Abstract
Considering the advantageous for the rectal administration of non-steroidal anti-inflammatory drugs, the objective of this study was to formulate and evaluate rectal mucoadhesive hydrogels loaded with diclofenac-sodium chitosan (DFS-CS) microspheres. Hydroxypropyl methylcellulose (HPMC; 5%, 6%, and 7% w/w) and Carbopol 934 (1% w/w) hydrogels containing DFS-CS microspheres equivalent to 1% w/w active drug were prepared. The physicochemical characterization revealed that all hydrogels had a suitable pH for rectal application (6.5–7.4). The consistency of HPMC hydrogels showed direct proportionality to the concentration of the gelling agent, while carbopol 934 gel showed its difficulty for rectal administration. Farrow’s constant for all hydrogels were greater than one indicating pseudoplastic flow. In vitro drug release from the mucoadhesive hydrogel formulations showed a controlled drug release pattern, reaching 34.6–39.7% after 6 h. The kinetic analysis of the release data revealed that zero-order was the prominent release mechanism. The mucoadhesion time of 7% w/w HPMC hydrogel was 330 min, allowing the loaded microspheres to be attached to the surface of rectal mucosa. Histopathological examination demonstrated the lowest irritant response to the hydrogel loaded with DFS-CS microspheres in response to other forms of the drug.
Key words: carbopol 934, diclofenac sodium–chitosan microspheres, histopathological study, HPMC, rectal mucoadhesive hydrogel
INTRODUCTION
Rectal route offers a noninvasive and useful route of drug administration when systemic or local effects are required. The rectum offers a relatively constant environment for drug delivery that allows a constant steady-state concentration of drug in plasma and partially avoids hepatic first-pass effect or gastrointestinal drug absorption difficulties (1). Dosage forms designed for rectal administration should be non-irritant, possesses good retention in the lower region of the large intestine, and acceptable by patient. These requirements could be met by using hydrogels rather than rectal solutions that tend to leak out of the rectum, leading to inaccurate dosing and treatment failure (2).
Hydrogels are composed of three-dimensional network of hydrophilic polymer chains that could be cross-linked by chemical or physical bonding. They physically entrap drug molecules for subsequent slow drug release by diffusion or erosion depending on their state of hydration (3). For highly hydrated hydrogels, the drug diffusion occurs through the pores of the gel network, whereas, lower hydration allows drug to dissolve in the polymer and transport between the chains. The polymers cross-linking was reported to increase the hydrophobicity of the gel and diminish diffusion rate of the drug, whereas its soft rubbery nature minimizes mechanical and frictional irritation to the surrounding tissues (4). These characteristics of hydrogels, as well as their biocompatibility and increased duration of action, could be considered a potentially beneficial approach in increasing therapeutical efficiency of rectal forms of drugs (5).
The use of rectal suppository for sustaining drug action had been successfully reported with a number of drugs such as nifedipine (6), Zidovudine (7), morphine (8), paracetamol (9), metoclopramide (10), diclofenac sodium (11), and aminophylline (12).
Conventional suppository has the disadvantage of providing an alien feeling, discomfort, and lowering patient compliance especially when treating a chronic disease. Furthermore, a suppository reaching the end of the colon might lose part of its drug at the colonic level or expose the drug to undergo the first-pass effect (13).
Recently, liquid suppositories (hydrogels) that exist as liquid in vitro but gel in vivo have been proposed as alternatives to conventional suppositories (14). Ibuprofen, quinine, diazepam, and propranolol are examples of drugs presented as rectal hydogels to improve drug bioavailability or patient compliance (5,15–17).
Carbopol 934 and hydroxypropyl methylcellulose (HPMC) are commonly applied for sustaining the drug release from its dosage forms. Carbopol 934 is polyacrylic acid polymer with highly ionized carboxyl groups after neutralization that lead to gel formation due to the electrostatic repulsion among the charged polymer chains (18). On the other hand, HPMC is an attractive nonionic water-soluble cellulose ether derivative available in grades containing 16.5–30% of methoxy and 4.0–32.0% of hydroxypropoxy groups, with specified viscosities for specific concentrations.
A number of studies have been performed on diclofenac sodium using chitosan (CS) as a matrix that has been reticulated covalently or ionically with different cross-linking methods (19,20).
In our previous study, diclofenac sodium (DFS) has been formulated as CS microspheres through a coacervation technique (21). The rectal application of hydrogels loaded with diclofenac sodium microspheres to overcome the gastrointestinal (GIT) side effects of the drug was not previously reported. Therefore, the present study aimed to evaluate rectal application of mucoadhesive hydrogels as a delivery system for diclofenac sodium (DFS-CS) microspheres to achieve long-term plasma level along with the minimization to the drug irritant effect on GIT. The rheological behaviors, mucoadhesiveness, in vitro drug release of drug were studied. Additionally, histopathological study was conducted to evaluate the extent of rectal irritation to the applied formulation.
