed. In this work, the solubility of PGZ-HCl in binary and ternary mixtures of water, PG, and PEGs 200, 400, and 600 at 298.2 K are reported. The generated data are predicted using numerical methods, and the accuracies of different methods are discussed.
EXPERIMENTAL METHODS
Materials
PGZ-HCl (99.8% w/w) was purchased from Osveh Pharmaceutical Company (Tehran, Iran). PEG 400 and PEG 600 were kindly gifted by Daana Pharmaceutical Co. (Tabriz, Iran); propylene glycol (99.5% w/w), and PEG 200 (99.5% w/w) were purchased from Merck (Germany); methanol (99.8% w/w) was purchased from Caledon (Canada), and double-distilled water was used for preparation of the solutions.
Apparatus and Procedures
The binary mixtures composed of the solvents with suitable masses of the solvents were prepared with the accuracy of 0.001 mass fraction. The solubilities of PGZ-HCl were determined by the saturation shake-flask method of Higuchi and Connors (19). Briefly, an excess amount of the drug was added to the prepared solvent mixtures. The resulting solutions were equilibrated for at least 3 days on a shaker (Behdad, Tehran, Iran) in an incubator equipped with a temperature-controlling system maintained constant at 298.2 (±0.2) K. The saturated solutions were filtered using hydrophilic Durapore filters (0.45 μm, Milipore, Ireland) and diluted with methanol. Diluted samples were then assayed at 267 nm (molar absorptivity of 7,464–7,537 L mol−1 cm−1) using a UV-Vis spectrophotometer (Beckman DU-650, Fullerton, USA). Preliminary investigations showed that the filter did not absorb the solute during the filtration process. The concentration of each solution was determined with an absorbance versus concentration calibration curve (Absorbance = 21.086 × Conc−0.0066, R2 = 0.999) after appropriate dilution, the standard deviations for R2, intercept and slope of the curve are 0.0132, 0.0130, and 0.4531, respectively. Each experimental data point represents the average of at least three repetitive measurements with the measured in moles per liter (M) solubilities being reproducible within ±2.8%. Densities of the saturated solutions were determined using a 5-mL pycnometer.
Computational Methods
The Jouyban-Acree model for calculating the solubilities of drugs in binary solvent mixtures at different temperatures is (18):
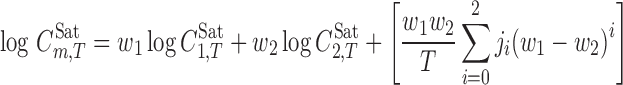 |
1 |
where  is the molar solute solubility in the solvent mixtures at temperature T, w1, and w2 are the mass fractions of the solvents 1 and 2 in the absence of the solute,
is the molar solute solubility in the solvent mixtures at temperature T, w1, and w2 are the mass fractions of the solvents 1 and 2 in the absence of the solute, 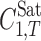 and
and 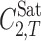 denote the molar solubility of the solute in the solvents 1 and 2, respectively, and Ji are the constants of the model computed by a regression analysis. Equation 1 could be converted to a simpler version at a given temperature; however, we recommend the presented version since it could be used to predict the solubility of drugs at other temperatures of interest as shown in previous papers (20,21). The regression constants represent differences in the various solute–solvent and solvent–solvent interactions in the solution. The Jouyban-Acree model has the advantage that it can be used to describe mole fraction or moles per liter solubilities of solutes dissolved in binary solvent mixtures as a function of either solvent mole fraction composition or solvent weight fraction composition. The generalized model can accommodate different units of solubility and different units of solvent composition.
denote the molar solubility of the solute in the solvents 1 and 2, respectively, and Ji are the constants of the model computed by a regression analysis. Equation 1 could be converted to a simpler version at a given temperature; however, we recommend the presented version since it could be used to predict the solubility of drugs at other temperatures of interest as shown in previous papers (20,21). The regression constants represent differences in the various solute–solvent and solvent–solvent interactions in the solution. The Jouyban-Acree model has the advantage that it can be used to describe mole fraction or moles per liter solubilities of solutes dissolved in binary solvent mixtures as a function of either solvent mole fraction composition or solvent weight fraction composition. The generalized model can accommodate different units of solubility and different units of solvent composition.
