Abstract
Although preformed polymers are commercially available for use in the design and development of drug delivery systems, in situ polymerization has also been employed. In situ polymerization affords the platform to tailor and optimize the drug delivery properties of polymers. This review brings to light the benefits of in situ polymerization for oral drug delivery and the possibilities it provides to overcome the challenges of oral route of administration.
KEY WORDS: drug loading, drug release, in situ polymerization, oral drug delivery, polymers
INTRODUCTION
It is a veritable truism that polymers are inevitable in drug delivery. Polymers have been employed and tailored to achieve the desired drug delivery properties. When the desired drug delivery properties are not obtained in one polymer, the polymer is modified, blended, interacted, or grafted with another polymer. Polymers have been used to control the rate of release of drug; prevent toxicity; protect drugs from degradation before delivery to site of action thereby enhancing their stability; and target drug to site of action to improve absorption, and subsequently bioavailability and therapeutic efficacy. The preformed and commercially available polymers can be subdivided into biodegradable and non-biodegradable polymers.
The biodegradable polymers that have been employed in drug delivery systems include natural polymers such as chitosan (1–4) and alginate (5–7); and synthetic polymers such as polyesters which include poly (lactic acid), poly(glycolic acid), poly(lactic-co-glycolic acid; 8,9), polyanhydrides (10,11), and polyamides (12,13). The non-biodegradable polymers include the conventional cellulose derivatives (14–17) and acrylic polymers (18,19). These above-mentioned polymers were purchased by the researchers for preparation of drug delivery systems with fixed polymer chain lengths, molecular weights, and drug delivery properties.
However, drug delivery systems are also developed by in situ polymerization. In situ polymerization in the context of drug delivery implies the development of drug delivery systems within the polymerization mixtures. Despite the numerous polymers available, quite a number of them are not employed for drug delivery because they are not biocompatible and do not possess excellent drug delivery properties. Hence, there is always the need to synthesize new polymers with improved drug delivery properties tailor-made to achieve specific drug delivery outcomes. Instead of purchasing preformed polymers for development of drug delivery, monomers are purchased and novel polymers are formed. One of the benefits of in situ polymerization is that the desired polymer chain length and molecular weight required for a specific drug delivery system can be actualized. The second benefit is that it can be employed to improve drug loading of particulate and matrix delivery systems. Furthermore, the desired monomers can be employed in the desired ratio and optimized to achieve certain drug delivery attributes. In situ polymerization is a method that can be employed to develop polymers that can withstand the harsh conditions of the gastric region in order to protect and deliver macromolecules through the oral route (20). In situ polymerization such as free radical and interfacial polymerizations are rapid and cross-linking density can be controlled to modulate drug release (21). The polymerization process of photopolymerization for instance can be undertaken at temperatures and pHs within physiological ranges (22).
The different in situ polymerization methods that have been used to prepare drug delivery systems include interfacial polymerization (23–25), free radical polymerization (26,27), anionic polymerization (28), ring-opening polymerization (29), frontal polymerization(30), micellar copolymerization, and network polymerization (31).
This review focuses on highlighting the different techniques of in situ polymerization for oral drug delivery systems with emphasis on drug incorporation during polymerization.
DRUG INCORPORATION AFTER IN SITU POLYMERIZATION
This section refers to drug delivery systems prepared by in situ polymerization of which the drugs are incorporated after polymerization. Such drugs include proteins which may be denatured by heat or UV-applied during polymerization.
In Situ Free Radical Solution Polymerization for Films, Microparticles, and Nanospheres
Peppas and co-workers prepared complexation polymers which protected proteins such as insulin and calcitonin from possible degradation in the gastric region by the ability of the polymers to respond to changes in pH (32–36). Complexation hydrogels of poly(methacrylic acid) grafted with poly(ethylene glycol) depicted by the authors as P(MAA-g-EG) were prepared by free radical solution UV-polymerization of methacrylic acid (MAA) and methoxy-terminated poly(ethylene glycol) monomethacrylate (PEGMA). Swelling, calcium binding, and enzyme inhibition studies were undertaken (33). Formation of complexation at lower pH due to hydrogen bonding between the PEG grafts and PMAA pendant groups yielded low swelling rate. However, as the pH increased, the swelling rate increased, as complexation did not occur at higher pHs due to dissociation of complexes. The complexation hydrogel exhibited good calcium binding ability which enabled it to inhibit calcium-dependent enzymes such as trypsin making it a potential carrier for peptides and proteins (33).
Peppas and co-workers prepared microparticles of P(MAA-g-EG) by free radical solution polymerization; and insulin and other drugs such as vancomycin and theophyline were incorporated individually by equilibrium partitioning. Initially, polymer films were obtained which were crushed into microparticles before drug incorporation. After drug incorporation, the microparticles were filtered, washed, dried under vacuum, and stored at 4°C for further studies (34). The loading efficiency of insulin was 87.4%, 14.5% of vancomycin while theophyline was lower (1.1%). The drug release studies indicated that P(MAA-g-EG) at 1:1 MAA/EG ratio protected insulin in acidic medium as only 6% was released. However, there was a rapid burst release of insulin in higher pH due to rapid swelling of the microparticles (34). Vancomycin and theophyline, on the contrary, released more in acidic medium due to the fact that the drug size decreased thereby increasing the ability of the drugs to move through the complexed network (34).
To control the rate of release of insulin which will improve on the amount absorbed, P(MAA-g-EG) was tailored to achieve this by altering the solvent content during network formation (36). The intestinal administration of the insulin-loaded polymer samples showed significant increase in bioavailability (4.6–7.4%) as opposed to insulin solutions (1.0%) (36). This is an indication that complexation hydrogels show promise for the delivery of proteins by protecting them in the stomach and releasing them in the intestine, and their release can be controlled by modifying the hydrogel network.
In another study, Foss and Peppas stated that the complexation nanospheres and microparticles especially at equimolar ratio of MAA/EG did not significantly decrease cell viability which was measured by nicotinamide adenine dinucleotide phosphate production, and the complexation hydrogels also contributed to the permeability of insulin (37).
