Abstract
This work aimed to develop a fast-dissolving film made of low dextrose equivalent maltodextrins (MDX) containing nicotine hydrogen tartrate salt (NHT). Particular attention was given to the selection of the suitable taste-masking agent (TMA) and the characterisation of the ductility and flexibility under different mechanical stresses. MDX with two different dextrose equivalents (DEs), namely DE 6 and DE 12, were selected in order to evaluate the effect of polymer molecular weight on film tensile properties. The bitterness and astringency intensity of NHT and the suppression effect of several TMA were evaluated by a Taste-Sensing System. The films were characterised in term of NHT content, tensile properties, disintegration time and drug dissolution test. As expected, placebo films made of MDX DE 6 appeared stiffer and less ductile than film prepared using MDX DE 12. The films disintegrated within 10 s. Among the tested TMA, the milk and mint flavours resulted particularly suitable to mask the taste of NHT. The addition of NHT and taste-masking agents affected film tensile properties; however, the effect of the addition of these components can be counterweighted by modulating the glycerine content and/or the MDX molecular weight. The feasibility of NHT loaded fast-dissolving films was demonstrated.
KEY WORDS: fast-dissolving film, maltodextrin, nicotine, plasticiser, tensile property
INTRODUCTION
Fast-dissolving oral delivery systems are solid dosage forms which disintegrate or dissolve rapidly (<1 min) when placed in the mouth, without drinking or chewing. In the development of these dosage forms, the main critical issues are represented by dissolution in the oral cavity, tensile properties required for packaging and handling procedures and taste masking. Maltodextrines (MDX) are water-soluble biopolymers containing linear amylase, branched amylopectin and a relatively small amount of dextrose and maltose. MDX is a non-sweet, nutritive saccharide mixture of polymers consisting of d-glucose units, with a dextrose equivalent (DE) less than 20 (1). It is prepared by the partial hydrolysis of a food grade starch with suitable acids and/or enzymes. DE is defined as a measure of the total reducing power of all sugars present in the hydrolysate material relative to glucose as 100 and expressed on a dry weight basis; DE is an indicator of the degree of depolymerisation of starch: the lower the average molecular weight, the higher the DE value of MDX. Several physical and functional characteristics are influenced by the DE value (2). The solubility, sweetness and hygroscopicity increase with increasing DE, whereas the viscosity (3,4), the anti-crystallising power and the freezing temperature decrease as the DE increases.
Recently, the use of MDX with a DE equal to 12 plasticised with 16–20% w/w glycerine was proposed to produce fast-dissolving films (5). In the same work, it was found that the loading of an active ingredient, such as piroxicam, can modify the film tenacity. Actually, the effect of the addition of taste-masking agents (TMA), namely sweeteners and flavours, on the mechanical characteristics of the fast-dissolving films, is scantly investigated, and no information is available.
This work aimed to investigate the effects of such formulation variables (MDX molecular weight and TMA) on the fast-dissolving film performances measured in terms of disintegration time and tensile properties. Nicotine was selected as model drug since it is well-known as astringent and bitter drug. The fast dissolving films were loaded with 0.5 mg nicotine. Preliminary, suitable flavours were selected using a human taste panel and an electronic tongue.
MATERIALS AND METHODS
Materials
Two types of maltodextrin having a DE equal to 12 (Glucidex® IT12, MDX12) or 6 (Glucidex® IT6, MDX6) were obtained by Roquette (F). Nicotine (N) and (−)-nicotine hydrogen tartrate (NHT) were supplied from Sigma–Aldrich (I). Sorbitan oleate (Span® 80, SO) was purchased by Uniqema (UK). Glycerol (GLY) and citric acid were obtained by Carlo Erba Reagenti (I). Mint, milk and liquorice flavours were kindly gifted by Kerry Ingredients & Flavours (I).
All solvents were of analytic grade, unless specified.
Film Preparation
Films were prepared by the solvent casting technique described in a previous work (5). Briefly, MDX, GLY and SO were dispersed in water at 80°C. Afterwards, the dispersion was cooled down to 40°C and, if required, the active ingredient and/or flavours were added in the specific proportion (Table I). The amount of water was adjusted in order to obtain a viscosity measured at 50 s−1 shear rate and 25°C of about 20 Pa (VT500, Hakee, I).
