Abstract
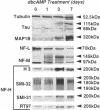
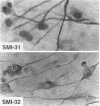
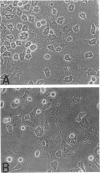
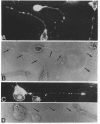
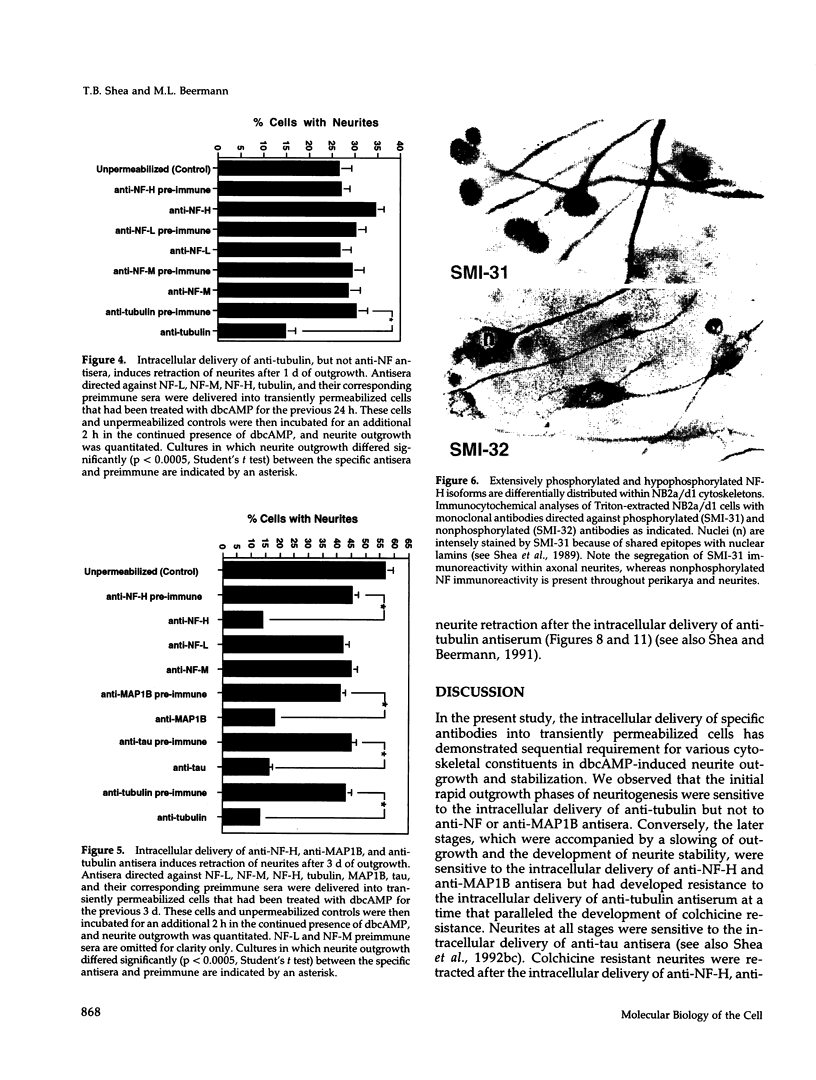
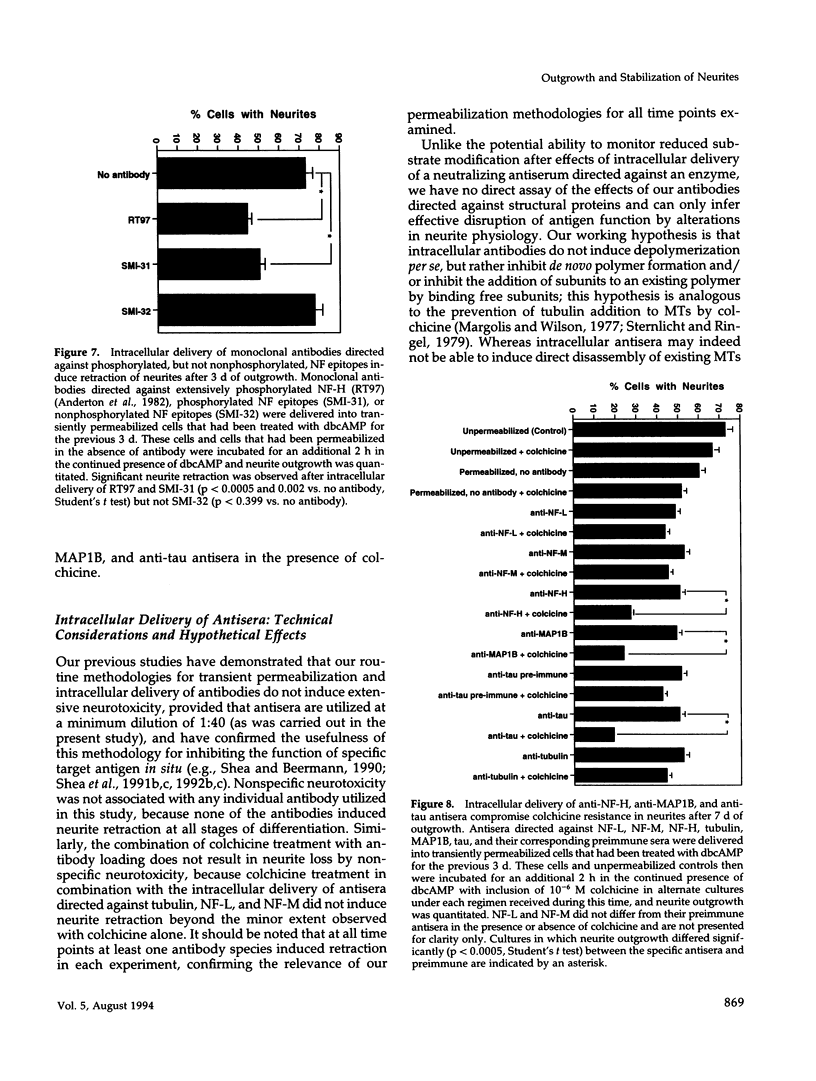
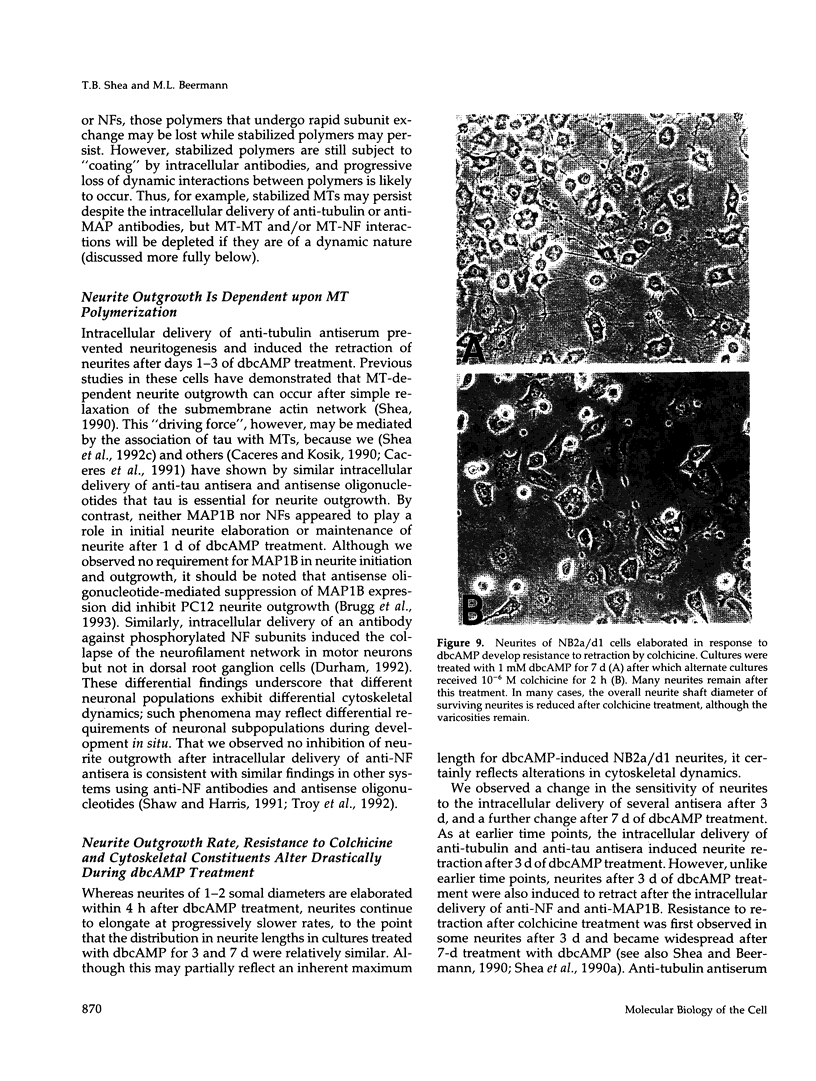
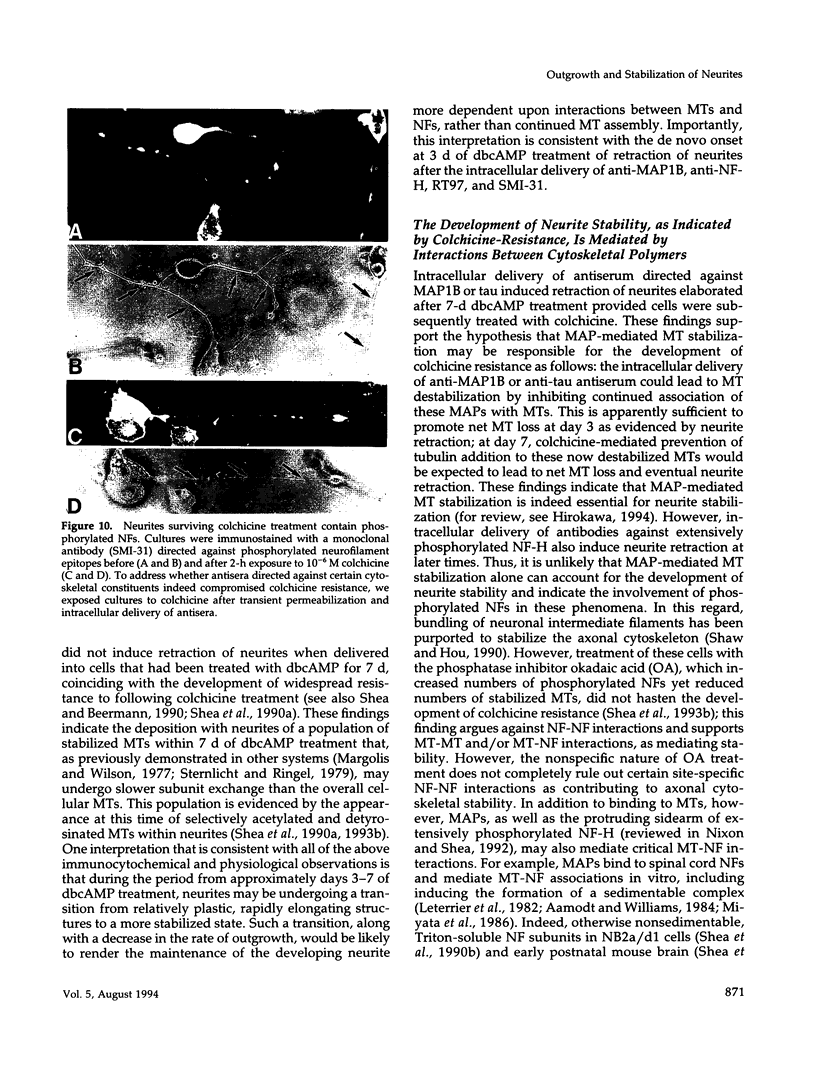
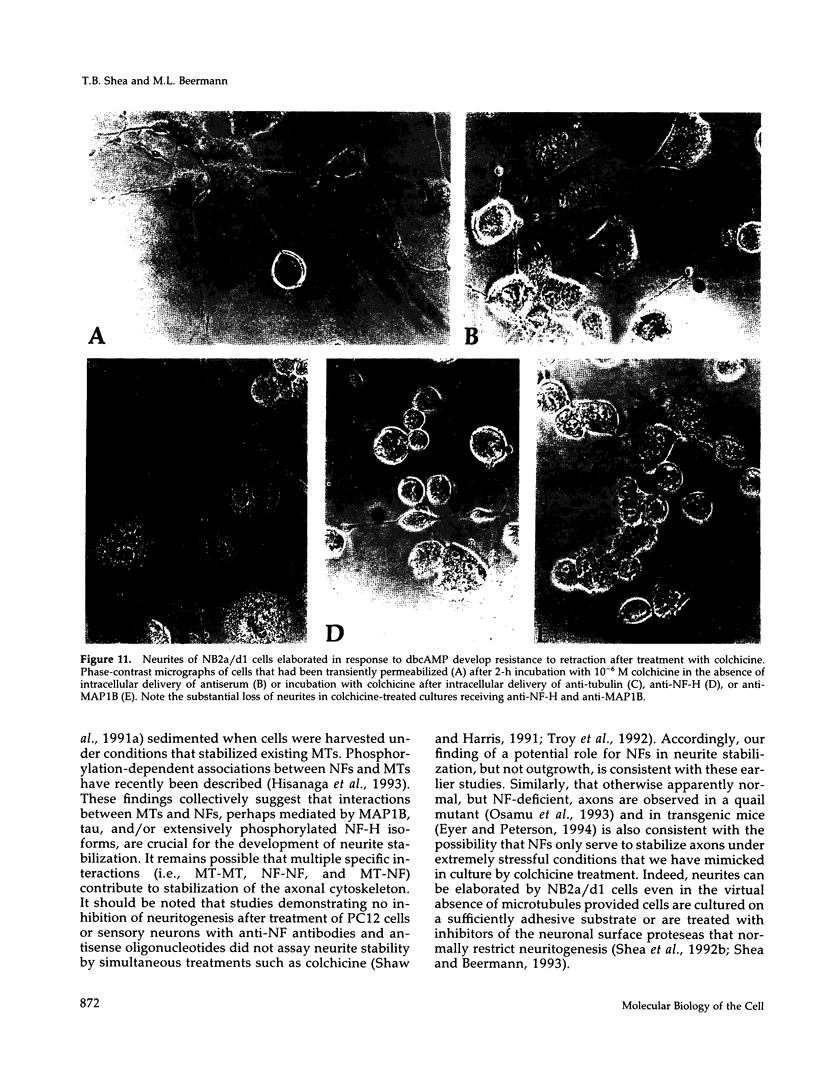
The respective roles of neurofilaments (NFs), microtubules (MTs), and the microtubule-associated proteins (MAPs) MAP 1B and tau on neurite outgrowth and stabilization were probed by the intracellular delivery of specific antisera into transiently permeabilized NB2a/d1 cells during treatment with dbcAMP. Intracellular delivery of antisera specific for the low (NF-L), middle (NF-M), or extensively phosphorylated high (NF-H) molecular weight subunits did not prevent initial neurite elaboration, nor did it induce retraction of existing neurites elaborated by cells that had been previously treated for 1 d with dbcAMP. By contrast, intracellular delivery of antisera directed against tubulin reduced the percentage of cells with neurites at both these time points. Intracellular delivery of anti-NF-L and anti-NF-M antisera did not induce retraction in cells treated with dbcAMP for 3 d. However, intracellular delivery of antisera directed against extensively phosphorylated NF-H, MAP1B, tau, or tubulin induced similar levels of neurite retraction at this time. Intracellular delivery of monoclonal antibodies (RT97 or SMI-31) directed against phosphorylated NF-H induced neurite retraction in cell treated with dbcAMP for 3 d; a monoclonal antibody (SMI-32) directed against nonphosphorylated NF-H did not induce neurite retraction at this time. By contrast, none of the above antisera induced retraction of neurites in cells treated with dbcAMP for 7 d. Neurites develop resistance to retraction by colchicine, first detectable in some neurites after 3 d and in the majority of neurites after 7 d of dbcAMP treatment. We therefore examined whether or not colchicine resistance