Ring-Opening Polymerization for Nanoparticles
Ring-opening polymerization is an addition polymerization in which the end of a polymer functions as a reactive center (an initiator) and cyclic monomers attached to form a higher molecular weight polymer. However, before the addition takes place, ring-opening of the cyclic monomers would occur and the polymer obtained after polymerization is a usually linear polymer. Ring-opening polymerization was employed for the synthesis of poly(lactide)-d-α-tocopheryl polyethylene glycol 1,000 succinate (PLA-TPGS) copolymer (29,38). The ring-opening polymerization of lactide monomer with TPGS occurred in the presence of stannous octoate as catalyst. Drug incorporation was undertaken by modified solvent extraction/evaporation method which produced Paclitaxel-loaded PLA-TPGS nanoparticles. The in vitro drug release studies indicated an initial burst release of 17% and sustained release of 51% by the thirty-first day (29). The cellular uptake efficiency of PLA-TPGS (ratio 89:11) nanoparticles was 55.9% while they showed inhibition of proliferation of HT-9 and Caco-2 cells after 48 and 72 h even at low drug concentration (<0.025 μg/ml; 38).
DRUG INCORPORATION DURING IN SITU POLYMERIZATION
In situ polymerization in the presence of a drug is only undertaken if the drug can withstand polymerization and does not react with the monomers (26). The various techniques of polymerization employed for in situ fabrication of oral drug delivery systems are discussed (Table I):
Table I.
A Representation of Applications of In Situ Polymerization for Oral Drug Delivery Systems
| Technique | Drug | Delivery system | Researchers | |
|---|---|---|---|---|
| Drug incorporation after in situ polymerization | Free radical solution polymerization | Trypsin, insulin, vancomycin, theophylline | Films, microparticles, nanospheres | Peppas et al. (32–36) |
| Ring-opening polymerization | Paclitaxel | Nanoparticles | Zhang and Feng (29, 38) | |
| Drug incorporation during in situ polymerization | Annealing | Metronidazole | Controlled release matrices | Limmatvapirat et al. (39) |
| Free radical polymerization | Theophylline, bleomycin, diltiazem, proxyphylline, vitamin B12, insulin | Coating of pellets, nanospheres | Peppas et al. (32, 40); Mayo-Pedrosa et al. (41, 42) | |
| Interfacial polymerization | Insulin | Nanocapsules | Watnasirichaikul et al. (43, 45) | |
| Frontal polymerization | Diclofenac sodium | Hydrogels | Gavin et al. (30) | |
| Anionic polymerization | Insulin | Nanoparticles | Mesiha et al. (28) | |
| Micellar copolymerization and network polymerization | Vitamin B, progesterone, insulin, interferon | Hydrogels | Yu and Grainger (31) |
In Situ Polymerization Precipitated by Annealing for Controlled Release Matrices
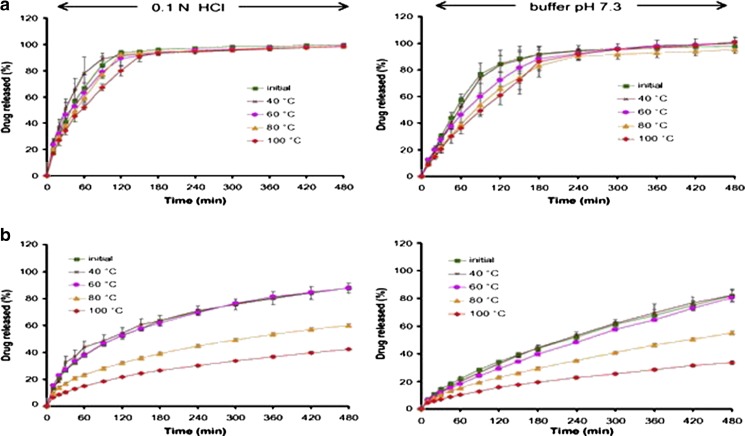
Limmatvapirat and co-workers developed controlled release matrices by employing a natural polymer, shellac (39). Shellac-based controlled release matrices were formulated by wet granulation and were subjected to temperatures 40°C, 60°C, 80°C, and 100°C over 24 h in an oven. The annealed shellac-based matrices were characterized in comparison with the native shellac-based matrices. Before shellac was utilized to formulate matrices, the polymer was subjected to temperatures as stated above. The impact of annealing on the polymer leading to polymerization was confirmed by differential scanning calorimetry and acid–base titration method which yielded decreased acid value and increased insoluble solid matter. It was reported that in situ polymerization due to annealing produced stronger tablets, delayed disintegration to as much as 120 min in acidic medium and 180 min in buffer pH 7.3 for a higher concentration of shellac (40%). However, higher temperature generated non-disintegrating tablets even at lower concentration of shellac. The metronidazole-loaded matrices were found to erode at pH 7.3; but as the annealing temperature increased, erosion of the matrices decreased which is an indication that in situ polymerization improved their mechanical strength. As the concentration and temperature increased, the drug release profile became linear with percentage drug released over time decreasing as the annealing temperature increased (Fig. 1; 39). In sum, in situ polymerization due to annealing modified the properties of shellac, improving its properties for controlled drug delivery.
Fig. 1.
Drug release profiles indicating the effect of annealing temperature employing a 5% w/w shellac-based matrix tablets and b 50% w/w shellac-based matrix tablets, in 0.1 N HCl and pH 7.3 buffer [Source: (39)]
In Situ Free Radical Polymerization for Oral Drug Delivery Systems
Free radical polymerization is one of the most versatile methods of polymerization. It involves an initiator and a monomer. First, the initiator molecules are converted to free radicals by different mechanisms such as heating, photolysis, and electrolysis and the free radicals, being highly active, are able to obtain electrons from the molecules of the monomers making them free radical and highly reactive. The polymer chain grows by the reactive monomers reacting with the monomers molecules by electron transfer.
Peppas and co-workers have utilized in situ free radical polymerization for preparation of oral delivery systems (32, 40). MAA monomers were grafted to PEGMA in a molar ratio of 23:1 to form P(MAA-g-EG) in the presence of tetraethylene glycol dimethacrylate as the cross-linking agent and Irgacure184®[1-hydroxy-cyclohexl-phenylketone] as the initiator with UV source to initiate free radical polymerization. The nanosphere suspension obtained was washed employing cellulose dialysis membrane to remove unreacted monomers. Bleomycin was loaded into the nanospheres prior to polymerization and after polymerization (imbibition). In situ polymerization achieved a drug loading efficiency of 76% as compared with bleomycin loading by imbibition (45%). The drug release studies undertaken indicated that more bleomycin is released at a higher pH of 7.0 and no cytotoxic effect was observed on Caco-2 cells. The nanospheres were also reported by the authors as a good permeability enhancer which will aid the transport of bleomycin and subsequently improve its bioavailability (40).