Table I.
Composition (%, w/w) of the Placebo, N and NHT-Loaded Films
| Form. | N | NHT | MDX12 | MDX6 | CA | GLY | SO | MT | MK |
|---|---|---|---|---|---|---|---|---|---|
| 1 | – | – | 82 | – | – | 18 | – | – | – |
| 2 | – | – | 79 | – | – | 18 | 3 | – | – |
| 3 | – | – | – | 82 | – | 18 | – | – | – |
| 4 | – | – | – | 79 | – | 18 | 3 | – | – |
| 5 | – | – | – | 80 | – | 20 | – | – | – |
| 6 | – | – | – | 78 | – | 22 | – | – | – |
| 7 | – | – | – | 76 | – | 24 | – | – | – |
| 8 | 0.5 | – | 77.5 | – | 1 | 18 | 3 | – | – |
| 9 | 0.5 | – | 80.5 | – | 1 | 15 | 3 | – | – |
| 10 | 0.5 | – | – | 77.5 | 1 | 18 | 3 | – | – |
| 11 | 0.5 | – | – | 80.5 | 1 | 15 | 3 | – | – |
| 12 | – | 1.5 | 80.5 | – | – | 15 | 3 | – | – |
| 13 | – | 1.5 | 74.5 | – | – | 15 | 3 | 6 | – |
| 14 | – | 1.5 | 74.5 | – | – | 15 | 3 | – | 6 |
| 15 | – | 1.5 | 74.5 | – | – | 15 | 3 | 3 | 3 |
| 16 | – | 1.5 | – | 80.5 | – | 15 | 3 | – | – |
| 17 | – | 1.5 | – | 74.5 | – | 15 | 3 | 6 | – |
| 18 | – | 1.5 | – | 74.5 | – | 15 | 3 | – | 6 |
| 19 | – | 1.5 | – | 74.5 | – | 15 | 3 | 3 | 3 |
N nicotine, NHT (–)-nicotine hydrogen tartrate, MDX12 maltodextrin with a DE of 12, MDX6 maltodextrin with a DE of 6, CA citric acid, GLY glycerol, SO sorbitan oleate, MT mint flavour, MK milk flavour
After a rest period of at least 24 h, the dispersion was cast over a silicone release liner by a laboratory-coating unit Mathis LTE-S(M) (CH). Operative conditions: coating rate 1 m/min; drying temperature 100°C; drying time 3 min; air circulation speed 1,200 rpm. These conditions were set to obtain films having a thickness of about 100 μm (MI 1,000 μm, ChemInstruments, USA).
Films were cut into suitable shape and size as required for testing, packed in individual air-tight seal packs immediately after preparation and stored at 25°C until use.
Tensile Properties
Tensile properties were determined using a texture analyzer AG/MC1 (Acquati, I), equipped with a 5-DaN load cell. The film was cut into 100 × 12.5 mm strips and equilibrated at 25°C for 1 week. Tensile tests were performed according to ASTM International Test Method for Thin Plastic Sheeting (D 882-02) as previously described (5). A variety of parameters was calculated from the texture profiles: tensile strength, percent elongation at break; elastic modulus or Young’s modulus and tensile energy to break.
An average of five measurements was taken for each specimen.
Stickiness Determination
The film stickiness was evaluated according to the texture method usually used for the measurement of the tack of pressure sensitive adhesives (6), using a dynamometer (AG/MC, Acquati, I) equipped with a 5-DaN cell.
Drug Content
A 6-cm2 sample was dissolved in an appropriate amount of the mobile phase, and the solution was filtered (Durapore® membrane, pore size 0.45 μm; Millex GV, Millipore Corporation, USA) and the active ingredient assayed by HPLC equipped with a Diode array UV–VIS detector (HP 1100, Chemstation, Hewlett Packard, USA) by adapting the Ph. Eur. 6.4 method reported below. A 25-μL sample was injected at room temperature on a reverse phase column (LiCrospher 100RP-18E, 125 × 4 mm-5 μm), and the flow rate was set at 1.5 mL/min. The mobile phase was prepared as follow: acetonitrile and 0.1 M potassium dihydrogen phosphate solution were mixed at the ratio 25:75 (%, v/v) and 2.3 g/L sodium lauryl sulphate was added. The drug content was determined at 254 nm. A standard calibration curve represented by five known concentrations of NHT ranging from 10 to 100 μg/mL (r2 = 0.999) was used.