The model for representing the solubility of drugs in ternary solvent mixtures based on sub-binary interaction terms is:
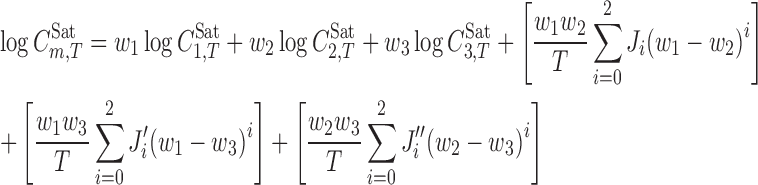 |
2 |
where  is the solute (moles per liter) solubility in the solvent 3 (water) at temperature T, and w3 is the mass fraction of the solvent 3 in the absence of the solute. The J′i and J″i terms are computed using the same procedure of Ji terms. The solvents numbers are defined as
is the solute (moles per liter) solubility in the solvent 3 (water) at temperature T, and w3 is the mass fraction of the solvent 3 in the absence of the solute. The J′i and J″i terms are computed using the same procedure of Ji terms. The solvents numbers are defined as 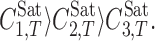
The model requires knowledge of the solubility of drug in mono-solvents and in several binary solvent mixtures in order to calculate the model constants. By assuming similar solute–solvent interactions for various drugs, trained versions of the Jouyban-Acree model have been reported for a number of aqueous and non-aqueous binary solvents at various temperatures (18,22). From these models, the trained version for PEG 400+ water mixtures is (23):
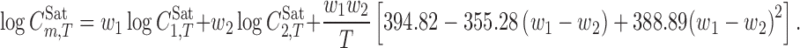 |
3 |
Although Eq. 3 was developed for PEG 400+ water mixtures, it provided reasonably accurate solubility predictions for drugs in ethylene glycol+water and PEG 200+water mixtures (24).
The mean percentage deviation (MPD) was used to check the accuracy of the fitted and predicted values and was calculated using:
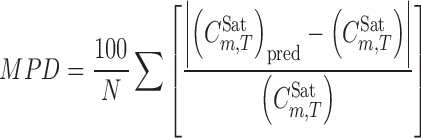 |
4 |
where N is the number of data points in each set.
RESULTS AND DISCUSSION
Table I lists the experimental solubilities of PGZ-HCl in aqueous binary mixtures of PEGs 200, 400, and 600 at 298.2 K. As noticed in a previous paper (8), converting base form of PGZ into its HCl salt form increases its aqueous solubility by ~16-fold. Seedher and Kanojia (7) investigated the solubility of PGZ in different pH values adjusted by glycine–HCl/glycine–NaOH where the minimum solubility of 0.014 mmol∙L−1 at pH 3.92, and the maximum solubilities at two extremes were observed as 0.165 and 0.157 mmol∙L−1 at pHs of 1.83 and 9.52, respectively (7). The PGZ solubility at pH 7.39 of glycine buffer was 0.020 mmol∙L−1 and that of phosphate buffer (pH 7.40) was 0.033 mmol∙L−1, revealing that the solubility of PGZ is affected by type of buffer as well as pH value. Aqueous solubility of PGZ was 0.044 mmol∙L−1 (7). The solubility behavior of drugs in their salt forms is more complicated when compared with their base forms and/or the solubility of non-electrolytes. There are some evidences of the effects of excess solid on the solubility of drugs and numerous mechanisms have been proposed including different dissolution and crystallization rates (25), protonation and deprotonation of weak acid/basic drugs (26), dimerization of some drugs (27), possible adsorption of the charged form of solutes onto the excess solid (11), and the common ion effect (26). To investigate the effect of excess solid on the aqueous solubility of PGZ-HCl, exact amount of the saturated solubility of PGZ-HCl, 1%, 5%, 10%, and 50% excess values of the drug were added to water and shaken for 3 days, and then the solubility of PGZ-HCl were determined. Figure 1 shows the results in which slight increase is observed with the increased excess solid in the solution.