In another study with P(MAA-g-EG), Peppas and co-workers loaded diltiazem during polymerization (32). Diltiazem was able to diffuse through the hydrogel networks and at least 90% of the drug was released in the first 90 min. However, the rate of release of diltiazem decreased with increasing fraction of poly(ethylene glycol) dimethacrylate. They also studied other drugs such as proxyphylline, vitamin B12, insulin, and calcitonin, and in sum, the rate of release of the drugs were dependent on the method of preparation, cross-linking density, molecular weight of the PEO chains, and the drug solubility (32).
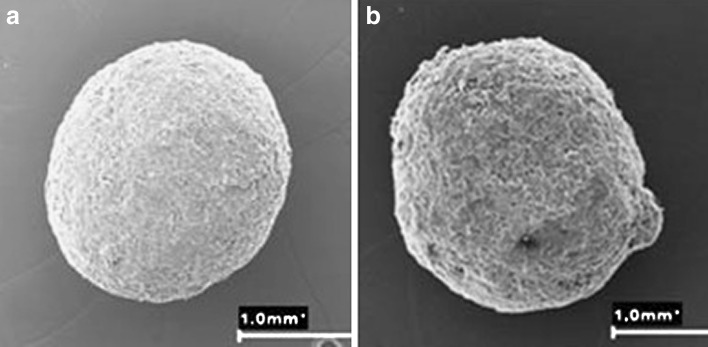
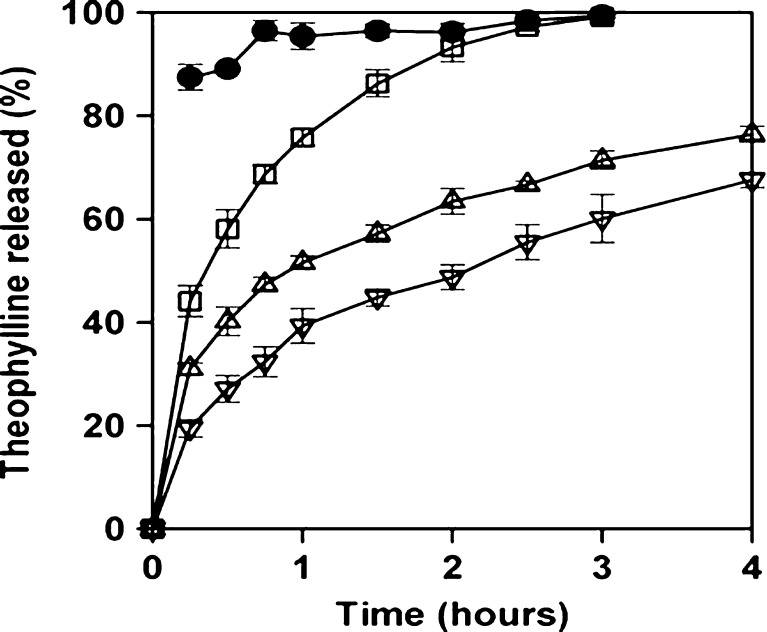
Furthermore, in situ polymerization has been employed to develop coatings of solid dosage forms that are temperature- and pH-responsive (41,42). Before coating in each case, pre-coating monitoring of the polymerization process was undertaken in order to assess the time required for complete polymerization. Then, characterization of the polymerization kinetics was undertaken to determine the best composition of the coating to achieve the desired results. Poly(N-isopropylacrylamide) (PNIPA) was utilized for the temperature-sensitive coating on pellets (41). The characteristic low critical solubility temperature at 33°C of PNIPA led to its utilization. PNIPA cross-linked hydrogels at swollen state can load drugs from their environment; however, above 33°C, the hydrogels become hydrophobic, collapse, and a prolonged drug release can be obtained. The pellets were formulated by extrusion–speronization process with or without PNIPA as one of the excipients. In situ polymerization was achieved by irradiation in the presence of a cross-linker, N,N-methylenebisacrylamide (MBAAm). The coating medium constitutes 20% N-isopropylacrylamide (NIPA) monomers, 6.25% MBAAm, and 3.75% Irgacure® 2959 (photoinitiator) ethanol/water 50:50 v/v solution. The surfaces of the pellets were covered with the coating medium and immediately subjected to irradiation with a UV-lamp at 366 nm (20 min per session). Four to eight coatings were undertaken and the pellets dried in an oven at 40°C. Theophylline release studies were carried out using USP apparatus II at 50 rpm, 900 mL of dissolution medium at 37°C. The quantity of drug released per time was analyzed spectrophotmetrically. Addition of MBAAm produced PNIPA hydrogel on the surface of the pellets which led to prolonged release of up to 24 h as the number of coatings increased to six or eight (41). The SEM photographs of a pellet before and after coating is shown in Fig. 2 while theophylline release profiles are shown in Fig. 3. Having PNIPA incorporated into the pellets and as coating on the surface of the pellets further retarded the rate of release of theophylline.
Fig. 2.
SEM photographs of pellet before coating a and after six coatings b employing in situ photopolymerization in the presence of a cross-linker [Source: (41)]
Fig. 3.
