Abstract
The aim of this work was to investigate the effects of formulation variables on development of carvedilol (CAR) proniosomal gel formulations as potential transdermal delivery systems. Different non-ionic surfactants; polyoxyethylene alkyl ethers, namely Brij 78, Brij 92, and Brij 72; and sorbitan fatty acid esters (Span 60) were evaluated for their applicability in preparation of CAR proniosomal gels. A 23 full factorial design was employed to evaluate individual and combined effects of formulation variables, namely cholesterol content, weight of proniosomes, and amount of CAR added on performance of proniosomes. Prepared proniosomes were evaluated regarding entrapment efficiency (EE%), vesicle size, and microscopic examination. Also, CAR release through cellulose membrane and permeation through hairless mice skin were investigated. Proniosomes prepared with Brij 72 and Span 60 showed better niosome forming ability and higher EE% than those prepared with Brij 78 and Brij 92. Higher EE% was obtained by increasing both weight of proniosomes and amount of CAR added, and decreasing cholesterol content. Release rate through cellulose membrane was inversely affected by weight of proniosomes. In Span 60 proniosomes, on increasing percent of cholesterol, a decrease in release rate was observed. While in Brij 72 proniosomes, an enhancement in release rate was observed on increasing amount of CAR added. Permeation experiments showed that skin permeation was mainly affected by weight of proniosomes and that Span 60 proniosomal gels showed higher permeation enhancing effect than Brij 72. Proniosomal gel could constitute a promising approach for transdermal delivery of CAR.
KEY WORDS: Brij, carvedilol, proniosomes, Span, transdermal delivery
INTRODUCTION
The use of colloidal particulate carriers such as liposomes (1) or niosomes (2–4) as drug delivery systems have distinct advantages over conventional dosage forms. These carriers can act as drug reservoirs, and modification of their composition or surface can adjust drug release rate and/or the affinity for the target site. Liposomes or niosomes can encapsulate both lipophilic and hydrophilic drugs and serve as local depot for sustained release of dermally active compounds, as permeation enhancers, or as a rate-limiting membrane barrier for controlling transdermal drug absorption (5).
Niosomes offer several advantages over liposomes such as higher chemical stability, intrinsic skin-penetration-enhancing properties, and lower costs (6). However, there may be problems of physical instability in niosome dispersions during storage like vesicles aggregation, fusion, leaking, or hydrolysis of entrapped drugs, which affect the shelf life of the dispersion (7). Proniosomes are semisolid liquid crystal (gel) products of non-ionic surfactants prepared by dissolving the surfactant in a minimal amount of an acceptable solvent namely ethanol and the least amount of water (8) which can be converted into niosomes upon hydration (9,10).
Carvedilol (CAR), ((±)-1-(carbazol-4-yloxy)-3-[[2-(o-methoxyphenoxy) ethyl] amino]-2-propanol) (11), is a compound displaying antioxidant properties used in clinical practice for the treatment of cardiovascular diseases (hypertension, congestive heart failure, or myocardial infarction) (12) being available in the market in 3.125, 6.25, 12.5, and 25 mg tablets. CAR is rapidly absorbed from the gastrointestinal tract but it is subjected to significant first-pass metabolism in the liver; its absolute bioavailability is about 25%. It has a short plasma half-life of about 6 h (13,14).
Transdermal delivery systems offer several distinct advantages. They avoid factors that affect the gastrointestinal absorption of drugs, such as pH, enzymatic activity, and drug–food interactions and bypass first-pass effect. Provide multiday sustained release delivery (useful for drugs with short biological half-lives requiring frequent oral or parenteral administration), hence improve patient compliance. Allow rapid termination of drug effects when necessary (15,16).
CAR is a good candidate for formulation in transdermal delivery systems because it has low molecular weight (406.5), a suitable logarithmic partition coefficient (log octanol/water, 0.58 ± 0.02; log octanol/buffer pH 7.4, 0.61 ± 0.06), small dose range, short plasma half-life, and poor oral bioavailability (14).
Proniosomes are used for drug delivery via the transdermal route. This would be possible if proniosomes form niosomes following topical application under occlusive conditions, due to hydration by water from the skin itself (8). Proniosomes have been used as a dosage form to enhance the transdermal delivery of flurbiprofen (17,18), estradiol (9), levonorgestrel (8), ketorolac (19), frusemide (20), and chlorpheniramine maleate (10). However, all the previous studies used the non-ionic surfactant sorbitan fatty acid esters (Span). To our knowledge, polyoxyethylene alkyl ether was not previously employed as a non-ionic surfactant in formulation of proniosomes although it was successfully used in preparation of niosomes (21–24).
The aim of this study is to investigate the feasibility of development of CAR transdermal proniosomal gel. Different non-ionic surfactants; polyoxyethylene alkyl ethers, namely Brij 78, Brij 92, and Brij 72; and sorbitan fatty acid esters (Span 60) will be evaluated for their applicability in preparation of CAR proniosomal gels. The influence of different formulation variables such as cholesterol content, weight of proniosomes, and amount of CAR added on the performance of CAR proniosomal gels will be explored using 23 full factorial design. Also, to investigate the possibility of using proniosomal gel systems for transdermal delivery of CAR, in vitro release through cellulose membrane and permeation through hairless mice skin studies of CAR will be studied.
MATERIALS AND METHODS
Materials
Carvedilol (CAR) was a gift from Chemipharm, Egypt. Sorbitan monostearate (Span 60), Brij 78 (polyoxyethylene 20 stearylether, C18EO20), Brij 92 (polyoxyethylene 2 oleylether, C9=9EO2), Brij 72 (polyoxyethylene 2 stearylether, C18EO2), and cholesterol were purchased from Sigma Chemical Co., St. Louis, MO, USA. All other chemicals and solvents were of pharmaceutical grade and obtained from El-Nasr Company for Pharmaceutical Chemicals, Cairo, Egypt.
Methods
Preparation of Proniosomes
Proniosomes were prepared by the method reported by Vora et al. (8) with some modifications. Stock solution of CAR in absolute alcohol was prepared by sonication. Accurately weighed amounts of the surfactant or surfactant/cholesterol mixtures were placed in glass vials. Alcoholic stock solution (0.5 ml) was added; the vial was closed with a tight lid and warmed in thermostatically controlled shaking water bath at 60°C, 75 strokes per minute till the complete dissolution of surfactant and cholesterol. Then 0.12 ml of distilled water at 60°C was added while warming in the water bath for 5 min till a clear or translucent solution was produced. The mixtures were allowed to cool down at room temperature and observed for their physical appearance. The glass vials were kept in the dark until further characterization. The compositions of additives of the preliminary trials are listed in Table 1.
Table 1.