was compromised by intracellular delivery of the above antisera. Colchicine treatment resulted in rapid neurite retraction after intracellular delivery of antisera directed against extensively phosphorylated NF-H, MAP1B, or tau into cells that had previously been treated with dbcAMP for 7 d. By contrast, colchicine resistance was not compromised by the intracellular delivery of antisera directed against NF-L, NF-M, or tubulin. These findings support previous studies indicating that MT polymerization mediates certain aspects of axonal neurite outgrowth and suggest that NFs do not directly participate in these events. These findings further suggest that NFs function in stabilization of the axonal cytoskeleton, apparently by interactions among NFs and MTs that are mediated by NF-H and MAPs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aamodt E. J., Williams R. C., Jr Microtubule-associated proteins connect microtubules and neurofilaments in vitro. Biochemistry. 1984 Dec 4;23(25):6023–6031. doi: 10.1021/bi00320a019. [DOI] [PubMed] [Google Scholar]
- Anderton B. H., Breinburg D., Downes M. J., Green P. J., Tomlinson B. E., Ulrich J., Wood J. N., Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982 Jul 1;298(5869):84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- Bignami A., Raju T., Dahl D. Localization of vimentin, the nonspecific intermediate filament protein, in embryonal glia and in early differentiating neurons. In vivo and in vitro immunofluorescence study of the rat embryo with vimentin and neurofilament antisera. Dev Biol. 1982 Jun;91(2):286–295. doi: 10.1016/0012-1606(82)90035-5. [DOI] [PubMed] [Google Scholar]
- Black M. M., Keyser P. Acetylation of alpha-tubulin in cultured neurons and the induction of alpha-tubulin acetylation in PC12 cells by treatment with nerve growth factor. J Neurosci. 1987 Jun;7(6):1833–1842. doi: 10.1523/JNEUROSCI.07-06-01833.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugg B., Reddy D., Matus A. Attenuation of microtubule-associated protein 1B expression by antisense oligodeoxynucleotides inhibits initiation of neurite outgrowth. Neuroscience. 1993 Feb;52(3):489–496. doi: 10.1016/0306-4522(93)90401-z. [DOI] [PubMed] [Google Scholar]