The results were expressed as mean of three determinations.
Disintegration Test
Disintegration test was performed in a Pharmacopoeia disintegrating test apparatus following the specifications for orodispersible tablet reported in Ph. Eur. 6.4 Ed. (2.9.1) by using 6-cm2 samples.
In Vitro Dissolution Test
The in vitro dissolution test was performed in a Ph. Eur. 6.4 Ed. paddle dissolution apparatus; 3 × 2 cm samples of the NHT loaded films, containing about 1.5 mg NHT, were exactly weighed in order to assure the sink condition. The dissolution medium consisted in 300 mL freshly deionized water, maintained at 37 ± 1°C and stirred at 50 rpm. NHT concentrations were assayed spectrophotometrically at 259 nm (DU-640 Beckman Coulter, I). The results were expressed as the average of three determinations.
Taste Evaluation
Human Taste Panel
The study, approved by Ethical Committee of the Università degli Studi di Milano (protocol n. 160310/4), was conducted in accordance with the ethical principles originating from the Declaration of Helsinki and followed the ICH-GCP guidelines of the 17-01-1997 and was in compliance with local regulatory requirements. All subjects were completely informed concerning the pertinent details and the purpose of the study. A written consent form was supplied, understood and signed by each subject prior to tasting test samples.
Samples of 1 mL for each solution (Table II) were randomly tasted by ten healthy volunteers. All samples were kept in the mouth for 15 s, afterwards subjects gargled well and waited for at least 15 min before tasting the next sample. After tasting, the volunteers were asked to value bitterness and irritating sensation of solutions using a score from 0 to 4. A score of zero corresponded to a low bitterness degree and palatable taste, while a score of four indicated high bitterness and high irritation.
Table II.
Composition (% w/v) of Aqueous Solutions Tasted by the Human Taste Panel
| Solutions | NHT | MK | MT | LQ |
|---|---|---|---|---|
| A | 0.15 | – | – | – |
| B | 0.15 | 0.60 | – | – |
| C | 0.15 | – | 0.60 | – |
| D | 0.15 | 0.30 | 0.30 | – |
| E | 0.15 | – | – | 0.60 |
| F | 0.15 | – | 0.30 | 0.30 |
NHT (–)-nicotine hydrogen tartrate, MT mint flavour, MK milk flavour, LQ liquorice flavour
In Vitro Tasting System
Analyses were performed with the Taste-Sensing System SA 402B (Intelligent Sensor Technology Co. Ltd, J), namely Electronic Tongue (ET). The detecting part of the system consists of sensors whose surface is attached with artificial lipid membranes having different response properties to chemical substances on the basis of their taste. For the present work, four detecting sensors were separated into two groups according to the membrane charge: two consisted of positively charged membranes (C00 and AE1) and two of negatively membranes (AC0 and AN0). The sensor C00 was specific for bitterness and acidic bitterness, the sensor AE1 was specific for astringency. The sensors AC0 and AN0 were both specific for basic bitterness. Two reference electrodes (Ag/AgCl) were also used.
The measurement principle of the electronic tongue is based on the capacity of taste substances to change the electric potential of the detecting sensors through electrostatic or hydrophobic interaction with the hydrophilic and hydrophobic groups of the lipid membranes. The response of each sensor, recorded as the difference between the potential detected by the sensor electrode and the potential of the reference electrode (Ag/AgCl), is elaborated by a computer and processed via a pattern recognition system.