Table I.
Millimole per Liter Solubility of Pioglitazone HCl in Various Polyethylene Glycols (1) + Water (2) Mixtures at 298.2 K
| w 1 | PEG 200 | PEG 400a | PEG 600b |
|---|---|---|---|
| 0.000 | 0.7 | 0.7 | 0.7 |
| 0.100 | – | – | 4.6 |
| 0.200 | 5.3 | 7.6 | 8.6 |
| 0.300 | – | – | 11.9 |
| 0.400 | 5.9 | 10.2 | 16.0 |
| 0.500 | – | – | 20.1 |
| 0.600 | 11.0 | 18.3 | 25.4 |
| 0.700 | – | 25.4 | 37.3 |
| 0.800 | 30.2 | 35.5 | 48.8 |
| 0.900 | – | 27.7 | 39.1 |
| 1.000 | 19.1 | 20.2 | 24.2 |
Fig. 1.
Effect of excess solid on the aqueous solubility of PGZ-HCl
Addition of the PEGs increased the solubility of PGZ-HCl with a similar pattern, and the maximum solubilities were observed at w1 = 0.800 of PEGs. For three PEGs investigated, the solubility values were PEG 600 > PEG 400 > PEG 200 aqueous mixtures, when the same mass fractions are considered. Considering the solubilization power definitions from the literature, i.e., Eqs. 5 (28) and 6 (29):
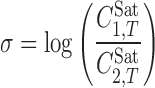 |
5 |
and
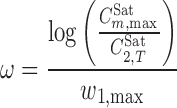 |
6 |
where 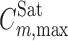 is the maximum observed solubility and w1,max denotes the fraction of the cosolvent producing the maximum solubility. Considering the numerical values of σ and ω for the PEGs (as listed in Table II), the solubilization power of PEG 600 is greater than that of PEG 400 and the lowest power is for PEG 200 when σ definition is concerned. The order of the solubilization power of the cosolvents is PEG 600, followed by PEG 400 and PEG 200, considering the ω definition. This order is confirmed by the experimental solubility data of PGZ-HCl in PEGs+water mixtures.
is the maximum observed solubility and w1,max denotes the fraction of the cosolvent producing the maximum solubility. Considering the numerical values of σ and ω for the PEGs (as listed in Table II), the solubilization power of PEG 600 is greater than that of PEG 400 and the lowest power is for PEG 200 when σ definition is concerned. The order of the solubilization power of the cosolvents is PEG 600, followed by PEG 400 and PEG 200, considering the ω definition. This order is confirmed by the experimental solubility data of PGZ-HCl in PEGs+water mixtures.
Table II.
The Numerical Values of σ and ω for PEG Cosolvents Investigated in This Work
| PEG 200 | PEG 400 | PEG 600 | |
|---|---|---|---|
| Water | |||
| σ | 1.50 | 1.53 | 1.61 |
| ω | 2.13 | 2.22 | 2.39 |
| PG | |||
| σ | 0.77 | 0.75 | 0.70 |
| ω | 0.77 | 1.17 | 1.03 |
PEG polyethylene glycol, PG propylene glycol
Table III lists the experimental solubility of PGZ-HCl in PG+PEGs binary mixtures at 298.2 K. Non-aqueous mixed solvents could be used to prepare liquid formulations of instable drugs in aqueous media and/or in the pharmaceutical formulations such as soft gels which water content could make difficulties in the formulations. In these sets of data, PEG 600 promises more solubilization capabilities when compared with PEGs 400 and 200 when ω values (listed in Table II) are considered.
Table III.