- Caceres A., Kosik K. S. Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature. 1990 Feb 1;343(6257):461–463. doi: 10.1038/343461a0. [DOI] [PubMed] [Google Scholar]
- Caceres A., Potrebic S., Kosik K. S. The effect of tau antisense oligonucleotides on neurite formation of cultured cerebellar macroneurons. J Neurosci. 1991 Jun;11(6):1515–1523. doi: 10.1523/JNEUROSCI.11-06-01515.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambray-Deakin M. A., Burgoyne R. D. Posttranslational modifications of alpha-tubulin: acetylated and detyrosinated forms in axons of rat cerebellum. J Cell Biol. 1987 Jun;104(6):1569–1574. doi: 10.1083/jcb.104.6.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden M. J., Trojanowski J. Q., Schlaepfer W. W., Lee V. M. Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J Neurosci. 1987 Nov;7(11):3489–3504. doi: 10.1523/JNEUROSCI.07-11-03489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu F. C., Norton W. T. Bulk preparation of CNS cytoskeleton and the separation of individual neurofilament proteins by gel filtration: dye-binding characteristics and amino acid compositions. J Neurochem. 1982 Nov;39(5):1252–1260. doi: 10.1111/j.1471-4159.1982.tb12562.x. [DOI] [PubMed] [Google Scholar]
- Cochard P., Paulin D. Initial expression of neurofilaments and vimentin in the central and peripheral nervous system of the mouse embryo in vivo. J Neurosci. 1984 Aug;4(8):2080–2094. doi: 10.1523/JNEUROSCI.04-08-02080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D. Early and late appearance of neurofilament phosphorylated epitopes in rat nervous system development: in vivo and in vitro study with monoclonal antibodies. J Neurosci Res. 1988 Aug;20(4):431–441. doi: 10.1002/jnr.490200405. [DOI] [PubMed] [Google Scholar]