MATERIALS AND METHODS
Materials
Diclofenac sodium was kindly donated from Novartis Pharma CO. (Cairo, Egypt). Chitosan (with 85% degree of deacetylation) was obtained from Sigma Chemical Co. (St. Louis, USA). Hydroxypropyl methylcellulose with viscosity of 2% aqueous solution at 20°C: 3,500–5,600 centipoises was purchased from Tama (Tokyo, Japan). Carbopol 934 was obtained from BF. Goodrich (USA). Analytical grades of acetic acid (96%), sodium citrate, glycerin, propylene glycol, and triethanolamine were obtained from El-Nasr Pharmaceutical Chemical CO. (Cairo, Egypt).
Preparation of Chitosan Microspheres
Diclofenac sodium-loaded chitosan microspheres were prepared by the coacervation method previously described by Berthold et al. (19) with certain modifications in the type of cross-linking agent and the technique of addition.
An aqueous solution of 1% w/v diclofenac sodium and 5% w/v sodium citrate was prepared. The solution was sprayed into an equal volume of magnetically stirred solution of 0.5% w/v chitosan in acetic acid (2% v/v). The spraying was performed using an atomizer with 0.7 mm inner diameter at rate of 1 ml/min. The microspheres formation by ionic cross-linking was allowed for 3 h followed by filtration, washing with distilled water and then drying at 40°C. The applied method allowed the use of DFS solution instead of its dispersion in an acidic solution of CS. It also permitted the use of high concentration of polymer solution without the possibility of plugging the spray nozzle with the viscous polymer solution. Based on a previous study, the formula composed of 1% w/v DFS, 0.5% w/v CS and cross-linked with 5% w/v sodium citrate for 3 h, (mean particle size of 22 μm and drug content 66.6% w/w of microspheres weight) was selected as the optimum formula to be incorporated in the hydrogels (21).
Preparation of Hydrogels Loaded with DFS-CS Microspheres
Hydroxypropyl methylcellulose and Carbopol 934 hydrogels were prepared by slowly dispersing the polymer powders into amount of distilled water under constant stirring until no lumps were observed. Carbopol solution kept at ambient temperature for 24 h was neutralized with few drops of 0.5% w/v triethanolamine solution to allow gel formation. A weighed amount of DFS-CS microspheres equivalent to 10 mg drug/g gel was levigated into a specified amount of glycerin/propylene glycol mixture (1:2). The wetted microspheres were incorporated into hydrogels that was completed to 100 g with distilled water. Afterwards, the microspheres was uniformly distributed into the structured vehicle of hydrogels by gentle mixing with a magnetic stirrer overnight in a closed container until translucent gels were formed, so the final concentration of DFS in hydrogels was 1% w/w (5). All the prepared formulations were packed in polyethylene containers and stored at room temperature for subsequent use. The composition of the prepared formulae was given in Table I.
Table I.
Composition of the Hydrogels Formulae
| Polymer | Polymer weight (g) | Drug loaded microspherea (g) | Glycerin (g) | Propylene glycol (g) | Distilled water (g) |
|---|---|---|---|---|---|
| HPMC | 5 | 1.65 | 10 | 20 | Up to 100 |
| 6 | 1.65 | 10 | 20 | Up to 100 | |
| 7 | 1.65 | 10 | 20 | Up to 100 | |
| Carbopol | 1 | 1.65 | 10 | 20 | Up to 100 |
aThe weight of the microspheres was equivalent to 1 g of drug
Evaluation of Hydrogel Formulations
pH Measurement
The values of the hydrogels pH were determined by dispersing 1 g of each formulated gel in 30 ml distilled water and recorded by a digital pH meter (pH meter 3,310, Jenway, UK).
Homogeneity Measurement
The hydrogels were visually inspected for general appearance and presence of any aggregates after they had been set in their final containers.
Measurement of Strength and Consistency of Hydrogels
The gel strength was determined according to a method reported by Choi et al. (13). A sample of 50 g of prepared hydrogel was placed in a 100-ml graduated cylinder. A standard weight of 35 g was placed onto the hydrogel surface. The strength of gels was determined by measuring the time in seconds taken by the weight to penetrate 5 cm down through the gel. A range of 10–50 s was acceptable for rectal application. A time less than 10 s was considered to cause gel leakage out from the rectum, whereas more than 50 s would be too viscous for rectal administration.
Rheological Studies
The viscosity of hydrogels was determined using Brookfield viscometer (Brookfield viscometer, Model DV-III, programmable rheometer, spindles 40, USA) at room temperature. The angular velocity was changed from 0.5 to 110 rpm at a controlled ramp speed. The average of three readings was used to calculate the viscosity. Flow behavior was further analyzed by regression analysis of the log shear stress vs. log shear rate and the following equation was applied:
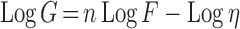 |
Where, G is shear rate (s−1), F is shear stress (dyne/cm2), η is viscosity, and n is the Farrow’s constant and is index of the deviation from Newtonian flow behavior. The more the value of n differs from unity, the more non-Newtonian is the flow behavior. For pseudoplastic flow, n > 1 while for dilatancy, n < 1.