Theophylline release profiles from pellets (without PNIPA incorporated in the formulation) before coating (solid circles) and after four (open squares) and eight (up triangles) coating sessions, and from pellets (containing PNIPA within the formulation) after six (down triangles) coating sessions [Source: (41)]
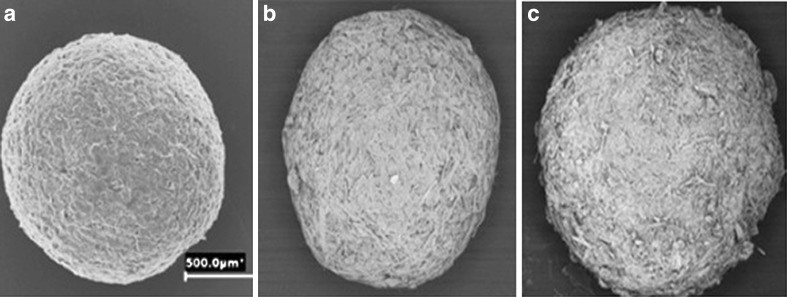
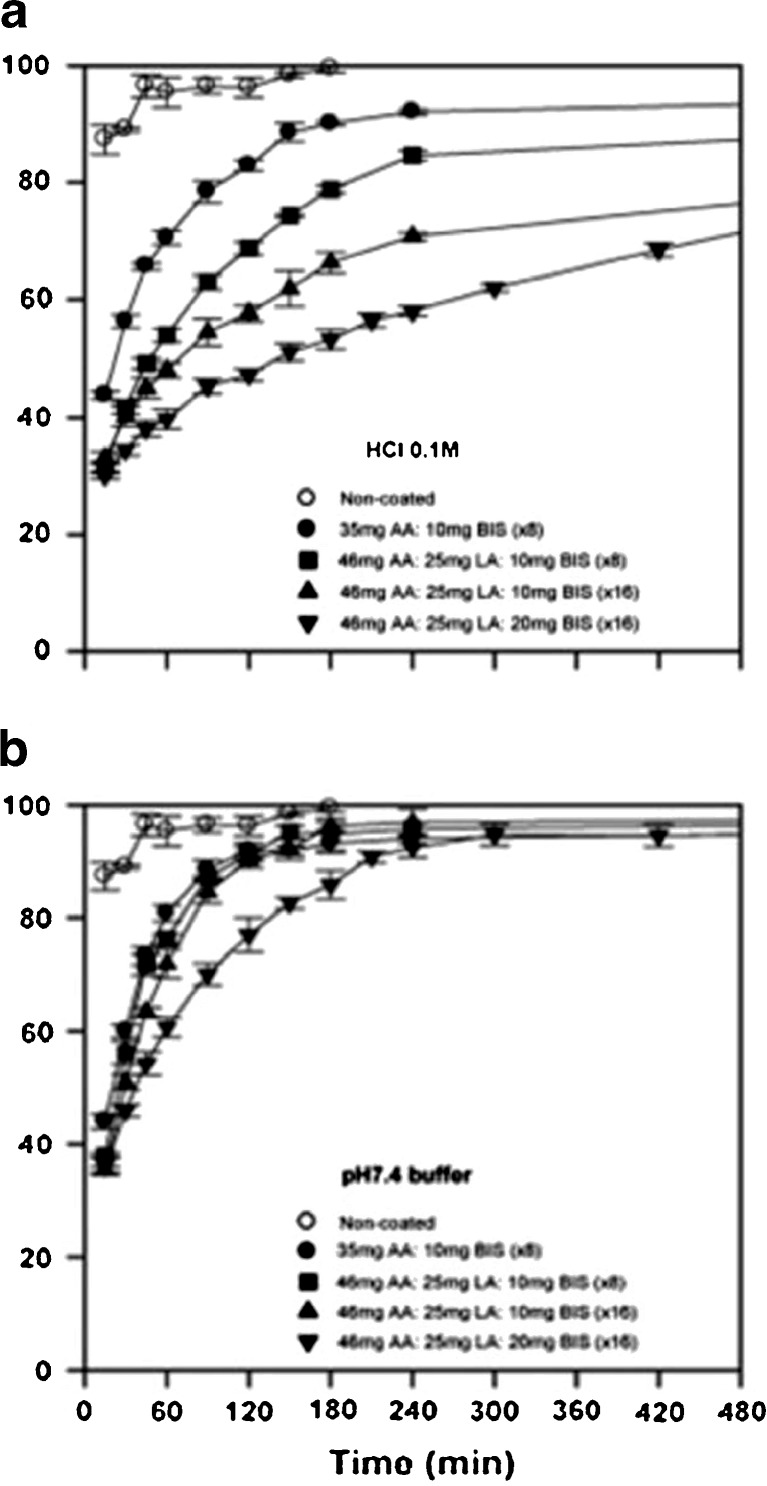
Mayo-Pedrosa and co-workers performed further in situ polymerization for pH-responsive coatings (42). Acrylic acid (AA) monomers were utilized as the pH-responsive component and to tailor the coatings to trigger drug release at different pH, hydrophobic monomers such as lauryl acrylate (LA), or octadecyl acrylate were copolymerized with AA which increased the pKa of AA and, in turn, pH which initiated swelling of the hydrogel formed after polymerization. The theophylline-loaded pellets composed of powdered cellulose and polyvinylpyrrolidone (PVP as a binder) was obtained by extrusion–spheronization process, sprayed with monomeric solutions of AA or AA/LA 88:12 M ratio in 50:50 ethanol/water medium containing MBAAm (0.2 or 0.4 g—added at two levels) and Irgacure® 2959 (0.2 g) and irradiated with a UV-lamp at 366 nm (20 min per application). The coating was done severally, and then the pellets were dried in an oven at 40°C. Uncoated pellets (Fig. 4a) disintegrated, and the drug was released in a few minutes. Coating with AA alone sustained release for 4 h in acidic medium, and the remaining drug released within 2 h in pH 7.4 (Fig. 5). The AA/LA coating further controlled the release of theophylline in acidic medium and enhanced its release in pH 7.4. An increased number of coatings (Fig. 4b, c) and doubling the cross-linker further retarded the release of theophylline in acidic medium and did not significantly affect its rapid release in pH 7.4 (42).
Fig. 4.
SEM photographs of a uncoated pellets, b pellets coated eight times, and c pellets coated 16 times with layers of AA/LA 88:12 M ratio [Source: (42)]
Fig. 5.
Drug release profiles in HCl 0.1 M and pH 7.4 phosphate buffer of core pellets and of pellets that were coated by in situ photopolymerization of different monomeric mixtures [Source: (42)]
In Situ Interfacial Polymerization for Nanocapsules
Interfacial polymerization is a method of polymerization that occurs at the interface of two immiscible liquids containing monomers. A film of polymer is formed at the interface which may slow down the reaction, but removal of the film improves the rate of the reaction. Unstirred interfacial polymerization produces membranes while agitated interfacial polymerization produces micro- and nanoparticles.