- Durham H. D. An antibody against hyperphosphorylated neurofilament proteins collapses the neurofilament network in motor neurons but not in dorsal root ganglion cells. J Neuropathol Exp Neurol. 1992 May;51(3):287–297. doi: 10.1097/00005072-199205000-00007. [DOI] [PubMed] [Google Scholar]
- Fischer I., Romano-Clarke G. Changes in microtubule-associated protein MAP1B phosphorylation during rat brain development. J Neurochem. 1990 Jul;55(1):328–333. doi: 10.1111/j.1471-4159.1990.tb08855.x. [DOI] [PubMed] [Google Scholar]
- Fischer I., Shea T. B. Differential appearance of extensively phosphorylated forms of the high molecular weight neurofilament protein in regions of mouse brain during postnatal development. J Neuroimmunol. 1991 Jan;31(1):73–81. doi: 10.1016/0165-5728(91)90089-p. [DOI] [PubMed] [Google Scholar]
- Foster G. A., Dahl D., Lee V. M. Temporal and topographic relationships between the phosphorylated and nonphosphorylated epitopes of the 200 kDa neurofilament protein during development in vitro. J Neurosci. 1987 Sep;7(9):2651–2663. doi: 10.1523/JNEUROSCI.07-09-02651.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Weeks P. R., Lang R. D. The alpha-tubulin of the growth cone is predominantly in the tyrosinated form. Brain Res. 1988 Jul 1;470(1):156–160. doi: 10.1016/0165-3806(88)90213-1. [DOI] [PubMed] [Google Scholar]
- Hanemaaijer R., Ginzburg I. Involvement of mature tau isoforms in the stabilization of neurites in PC12 cells. J Neurosci Res. 1991 Sep;30(1):163–171. doi: 10.1002/jnr.490300117. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Glicksman M. A., Willard M. B. Organization of mammalian neurofilament polypeptides within the neuronal cytoskeleton. J Cell Biol. 1984 Apr;98(4):1523–1536. doi: 10.1083/jcb.98.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr Opin Cell Biol. 1994 Feb;6(1):74–81. doi: 10.1016/0955-0674(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Hisanaga S., Yasugawa S., Yamakawa T., Miyamoto E., Ikebe M., Uchiyama M., Kishimoto T. Dephosphorylation of microtubule-binding sites at the neurofilament-H tail domain by alkaline, acid, and protein phosphatases. J Biochem. 1993 Jun;113(6):705–709. doi: 10.1093/oxfordjournals.jbchem.a124107. [DOI] [PubMed] [Google Scholar]