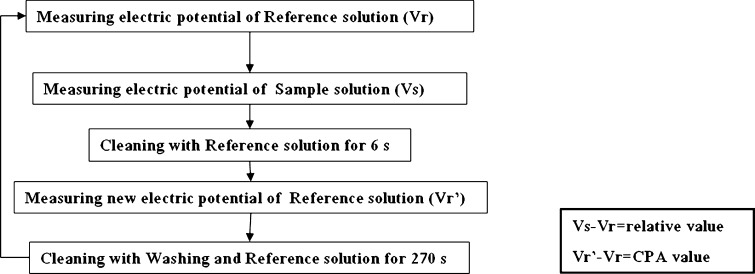
Figure 1 shows the measuring process applied in this study. Detecting sensors and reference electrodes were firstly dipped into the reference solution (30 mM potassium chloride and 0.3 mM tartaric acid), and the electric potential measured for each sensor was defined as Vr. Then, the sensors were dipped for 30 s into the sample solution. For each sensor, the measured potential was defined as Vs. For each sensor, the “relative value” was represented by the difference (Vs–Vr) between the potential of the sample and the reference solution. Sensors were rinsed with fresh reference solution for 6 s and then dipped into the reference solution again. The new potential of the reference solution was defined as Vr′. For each sensor, the difference (Vr′–Vr) between the potentials of the reference solution before and after sample measurement is the CPA (Change of Membrane Potential caused by Absorption) value and corresponds to the ET “aftertastes”. Before a new measurement cycle started, electrodes were rinsed for 90 s with a washing solution and then for 180 s with the reference solution.
Fig. 1.
Measuring process by the electronic tongue
Measurements with the ET were performed aiming to detect the decrease of nicotine bitter intensity after the addition of taste-masking agents or also identify the effect of flavouring. For each tasted solution, the sensor outputs were collected and converted to “taste information”. The “taste values” were calculated by multiplying sensor outputs for appropriate coefficients based of Weber–Fechner law which gives an approximately accurate generalisation of the intensity of sensation considering the sensor properties for tastes. In particular, the “taste values” were estimated as:
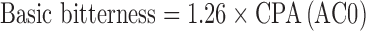 |
2 |
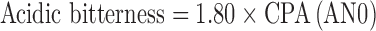 |
3 |
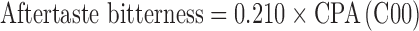 |
4 |
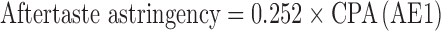 |
5 |
The tasted solutions and their composition are reported in Table III. Each solution was evaluated twice, and the average of the results was used for data analysis. Moreover, bitter standard solution consisting of 30 mM potassium chloride + 0.3 mM tartaric acid + 0.1 mM quinine hydrochloride was analysed.
Table III.
Composition (%, w/v) of Aqueous Solutions Tasted by the Electronic Tongue
| Solutions | NHT | MDX12 | GLY | SO | MT | MK |
|---|---|---|---|---|---|---|
| α | 0.15 | – | – | – | – | – |
| β | – | 8.20 | 1.50 | 0.30 | – | – |
| χ | 0.15 | 8.05 | 1.50 | 0.30 | – | – |
| δ | 0.15 | 7.45 | 1.50 | 0.30 | 0.60 | – |
| ε | 0.15 | 7.45 | 1.50 | 0.30 | – | 0.60 |
| ϕ | 0.15 | 7.45 | 1.50 | 0.30 | 0.30 | 0.30 |
NHT (−)-nicotine hydrogen tartrate, MDX12 maltodextrin with a DE of 12, GLY glycerol, SO sorbitan oleate, MT mint flavour, MK milk flavour
Principal component analysis was applied on electronic tongue data and was performed by SCAN software (v. 1.1 Minitab Inc., PA, USA).
RESULTS AND DISCUSSION
Selection of the Taste-Masking Agent
Human Taste Panel
Results obtained by human taste panel are shown in Table IV.
Table IV.
Bitterness Score and Irritation Score of Solutions Tasted by Volunteers
| Solutions | Human taste panel results | |
|---|---|---|
| Bitterness score | Irritation score | |
| A | 0.7 | 3.4 |
| B | 0.6 | 2.2 |
| C | 1.8 | 1.8 |
| D | 1.2 | 1.2 |
| E | 0.6 | 2.2 |
| F | 0.7 | 2.0 |
Volunteers described the reference solution (A) not very bitter but highly irritating. Generally speaking, the addition of flavours reduced only irritation, without modifying bitterness. In particular, both mint (sol. C) and liquorice (sol. E) could immediately mask unpleasant taste of nicotine at the moment of film disintegration and NHT dissolution, but their effect was not prolonged overtime. Milk (sol. B), instead, was able to mask irritation in mouth and throat until few minutes after complete swallowing. The combination of mint and milk in solution D appeared the most suitable solution to mask nicotine taste.