Watnasirichaikul and co-workers employed agitated interfacial polymerization to fabricate insulin-loaded nanocapsules (43). Insulin poly(ethyl 2-cyanoacrylate) nanocapsules were formed within a microemulsion by utilizing ethyl 2-cyanoacrylate for oral drug delivery. The microemulsion comprised of surfactant blend (1.4 g), oil mixture (7.6 g), and aqueous insulin (100 units/mL) at pH 7.4 (Humulin R®) at 4°C. Into the microemulsion was slowly added a solution of 100 mg ethyl 2-cyanoacrylate in 300 mL of chloroform under agitation and left for at least 4 h at 4°C for polymerization to occur. The nanocapsules were collected and characterized for size, morphology, entrapment, and drug release. The poly(ethyl 2-cyanoacrylate) nanocapsules formed by in situ interfacial polymerization has an average particle size of 150.9 nm with a narrow distribution having a polydispersity of 0.101, and the particles were observed to be spherical with a smooth surface having an inner cavity surrounded by a polymer wall. Entrapment of insulin was 86%, and release was in acidic medium with an initial rapid release of 14% in 30 min followed by a constant release rate between 60 and 180 min and then a decline in the rate after 180 min. The use of biocompatible oils and surfactants negates the need for isolation of the nanocapsules from the polymerization vehicle which can be used for the administration which, in turn, would enhance the absorption of insulin (43,44).
Watnasirichaikul and co-workers went ahead to evaluate insulin nanocapsules in vivo using diabetic rats (45) employing microemulsion as a template for in situ interfacial polymerization; however, the monomer was iso-butyl cyanoacrylate. The oral delivery of insulin nanocapsules formed by in situ interfacial polymerization of microemulsion by alkyl cyanoacrylate when compared with aqueous insulin solution and a water-in-oil insulin microemulsion increased the oral bioavailability of insulin. Furthermore, varying the mass of the monomer–iso-butyl cyanoacrylate controlled the rate of release of insulin. The release of insulin was suppressed in the stomach (acidic medium) and enhanced in the intestinal region (neutral pH; 45).
Frontal Polymerization for Hydrogels
Although frontal polymerization dates back to the 70 s (46) and has been employed in engineering, chemistry, and astrophysics (47–49), its use in drug delivery is recent (30). Frontal polymerization is a process of polymerization whereby the reaction is initiated from one end of the sample and then it propagates throughout the sample. Frontal polymerization could be temperature-activated or photo-activated. After an initial ignition, hot polymerization front is formed and is self-sustaining with no further energy required once the reaction reaches a steady state converting monomer to polymer at a fast rate (30).
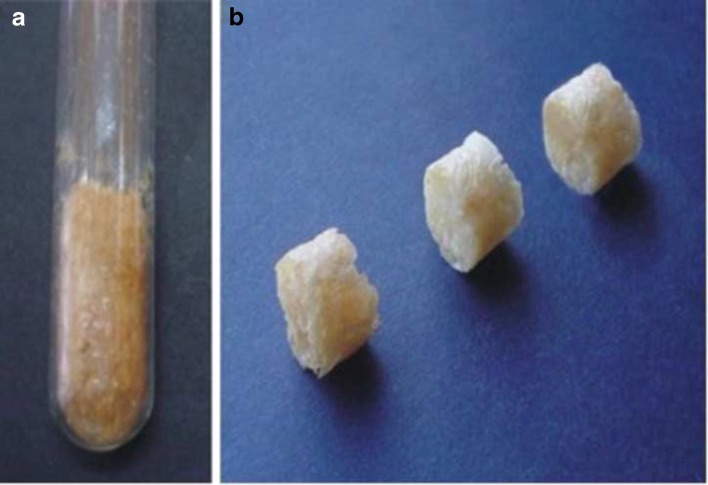
Gavin et al. employed acrylamide as the monomer and diclofenac sodium as the model drug. Frontal polymerization was undertaken in glass tubes containing acrylamide, MBAAm as the cross-linker, peroxodisulfate as the initiator, and water as the solvent (30). The tubes were heated at the bottom level of the solution using a soldering iron as the heat source until the formation of a propagating front became apparent. The polymerization period only took a few minutes as compared with the batch polymerization which took 4 h. In batch polymerization, the tubes were immersed in an oil bath at 70°C. Cylindrical hydrogels (Fig. 6) were obtained which were divided into disks of uniform sizes and dried in an oven until constant weights were obtained. In the study, the hydrogels were characterized for morphology, drug content, loading and stability, swelling, and drug release. The results indicated that the lower the concentration of the cross-linker, the higher the drug content, drug loading capacity, and the degree of hydrogel swelling. The infrared spectroscopy confirmed the stability of diclofenac sodium during in situ polymerization while in vitro drug release profiles showed that 100% of the incorporated drug was released after 2 h (30). Diclofenac sodium loaded hydrogels obtained by frontal and batch polymerization exhibited similar properties; however, frontal polymerization was found to be faster and hence, more economical for developing controlled drug delivery systems.
Fig. 6.
Cylindrical hydrogel synthesized by frontal polymerization with acrylamide as monomer. a Hydrogel in a glass tube; b Hydrogel divided into three single-dosage units [Source: (30)]
Anionic Polymerization for Fabrication of Nanoparticles
Anionic polymerization is an addition polymerization which can occur when the monomers have anion stabilizing substituents such as phenyl, cyano, carbonyl, and vinyl groups. The cyano groups are quite efficient as stabilizing substituents such that even water can initiate the polymerization of cyanoacrylate. Mesiha et al. developed insulin-loaded poly(isobutylcyanoacrylate) nanoparticles by in situ anionic polymerization (28). Insulin solution (pH 7.42) was added to an aqueous solution of pluronic acid and 5% w/v citric acid; isobutylcyanoacrylate was added dropwise into the solution and agitated for 4 h at ambient temperature. Different concentrations of pluronic acid (at the lowest pH 1.7) were employed to attempt to reduce the average particle size of the nanoparticles below 100 nm in order to optimize bioavailability of insulin. The resultant dispersions after polymerization were neutralized with 1.0 N sodium hydroxide to pH 7.4. Nanoparticles with average particle size below 100 nm obtained in the presence of 2.5% pluronic acid were utilized for in vivo bioavailability studies in streptozocin-induced diabetic rats. Fast hypoglycemic effect was achieved 2 h after administration and was sustained over 40 h (28).