- Julien J. P., Mushynski W. E. The distribution of phosphorylation sites among identified proteolytic fragments of mammalian neurofilaments. J Biol Chem. 1983 Mar 25;258(6):4019–4025. [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Bakalis S., Neve R. L. Developmentally regulated expression of specific tau sequences. Neuron. 1989 Apr;2(4):1389–1397. doi: 10.1016/0896-6273(89)90077-9. [DOI] [PubMed] [Google Scholar]
- Lee G., Cowan N., Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988 Jan 15;239(4837):285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- Lendahl U., Zimmerman L. B., McKay R. D. CNS stem cells express a new class of intermediate filament protein. Cell. 1990 Feb 23;60(4):585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Leterrier J. F., Liem R. K., Shelanski M. L. Interactions between neurofilaments and microtubule-associated proteins: a possible mechanism for intraorganellar bridging. J Cell Biol. 1982 Dec;95(3):982–986. doi: 10.1083/jcb.95.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieska N., Yang H. Y., Goldman R. D. Purification of the 300K intermediate filament-associated protein and its in vitro recombination with intermediate filaments. J Cell Biol. 1985 Sep;101(3):802–813. doi: 10.1083/jcb.101.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall G., Cole R. D. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984 Apr 25;259(8):5301–5305. [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Addition of colchicine--tubulin complex to microtubule ends: the mechanism of substoichiometric colchicine poisoning. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3466–3470. doi: 10.1073/pnas.74.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y., Hoshi M., Nishida E., Minami Y., Sakai H. Binding of microtubule-associated protein 2 and tau to the intermediate filament reassembled from neurofilament 70-kDa subunit protein. Its regulation by calmodulin. J Biol Chem. 1986 Oct 5;261(28):13026–13030. [PubMed] [Google Scholar]