In vitro Drug Release Studies
A modified method was adopted (22), where a known quantity of the hydrogel (1 g) was introduced into the glass tube of 2.5 cm diameter and 3 cm length opened from both ends. The lower end of the tube was tightly covered with a cellophane membrane (Spectrapor membrane tubing No. 2 Spectrum medical industries, USA) and the upper was hanged to the shaft of a USP dissolution apparatus I (Hanson Research Dissolution tester, Chatsworth, USA) rotating at 100 rpm. The tubes were adjusted so that the cellophane membrane was below the surface of 100 ml phosphate buffer (pH 6.8) at 37°C. Aliquots were taken at regular time intervals and replaced by an equal volume of prewarmed phosphate buffer. Withdrawn aliquots were diluted with phosphate buffer, filtered through microfilter (0.45 μm), and then analyzed spectrophotometrically (Lambda EZ 201, Perkin Elmer, USA) at 276 nm (23). All experiments were carried out in triplicates, and the mean values were presented. In vitro release study was also carried out with the selected hydrogel formula containing equivalent amount of pure drug for comparison.
To study the drug release mechanism, the release data were fitted to zero-order (Eq. 1), first-order (Eq. 2), and Higuchi model (Eq. 3). For further evaluation to the mechanism of drug release, Korsmeyer–Peppas model (Eq. 4) was used to describe the release behavior from polymeric systems.
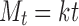 |
1 |
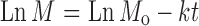 |
2 |
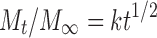 |
3 |
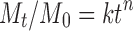 |
4 |
Where M0 is the initial amount of drug released at zero time and Mt is the amount of released at time t, n is a diffusion exponent characteristic of the release mechanism, and k is a kinetic constant characteristics for the drug/polymer. For Korsmeyer–Peppas equation, if the n value is equal to 0.5, the release mechanism is represented by Fickian diffusion whereas for n = 1, the release is zero-order. Values of 0.5 < n < 1 indicate non-Fickian or anomalous mechanism due to both drug diffusion and polymer chain relaxation (24).
Statistical Analysis
The Student t test was applied for comparing the release data of the drug after 6 h among the formulae containing different concentrations of HPMC as well as between the two types of polymers. A JMP software version 4.0.4 (SAS Institute, Cary, NC).
In vitro Mucoadhesion Performance of Hydrogels
The mucoadhesive properties of hydrogels loaded with DFS-CSS microspheres were determined according to the method reported by El-Samaligy et al. (25). The method was based on assessing the time required for detachment of the hydrogel spread on the rabbit rectum. Sections of the rabbit rectum tissues were surgically removed and placed in saline solution. The tissues were stored frozen in phosphate buffer (pH 7.4) and thawed to room temperature before use. At the time of testing, a section of rabbit rectum tissue was fixed with the mucosal surface outwards onto a glass beaker using a rubber band. A known quantity of hydrogel (1 g) was placed onto the rectal mucosa. The beaker was filled with 100 ml phosphate buffer pH 6.8 at 37°C and magnetically stirred at 100 rpm. The time for complete erosion of the hydrogels from the mucosal surface was determined visually and recorded as an indication of the in vitro adhesion time.
Histopathological Examination
The hydrogels loaded with DFS-CSS microspheres and pure drug were tested for abnormal irritability on the rat rectal mucosa. Fifteen male Wistar rats weighing 150–200 g each were divided into five groups (three animals per group). Group I was the control group and given a score of zero, whereas group II was for the pure drug powder and given a score of 5. Group III was for pure drug incorporated in a 7% w/w HPMC hydrogel. Group IV was for DFS-CSS microspheres. Group V was considered for 7% w/w HPMC hydrogel loaded with DFS-CSS microspheres equivalent to pure drug. The amount of drug for all tested groups was calculated to be equivalent to 1% w/w. The animals were fasted for 24 h prior to the experiment. After 8 h of rectal administration for the different dosage forms, the animals were killed, the rectal segments were isolated, and then immersed in 10% v/v formalin buffer for 24 h (the animal experiments were conducted in full compliance with local, national, ethical, and regulatory principles and local licensing regulations per the spirit of AAALAC International’s expectations for animal care and approved by the National Research Institute animal care committee in Egypt). The segments prepared for examination by washing with distilled water, ethyl alcohols for their dehydration, and stained by hematoxylin and eosin stains. A light microscope was used for histopathological abnormalities examination such as inflammatory cell infiltration. The abnormality was quantified on an arbitrary scale from 0 (no effect) to 5 (severe effect) (26).
RESULTS AND DISCUSSION
Evaluation of the Hydrogels Loaded with DFS-CSS Microspheres
The formulated hydrogels had pH values in range of 6.5–7.4, which were close to the pH of rectum (6.8). Hence, these values indicated the suitability of the hydrogels for rectal application with minimal risk of tissue irritation. Among the prepared formulae the HPMC hydrogels loaded with DFS-CS microspheres showed good homogeneity with absence of lumps compared to the Carbopol hydrogel.
The gels properties are important for rectal administration to allow retaining of the gel with no leakage from the anus (9). The strength is the ability of a material to withstand a load without rupture and was taken as a marker for the suitability of the gel viscosity for rectal administration. The measured gel strengths of the prepared formulae were presented in Table II. The strengths of HPMC hydrogels containing 5%, 6%, and 7% w/w were 40.8, 45.6, and 48 s, respectively. Carbopol hydrogel (1% w/w) showed a higher strength reaching 82.2 s. These values clearly revealed that the strength of HPMC hydrogels can be considered reasonable for rectal application, whereas, Carbopol gel was too far from the acceptable range (10–50 s).