Millimole per Liter Solubility of Pioglitazone HCl in Various Propylene Glycol (1) + Polyethylene Glycols (2) Mixtures at 298.2 K
| w 2 | PEG 200 | PEG 400a | PEG 600 |
|---|---|---|---|
| 0.000 | 113.1 | 113.1 | 113.1 |
| 0.100 | – | – | 127.0 |
| 0.200 | 105.0 | 131.6 | 135.2 |
| 0.300 | – | 133.0 | 134.8 |
| 0.400 | 100.0 | 130.6 | 132.0 |
| 0.500 | – | – | 122.4 |
| 0.600 | 65.6 | 102.9 | 108.6 |
| 0.700 | – | 80.9 | 84.4 |
| 0.800 | 39.7 | 46.1 | 49.0 |
| 0.900 | – | – | 36.0 |
| 1.000 | 19.1 | 20.2 | 24.2 |
aData taken from a previous work (10)
The measured experimental solubility data of PGZ-HCl in binary solvents were fitted to Eq. 1, the model constants computed, and the back-calculated solubility data used to compute the MPD values. The calculated MPDs along with the model constants are listed in Table IV. The model provides a very good mathematical description of the experimental solubility data and the overall MPD is 5.0%. Using the model constants (J0, J1, and J2) listed in Table IV, it is possible to predict the solubility of PGZ-HCl in binary mixtures of solvents 1 and 2 at all composition ranges and by employing the solubility in the mono-solvents at other temperatures, one can extend this prediction method for other temperatures as shown in previous works (20,21). As noted in INTRODUCTION, using generally trained version of the model for PEG 400 + water mixtures and employing 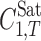 and
and  data, it is possible to predict the solubility of PGZ-HCl in PEGs+water mixtures. The predictions using Eq. 3 resulted in MPDs of 18.0%, 34.7%, and 46.5%, respectively, for aqueous mixtures of PEGs 200, 400, and 600 with the overall MPD of 33.1%. The MPDs for different PEGs lies in the range of MPDs of a previous data set (23) where the MPD range varied between 3.5% and 269.3% and the overall MPD for predicted solubilities in this work is less than that of the reported value in our previous paper, i.e., 39.8% (23).
data, it is possible to predict the solubility of PGZ-HCl in PEGs+water mixtures. The predictions using Eq. 3 resulted in MPDs of 18.0%, 34.7%, and 46.5%, respectively, for aqueous mixtures of PEGs 200, 400, and 600 with the overall MPD of 33.1%. The MPDs for different PEGs lies in the range of MPDs of a previous data set (23) where the MPD range varied between 3.5% and 269.3% and the overall MPD for predicted solubilities in this work is less than that of the reported value in our previous paper, i.e., 39.8% (23).
Table IV.
The Numerical Values of the Constants of the Jouyban-Acree Model, the Mean Percentage Deviations (MPDs) for the Fitted Model
| Solvent system | J 0 | J 1 | J 2 | Eq. 1 | N |
|---|---|---|---|---|---|
| PG+PEG 200 | 288.959 | NS | NS | 2.7 | 6 |
| PG+PEG 400 | 461.517 | NS | NS | 5.8 | 8 |
| PG+PEG 600 | 425.700 | NS | NS | 4.9 | 11 |
| PG+watera | 923.341 | −672.289 | 578.186 | 7.7 | 9 |
| PEG 200 + water | 355.814 | −146.604 | 1804.685 | 1.6 | 6 |
| PEG 400 + water | 688.422 | −331.664 | 1253.150 | 6.5 | 8 |
| PEG 600 + water | 802.666 | −334.631 | 1374.847 | 5.8 | 11 |
| Overall MPD | 5.0 |
MPD mean percentage deviation, NS not significant, PEG polyethylene glycol, PG propylene glycol
aVolume fraction based data taken from a previous work (8) and data was converted to mass fraction using density data of water and PG
Table V lists the experimental solubility of PGZ-HCl in ternary solvent mixtures of PG+PEGs+water. The data could be used in the preparation of liquid formulation when binary solvents are not able to dissolve the desired amount of drug in a given volume. As shown, the highest solubility of PGZ-HCl (290.9 mM) in the investigated mixtures was observed in PG+PEG 400+water with the mass fraction composition of 0.600 + 0.200 + 0.200. This data could be predicted using the model constant of sub-binary solvents using Eq. 2 by incorporating the J terms from Table IV. The obtained MPD values for PEGs 200, 400, and 600 were 36.3% (N = 15), 47.0% (N = 14), and 38.5% (N = 33), respectively. The main advantage of this prediction method is that it is based on just mono-solvent and sub-binary data, and no further experimental efforts are required.