- Nixon R. A., Shea T. B. Dynamics of neuronal intermediate filaments: a developmental perspective. Cell Motil Cytoskeleton. 1992;22(2):81–91. doi: 10.1002/cm.970220202. [DOI] [PubMed] [Google Scholar]
- Nixon R. A., Sihag R. K. Neurofilament phosphorylation: a new look at regulation and function. Trends Neurosci. 1991 Nov;14(11):501–506. doi: 10.1016/0166-2236(91)90062-y. [DOI] [PubMed] [Google Scholar]
- Scott C. W., Blowers D. P., Barth P. T., Lo M. M., Salama A. I., Caputo C. B. Differences in the abilities of human tau isoforms to promote microtubule assembly. J Neurosci Res. 1991 Sep;30(1):154–162. doi: 10.1002/jnr.490300116. [DOI] [PubMed] [Google Scholar]
- Shaw G., Hou Z. C. Bundling and cross-linking of intermediate filaments of the nervous system. J Neurosci Res. 1990 Apr;25(4):561–568. doi: 10.1002/jnr.490250414. [DOI] [PubMed] [Google Scholar]
- Shea T. B., Beermann M. L. Alterations in dynamics of microtubule assembly during axonal neuritogenesis in NB2a/d1 cells. Cell Biol Int Rep. 1990 Dec;14(12):1093–1098. doi: 10.1016/0309-1651(90)90017-s. [DOI] [PubMed] [Google Scholar]
- Shea T. B., Beermann M. L., Fischer I. Transient requirement for vimentin in neuritogenesis: intracellular delivery of anti-vimentin antibodies and antisense oligonucleotides inhibit neurite initiation but not elongation of existing neurites in neuroblastoma. J Neurosci Res. 1993 Sep 1;36(1):66–76. doi: 10.1002/jnr.490360108. [DOI] [PubMed] [Google Scholar]
- Shea T. B., Beermann M. L., Nixon R. A. Aluminum alters the electrophoretic properties of neurofilament proteins: role of phosphorylation state. J Neurochem. 1992 Feb;58(2):542–547. doi: 10.1111/j.1471-4159.1992.tb09753.x. [DOI] [PubMed] [Google Scholar]
- Shea T. B., Beermann M. L., Nixon R. A. Appearance and localization of phosphorylated variants of the high molecular weight neurofilament protein in NB2a/d1 cytoskeletons during differentiation. Brain Res Dev Brain Res. 1989 Nov 1;50(1):142–146. doi: 10.1016/0165-3806(89)90134-x. [DOI] [PubMed] [Google Scholar]