Composition, Appearance, Noisome Forming Ability (NFA), and% Entrapment Efficiency (EE%) of CAR Proniosomal Gel Formulations (Concentration of CAR was 3.125/400 mg Total Lipid Weight)
| Surf:chola | Brij 72 (HLB 4.9) | Brij 92 (HLB 4.9) | Brij 78 (HLB 15.3) | Span 60 (HLB 4.7) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NFAb | Appearance | %EE | NFA | Appearance | %EE | NFA | Appearance | %EE | NFA | Appearance | %EE | |
| 10:0 | + | White gel | 26.42 | − | Yellowish clear liquid | − | − | Transparent liquid | − | + | White creamy gel | 44.74 |
| 9:1 | + | White gel | 21.95 | − | Yellowish clear liquid | − | − | Transparent liquid | − | + | White creamy gel | 16.30 |
| 7:3 | + | White gel | 0 | + | Translucent gelc | 4.06 | − | Translucent gele | − | + | White creamy gel | 8.48 |
| 6:4 | + | White gelc | 0 | − | NAd | − | − | NA | − | − | NA | − |
| 5:5 | − | NA | − | − | NA | − | − | NA | − | − | NA | − |
aSurf:chol is surfactant–cholesterol molar ratio
bNoisome forming ability (it is measured only for gel formulations)
cObvious cholesterol crystals present
dNA (not applicable): either cholesterol hardly dissolved and re- precipitated after first addition of water or it did not dissolve at all
eThe proniosomal gel was unstable where liquefaction of the gel occurred within 24 h of storage
Hydration Step and Formation of Niosomes
Proniosome-derived niosome dispersions were obtained by hydrating the proniosomal gel preparations with 7 ml of Sorensen’s phosphate buffer pH 5.5 containing 20% propylene glycol at 60°C followed by vortex mixing for 2 min, heating for 10 min at a temperature of 60°C in a water bath, and further 2 min vortex mixing. The final volume was adjusted to 10 ml by the same buffer system.
Determination of Entrapment Efficiency of CAR in Niosomes
Free CAR was separated from niosomes entrapped CAR by centrifugation. One milliliter aliquots of the previously prepared niosome dispersions were centrifuged (Megafuge 1.0 R, Heraeus, Germany) at 18,000 rpm for 1 h at 0°C. A 0.1 ml aliquot of supernatant was diluted with Sorensen’s phosphate buffer pH 5.5 containing 20% propylene glycol. The CAR concentration in the resulting solution was assayed spectrophotometrically at 242 nm. The percentage entrapment efficiency of the drug was calculated as follows:
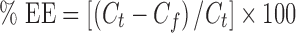 |
1 |
Where Ct is the total concentration of CAR, and Cf is the free concentration of CAR.
Experimental Design
A 23 full factorial design was employed to evaluate the individual and combined effects of three formulation variables on proniosomes performance and characteristics. In this design, three factors were evaluated, each at two levels, and experimental trials were performed at all eight possible combinations with replication for each proniosome system using either Brij 72 or Span 60. The % of cholesterol (XA), the weight of proniosome (XB), and amount of CAR added (XC) were selected as independent variables and the percentage entrapment efficiency (EE%) and the CAR release rate through synthetic membrane were selected as dependent variables.
Another 22 factorial design was designed to evaluate main effects and interactions of two chosen factors, XA and XB on the permeation of CAR through hairless mice skin. The response (dependent variable) was the cumulative amount of CAR permeated hairless mice skin per unit area after 24 h Q24 (μg/cm2; Table 2). The levels of the independent variables were chosen based on the preliminary experiments.
Table 2.
The Formulations of the Factorial Design and Their Characterization Results
| Formula number | Independent variables levels in coded form | Surfactant | % Entrapment efficiency (EE%) ± SD | Release ratea (μg/cm2/h) ± SD | Q 24 b (μg/cm2) ± SD | Mean vesicle size (nm) | ||
|---|---|---|---|---|---|---|---|---|
| X A | X B | X C | ||||||
| 1B | −1 | −1 | −1 | Brij 72 | 10.69 ± 0.72 | 11.68 ± 0.79 | – | 688 |
| 2B | 1 | −1 | −1 | 10.79 ± 0.73 | 19.41 ± 1.31 | – | 642 | |
| 3B | −1 | 1 | −1 | 26.42 ± 1.78 | 8.60 ± 0.58 | – | 574 | |
| 4B | 1 | 1 | −1 | 21.95 ± 1.48 | 7.36 ± 0.50 | – | 707 | |
| 5B | −1 | −1 | 1 | 43.27 ± 2.91 | 15.26 ± 1.03 | 51.62 ± 1.78 | 730 | |
| 6B | 1 | −1 | 1 | 37.02 ± 2.49 | 17.84 ± 1.20 | 52.82 ± 1.82 | 650 | |
| 7B | −1 | 1 | 1 | 45.27 ± 3.05 | 15.00 ± 1.01 | 37.11 ± 1.28 | 745 | |
| 8B | 1 | 1 | 1 | 43.13 ± 2.90 | 9.00 ± 0.61 | 38.15 ± 1.32 | 578 | |
| 1S | −1 | −1 | −1 | Span 60 | 30.66 ± 2.06 | 25.86 ± 1.74 | – | 578 |
| 2S | 1 | −1 | −1 | 8.23 ± 0.55 | 16.97 ± 1.14 | – | 657 | |
| 3S | −1 | 1 | −1 | 44.74 ± 3.01 | 16.11 ± 1.08 | – | 554 | |
| 4S | 1 | 1 | −1 | 16.30 ± 1.10 | 9.58 ± 0.64 | – | 650 | |
| 5S | −1 | −1 | 1 | 47.91 ± 3.23 | 26.54 ± 1.79 | 54.03 ± 1.86 | 410 | |
| 6S | 1 | −1 | 1 | 38.70 ± 2.61 | 11.67 ± 0.79 | 55.01 ± 1.90 | 280 | |
| 7S | −1 | 1 | 1 | 64.18 ± 4.32 | 16.28 ± 1.10 | 61.30 ± 2.11 | 245 | |
| 8S | 1 | 1 | 1 | 35.00 ± 2.36 | 11.66 ± 0.78 | 64.65 ± 2.23 | 240 | |
| The codes in the table symbolize | ||||||||
| Factor | Level used | |||||||
| −1 | 1 | |||||||
| X A:% of cholesterol | 0% | 10% | ||||||
| X B: weight of proniosome (mg) | 200 | 400 | ||||||
| X C: amount of CAR added (mg) | 3.125 | 9 | ||||||
aRelease rate through cellulose membrane
bCumulative amount of CAR permeated hairless mice skin per unit area after 24 h
A first-order polynomial regression equation was generated between the factors and responses and described by the following equation:
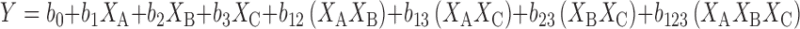 |
2 |
Where Y is the dependant variable (response), b0 is the intercept representing the arithmetic averages of all the quantitative outcomes of all experimental runs; b1–b3 are the coefficients computed from the observed experimental values of Y; and XA, XB, and XC are the coded levels of factors. The terms b12, b13, b23, and b123 represent the interaction terms. Coefficients with one factor represent the effect of that particular factor while the coefficients with more than one factor represent the interaction between those factors. The polynomial equations can be used to draw conclusions after considering the magnitude of coefficient and the mathematical sign it carries. A positive sign in front of the terms indicates synergistic effect while negative sign indicates antagonistic effect of the factors (25).
Contour plots were obtained by fixing the XC factor at its high and low level and varying (XA) and (XB) over the range used in the factorial study to determine the influence of the formulation factors on CAR entrapment efficiency and release rate. A significant level of 5% was used as the criterion to reject the null hypothesis. The statistical and factorial analyses were performed by using Design-Expert® v. 7.1.5 (Stat-Ease, Inc., Minneapolis, MN, USA).