Table V.
Millimole per Liter Solubility of Pioglitazone HCl in Various Propylene Glycol (1)+Polyethylene Glycols (2)+Water (3) Mixtures at 298.2 K
| w 1 | w 2 | PEG 200 | PEG 400 | PEG 600 |
|---|---|---|---|---|
| 0.300 | 0.100 | – | – | 15.5 |
| 0.400 | 0.100 | – | – | 27.9 |
| 0.500 | 0.100 | 67.8 | 135.3 | 78 |
| 0.600 | 0.100 | 85.6 | 126.8 | 94.9 |
| 0.700 | 0.100 | – | – | 148.1 |
| 0.800 | 0.100 | – | – | 145.5 |
| 0.100 | 0.200 | – | – | 11.4 |
| 0.300 | 0.200 | 22.6 | 29.7 | 33.3 |
| 0.400 | 0.200 | – | – | 74.1 |
| 0.500 | 0.200 | – | – | 163.2 |
| 0.600 | 0.200 | 140.1 | 290.9 | 175.6 |
| 0.700 | 0.200 | 65.6 | 150.8 | 164.1 |
| 0.100 | 0.300 | – | – | 17.1 |
| 0.200 | 0.300 | – | – | 40.4 |
| 0.300 | 0.300 | 46.1 | 127.3 | 74.1 |
| 0.400 | 0.300 | 94.5 | 167.2 | 101.6 |
| 0.500 | 0.300 | – | – | 139.2 |
| 0.600 | 0.300 | – | – | 175.6 |
| 0.100 | 0.400 | 20.4 | 39.0 | 44.3 |
| 0.200 | 0.400 | – | – | 61.6 |
| 0.300 | 0.400 | – | – | 125.1 |
| 0.400 | 0.400 | 105.5 | 229.7 | 174.7 |
| 0.500 | 0.400 | 62.1 | 158.8 | 171.2 |
| 0.100 | 0.500 | 29.3 | 40.8 | 45.7 |
| 0.200 | 0.500 | 65.2 | 126.8 | 69.6 |
| 0.300 | 0.500 | – | – | 84.2 |
| 0.400 | 0.500 | – | – | 169.4 |
| 0.100 | 0.600 | – | – | 95.3 |
| 0.200 | 0.600 | 99.3 | 166.7 | 120.6 |
| 0.300 | 0.600 | 59.4 | – | 69.2 |
| 0.100 | 0.700 | – | – | 108.2 |
| 0.200 | 0.700 | – | – | 105.5 |
| 0.100 | 0.800 | 80.7 | 94.0 | 86.9 |
PEG polyethylene glycol
SUMMARY AND CONCLUSION
Experimental molar solubility of PGZ-HCl in binary and ternary mixtures of PG; PEGs 200, 400, 600; and water at 298.2 K are reported. The solubility of PGZ-HCl was increased with the addition of PG and PEGs in which the maximum solubility is observed at 0.600 + 0.200 + 0.200 mass fractions of the PG+PEG 400+ water ternary mixture. In order to provide a computational method to calculate the solubilities, the Jouyban-Acree model was fitted to the results of these measurements, and solubilities were back-calculated with employing the solubility data in mono-solvents in which the overall mean deviation of the models was 5.0% and 40.6%, respectively, for correlated data of binary and predicted data of ternary solvents. A previously trained version of the model was used to predict the solubility of PGZ-HCl in PEGs+water mixtures employing the experimental solubility data in mono-solvents in which the overall prediction error was 33.1%. In practical applications of the cosolvency models, when the solubilities of a drug in water and PEG are determined by experiment, it is possible to predict the solubility in PEG+water mixtures using Eq. 3. The expected prediction error for this prediction is ~33% as noticed above. If the solubility data in PEG+water binary mixtures were determined by experiments and the desired solubility is not achieved, then it is possible to use the binary data for predicting the solubility in ternary solvent mixtures. The expected prediction error for this prediction is ~41%.