- Shea T. B., Beermann M. L., Nixon R. A., Fischer I. Microtubule-associated protein tau is required for axonal neurite elaboration by neuroblastoma cells. J Neurosci Res. 1992 Jul;32(3):363–374. doi: 10.1002/jnr.490320308. [DOI] [PubMed] [Google Scholar]
- Shea T. B., Beermann M. L., Nixon R. A. Post-translational modification of alpha-tubulin by acetylation and detyrosination in NB2a/d1 neuroblastoma cells. Brain Res Dev Brain Res. 1990 Feb 1;51(2):195–204. doi: 10.1016/0165-3806(90)90276-5. [DOI] [PubMed] [Google Scholar]
- Shea T. B., Fischer I., Sapirstein V. S. Effect of retinoic acid on growth and morphological differentiation of mouse NB2a neuroblastoma cells in culture. Brain Res. 1985 Aug;353(2):307–314. doi: 10.1016/0165-3806(85)90220-2. [DOI] [PubMed] [Google Scholar]
- Shea T. B. Neuritogenesis in mouse NB2a/d1 neuroblastoma cells: triggering by calcium influx and involvement of actin and tubulin dynamics. Cell Biol Int Rep. 1990 Nov;14(11):967–979. doi: 10.1016/0309-1651(90)90109-c. [DOI] [PubMed] [Google Scholar]
- Shea T. B., Paskevich P. A., Beermann M. L. The protein phosphatase inhibitor okadaic acid increases axonal neurofilaments and neurite caliber, and decreases axonal microtubules in NB2a/d1 cells. J Neurosci Res. 1993 Aug 1;35(5):507–521. doi: 10.1002/jnr.490350507. [DOI] [PubMed] [Google Scholar]
- Shea T. B., Perrone-Bizzozero N. I., Beermann M. L., Benowitz L. I. Phospholipid-mediated delivery of anti-GAP-43 antibodies into neuroblastoma cells prevents neuritogenesis. J Neurosci. 1991 Jun;11(6):1685–1690. doi: 10.1523/JNEUROSCI.11-06-01685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea T. B., Sihag R. K., Nixon R. A. Dynamics of phosphorylation and assembly of the high molecular weight neurofilament subunit in NB2a/d1 neuroblastoma. J Neurochem. 1990 Nov;55(5):1784–1792. doi: 10.1111/j.1471-4159.1990.tb04969.x. [DOI] [PubMed] [Google Scholar]