Table II.
Physicochemical Properties of Hydrogels Loaded with Diclofenac Sodium/Chitosan Microspheres
| Hydrogels formulae | pH | Gel strength (time in sec) | Viscosity (cps) | Farrow’s constant (n) | Area of hysteresis loop (dyne/s cm2) | Flow behavior |
|---|---|---|---|---|---|---|
| 5% w/v HPMC | 6.5 ± 0.3 | 40.8 ± 2.3 | 93528 ± 235 | 1.12 | 2818.5 | Psuedoplastic with thixotropy |
| 6% w/v HPMC | 6.7 ± 0.05 | 45.6 ± 0.8 | 98242 ± 178 | 1.4 | 3298 | |
| 7%w/v HPMC | 6.8 ± 0. | 48 ± 0.6 | 113768 ± 67 | 1.19 | 3383.95 | |
| 1% w/v Carbopol | 7.4 ± 0. | 82.17 ± 1.2 | 100832 ± 548 | 1.12 | 3154.5 |
Mean values±SD, n = 3
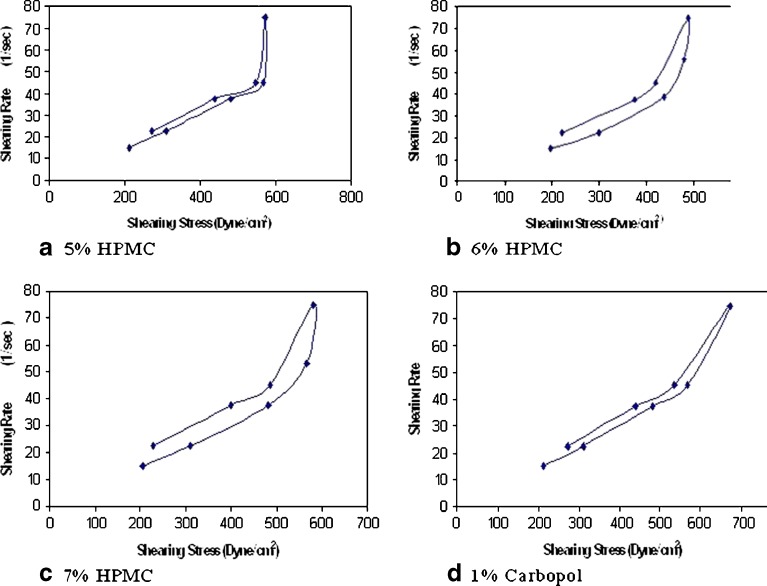
The viscosity of rectal hydrogels had an influence on the rate of drug release and distribution in the distal portion of the large intestine. In addition, the relative viscosity could provide some insight about the expected mucoadhesive strength of the gel and its retention time (27). The results presented in Table II showed that the viscosity of HPMC hydrogels was directly proportional to the polymer concentration and greatly increased with 7% w/w HPMC. The viscosities of 5%, 6% and 7% w/w HPMC hydrogels were 93,528, 98,242, and 113,768 cps, respectively, while that of 1% w/w Carbopol was 100,832 cps. Rheograms of the prepared gels were presented in Fig. 1a, b, c and d. The prepared formulations exhibited pseudoplastic rheology, as evidenced by shear thinning and an increase in the shear stress with increasing the angular velocity. The calculated values of Farrow’s constant (Table II) were greater than one, which confirmed the hydrogels pseudoplastic properties. This result correlated well with the behavior of HPMC hydrogels reported by Dodovi et al. (5). Upon comparing the different formulae, it was observed that the one prepared with 7% w/w HPMC had the highest shear thinning effect. In addition, the area of its hysteresis loop was the largest (3,384 dyn s−1 cm−2) indicating a better thixotropic behavior. Thus, this formula was selected for rectal administration and for further histopathological evaluation.
Fig. 1.
Rheological behavior DFS-microspheres loaded hydrogels prepared by: a 5% HPMC, b 6% HPMC, c 7% HPMC, and d 1% Carbopol
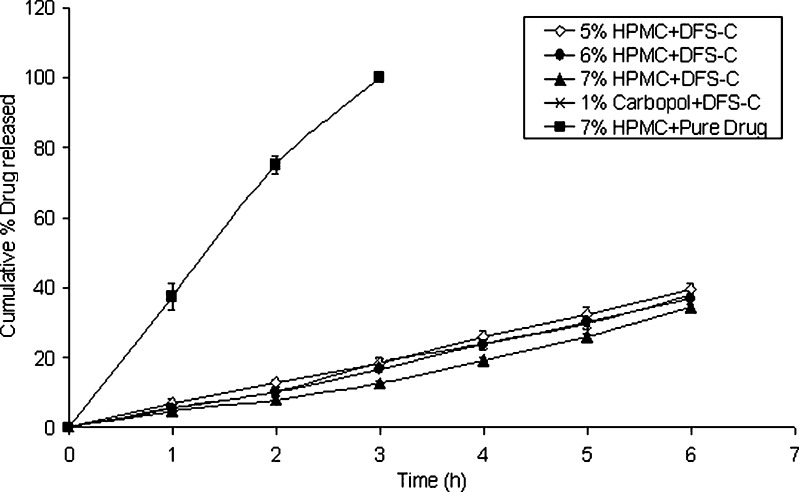
Release profiles of Diclofenac sodium from the hydrogels loaded with DFS-CS microspheres or the pure drug were presented in Fig. 2. The results clearly revealed that the hydrogel containing drug powder allowed for complete drug release (100%) within the first 3 h. Such results illustrated the inability of the hydrogel matrix by itself to control the drug release. On the other hand, the drug release from all hydrogel formulations loaded with DFS-CS microspheres showed a slower linear release with a cumulative percentage drug release reaching 34.6–39.7% after 6 h. This result showed the remarkable advantage of formulating the drug as CS microspheres in suppressing and controlling the DFS release from the hydrogel matrix. However, at the 6 h time interval, no significant effect was observed for the HPMC concentration or the polymer type on the cumulative drug release (p < 0.01). Bravo et al. (28) recorded a similar behavior for the HPMC concentration, where there was no significant difference in the release profiles with slight variations in the percent polymer used.