Acknowledgments
We thank Osveh Pharmaceutical Company for supplying the drug powder. The financial support under Grant No. NSM63-50 of Research Center for Pharmaceutical Nanotechnology is gratefully acknowledged.
References
- 1.Bradbury J. Explanation found for “glitazone paradox”. Lancet. 2002;360:1003. doi: 10.1016/S0140-6736(02)11125-1. [DOI] [Google Scholar]
- 2.Prashantha Kumar BR, Nanjan MJ. Novel glitazones: Design, synthesise, glucose uptake and structure-activity relationships. Bioorg Med Chem Lett. 2010;20:1953–6. doi: 10.1016/j.bmcl.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 3.Cohen A, Rabbani A, Shah N, Caleb Alexander G. Changes in glitazone use among office-based physicians in the US, 2003–2009. Diabetes Care. 2010;33:823–5. doi: 10.2337/dc09-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakatsuji H, Kishida K, Shimomura I. Three-month treatment with pioglitazone reduces circulating levels of thiobarbituric acid-reacting substances, a marker of reactive oxidative stress, without change in body mass index, in Japanese patients with type 2 diabetes. Atherosclerosis. 2010;212:243–5. doi: 10.1016/j.atherosclerosis.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai H, Iwamoto T. Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging 2009; doi:10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed]
- 6.Seedher N, Kanojia M. Micellar solubilization of some poorly soluble drugs: A technical note. AAPS PharmSciTech. 2008;9:431–6. doi: 10.1208/s12249-008-9057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seedher N, Kanojia M. Co-solvent solubilization of some poorly soluble antidiabetic drugs. Pharm Develop Tech. 2009;14:185–92. doi: 10.1080/10837450802498894. [DOI] [PubMed] [Google Scholar]
- 8.Soltanpour Sh, Acree WE, Jr, Jouyban A. Solubility of pioglitazone hydrochloride in aqueous solutions of ethanol, propylene glycol, and N-methyl-2-pyrrolidone at 298.2 K. AAPS PharmSciTech. 2009;10:1153–7. doi: 10.1208/s12249-009-9322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jouyban A, Soltanpour Sh. Solubility of pioglitazone hydrochloride in polyethylene glycol 600—ethanol—water mixtures at 25°C. Latin Am J Pharm. 2010;29:825–9. [Google Scholar]
- 10.Jouyban A, Soltanpour Sh. Solubility of pioglitazone hydrochloride in binary mixtures of polyethylene glycol 400 with ethanol, propylene glycol, N-methyl-2-pyrrolidone, and water at 25°C. Chem Pharm Bull. 2010;58:1132–5. doi: 10.1248/cpb.58.1132. [DOI] [PubMed] [Google Scholar]
- 11.Avdeef A. Solubility of sparingly-soluble ionizable drugs. Adv Drug Delivery Rev. 2007;59:568–90. doi: 10.1016/j.addr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Myrdal PB, Yalkowsky SH. Solubilization of Drugs. Encyclopedia of Pharmaceutical Technology. New York: Mercel Dekker Inc; 1999. [Google Scholar]
- 13.Harris JM. Poly (ethylene glycol) Chemistry, Biotechnical and Biomedical Application. New York: Plenum; 1992. [Google Scholar]