Vesicle Size Analysis
The mean vesicle size of the prepared niosomes was determined based on photon correlation spectroscopy using Zetasizer 3000 (Malvern Instruments, UK) capable of measuring sizes between 10 and 5,000 nm at 25°C and keeping angle of detection at 90°.
Microscopic Examination
Optical Light Microscopy
A thin layer of the diluted hydrated proniosomal formulation was spread on a slide and after placing the cover slip, was examined using ordinary light microscope (Leica Imaging Systems, Cambridge, UK). Photomicrographs were taken using a digital camera (JVC, Victor Co, Yokohama, Japan).
Transmission Electron Microscopy
Morphological characteristics of the vesicles were studied by a negative stain electron microscopy using the Jeol JEM 1230 (Tokyo, Japan) electron microscope. Samples were diluted five times with distilled water to reduce the concentration of the vesicles. Equal volumes of the diluted sample and a 2% ammonium molybdate solution were mixed together and left for 3 min at room temperature. A drop of the diluted hydrated proniosome was pulled on a copper grid (200 mesh, Science Services, Munich, Germany), and the excess material was removed with a filter paper leaving a thin film stretched over the holes, then the grid was examined under a transmission electron microscopy (TEM) at 80 kV.
In Vitro Release Study
In vitro release studies were performed using vertical diffusion Franz cells with an effective diffusion area of 3.14 cm2. Proniosomal gel of different formulations was placed in the donor compartment and the top of the donor compartment was covered with paraffin film. A 25 ml of 15%:40%:45% (v:v:v) propylene glycol–ethanol–phosphate buffer pH 7.4 was used as receptor medium to ensure a sink condition (26). The receptor compartment was maintained at 37°C and stirred by a magnetic bar at 600 rpm. The donor compartment was separated from the receptor compartment by cellulose dialyzing membrane with molecular weight cut-off of 12,000–14,000 (Spectrum Medical Inc., Los Angeles, CA, USA) which was soaked in the receptor medium overnight. At predetermined time intervals (0.5, 1, 2, 3, 4, 5, 6, 12, and 24 h), 1 ml aliquots were withdrawn from the sampling port and were replaced with an equal volume of fresh solvent mixture to maintain constant volume. The samples were analyzed spectrophotometrically at 242 nm in reference with the constructed calibration curve (R2, 0.999). For each formula, drug release was studied in triplicate and the cumulative amount of drug released was determined. The results of the cumulative amount of drug released from different proniosomal formulations were compared to a control solution which was prepared by dissolving CAR (either 3.125 or 9 mg) in 1 ml of propylene glycol/ethanol solution (50%/50%, v/v). The cumulative amount of drug released per unit area (μg/cm2) was plotted as a function of time (h) for each formulation. The rate of drug release was calculated from the slope of the linear portion of the graph.
In Vitro Skin Permeation
The same procedure as in in vitro release studies was carried out except replacing the cellulose membrane with natural mice skin. Fresh hairless skin specimens of newly born mice were sandwiched securely between the donor and receptor compartments of vertical Franz diffusion cell with the epidermis side facing the donor compartment. The experiment was done under occlusion by covering the preparation by paraffin film to simulate the application conditions. For each formula, drug permeation through hairless mouse skin was studied in triplicate. The cumulative amount of drug permeated through the skin per unit area (μg/cm2) was plotted as a function of time (h) for each formulation.
RESULTS AND DISCUSSION
Preparation of Proniosomes (Preliminary Trials)
CAR is practically insoluble in water and exhibits pH-dependent solubility. Its solubility is <1 μg/ml above pH 9.0, 23 μg/ml at pH 7, and about 100 μg/ml at pH 5 at room temperature (11). The use of Sorensen’s phosphate buffer pH 7.4 for the hydration step caused the precipitation of colloidal white CAR particles which was difficult to be separated from the formed niosomes by centrifugation. Therefore, Sorensen’s phosphate buffer pH 5.5 containing 20% propylene glycol (saturation solubility = 276.4 μg/ml) was used as the hydration medium. Moreover, the use solution of pH 5.5 is expected to give better simulation of in vivo condition where it is similar to the pH of skin.
Our objective in this study is to prepare concentrated proniosomal gel formed of non-ionic surfactant, cholesterol, ethanol, and water which can be converted to a stable niosome suspension by hydration. As shown in Table 1, Brij 92 (hydrophilic–lipophilic balance (HLB) = 4.9) which is liquid at room temperature, could not form gel at cholesterol concentration lower than 30%. Similarly, Brij 78 (HLB = 15.3) although being solid at room temperature (melting point = 38°C); it did not form proniosomal gel at cholesterol concentration lower than 30%; this might be due to its high HLB value and being dispersible in water (27).
On the other hand, Brij 72 (HLB 4.9, Tc = 44°C) and Span 60 (HLB 4.7, Tc = 53°C) produced proniosomal gels in the absence or presence of cholesterol because they are solid at room temperature. In addition, they have high transition temperatures and proper low HLB value. These results were in accordance with Ibrahim et al. (18) who found that Span 20 and Span 80 that are liquids at room temperature were only able to form proniosomal gels in the presence of not less than 20% cholesterol. On the other hand, Span 40 and Span 60 that are solid at room temperature produced gels in the presence or absence of cholesterol.
The ability of the studied surfactants to form niosomes by hydration of the prepared proniosomes is summarized in Table 1. The ability of non-ionic surfactant to form vesicles depends on its structure, critical packing parameter (CPP), hydrophilic–lipophilic balance, and presence of cholesterol (28). The critical packing parameter (v/lcao), CPP, depends on the balance between the hydrophobic group volume (v), the critical hydrophobic group length (lc), and the area of the hydrophilic head group (ao). A CPP between 0.5 and 1 indicates that the surfactant is likely to form vesicles. A CPP below 0.5 indicates the spherical micelle formation and a CPP of above one would predispose a compound to form inverted micelles (29). Hydrophilic–lipophilic balance is a good indicator of the vesicle-forming ability of any surfactant. With the sorbitan monostearate (Span) surfactants, a HLB number of between 4 and 8 was found to be compatible with vesicle formation (28).
Brij 72 and Span 60 proniosomes were able to form niosomes upon hydration in absence of cholesterol because of their proper HLB and CPP. Manosroi et al. (24) reported that Brij 72 and span 60 can form vesicular structure because they have relatively large hydrophobic moieties with low water solubility. On the other hand, Brij 78 was not able to form niosome even in the presence of high concentration of cholesterol. This may be attributed to its solubilizing property, high HLB values, and therefore its micelle formation ability that dissolves the small amounts of cholesterol (21).
To study the effect of increasing cholesterol molar ratio on the amount of CAR entrapped in niosomes, a series of formulations were prepared with increasing cholesterol molar ratio (from 0 to 5 parts) and fixing both the amount of CAR (3.125 mg) and total weight of proniosomes (400 mg). The entrapment efficiency (EE%) was then determined. As shown in Table 1, for Brij 72, EE% of CAR was decreased from 26.42 to 21.95 with increasing cholesterol molar ratio from 0 to 1. Further increase in cholesterol molar ratio from 1 to 3 or 4 resulted in a sharp decrease in CAR EE% from 21.95 to 0. For Span 60, on increasing the cholesterol molar ratio from 0 to 1 to 3, a remarkable decrease in CAR EE% from 44.74 to 16.30 to 8.48, respectively, was observed.