In Situ Micellar Copolymerization and Network Polymerization for Hydrogels
Micellar copolymerization is a process of modifying water soluble polymers to possess hydrophobic groups. It consists of copolymerizing a mixture of hydrophilic and hydrophobic monomers whereby the hydrophobic monomers are within the surfactant micelles and the hydrophilic monomers are within the aqueous continuous phase containing an initiator (50,51). In comparison with conventional methods, micellar coplymerization is a more efficient process of incorporating hydrophobic moieties to hydrophilic polymers (52). Furthermore, the method permits the manipulation of the monomer distribution which enables the production of a polymer with tailored properties (51). The fact that micellar copolymerization can be undertaken at low temperatures enables incorporation of proteins, polypeptides, and heat-labile drugs, whose stability and bioactivity can be augmented by mild encapsulating approach (52). Amphiphilic polymers thereby produced have extended-release properties due to the hydrophobic groups within the polymers (52).
Network polymerization produces a polymer network where all polymer chains are interconnected such that they can form one molecule due to high degree of cross-linking.
In situ micellar copolymerization and network polymerization were employed to modulate drug release to display zero-order kinetics (31). First, the hydrophobic monomers, n-N-alkylacrylamide was synthesized by the reaction of acryloyl chloride with the appropriate amine; then the cross-linker, N,N’-bisacryloyl-cystamine (BAC) was synthesized by the reaction of acryloyl chloride with cystamine. Next, the hydrophilic polymer, poly(N-isopropylacrylamide-co-sodium acrylate) (NIPA-co-SA) was synthesized by aqueous redox polymerization with MBAAm as cross-linker, tetramethylethylenediamine (TEMED), and ammonium persulfate as initiators. Finally, NIPA-co-SA-co-n-N-alkylacrylamide was synthesized by micellar copolymerization. Hydrophobic monomers, n-N-alkylacrylamide was stabilized with sodium dodecyl sulfate under agitation and temperature control to form a micelle solution into which aqueous solutions of various amounts of mixed comonomers (NIPA and SA), cross-linker (BAC), and TEMED were carefully added with stirring and N2 bubbling for 15 min. In this study, vitamin B12, progesterone, insulin, and interferon were chosen as model drugs; however, all drugs were loaded by solvent sorption methods while insulin and interferon were loaded during the polymerization process. The incorporation of the hydrophobic monomer improved the mechanical strength of the hydrophilic polymer. The amphiphilic polymer obtained was thermo- and pH-sensitive which would enable drugs such as vitamin B12, unstable in the gastric region, to be released more in the intestine. The polymer exhibited higher swelling ratio in neutral or basic media than in acidic media. Incorporating insulin during polymerization prevented the initial burst effect observed with solvent sorption method and extended zero-order release profile was obtained. In sum, the rate of release of all the drugs as envisaged decreased with increasing degree of network cross-linking (31).
CONCLUSION
In situ polymerization has shown to be a method that can be employed to improve oral delivery of drugs that otherwise could not be delivered optimally. It improves the mechanical strength of polymers while drug loading and rate of release can be optimized at the level of monomers and ultimately, bioavailability is enhanced. It has also shown to help prevent the degradation of drugs because they are entrapped within the network of the polymers and the polymers have the abilities to inhibit the activities of protein degrading enzymes and are environmentally sensitive in favor of the drugs. Apparently, in situ polymerization has not been extensively employed in development of oral delivery systems, but it shows promise of overcoming the challenges of oral route of administration.
REFERENCES
- 1.Chandy T, Sharma CP. Chitosan matrix for oral sustained delivery of ampicillin. Biomaterials. 1993;14(12):939–44. doi: 10.1016/0142-9612(93)90136-P. [DOI] [PubMed] [Google Scholar]
- 2.Ilium L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15(9):1326–31. doi: 10.1023/A:1011929016601. [DOI] [PubMed] [Google Scholar]
- 3.Tozaki H, Odoriba T, Okada N, Fujita T, Terabe A, Suzuki T, et al. Chitosan capsules for colon-specific drug delivery: enhanced localization of 5-aminosalicylic acid in the large intestine accelerates healing of TNBS-induced colitis in rats. J Control Release. 2002;82(1):51–61. doi: 10.1016/S0168-3659(02)00084-6. [DOI] [PubMed] [Google Scholar]
- 4.Trapani A, Lopedota A, Franco M, Cioffi N, Ieva E, Garcia-Fuentes M, et al. A comparative study of chitosan and chitosan/cyclodextrin nanoparticles as potential carriers for the oral delivery of small peptides. Eur J Pharm Biopharm. 2010;75(1):26–32. doi: 10.1016/j.ejpb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Gombotz WR, Wee S. Protein release from alginate matrices. Adv Drug Deliv Rev. 1998;31(3):267–85. doi: 10.1016/S0169-409X(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 6.Tønnesen HH, Karlsen J. Alginate in drug delivery systems. Drug Dev Ind Pharm. 2002;28(6):621. doi: 10.1081/DDC-120003853. [DOI] [PubMed] [Google Scholar]
- 7.Qurrat-ul-Ain, Sharma S, Khuller GK, Garg SK. Alginate-based oral drug delivery system for tuberculosis: pharmacokinetics and therapeutic effects. J Antimicrob Chemother. 2003;51(4):931–8. doi: 10.1093/jac/dkg165. [DOI] [PubMed] [Google Scholar]