Fig. 2.
Release profiles of Diclofenac sodium from the hydrogels (each point presents the mean±SD)
The regression constants (r2) resulted from fitting the data to the different kinetic models were shown in Table III. The r2 of 5% and 6% HPMC were fitted to zero-order kinetics (0.997 and 0.992, respectively). Fitting of the drug release data for the 7%HPMC hydrogel to zero-order (r2 = 0.956) or first-order kinetics (r2 = 0.959) was good to almost the same extent. The increase in the polymer concentration might have led to a change in the release behavior from the matrix with more tendency to be dependent on the drug concentration. The hydrogel prepared with 1% Carbopol showed a controlled drug release pattern and high linearity with zero-order kinetic (r2 = 0.991). The values of exponent “n” obtained from plotting log cumulative percentage drug release vs log time (the Korsmeyer–Peppas equation) were equal to one, confirming the zero-order for the drug released from 5% to 6% HPMC hydrogels. For 7% HPMC hydrogel, the n value was 1.1, revealing a change in the mechanism of drug release to Super Case II transport. This result indicated that for 7% HPMC the release might be due to a combination of both drug diffusion and polymer relaxation. This type of release mechanism has also been reported by Sujja-areevath et al. (29).
Table III.
Kinetics Parameters of Hydrogel Formulations Loaded with DFS-CS Microspheres and Pure Drug
| Gel Formulae | Zero-order | First-order | Higuchi-model | Korsemeyer-model | ||
|---|---|---|---|---|---|---|
| r 2 | K (% h−1) | r 2 | r 2 | r 2 | n | |
| 5% HPMC+DFS-CS Microspheres | 0.9967 | 6.50 | 0.9879 | 0.9704 | 0.9957 | 0.971 |
| 6% HPMC+DFS-CS Microspheres | 0.9921 | 5.97 | 0.9906 | 0.7622 | 0.992 | 1.077 |
| 7% HPMC+DFS-CS Microspheres | 0.9557 | 5.20 | 0.9593 | 0.926 | 0.979 | 1.11 |
| 1% Carbopol+DFS-CS Microspheres | 0.9911 | 6.10 | 0.9866 | 0.9713 | 0.993 | 1.069 |
| 7% HPMC+drug powder | 0.9713 | 34.84 | – | 0.858 | 0.9927 | 0.905 |
r 2 coefficient of determination, K rate constant according to zero-order analysis, n diffusional exponent Krosemeyer model indicative of the mechanism of drug release
aAnalyzed by the regression coefficient method
The K values of the zero-order kinetic analysis were 6.498, 5.972, and 5.201 (% drug released/h) form the hydrogels containing 5%, 6%, and 7% HPMC, respectively. This reflects a slight decrease in the drug release rate with increasing the HPMC concentration, in turn, the matrix viscosity.
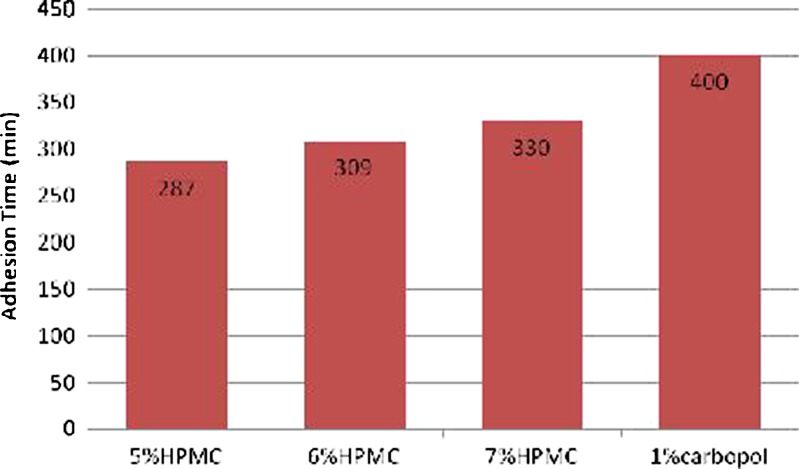
Figure 3 presented the bioadhesion time taken by gel formulae to erode from the rectal mucosal tissue. It was observed that Carbopol had the highest in-vitro adhesion time (400 min), whereas, the adhesion time of HPMC hydrogels was 287, 309, and 330 min for 5%, 6%, and 7% w/w, respectively. The higher the polymer concentration is, the higher the bioadhesion time of the cellulose derivatives. The reinforcement of the mucoadhesive forces was attributed to the presence of secondary bond forming groups such as hydroxyl, ether oxygen or amine groups that increase with increasing the HPMC concentration (30). The high residence time shown by the prepared hydrogels gave a chance to the loaded microspheres to be attached to the mucosal surface.