Electronic Tongue
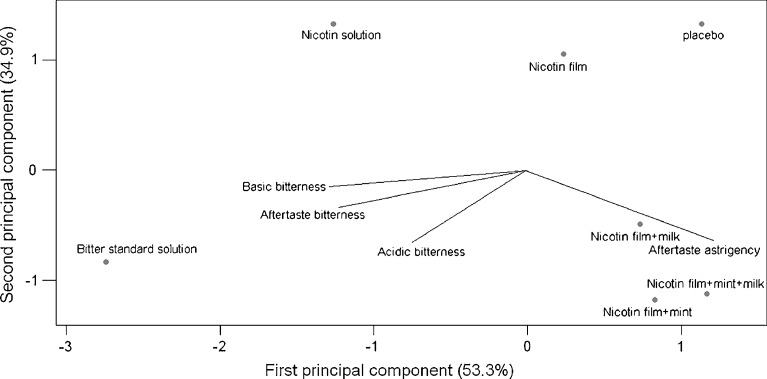
The “taste values” were elaborated by principal component analysis and Fig. 2 reports the bi-plot which represents, in the plane defined by the first two principal components, both the variables (tastes) and the samples.
Fig. 2.
Bi-plot of tastes (variables) and tasted solutions
Moving from the left to the right of the plot, the first principal component coincides with the direction in which the change of bitterness of the samples occurs. The standard bitter solution located at left in the negative part of first and second principal components is discriminated by the three variables specific for bitterness, and it is associated to the highest bitter intensity. Along the first principal component, the intensity of bitterness as perceived by the electronic tongue is: bitter standard solution > nicotine solution (sol α) > nicotine-film (sol χ) > placebo (sol β).
With respect to nicotine solution, nicotine films located in the positive part of the first principal component, exhibited less perceived bitterness showing that the formulation of the nicotine film is adequate to improve the taste and to reduce the bitter intensity. The nicotine films added with flavours are located right in the positive part of the first principal component and in the negative part of the second principal component; like placebo, they are less discriminated by the bitterness variables, while on the second principal component, they are characterised by the aftertaste astringency variable that probably reveals the increase of “irritant sensation” as perceived by the panellists. In agreement with sensory analysis, the binary combination of the two flavours (mint–milk, sol ϕ) masks the bitter taste of nicotine most effectively, but in any case, this formulation is perceived as irritant in mouth.
Formulation Study
The lowest amount of GLY which permitted to obtain flexible film made of MDX6 was equal to 18% w/w, and consequently, this amount was chosen to compare the disintegration time and tensile properties of the two selected types of MDX.
As previously demonstrated for MDX12 (5), also in the case of MDX6, the addition of SO was necessary to uniformly spread the polymeric mixture onto the silicone release liner and decrease the peel force required to separate the dried film from the release liner.
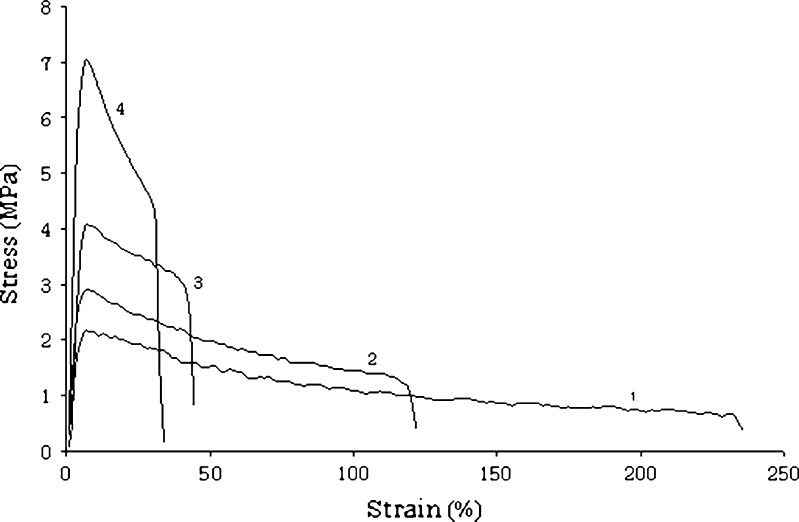
Figure 3 shows force–deformation curves for films made of MDX6 and MDX12 with or without added SO (formulations nos. 1–4).