- 8.Rafati H, Coombes AGA, Adler J, Holland J, Davis SS. Protein-loaded poly(-lactide-co-glycolide) microparticles for oral administration: formulation, structural and release characteristics. J Control Release. 1997;43(1):89–102. doi: 10.1016/S0168-3659(96)01475-7. [DOI] [Google Scholar]
- 9.Yin Win K, Feng S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26(15):2713–22. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 10.Chickering D, Jacob J, Mathiowitz E. Poly(fumaric-co-sebacic) microspheres as oral drug delivery systems. Biotechnol Bioeng. 1996;52(1):96–101. doi: 10.1002/(SICI)1097-0290(19961005)52:1<96::AID-BIT9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 11.Furtado S, Abramson D, Burrill R, Olivier G, Gourd C, Bubbers E, et al. Oral delivery of insulin loaded poly(fumaric-co-sebacic) anhydride microspheres. Int J Pharm. 2008;347(1–2):149–55. doi: 10.1016/j.ijpharm.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 12.Crespy D, Landfester K. Preparation of nylon 6 nanoparticles and nanocapsules by two novel miniemulsion/solvent displacement hybrid techniques. Macromol Chem Phys. 2007;208(5):457–66. doi: 10.1002/macp.200600487. [DOI] [Google Scholar]
- 13.Zhang H, Li S, Branford White CJ, Ning X, Nie H, Zhu L. Studies on electrospun nylon-6/chitosan complex nanofiber interactions. Electrochim Acta. 2009;54(24):5739–45. doi: 10.1016/j.electacta.2009.05.021. [DOI] [Google Scholar]
- 14.Choy YB, Choi H, Kim K. Uniform ethyl cellulose microspheres of controlled sizes and polymer viscosities and their drug-release profiles. J Appl Polym Sci. 2009;112(2):850–7. doi: 10.1002/app.29473. [DOI] [Google Scholar]
- 15.Conti S, Maggi L, Segale L, Ochoa Machiste E, Conte U, Grenier P, et al. Matrices containing NaCMC and HPMC: 1. Dissolution performance characterization. Int J Pharm. 2007;333(1–2):136–42. doi: 10.1016/j.ijpharm.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 16.Fu XC, Wang GP, Liang WQ, Chow MSS. Prediction of drug release from HPMC matrices: effect of physicochemical properties of drug and polymer concentration. J Control Release. 2004;95(2):209–16. doi: 10.1016/j.jconrel.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Hussain MA, Badshah M, Iqbal MS, Tahir MN, Tremel W, Bhosale SV, et al. HPMC-salicylate conjugates as macromolecular prodrugs: design, characterization, and nano-rods formation. J Polym Sci, A: Polym Chem. 2009;47(16):4202–8. doi: 10.1002/pola.23463. [DOI] [Google Scholar]
- 18.Eerikäinen H, Peltonen L, Raula J, Hirvonen J, Kauppinen E. Nanoparticles containing ketoprofen and acrylic polymers prepared by an aerosol flow reactor method. AAPS PharmSciTech. 2004;5(4):129–37. doi: 10.1208/pt050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta KA, Kislalioglu MS, Phuapradit W, Malick AW, Shah NH. Release performance of a poorly soluble drug from a novel, Eudragit®-based multi-unit erosion matrix. Int J Pharm. 2001;213(1–2):7–12. doi: 10.1016/S0378-5173(00)00594-9. [DOI] [PubMed] [Google Scholar]
- 20.Mahkam M. New terpolymers as hydrogels for oral protein delivery application. J Drug Target. 2009;17(1):29–35. doi: 10.1080/10611860802438728. [DOI] [PubMed] [Google Scholar]
- 21.Ramanan RMK, Chellamuthu P, Tang L, Nguyen KT. Development of a temperature-sensitive composite hydrogel for drug delivery applications. Biotechnol Prog. 2006;22(1):118–25. doi: 10.1021/bp0501367. [DOI] [PubMed] [Google Scholar]
- 22.Lu S, Anseth KS. Photopolymerization of multilaminated poly(HEMA) hydrogels for controlled release. J Control Release. 1999;57(3):291–300. doi: 10.1016/S0168-3659(98)00125-4. [DOI] [PubMed] [Google Scholar]
- 23.Krause H, Schwarz A, Rohdewald P. Interfacial polymerization. A useful method for the preparation of polymethylcyanoacrylate nanoparticles. Drug Dev Ind Pharm. 1986;12(4):527–52. doi: 10.3109/03639048609048026. [DOI] [Google Scholar]
- 24.Krauel K, Davies NM, Hook S, Rades T. Using different structure types of microemulsions for the preparation of poly(alkylcyanoacrylate) nanoparticles by interfacial polymerization. J Control Release. 2005;106(1–2):76–87. doi: 10.1016/j.jconrel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Lee H, Hyung W, Park S, Haam S. Magnetic PECA nanoparticles as drug carriers for targeted delivery: synthesis and release characteristics. J Microencapsul Micro Nano Carriers. 2006;23(2):203–12. doi: 10.1080/02652040500435444. [DOI] [PubMed] [Google Scholar]
- 26.Ward JH, Peppas NA. Preparation of controlled release systems by free-radical UV polymerizations in the presence of a drug. J Control Release. 2001;71(2):183–92. doi: 10.1016/S0168-3659(01)00213-9. [DOI] [PubMed] [Google Scholar]
- 27.Elvira C, Mano JF, San Román J, Reis RL. Starch-based biodegradable hydrogels with potential biomedical applications as drug delivery systems. Biomaterials. 2002;23(9):1955–66. doi: 10.1016/S0142-9612(01)00322-2. [DOI] [PubMed] [Google Scholar]
- 28.Mesiha MS, Sidhom MB, Fasipe B. Oral and subcutaneous absorption of insulin poly(isobutylcyanoacrylate) nanoparticles. Int J Pharm. 2005;288(2):289–93. doi: 10.1016/j.ijpharm.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Feng S. Nanoparticles of poly(lactide)/vitamin E TPGS copolymer for cancer chemotherapy: synthesis, formulation, characterization and in vitro drug release. Biomaterials. 2006;27(2):262–70. doi: 10.1016/j.biomaterials.2005.05.104. [DOI] [PubMed] [Google Scholar]
- 30.Gavini E, Mariani A, Rassu G, Bidali S, Spada G, Bonferoni MC, et al. Frontal polymerization as a new method for developing drug controlled release systems (DCRS) based on polyacrylamide. Eur Polym J. 2009;45(3):690–9. doi: 10.1016/j.eurpolymj.2008.12.017. [DOI] [Google Scholar]