Because of poor proniosomal gel forming ability of Brij 92 and Brij 78 and low entrapment efficiency of Brij 92 (4.06% at 7:3 surfactant to cholesterol ratio), they were excluded from further studies. Brij 72 and Span 60 were chosen as candidates for CAR proniosomal gel formulation.
Microscopic Examination
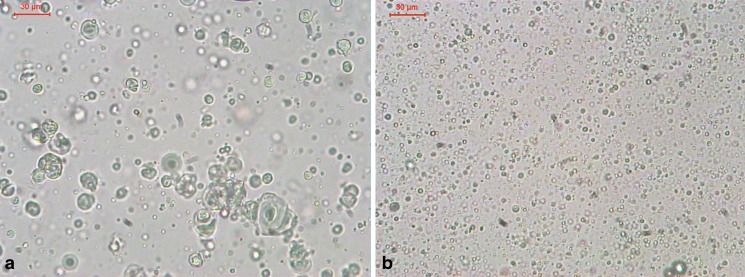
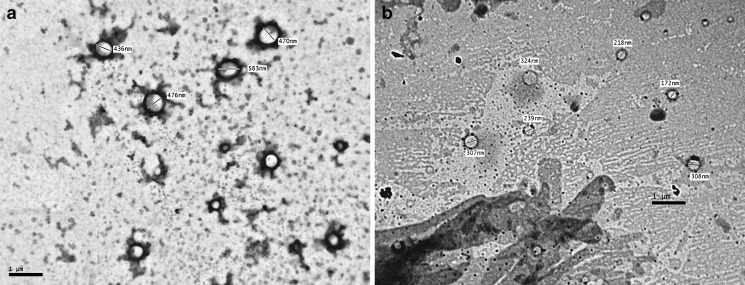
The photomicrographs and the TEM micrographs of formulations 5B and 5S are illustrated in Figs. 1 and 2, respectively. It was observed that the vesicles of the niosomes formed by hydration of the proniosomal gels are almost spherical shape and some are bilayered. It could be noticed also that the vesicles produced from formula 5S have smaller size than those of 5B.
Fig. 1.
Photomicrographs of hydrated proniosomes. a Formula 5B; b formula 5S. Bar = 30 μm
Fig. 2.
Transmission electron micrographs of hydrated proniosomes. a Formula 5B; b formula 5S. Bar = 1 μm
Vesicle Size Analysis
The proniosomal gel formulations were hydrated and the mean vesicle size of the produced niosomes was identified. Table 2 lists the mean vesicle size of the different proniosomes prepared. The mean vesicle size of Brij 72 proniosomes ranged from 574 nm (formula 3B) to 745 nm (formula 7B). While the mean vesicle size of Span 60 proniosomes ranged from 240 nm (formula 8S) to 657 nm (formula 2S). It could be noticed that the mean vesicle size of proniosomes prepared with Brij 72 was comparable. In addition, generally the mean vesicle size of proniosomes prepared with Brij 72 was greater than those prepared with Span 60. Also, the mean vesicle size of proniosomes prepared with Span 60 and containing 9 mg CAR was noticeably lower than that prepared with Span 60 and containing 3.125 mg CAR. Manconi et al. (6) suggested that the intercalation of the drug into the bilayers of niosomes leads to an increase in the cohesion among the apolar portions of the membrane, thus the vesicle diameter is reduced.
Entrapment Efficiency
The entrapment efficiency is one of the most important parameters from pharmaceutical viewpoint in the evaluation of niosomal formulations (30). Equations 3 and 4 represent the linear regression models for the Brij 72 and Span 60 proniosomes, respectively, as obtained from the factorial study.
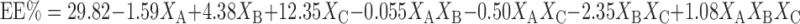 |
3 |
Where F = 85.36, p < 0.0001, and adjusted R2 = 0.9752
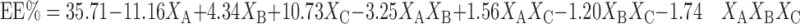 |
4 |
Where F = 89.64, p < 0.0001, and adjusted R2 = 0.9764.
From Table 3, it could be inferred that the entrapment efficiency is significantly affected by% cholesterol (XA), weight of proniosomes (XB), and amount of CAR added (XC) for Brij 72 and Span 60 proniosomes.
Table 3.
Effects, % Contribution, and p Values of the Model Coefficients Estimated from the Factorial Study for the Responses: Entrapment Efficiency (EE%) and CAR Release Rate Through Synthetic Cellulose Membrane
| Term | EE% | Release rate | ||||
|---|---|---|---|---|---|---|
| Effect | % contribution | p values for coefficients of factors | Effect | % contribution | p values for coefficients of factors | |
| Brij 72 | ||||||
| X A | −3.19 | 1.38 | 0.0200* | 0.77 | 0.81 | 0.1344 |
| X B | 8.75 | 10.42 | <0.0001* | −6.06 | 50.30 | <0.0001* |
| X C | 24.71 | 83.09 | <0.0001* | 2.51 | 8.62 | 0.0006* |
| X AB | −0.11 | 0.00 | 0.9229 | −4.39 | 26.38 | <0.0001* |
| X AC | −1.01 | 0.14 | 0.3880 | −2.48 | 8.43 | 0.0007* |
| X BC | −4.70 | 3.00 | 0.0028* | 1.51 | 3.12 | 0.0113* |
| X ABC | 2.17 | 0.64 | 0.0200 | 0.09 | 0.01 | 0.8419 |
| Span 60 | ||||||
| X A | −22.31 | 44.55 | <0.0001* | −8.73 | 52.82 | <0.0001* |
| X B | 8.68 | 6.74 | 0.0002* | −6.85 | 32.59 | <0.0001* |
| X C | 21.46 | 41.21 | <0.0001* | −0.59 | 0.24 | 0.3554 |
| X AB | −6.49 | 3.77 | 0.0012* | 3.15 | 6.89 | 0.0008* |
| X AC | 3.12 | 0.87 | 0.0465* | −1.02 | 0.72 | 0.1291 |
| X BC | −2.39 | 0.51 | 0.1091 | 1.72 | 2.04 | 0.0213* |
| X ABC | −3.49 | 1.09 | 0.0301* | 1.97 | 2.69 | 0.0112* |
*p < 0.05
On increasing the amount of CAR added, an increase in EE% was observed for both Brij 72 and Span 60 systems. This was deduced from the positive coefficients of (XC) in Eqs. 3 and 4. This result could be attributed to the saturation of the media with CAR at higher concentration that forces the drug to be encapsulated into niosomes (31). Increasing the weight of proniosomes augments EE% as confirmed by the positive coefficient of XB in Eqs. 3 and 4. Similar results were obtained by Yoshioka et al. (32) who reported a linear increase in the entrapment efficiency of 5(6)-carboxyfluorescein with increasing total lipid concentration.
The negative coefficient of XA in Eqs. 3 and 4 reveals that the% of cholesterol is inversely affecting the EE%. This could be explained on the basis that cholesterol may compete with the drug for packing space within the bilayer, hence excluding the drug as the amphiphiles assemble into the vesicles (30).