Fig. 3.
The in vitro adhesion time of the hydrogels loaded with DFS-C microspheres
The histopathological examination was performed to study the effect of DFS as a powder and in CS microspheres on the rat rectal mucosa when applied as free forms or incorporated in 7% w/w HPMC hydrogel.
Visual inspection of the rectal mucosa of the rat groups 8 h after administration of the hydrogel formulae revealed attachment of a part of the inserted dose to the mucosa. This adhesion property of the hydrogels to the site of application prevented the hydrogels from reaching the upper part of the rectum, thus, considered an advantage in avoiding the first-pass effect.
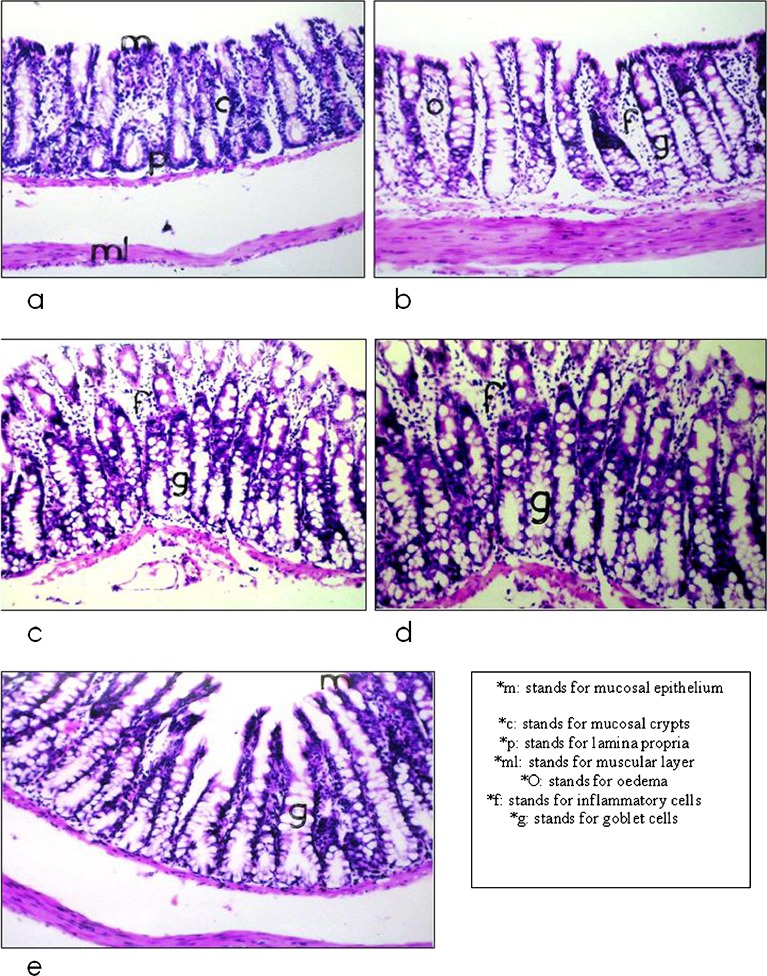
The results of the histopathological evaluation were shown in Table IV and Fig. 4. Histopathological findings of Group I (untreated) showed a normal rectal mucosa (Fig. 4a) with a series of small longitudinal folds of large and broad mucosal crypts. The mucosal layer was also lined with simple columnar epithelial cells containing few amounts of goblet cells. Furthermore, an adequate number of invading lymphocytes was present between the columnar cells. For group II (administered pure drug), the mucosal layer was characterized by the presence of goblet cells hyperplasia in a diffuse manner, which was associated with edema and massive number of inflammatory cells infiltration in the lamina propria (Fig. 4b). These results may be attributed to the highest inflammatory response caused by the DFS crystal structure.
Table IV.
Histopathological Effects of the Different Formulae of Diclofenac Sodium in on the Rat Rectal Mucosa
| Group code | I | II | III | IV | V |
|---|---|---|---|---|---|
| Formula administered | No administration | DFS | DFS incorporated in 7%(w/w) HPMC hydrogel | DFS loaded CS microspheres | DFS-CS microspheres incorporated in 7% (w/w) HPMC hydrogel |
| Mucosal layer | Mild goblet cells | Hyperplasia, goblet cells | diffusion of goblet cells | Heavy goblet cells | Mild goblet cells |
| Lamina propria | No goblet cell diffusion | Inflammatory cells and oedema | Infiltration of inflammatory cells | Mild inflammatory cells without diffusion | No inflammatory cells |
| Grade of irritation | 0 | 5 | 4 | 3 | 2 |
Fig. 4.