Fig. 3.
Stress–strain curves of formulations nos. 1–4
Although samples had different mechanical properties, the pattern of their stress–strain curves presented similar features. All samples exhibited a linear region at low strain; raising the strain, the behaviour shifted from elastic to plastic, the curve lost linearity and the deformation became irreversible, until the maximum force was achieved. Afterwards, the stress–strain curves evidenced that the deformation increased with an apparent lower stress. This was due to a local reduction of the cross section, namely tear, which propagated along the length of the sample until rupture.
The comparison of formulations nos. 1 and 3 showed that the molecular weight of MDX influenced the tensile properties of films (Table V). A significant decrease of plasticity was evident: film made of MDX with DE value of 6 (formulation no. 3), although flexible and not brittle, resulted stiffer than formulation no. 1. Indeed, the modulus of elasticity and tensile strength increased using a MDX with a lower DE, while elongation at break, that is a value of film ductility, significantly decreased. The force–deformation curve of the formulation no. 3 was not very evident, and the fracture mechanism changed from a slow tear failure of the formulation no. 1 to a more rapid and brittle fracture of the formulation no. 3. This characteristic should be favourably exploited during the packaging procedures since the films are subjected to high stress.
Table V.
Thickness, Drug Content and Tensile Properties of Films
| Form. | Thickness (μm) | Drug contenta (μg) | Tensile properties | |||
|---|---|---|---|---|---|---|
| Y (MPa) | TS (Mpa) | E (%) | TBE (J) | |||
| 1 | 127 ± 5 | – | 0.52 ± 0.08 | 2.25 ± 0.19 | 237.4 ± 5.46 | 0.27 ± 0.03 |
| 2 | 128 ± 3 | – | 0.71 ± 0.04 | 2.38 ± 0.16 | 131.1 ± 34.3 | 0.21 ± 0.02 |
| 3 | 120 ± 2 | – | 1.06 ± 0.07 | 4.17 ± 0.34 | 37.8 ± 13.0 | 0.13 ± 0.02 |
| 4 | 123 ± 7 | – | 1.96 ± 0.13 | 7.20 ± 0.31 | 37.0 ± 3.56 | 0.18 ± 0.03 |
| 5 | 125 ± 3 | – | 0.29 ± 0.09 | 2.08 ± 0.58 | 220.9 ± 60.55 | 0.29 ± 0.09 |
| 6 | 132 ± 2 | – | 0.23 ± 0.01 | 1.37 ± 0.17 | 302.5 ± 74.86 | 0.24 ± 0.07 |
| 7 | 135 ± 6 | – | 0.24 ± 0.11 | 1.66 ± 0.16 | 468.3 ± 93.4 | 0.34 ± 0.03 |
| 12 | 128 ± 5 | 0.44 ± 0.05 | 0.26 ± 0.07 | 1.03 ± 0.22 | 91.8 ± 4.5 | 0.06 ± 0.01 |
| 13 | 130 ± 5 | 0.51 ± 0.04 | 0.99 ± 0.32 | 3.95 ± 0.5 | 40.6 ± 1.25 | 0.11 ± 0.02 |
| 14 | 131 ± 10 | 0.38 ± 0.03 | 0.28 ± 0.03 | 1.22 ± 0.11 | 81.5 ± 9.71 | 0.07 ± 0.08 |
| 15 | 124 ± 8 | 0.57 ± 0.03 | 0.41 ± 0.10 | 1.77 ± 0.32 | 134.96 ± 9.53 | 0.16 ± 0.03 |
| 16 | 125 ± 2 | 0.44 ± 0.01 | 1.25 ± 0.36 | 5.23 ± 1.39 | 69.9 ± 22.8 | 0.21 ± 0.01 |