Generally, Span 60 showed higher entrapment efficiency compared to Brij 72 (Table 2) which might be due to the fact that Span has higher phase transition temperature (32). EE% of Span 60 niosomes was mainly affected by XA (44.55%) and XC (41.21%), while Brij 72 niosomes showed relatively lower contribution of XA (1.38%) but an extremely large XC effect (83%) as reported in Table 3 and confirmed by the values of XA and XC coefficients in Eqs. 3 and 4.
In Vitro Release Study
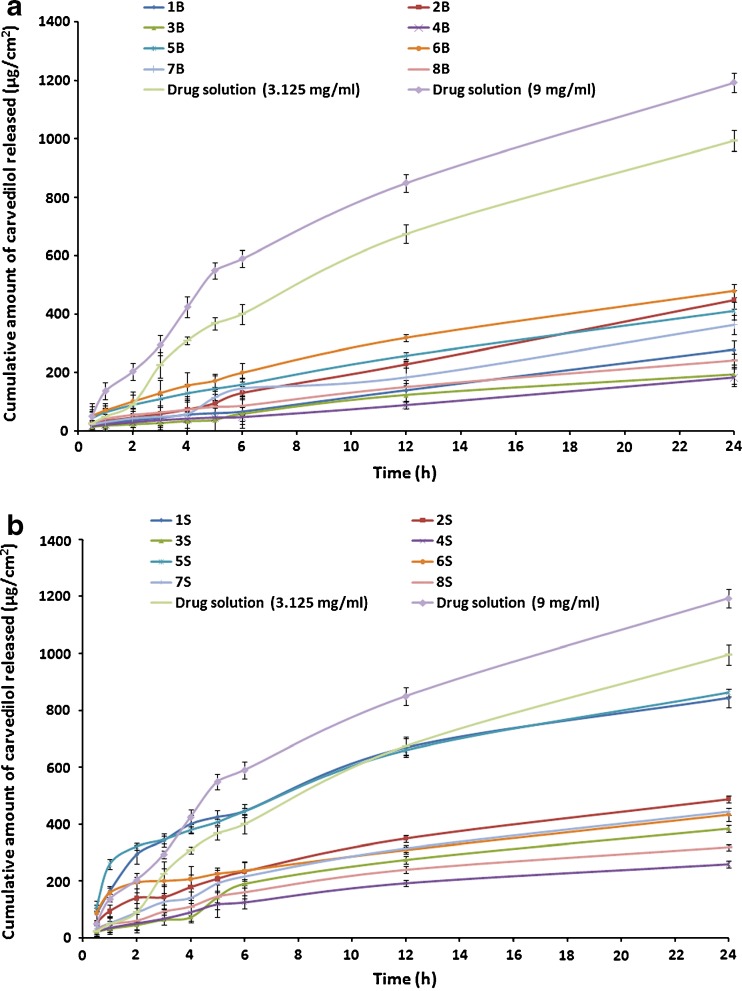
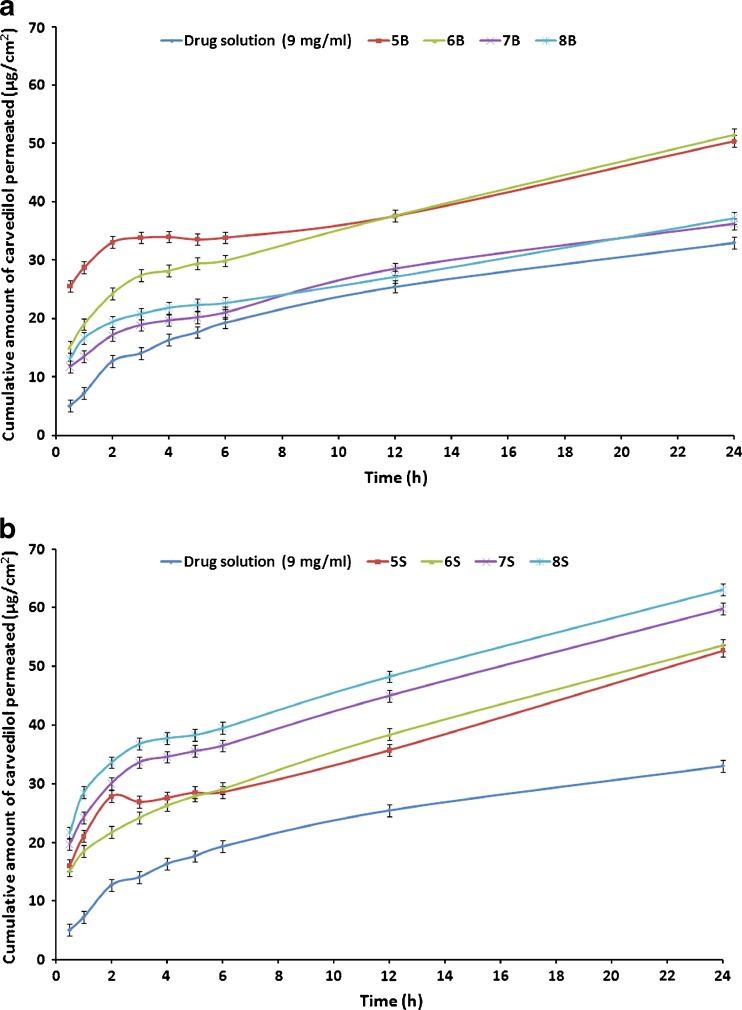
The release profiles of CAR from the proniosomal formulations prepared using either Brij or Span as surfactants are shown in Fig. 3.
Fig. 3.
In vitro release of CAR through cellulose membrane from different proniosomal gel formulations in 15%:40%:45% (v:v:v) propylene glycol–ethanol–phosphate buffer pH 7.4 at 37°C. a Brij 72 proniosomes; b Span 60 proniosomes
As shown in Table 2, the release rates of all proniosomes perpetrations were lower than those of the corresponding control solution composed of either 3.125 or 9 mg in 1 ml of propylene glycol/ethanol solution (50%/50%, v/v). This could be explained by the fact that the mean pore size of the cellulose membrane used in this study (molecular weight cut-off, 12,000–14,000) was less than 10 nm and only very small vesicles could diffuse intact across this membrane and the ability of the prepared proniosomes to retard the release of CAR (9).
From the release profiles of all proniosomes formulations, it could be noticed that the rate of CAR release from most proniosomal gels is a biphasic process containing of an initial relatively fast release and an equilibrium state or a slower release phase. The rapid initial release may be resulted from drug expulsion due the limited capacity of lipids to accommodate large amounts of drug and desorption of drug from the surface of the vesicles after niosomes formation. Then, the slower release phase may originate from CAR diffusion through the lipid bilayers of the vesicles (17,21).
Equations 5 and 6 represent the linear regression models for the Brij 72 and Span 60 proniosomes, respectively, as obtained from the factorial study.
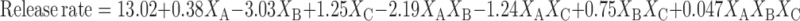 |
5 |
Where F = 47.93, p < 0.0001, and adjusted R2 = 0.9563
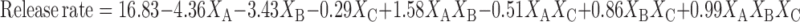 |
6 |
Where F = 55.87, p < 0.0001, and adjusted R2 = 0.9624.