Histopathological examination of a untreated rat rectal mucosa in comparison to 8 h after administration of b DFS powder, c DFS powder incorporated in 7%HPMC hydrogel, d DFS-CS microspheres, and e 7%HPMC hydrogel loaded with DFS-C microspheres
On the other hand, Fig. 4c exhibited reactions occurred in rectal mucosa after the application of drug powder incorporated into 7% w/w HPMC rectal hydrogel. The examination revealed the diffusion of goblet cells hyperplasia in the mucosal layer with the infiltration of few mononuclear leucocytes inflammatory cells all over the lamina propria layer. Group IV was given the drug loaded within CS microspheres. The histopathological examination (Fig. 4d) showed a heavy diffusion of goblet cells hyperplasia in the mucosal layer with the absence of inflammatory cells. Examination of rectal mucosa of group V that administered DFS-CS microspheres incorporated in 7% w/w HPMC hydrogel was presented in Fig. 4e. The mucosal layer was lined by columnar epithelial cells forming mucosal crypts with few goblet cells formation. The underlying lamina propria showed no inflammatory cells infiltration and the muscular layer was intact.
Table IV summarizes the histopathological findings associated with the rat rectal mucosa in response to administered formulae of the Diclofenac sodium. The results clearly revealed that rectal administration of the drug in the form of DFS-CS microspheres incorporated in 7%HPMC hydrogel gave the mildest histological changes to rectal tissues and was given score of 2. However, group II administered drug powder showed the most severe changes in the tissues and was given score of 5. Groups III and IV were given scores of 4 and 3, respectively.
CONCLUSION
The HPMC and carbopol 934 hydrogels containing DFS-CS microspheres have been prepared and evaluated. The formula of 7% w/w HPMC loaded with DFS-CS microspheres showed adequate rheological characteristics and mucoadhesive properties. The suggested formula allowed the adhesion of the microspheres loaded with the drug to the rectal mucosa for subsequent controlled release behavior with no burst effect. Histopathological evaluation of the selected formula revealed the effectiveness of encapsulation of anti-inflammatory drugs within chitosan microspheres prior to their incorporation into the hydrogel delivery system in reducing the irritation to the mucosal tissues.
Acknowledgments
The authors would like to thank Dr. Kholosy A. B., Professor of Pathology, Cairo University for his support.
References
- 1.Matsuda H, Arima H. Cyclodextrins in transdermal and rectal delivery. Adv Drug Deliv Rev. 1999;36:81–99. doi: 10.1016/S0169-409X(98)00056-8. [DOI] [PubMed] [Google Scholar]
- 2.Uchida T, Toida Y, Sakakibara S, Miyanaga Y, Tanaka H, Nishikata M, et al. Preparation and characterization of insulin-loaded acrylic hydrogels containing absorption enhancers. Chem Pharm Bull Tokyo. 2001;49:1261–6. doi: 10.1248/cpb.49.1261. [DOI] [PubMed] [Google Scholar]
- 3.Peppas NA, Buri PA. Surface interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Control Release. 1985;2:257–75. doi: 10.1016/0168-3659(85)90050-1. [DOI] [Google Scholar]
- 4.Nagaoka S, Akashi R. Low friction hydrophilic surface for medical devices. Biomaterials. 1990;11:419–24. doi: 10.1016/0142-9612(90)90098-B. [DOI] [PubMed] [Google Scholar]
- 5.Dodov MG, Goracinova K, Simonoska M, Trajkovic-Jolevska S, Ribarska JT, Mitevska MD. Formulation and evaluation of diazepam hydrogel for rectal administration. Acta Pharm. 2005;55:251–61. [PubMed] [Google Scholar]
- 6.Kurosawa N, Owada E, Ito K, Ueda K, Takahashi A, Kikuiri T. Bioavailability of nifedipine suppository in healthy subjects. Int J Pharm. 1985;27:81–8. doi: 10.1016/0378-5173(85)90187-5. [DOI] [Google Scholar]
- 7.Kawaguchi T, Hasegawa T, Juni K, Seki T. Rectal absorption of Zidovudine. Int J Pharm. 1991;77:71–4. doi: 10.1016/0378-5173(91)90303-6. [DOI] [Google Scholar]
- 8.Morgan DJ, McCormick Y, Cosolo W, Roller L, Zalcberg J. Prolonged release of morphine alkaloid from a lipophilic suppository base in vitro and in vivo. Int J Clin Pharmacol Ther Toxicol. 1992;30:576–81. [PubMed] [Google Scholar]
- 9.Kim C, Lee S, Choi H, Lee M, Gao Z, Kim I, et al. Trials of in situ-gelling and mucoadhesive acetaminophen liquid suppository in human subjects. Int J Pharm. 1998;174:201–7. doi: 10.1016/S0378-5173(98)00258-0. [DOI] [Google Scholar]
- 10.Schneeweis A, Müller-Goymann CC. Controlled release of solid-reversed-micellar-solution (SRMS) suppositories containing metoclopramide-HCl. Int J Pharm. 2000;196:193–6. doi: 10.1016/S0378-5173(99)00419-6. [DOI] [PubMed] [Google Scholar]