- 14.Ballantyne B, Leung HW, Hermansky SJ, Frantz SW. Subchronic, chronic, pharmacokinetic and genotoxicity studies with polyox water soluble resin. Toxicol Lett. 1998;1:46–7. doi: 10.1016/S0378-4274(98)80183-5. [DOI] [Google Scholar]
- 15.Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21:201–30. doi: 10.1023/B:PHAM.0000016235.32639.23. [DOI] [PubMed] [Google Scholar]
- 16.Yalkowsky SH, Rubino JT. Solubilization by cosolvents I: Organic solutes in propylene glycol-water mixtures. J Pharm Sci. 1985;74:416–21. doi: 10.1002/jps.2600740410. [DOI] [PubMed] [Google Scholar]
- 17.Jouyban A. Handbook of Solubility Data for Pharmaceuticals. Boca Raton: CRC Press; 2009. [Google Scholar]
- 18.Jouyban A. Review of the cosolvency models for predicting solubility of drugs in water-cosolvent mixtures. J Pharm Pharmaceut Sci. 2008;11:32–58. doi: 10.18433/j3pp4k. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–212. [Google Scholar]
- 20.Jouyban A, Acree WE., Jr Comments on “Solubility of ethyl maltol in aqueous ethanol mixtures”. J Chem Eng Data. 2009;54:1168–70. doi: 10.1021/je800798d. [DOI] [Google Scholar]
- 21.Jouyban A, Shokri J, Barzegar-Jalali M, Hassanzadeh D, Acree WE, Jr, Ghafourian T, Nokhodchi A. Solubility of chlordiazepoxide, diazepam, and lorazepam in ethanol+water mixtures at 303.2 K. J Chem Eng Data. 2009;54:2142–5. doi: 10.1021/je900200k. [DOI] [Google Scholar]
- 22.Jouyban A, Acree WE., Jr Prediction of drug solubility in ethanol–ethyl acetate mixtures at various temperatures using the Jouyban-Acree model. J Drug Del Sci Tech. 2007;17:159–60. [Google Scholar]
- 23.Jouyban A. Solubility prediction of drugs in water–polyethylene glycol using Jouyban-Acree model. Chem Pharm Bull. 2006;54:1561–6. doi: 10.1248/cpb.54.1561. [DOI] [PubMed] [Google Scholar]
- 24.Jouyban A, Soltanpour Sh, Tamizi E. Solubility prediction of solutes in aqueous mixtures of ethylene glycols. Pharmazie. 2008;63:548–50. [PubMed] [Google Scholar]
- 25.Kawakami A, Miyoshi K, Ida Y. Impact of the amount of excess solids on apparent solubility. Pharm Res. 2005;22:1537–43. doi: 10.1007/s11095-005-6247-7. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Burrell LS, Lambert WJ. Solubility of E2050 at various pH: A case in which apparent solubility is affected by the amount of excess solid. J Pharm Sci. 2002;91:1445–55. doi: 10.1002/jps.10107. [DOI] [PubMed] [Google Scholar]
- 27.Avdeef A, Voloboy D, Foreman A. Dissolution–solubility: pH, buffer, salt, dual-solid, and aggregation effects. In: Testa B, van de Waterbeemd H, editors. Comprehensive medicinal chemistry II, Vol. 5, ADME-TOX Approaches. Oxford: Elsevier; 2007. pp. 399–423. [Google Scholar]
- 28.Li A, Yalkowsky SH. Predicting cosolvency. 1. Solubility ratio and solute logKow. Ind Eng Chem Res. 1998;37:4470–5. doi: 10.1021/ie980232v. [DOI] [Google Scholar]
- 29.Jouyban A, Fakhree MAA. A new definition of solubilization power of a cosolvent. Pharmazie. 2008;63:317–19. [PubMed] [Google Scholar]