From Table 3, it is clear that the significant factors in controlling CAR release are XB and XC from Brij 72 proniosomes and XA and XB from Span 60 proniosomes. The negative coefficients of XB in Eqs. 5 and 6 reveal that the weight of proniosomes is inversely affecting the release rate. This may be attributed to the fact that increasing weight of proniosomes leads to an increase in both CAR encapsulation (Table 3) and the diffusion path of CAR in the proniosomal gel, hence, a slower release rate is observed.
In Span 60 proniosomes, on increasing % of cholesterol, a decrease in the release rate was observed as indicated by the negative coefficient of XA in Eq. 6. This result could be explained by the decreased leakage and permeability of niosomal formulations in presence of cholesterol which led to lower drug elution from the vesicles (33,34).
In Brij 72 proniosomes, an enhancement in release rate was observed on increasing the amount of CAR added as demonstrated by the positive coefficient of XC in Eq. 5. This could be ascribed to the relatively lower EE% of CAR in Brij 72 compared to Span 60 niosomes (Table 2). Consequently, on increasing the amount of CAR added, not only the EE% is increased but also the amount of free CAR is increased, hence, the CAR release rate is raised.
In general, Span 60 proniosomes showed faster release rate relative to Brij 72 proniosomes (Table 2). The release rate is dominantly affected by XA (52.82%) and XB (32.59%) in case of Span 60 proniosomes and by XB (50.30%) and XAB (26.38%) in case of Brij 72 proniosomes as demonstrated by the relatively large % contribution of these factors in Table 3 and their coefficients in Eqs. 5 and 6. Again, Span 60 and Brij 72 exhibited different behaviors, here regarding CAR release rate; also they showed different vesicle size and EE% as mentioned before. These different behaviors may be ascribed to different attitudes of the non-ionic surfactants. Brij 72 and Span 60 have basically different structures, and thus different properties, bilayer flexibility, capacity of reorganization during hydration, transition temperature, and affinity to CAR which possibly led to the differences observed.
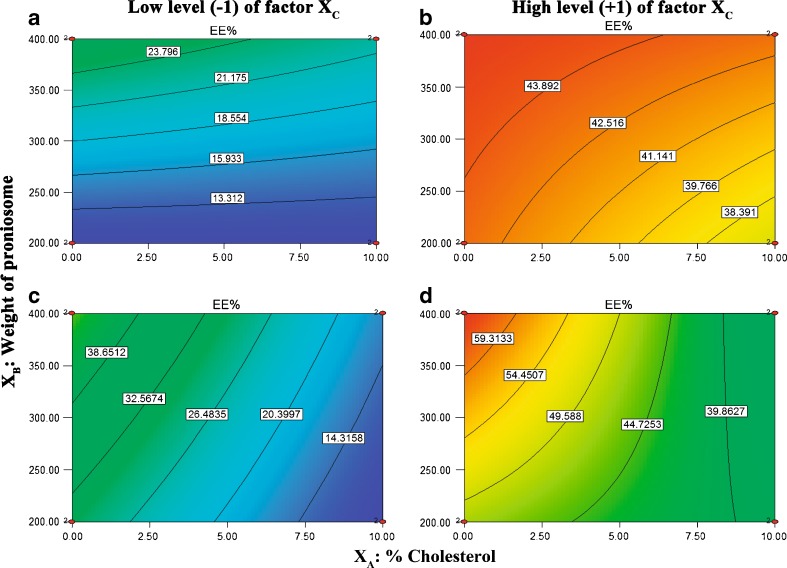
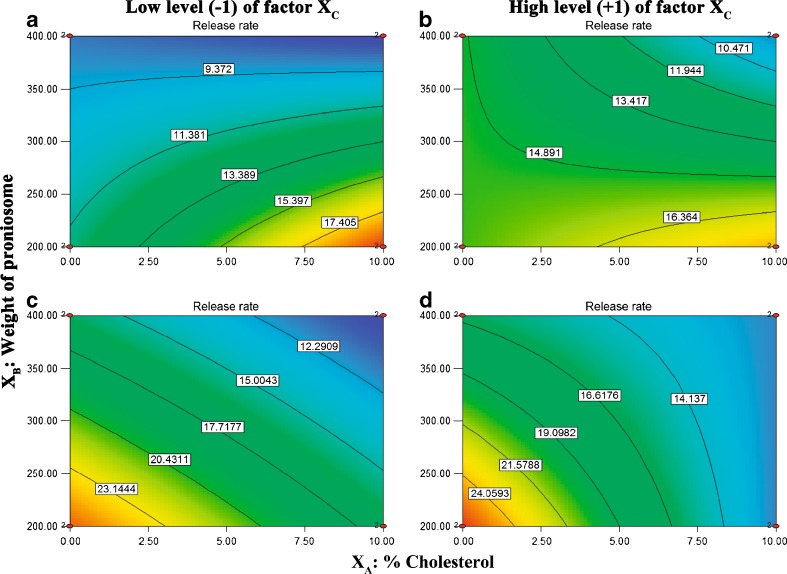
Contour Plots Interpretation
Contour plots were obtained by fixing the XC factor at its high and low level and varying (XA) and (XB) over the range used in the factorial study. Figure 4 depicts contour plots which show the effects of XA and XB on EE%. Analysis of the contour plot reveals that increasing amount of CAR added from 3.125 mg to 9 mg resulted in remarkable higher EE%. In addition, an increased EE% can be achieved by reducing % cholesterol and increasing weight of proniosomes at low and high XC levels for both Brij 72 and Span 60 proniosomes.
Fig. 4.
Contour plots of entrapment efficiency (EE%). a and b for Brij 72 proniosomal gels; c and d for Span 60 proniosomal gels
Analysis of Fig. 5 demonstrate the effects of XA and XB on CAR release rate revealed that the release rate was not greatly affected by increasing the amount of CAR added. Moreover, at low and high XC levels, in case of Brij 72 proniosomes faster release rate can be obtained by increasing% cholesterol and reducing weight of proniosomes. While in case of Span 60 proniosomes, enhanced released rate can be achieved by decreasing % cholesterol and reducing proniosomes weight. Accordingly, only formulations containing 9 mg CAR were selected for the in vitro skin permeation study.
Fig. 5.
Contour plots of release rate. a and b for Brij 72 proniosomal gels; c and d for Span 60 proniosomal gels
In Vitro Skin Permeation
The permeation profiles of CAR from the proniosomal formulations prepared using either Brij 72 or Span 60 as surfactants are shown in Fig. 6. By comparing the results obtained from permeation of CAR through hairless mice skin to those of release from cellulose membrane, it was found that the release of CAR through cellulose membrane was significantly higher than its permeation across skin (p < 0.05), indicating that the skin is a truly barrier controlling the permeation of the drug.
Fig. 6.