| 17 | 128 ± 7 | 0.48 ± 0.03 | 1.68 ± 0.21 | 6.51 ± 0.06 | 47.3 ± 3.94 | 0.19 ± 0.02 |
| 18 | 135 ± 6 | 0.52 ± 0.05 | 1.07 ± 0.30 | 3.85 ± 0.86 | 50.3 ± 11.0 | 0.17 ± 0.05 |
| 19 | 130 ± 3 | 0.45 ± 0.02 | 0.97 ± 0.30 | 3.72 ± 0.96 | 53.8 ± 14.6 | 0.15 ± 0.05 |
TS tensile strength, E% percent elongation at break, Y elastic modulus or Young’s modulus, TBE tensile energy to break
aExpressed as nicotine-free base
In general, the addition of SO similarly affected tensile properties of films obtained with the two types of MDX decreasing plasticity of the films. Elastic modulus and tensile strength increased for the formulations nos. 2 and 4, while elongation at break significantly decreased only for films made of MDX12. As far as MDX6 film was concerned, ductility, expressed by elongation at break, was not further reduced.
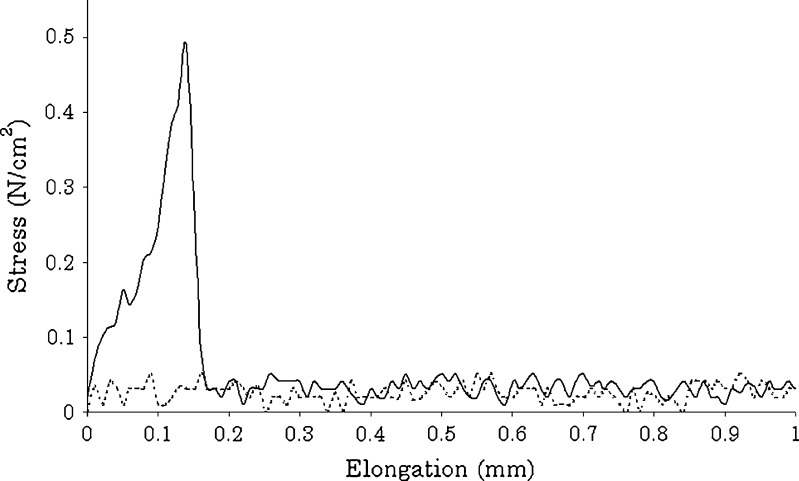
The increase of the MDX molecular weight also influenced the film stickiness that could affect the packaging and handling procedures. Figure 4 shows the texture profile of formulations nos. 2 and 4: the films obtained with MDX12 were stick, and the maximum detached force was 50 cN/cm2, while in the case of films prepared with MDX6, no signal was detected, index of a complete lack of tackiness.
Fig. 4.
Tack stress–strain curves of film formulations no. 2 (solid line) and no. 4 (dotted line)
As expected, the increase in GLY concentration to 20% w/w (formulation no. 5) raised significantly film plasticity, but tensile properties of films containing GLY percentage higher than 20% w/w (formulations nos. 6 and 7) did not significantly change (Table V).
All placebo formulations disintegrated within 15 s; therefore, the MDX molecular weight did not affect film disintegration time.
Considering that the formulations prepared using the lowest amount of GLY exhibited the best mechanical properties, they were selected for the nicotine loading.
The mixtures used for the preparation of fast-dissolving film had a pH value of about six and, in this condition, the required amount of nicotine-free base, which has a pKa of 8.5, was not completely soluble. Indeed, after the mixture preparation, a yellow-brownish oily layer appeared on its surface. Therefore, the pH of the mixtures was decreased to pH 4 by adding citric acid, and a clear solution was obtained using both types of MDX (formulations nos. 8 and 10). Nevertheless, the film oozed with GLY during the evaporation of the solvent. To avoid squeezing out of GLY, its concentration was reduced to 15% (formulations nos. 9 and 11). The formulations containing 1.5% w/w NHT were prepared with the same amount of GLY without the addition of citric acid (formulations nos. 12 and 16).