- 11.Azechi Y, Ishikawa K, Mizuno N, Takahashi K. Sustained release of diclofenac from polymer-containing suppository and the mechanism involved. Drug Dev Ind Pharm. 2000;26:1177–83. doi: 10.1081/DDC-100100989. [DOI] [PubMed] [Google Scholar]
- 12.Shiohira H, Fujii M, Koizumi N, Kondoh M, Watanabe Y. Novel chronotherapeutic rectal aminophylline delivery system for therapy of asthma. Int J Pharm. 2009;379:119–24. doi: 10.1016/j.ijpharm.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Hk C, Jung JH, Ryu JM, Yoon SJ, Oh YK, Kim CK. Development of in-situ gelling and mucoadhesive acetaminophen liquid suppository. Int J Pharm. 1998;165:33–44. doi: 10.1016/S0378-5173(97)00386-4. [DOI] [Google Scholar]
- 14.Yun M, Choi H, Jung J, Kim C. Development of a thermo-reversible insulin liquid suppository with bioavailability enhancement. Int J Pharm. 1999;189:137–45. doi: 10.1016/S0378-5173(99)00227-6. [DOI] [PubMed] [Google Scholar]
- 15.Fawaz F, Koffi A, Guyot M, Millet P. Comparative in vitro—in vivo study of two quinine rectal gel formulations. Int J Pharm. 2004;280:151–62. doi: 10.1016/j.ijpharm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Yong CS, Yang CH, Rhee J, Lee B, Kim D, Kim D, et al. Enhanced rectal bioavailability of ibuprofen in rats by poloxamer 188 and menthol. Int J Pharm. 2004;269:169–76. doi: 10.1016/j.ijpharm.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Ryu J, Chung S, Lee M, Kim C, Shim C. Increased bioavailability of propranolol in rats by retaining thermally gelling liquid suppositories in the rectum. J Control Release. 1999;59:163–72. doi: 10.1016/S0168-3659(98)00189-8. [DOI] [PubMed] [Google Scholar]
- 18.French DL, Häglund BO, Himmelstein KJ, Mauger JW. Controlled release of substituted benzole and naphthoic acids using carbopol® gels: measurement of drug concentration profiles and correlation to release rate kinetics. Pharm Res. 1995;12:1513–20. doi: 10.1023/A:1016295723253. [DOI] [PubMed] [Google Scholar]
- 19.Berthold A, Cremer K, Kreuter J. Preparation and characterization of chitosan microspheres as drug carrier for prednisolone sodium phosphate as model for anti-inflammatory drugs. J Contemp Relig. 1996;39:17–25. [Google Scholar]
- 20.Gonçalves VL, Laranjeira MCM, Fávere VT, Polímeros Effect of crosslinking agents on chitosan microspheres in controlled release of diclofenac sodium. Ciênc Tecnol. 2005;15(1):6–12. [Google Scholar]
- 21.El-Leithy ES, Shaker DS, Ghorab MK, Abdel-Rashid D. Optimization and characterization of Diclofenac sodium microspheres prepared by modified coacervation. Drug Discov Ther. 2010;4(3):208–16. [PubMed] [Google Scholar]
- 22.Abd El Hady SS, Mortada ND, Awad GA, Zaki NM, Taha RA. Development of in situ gelling and mucoadhesive mebeverine hydrochloride solution for rectal administration. Saudi Pharm J. 2003;11(4):159–71. [Google Scholar]
- 23.Gupta KC, Ravi Kumar MNV. Drug release behavior of beads and microgranules of chitosan. Biomaterials. 2000;21:1115–9. doi: 10.1016/S0142-9612(99)00263-X. [DOI] [PubMed] [Google Scholar]
- 24.Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC) Adv Drug Deliv Rev. 2001;48:139–57. doi: 10.1016/S0169-409X(01)00112-0. [DOI] [PubMed] [Google Scholar]
- 25.El-Samaligy MS, Yahia SA, Basalious EB. Formulation and evaluation of diclofenac sodium buccoadhesive discs. Int J Pharm. 2004;286:27–39. doi: 10.1016/j.ijpharm.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 26.van Hoogdalem EJ, Vermeij-Kerrs C, de Boer AG, Breimer DD. Topical effects of absorption enhancing agents on the rectal mucosa of rats in vivo. J Pharm Sci. 1990;79:866–70. doi: 10.1002/jps.2600791004. [DOI] [PubMed] [Google Scholar]
- 27.Hassan EE, Gallo JM. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharm Res. 1990;7:491–5. doi: 10.1023/A:1015812615635. [DOI] [PubMed] [Google Scholar]
- 28.Bravo SA, Lamas MC, Salomón CJ. In-vitro studies of diclofenac sodium controlled-release from biopolymeric hydrophilic matrices. J Pharm Pharm Sci. 2002;5:213–9. [PubMed] [Google Scholar]
- 29.Sujja-areevath J, Munday DL, Cox PJ, Khan KA. Release characteristics of diclofenac sodium from encapsulated natural gum mini-matrix formulations. Int J Pharm. 1996;139:53–62. doi: 10.1016/0378-5173(96)04573-5. [DOI] [Google Scholar]
- 30.Hunt G, Kearney P, Kellaway IW. Mucoadhesive polymers in drug delivery systems. In: Johnson P, Lloyd-Jones JG, editors. Drug delivery systems: fundamentals and techniques. Ann Arbor: VCH; 1987. pp. 180–99. [Google Scholar]