In vitro permeation of CAR through hairless mice skin from different proniosomal gel formulations in 15%:40%:45% (v:v:v) propylene glycol–ethanol–phosphate buffer pH 7.4 at 37°C. a Brij 72 proniosomes; b Span 60 proniosomes
It is clear that the developed proniosome gels exhibited higher skin permeation compared to the control solution containing equivalent amount of CAR (Fig. 6). This difference in the drug permeation patterns could be related to lower ability of CAR to penetrate skin layers and the permeation enhancing effect of the prepared CAR proniosomal gels. This observed penetration enhancement effect may be attributed to both the presence of non-ionic surfactants and the formation of niosomes on hydration of proniosomes. There are two possible mechanisms by which skin permeation is improved by surfactants. Firstly, surfactants may increase fluidity, solubilize, and extract lipid component in the stratum corneum. Secondly, they may interact and bind with keratin filaments resulting in a disruption within the corneocyte (35). Niosomes may enhance the permeability of drugs through structure modification of stratum corneum, previous researchers reported that the intercellular lipid barrier in the stratum corneum would be more permeable following treatment with niosomes (36,37). Additionally, adsorption and fusion of drug load niosomes onto the surface of the skin leads to a high thermodynamic activity gradient of the drug in upper part of the stratum corneum facilitating drug permeation (5,36).
As shown in Fig. 6, CAR was detected in the receptor compartment in the first 0.5 h for all proniosomal gel formulations. This could suggest that water penetration through skin from receptor compartment, hydration of CAR proniosomal gels to form niosomes, and eventually the permeation of CAR across the skin occurred very rapidly (9).
Equations 7 and 8 represent the linear regression models for the Brij 72 and Span 60 proniosomes, respectively, as obtained from the factorial study.
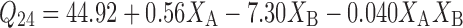 |
7 |
Where F = 57.97, p = 0.0009, and adjusted R2 = 0.9607
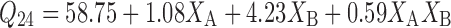 |
8 |
Where F = 12.54, p = 0.0167, and adjusted R2 = 0.8319
From Table 4, it can be inferred that % cholesterol had insignificant effect on the cumulative amount of CAR permeated hairless mice skin per unit area after 24 h (Q24). The result is in agreement with other reports (9,19). However, other report (18) suggested that reducing cholesterol content caused an increase in the drug permeation. These results may indicate that drugs permeate through the skin by different mechanisms depending on the composition of proniosomes and the drug used (19).
Table 4.
Effects, % Contribution, and p Values of the Model Coefficients Estimated from the Factorial Study for the Response: Cumulative Amount of CAR Permeated Hairless Mice Skin per Unit Area After 24 h (Q 24)
| Term | Q 24 | ||
|---|---|---|---|
| Effect | % contribution | p values for coefficients of factors | |
| Brij 72 | |||
| X A | 1.12 | 0.57 | 0.3693 |
| X B | −14.60 | 97.17 | 0.0002* |
| X AB | −0.08 | 0.00 | 0.9462 |
| Span 60 | |||
| X A | 2.17 | 5.46 | 0.2061 |
| X B | 8.46 | 83.29 | 0.0042* |
| X AB | 1.19 | 1.64 | 0.4554 |
*p < 0.05
Generally, Span 60 proniosomes demonstrated higher CAR skin permeation relative to Brij 72 proniosomes (Table 2). Moreover, as shown in Table 4, cumulative amount of CAR permeated hairless mice skin per unit area after 24 h (Q24) was only significantly affected by the weight of proniosomes (XB). This is confirmed by the extremely large % contribution of XB in Table 4 for both Brij 72 (97.14%) and Span 60 (83.29%) proniosomal gels. Interestingly, by examining the signs of the coefficients of XB in Eqs. 7 and 8, it is clear that the weight of proniosomes exerts opposite effects on Q24 in Brij 72 and Spans 60 proniosomes. On increasing the weight of proniosomes, Q24 was decreased in case of Brij 72 proniosomes but increased in case of Span 60 proniosomes. This outcome could be explained by combining the results of both the in vitro release study through synthetic cellulose membrane and the in vitro permeation study through hairless mice skin. It could be suggested that the net effect of the weight of proniosomes depends on the permeation enhancement effect of the used proniosomal gel and the release retardant effect proved in the in vitro release study through synthetic membrane. Consequently, it could be concluded that the CAR permeation enhancement effect of Span 60 proniosomal gels under the conditions of the study is greater than that of Brij 72 to an extent that it can overcome its release retardant effect.
CONCLUSION
Concluding the abovementioned results, polyoxyethylene alkyl ether (Brij 72) and sorbitan fatty ester (Span 60) types of non-ionic surfactants can be used for preparation of carvedilol-entrapping proniosomes. The type of surfactant, its HLB, transition temperature, and cholesterol content affect the capability of proniosomal gel formation and the niosome-forming ability from proniosomes. The release rate of carvedilol through synthetic cellulose membrane and its EE% in the niosomes prepared by the proniosomal method are affected by formulation variables; such as cholesterol content, total proniosomes weight, and drug concentration. The investigations also revealed the potentiality of proniosomes in enhancing transdermal delivery of carvedilol. Permeation experiments of the proniosomes showed that the permeation of carvedilol through mice skin is mainly affected by the weight of proniosomes and that Span 60 CAR proniosomal gels exhibited higher skin permeation than those of Brij 72.
References
- 1.Couvreur P, Fattal E, Andremont A. Liposomes and nanoparticles in the treatment of intracellular bacterial infections. Pharm Res. 1991;8:1079–1086. doi: 10.1023/A:1015885814417. [DOI] [PubMed] [Google Scholar]
- 2.Arunothayanun P, Uchegbu IF, Craig DQ, Turton JA, Florence AT. In vitro/in vivo characterisation of polyhedral niosomes. Int J Pharm. 1999;183:57–61. doi: 10.1016/S0378-5173(99)00044-7. [DOI] [PubMed] [Google Scholar]
- 3.Baroli B, Delogu G, Fadda AM, Podda G, Sinico C. Vesicle formation from hexasubstituted cyclophosphazenic derivatives. Int J Pharm. 1999;183:101–107. doi: 10.1016/S0378-5173(99)00024-1. [DOI] [PubMed] [Google Scholar]
- 4.Schreier H, Bouwstra J. Liposomes and niosomes as topical drug carriers: dermal and transdermal drug delivery. J Control Release. 1994;30:1–15. doi: 10.1016/0168-3659(94)90039-6. [DOI] [Google Scholar]
- 5.Fang J-Y, Hong C-T, Chiu W-T, Wang Y-Y. Effect of liposomes and niosomes on skin permeation of enoxacin. Int J Pharm. 2001;219:61–72. doi: 10.1016/S0378-5173(01)00627-5. [DOI] [PubMed] [Google Scholar]
- 6.Manconi M, Sinico C, Valenti D, Loy G, Fadda AM. Niosomes as carriers for tretinoin. I. Preparation and properties. Int J Pharm. 2002;234:237–248. doi: 10.1016/S0378-5173(01)00971-1. [DOI] [PubMed] [Google Scholar]