The formulations nos. 9, 11, 12 and 16 were in vivo tested to verify their taste. The astringency resulted slightly lower when NHT was loaded; therefore, this active ingredient was used to evaluate the impact of addition of the selected taste-masking agents on the tensile properties of fast dissolving films.
The NHT-loaded films complied with the uniformity of content assay (Table V). All films disintegrated within 10 s and the active ingredient was completely dissolved in less than 20 s.
The comparison of formulations nos. 4 and 16 shows that the addition of NHT to the film made of MDX6 slightly increased film plasticity. In particular, elastic modulus and tensile strength decreased symptomatic of a lower stiffness, while ductility and toughness did not significantly change. Although in formulation no. 16, the ratio MDX/GLY is higher than that of formulation no. 4, the film containing NHT appeared more plastic indicating that NHT acted as a plasticiser of MDX. Instead, the behaviour of NHT on films made of MDX12 could not be evaluated, because the effect of the different plasticiser concentrations in the two formulations resulted much more relevant.
In the case of films obtained with MDX12, the addition of mint and milk flavours had a different effect on mechanical properties of films. Indeed, film containing mint (formulation no. 13) was stiffer and less ductile than film lacking in flavours (formulation no. 12). The presence of milk flavour did not significantly affect tensile properties. In the formulation no. 15, both flavours influenced the film mechanical properties: elastic modulus, tensile strength and elongation at break were higher than those measured for formulation no. 12. The increasing of stiffness, expressed by elastic modulus and tensile strength, was probably due to the presence of mint, but its effect was counteracted by milk flavour that caused the increasing of ductility.
The addition of flavours in the formulation no. 16, obtained with MDX6, did not significantly influenced tensile properties of films.
In all cases, flavours did not affect disintegration time of films.
CONCLUSIONS
Both the addition of TMAs and/or small amount of drugs, such as NHT, to fast-dissolving films made of MDX can affect the film tensile properties and, therefore, compromise the film integrity during the packaging procedures. The effects of TMAs and NHT on film tensile properties are unpredictable. Indeed, the mint flavour increased the stiffness of MDX12 films, while milk flavour had an opposite behaviour. In the case of MDX6 films, neither mint nor milk flavour significantly affected tensile properties. Nevertheless, the effect of these components on film tensile properties can be easily counterweighted by modulating the GLY content and/or the MDX molecular weight. Indeed, the results of the formulation study evidenced that decreasing the DE value of MDX the tenacity of the film improved. The study also led to identify a NHT formulation with a satisfactory taste.
REFERENCES
- 1.United States Pharmacopoeia 32 National Formulary 27, Official Monographs, Maltodextrin; 2009. p. 1276
- 2.Chronakis IS. On the molecular characteristics, compositional properties, and structural-functional mechanisms of maltodextrins: a review. Crit Rev Food Sci. 1998;38(7):599–637. doi: 10.1080/10408699891274327. [DOI] [PubMed] [Google Scholar]
- 3.Dokic L, Jakovljevic J, Dokic P. Relation between viscous characteristics and dextrose equivalent of maltodextrins. Starch. 2004;56:520–525. doi: 10.1002/star.200400294. [DOI] [Google Scholar]
- 4.Avaltroni F, Bouquerand PE, Normand V. Maltodextrin molecular weight distribution influence on the glass transition temperature and viscosity in aqueous solutions. Carbohydr Polym. 2004;58:323–334. doi: 10.1016/j.carbpol.2004.08.001. [DOI] [Google Scholar]
- 5.Cilurzo F, Cupone IE, Minghetti P, Selmin F, Montanari L. Fast dissolving films made of maltodextrin. Eur J Pharm Biopharm. 2008;70(3):895–900. doi: 10.1016/j.ejpb.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Craton C, Fabre P. Tack of PSAs. In: Pocius A, Dillard D, editors. Comprehensive adhesion science: the mechanics of adhesion, rheology of adhesives, and strength of adhesive bonds. San Diego: Elsevier; 2002. [Google Scholar]