- Shea T. B., Sihag R. K., Nixon R. A. Neurofilament triplet proteins of NB2a/d1 neuroblastoma: posttranslational modification and incorporation into the cytoskeleton during differentiation. Brain Res. 1988 Sep 1;471(1):97–109. doi: 10.1016/0165-3806(88)90155-1. [DOI] [PubMed] [Google Scholar]
- Skalli O., Chou Y. H., Goldman R. D. Cell cycle-dependent changes in the organization of an intermediate filament-associated protein: correlation with phosphorylation by p34cdc2. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11959–11963. doi: 10.1073/pnas.89.24.11959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht H., Ringel I. Colchicine inhibition of microtubule assembly via copolymer formation. J Biol Chem. 1979 Oct 25;254(20):10540–10550. [PubMed] [Google Scholar]
- Takemura R., Kanai Y., Hirokawa N. In situ localization of tau mRNA in developing rat brain. Neuroscience. 1991;44(2):393–407. doi: 10.1016/0306-4522(91)90064-u. [DOI] [PubMed] [Google Scholar]
- Tapscott S. J., Bennett G. S., Toyama Y., Kleinbart F., Holtzer H. Intermediate filament proteins in the developing chick spinal cord. Dev Biol. 1981 Aug;86(1):40–54. doi: 10.1016/0012-1606(81)90313-4. [DOI] [PubMed] [Google Scholar]
- Tashiro T., Kurokawa M., Komiya Y. Two populations of axonally transported tubulin differentiated by their interactions with neurofilaments. J Neurochem. 1984 Nov;43(5):1220–1225. doi: 10.1111/j.1471-4159.1984.tb05376.x. [DOI] [PubMed] [Google Scholar]
- Troy C. M., Greene L. A., Shelanski M. L. Neurite outgrowth in peripherin-depleted PC12 cells. J Cell Biol. 1992 Jun;117(5):1085–1092. doi: 10.1083/jcb.117.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa L., Avila J., Díaz-Nido J. Heterogeneity in the phosphorylation of microtubule-associated protein MAP1B during rat brain development. J Neurochem. 1993 Sep;61(3):961–972. doi: 10.1111/j.1471-4159.1993.tb03609.x. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Lieska N., Goldman A. E., Goldman R. D. A 300,000-mol-wt intermediate filament-associated protein in baby hamster kidney (BHK-21) cells. J Cell Biol. 1985 Feb;100(2):620–631. doi: 10.1083/jcb.100.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]