- 7.Hu C, Rhodes DG. Proniosomes: a novel drug carrier preparation. Int J Pharm. 2000;206:110–122. doi: 10.1016/S0378-5173(00)00513-5. [DOI] [PubMed] [Google Scholar]
- 8.Vora B, Khopade AJ, Jain NK. Proniosome based transdermal delivery of levonorgestrel for effective contraception. J Control Release. 1998;54:149–165. doi: 10.1016/S0168-3659(97)00100-4. [DOI] [PubMed] [Google Scholar]
- 9.Fang JY, Yu SY, Wu PC, Huang YB, Tsai YH. In vitro skin permeation of estradiol from various proniosome formulations. Int J Pharm. 2001;215:91–99. doi: 10.1016/S0378-5173(00)00669-4. [DOI] [PubMed] [Google Scholar]
- 10.Varshosaz J, Pardakhty A, Baharanchi SM. Sorbitan monopalmitate-based proniosomes for transdermal delivery of chlorpheniramine maleate. Drug Deliv. 2005;12:75–82. doi: 10.1080/10717540490446044. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty S, Shukla D, Jain A, Mishra B, Singh S. Assessment of solubilization characteristics of different surfactants for carvedilol phosphate as a function of pH. J Colloid Interface Sci. 2009;335:242–249. doi: 10.1016/j.jcis.2009.03.047. [DOI] [PubMed] [Google Scholar]
- 12.Wen X, Tan F, Jing Z, Liu Z. Preparation and study the 1:2 inclusion complex of carvedilol with beta-cyclodextrin. J Pharm Biomed Anal. 2004;34:517–523. doi: 10.1016/S0731-7085(03)00576-4. [DOI] [PubMed] [Google Scholar]
- 13.Tanwar YS, Chauhan CS, Sharma A. Development and evaluation of carvedilol transdermal patches. Acta Pharm. 2007;57:151–159. doi: 10.2478/v10007-007-0012-x. [DOI] [PubMed] [Google Scholar]
- 14.Ubaidulla U, Reddy MV, Ruckmani K, Ahmad FJ, Khar RK. Transdermal therapeutic system of carvedilol: effect of hydrophilic and hydrophobic matrix on in vitro and in vivo characteristics. AAPS PharmSciTech. 2007;8:2. doi: 10.1208/pt0801002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Kattan A, Asbill CS, Haidar S. Transdermal testing: practical aspects and methods. Pharm Sci Technol Today. 2000;3:426–430. doi: 10.1016/S1461-5347(00)00316-3. [DOI] [PubMed] [Google Scholar]
- 16.Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin’s barrier function. Pharm Sci Technol Today. 2000;3:318–326. doi: 10.1016/S1461-5347(00)00295-9. [DOI] [PubMed] [Google Scholar]
- 17.Mokhtar M, Sammour OA, Hammad MA, Megrab NA. Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Int J Pharm. 2008;361:104–111. doi: 10.1016/j.ijpharm.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim MM, Sammour OA, Hammad MA, Megrab NA. In vitro evaluation of proniosomes as a drug carrier for flurbiprofen. AAPS PharmSciTech. 2008;9:782–790. doi: 10.1208/s12249-008-9114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alsarra IA, Bosela AA, Ahmed SM, Mahrous GM. Proniosomes as a drug carrier for transdermal delivery of ketorolac. Eur J Pharm Biopharm. 2005;59:485–490. doi: 10.1016/j.ejpb.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Azeem A, Jain N, Iqbal Z, Ahmad FJ, Aqil M, Talegaonkar S. Feasibility of proniosomes-based transdermal delivery of frusemide: formulation optimization and pharmacotechnical evaluation. Pharm Dev Technol. 2008;13:155–163. doi: 10.1080/10837450701831211. [DOI] [PubMed] [Google Scholar]
- 21.Pardakhty A, Varshosaz J, Rouholamini A. In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. Int J Pharm. 2007;328:130–141. doi: 10.1016/j.ijpharm.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Mura S, Pirot F, Manconi M, Falson F, Fadda AM. Liposomes and niosomes as potential carriers for dermal delivery of minoxidil. J Drug Target. 2007;15:101–108. doi: 10.1080/10611860600991993. [DOI] [PubMed] [Google Scholar]
- 23.Bayindir ZS, Yuksel N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J Pharm Sci. 2010;99:2049–2060. doi: 10.1002/jps.21944. [DOI] [PubMed] [Google Scholar]
- 24.Manosroi A, Wongtrakul P, Manosroi J, Sakai H, Sugawara F, Yuasa M, et al. Characterization of vesicles prepared with various non-ionic surfactants mixed with cholesterol. Colloids Surf, B. 2003;30:129–138. doi: 10.1016/S0927-7765(03)00080-8. [DOI] [Google Scholar]
- 25.Pund S, Joshi A, Vasu K, Nivsarkar M, Shishoo C. Multivariate optimization of formulation and process variables influencing physico-mechanical characteristics of site-specific release isoniazid pellets. Int J Pharm. 2010;388:64–72. doi: 10.1016/j.ijpharm.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 26.Vishnu YV, Chandrasekhar K, Ramesh G, Rao YM. Development of mucoadhesive patches for buccal administration of carvedilol. Curr Drug Deliv. 2007;4:27–39. doi: 10.2174/156720107779314785. [DOI] [PubMed] [Google Scholar]
- 27.Rowe RC, Sheskey PJ, Owen SC. Handbook of Pharmaceutical Excipients. Sixith edition ed: Pharmaceutical Press and the American Pharmacists Association; 2009
- 28.Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172:33–70. doi: 10.1016/S0378-5173(98)00169-0. [DOI] [Google Scholar]
- 29.Uchegbu IF, Florence AT. Non-ionic surfactant vesicles (niosomes): physical and pharmaceutical chemistry. Adv Colloid Interface Sci. 1995;58:1–55. doi: 10.1016/0001-8686(95)00242-I. [DOI] [Google Scholar]
- 30.Balakrishnan P, Shanmugam S, Lee WS, Lee WM, Kim JO, Oh DH, et al. Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int J Pharm. 2009;377:1–8. doi: 10.1016/j.ijpharm.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 31.El-Samaligy MS, Afifi NN, Mahmoud EA. Increasing bioavailability of silymarin using a buccal liposomal delivery system: preparation and experimental design investigation. Int J Pharm. 2006;308:140–148. doi: 10.1016/j.ijpharm.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Yoshioka T, Sternberg B, Florence AT. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60 and 80) and a sorbitan triester (Span 85) Int J Pharm. 1994;105:1–6. doi: 10.1016/0378-5173(94)90228-3. [DOI] [Google Scholar]
- 33.Vemuri S, Yu C-D, Degroot JS, Roosdorp N. In vitro interaction of sized and unsized liposome vesicles with high density lipo proteins. Drug Dev Ind Pharm. 1990;16:1579–1584. doi: 10.3109/03639049009074385. [DOI] [Google Scholar]
- 34.Virtanen JA, Ruonala M, Vauhkonen M, Somerharju P. Lateral organization of liquid-crystalline cholesterol-dimyristoylphosphatidylcholine bilayers. Evidence for domains with hexagonal and centered rectangular cholesterol superlattices. Biochemistry. 1995;34:11568–11581. doi: 10.1021/bi00036a033. [DOI] [PubMed] [Google Scholar]
- 35.Park ES, Chang SY, Hahn M, Chi SC. Enhancing effect of polyoxyethylene alkyl ethers on the skin permeation of ibuprofen. Int J Pharm. 2000;209:109–119. doi: 10.1016/S0378-5173(00)00559-7. [DOI] [PubMed] [Google Scholar]
- 36.Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci. 2001;14:101–114. doi: 10.1016/S0928-0987(01)00167-1. [DOI] [PubMed] [Google Scholar]
- 37.Ogiso T, Niinaka N, Iwaki M. Mechanism for enhancement effect of lipid disperse system on percutaneous absorption. J Pharm Sci. 1996;85:57–64. doi: 10.1021/js950178x. [DOI] [PubMed] [Google Scholar]