- 31.Yu H, Grainger DW. Modified release of hydrophilic, hydrophobic and peptide agents from ionized amphiphilic gel networks. J Control Release. 1995;34(2):117–27. doi: 10.1016/0168-3659(94)00127-G. [DOI] [Google Scholar]
- 32.Peppas NA, Keys KB, Torres-Lugo M, Lowman AM. Poly(ethylene glycol)-containing hydrogels in drug delivery. J Control Release. 1999;62(1–2):81–7. doi: 10.1016/S0168-3659(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 33.Madsen F, Peppas NA. Complexation graft copolymer networks: swelling properties, calcium binding and proteolytic enzyme inhibition. Biomaterials. 1999;20(18):1701–8. doi: 10.1016/S0142-9612(99)00071-X. [DOI] [PubMed] [Google Scholar]
- 34.Morishita M, Lowman AM, Takayama K, Nagai T, Peppas NA. Elucidation of the mechanism of incorporation of insulin in controlled release systems based on complexation polymers. J Control Release. 2002;81(1–2):25–32. doi: 10.1016/S0168-3659(02)00019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torres-Lugo M, García M, Record R, Peppas NA. pH-sensitive hydrogels as gastrointestinal tract absorption enhancers: transport mechanisms of salmon calcitonin and other model molecules using the caco-2 cell model. Biotechnol Prog. 2002;18(3):612–6. doi: 10.1021/bp0101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura K, Murray RJ, Joseph JI, Peppas NA, Morishita M, Lowman AM. Oral insulin delivery using P(MAA-g-EG) hydrogels: effects of network morphology on insulin delivery characteristics. J Control Release. 2004;95(3):589–99. doi: 10.1016/j.jconrel.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Foss AC, Peppas NA. Investigation of the cytotoxicity and insulin transport of acrylic-based copolymer protein delivery systems in contact with caco-2 cultures. Eur J Pharm Biopharm. 2004;57(3):447–55. doi: 10.1016/j.ejpb.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Feng S. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)–tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials. 2006;27(21):4025–33. doi: 10.1016/j.biomaterials.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Limmatvapirat S, Limmatvapirat C, Puttipipatkhachorn S, Nunthanid J, Luangtana-anan M, Sriamornsak P. Modulation of drug release kinetics of shellac-based matrix tablets by in-situ polymerization through annealing process. Eur J Pharm Biopharm. 2008;69(3):1004–13. doi: 10.1016/j.ejpb.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 40.Blanchette J, Peppas NA. Oral chemotherapeutic delivery: design and cellular response. Ann Biomed Eng. 2005;33(2):142–9. doi: 10.1007/s10439-005-8973-8. [DOI] [PubMed] [Google Scholar]
- 41.Mayo-Pedrosa M, Alvarez-Lorenzo C, Lacík I, Martinez-Pacheco R, Concheiro A. Sustained release pellets based on poly(N-isopropyl acrylamide): matrix and in situ photopolymerization-coated systems. J Pharm Sci. 2007;96(1):93–105. doi: 10.1002/jps.20708. [DOI] [PubMed] [Google Scholar]
- 42.Mayo-Pedrosa M, Cachafeiro-Andrade N, Alvarez-Lorenzo C, Martinez-Pacheco R, Concheiro A. In situ photopolymerization-coated pellets for pH-dependent drug delivery. Eur Polym J. 2008;44(8):2629–38. doi: 10.1016/j.eurpolymj.2008.05.023. [DOI] [Google Scholar]
- 43.Watnasirichaikul S, Davies N, Rades T, Tucker I. Preparation of biodegradable insulin nanocapsules from biocompatible microemulsions. Pharm Res. 2000;17(6):684–9. doi: 10.1023/A:1007574030674. [DOI] [PubMed] [Google Scholar]
- 44.Damgé C, Vranckx H, Balschmidt P, Couvreur P. Poly(alkyl cyanoacrylate) nanospheres for oral administration of insulin. J Pharm Sci. 1997;86(12):1403–9. doi: 10.1021/js970124i. [DOI] [PubMed] [Google Scholar]
- 45.Watnasirichaikul S, Rades T, Tucker I, Davies N. In-vitro release and oral bioactivity of insulin in diabetic rats using nanocapsules dispersed in biocompatible microemulsion. J Pharm Pharmacol. 2002;54(8):473–80. doi: 10.1211/0022357021778736. [DOI] [PubMed] [Google Scholar]
- 46.Tredici A, Pecchini R, Sliepcevich A, Morbidelli M. Polymer blends by self-propagating frontal polymerization. J Appl Polym Sci. 1998;70(13):2695–702. doi: 10.1002/(SICI)1097-4628(19981226)70:13<2695::AID-APP14>3.0.CO;2-E. [DOI] [Google Scholar]
- 47.Chekanov Y, Arrington D, Brust G, Pojman JA. Frontal curing of epoxy resins: comparison of mechanical and thermal properties to batch-cured materials. J Appl Polym Sci. 1997;66(6):1209–16. doi: 10.1002/(SICI)1097-4628(19971107)66:6<1209::AID-APP20>3.0.CO;2-V. [DOI] [Google Scholar]
- 48.Pojman JA, Gunn G, Patterson C, Owens J, Simmons C. Frontal dispersion polymerization. J Phys Chem B. 1998;102(20):3927–9. doi: 10.1021/jp9814911. [DOI] [Google Scholar]
- 49.Proietti N, Capitani D, Cozzolino S, Valentini M, Pedemonte E, Princi E, et al. In situ and frontal polymerization for the consolidation of porous stones: a unilateral NMR and magnetic resonance imaging study. J Phys Chem B. 2006;110(47):23719–28. doi: 10.1021/jp063219u. [DOI] [PubMed] [Google Scholar]
- 50.Candau F, Selb J. Hydrophobically-modified polyacrylamides prepared by micellar polymerization. Adv Colloid Interface Sci. 1999;79(2–3):149–72. doi: 10.1016/S0001-8686(98)00077-3. [DOI] [Google Scholar]
- 51.Kujawa P, Audibert-Hayet A, Selb J, Candau F. Compositional heterogeneity effects in multisticker associative polyelectrolytes prepared by micellar polymerization. J Polym Sci, A: Polym Chem. 2003;41(21):3261–74. doi: 10.1002/pola.10866. [DOI] [Google Scholar]
- 52.Yu H, Grainger DW. Amphiphilic thermosensitive N-isopropylacrylamide terpolymer hydrogels prepared by micellar polymerization in aqueous media. Macromolecules. 1994;27(16):4554–60. doi: 10.1021/ma00094a019. [DOI] [Google Scholar]