INTRODUCTION
In drug discovery and formulation, drug solubility in water and organic solvent plays an important role and affects many pharmaceutical processes including design, synthesis, extraction, purification, formulation, absorption, and distribution in body fluids (1). In most cases, aqueous solubility of a chemical compound as a medicine is not enough to be applicable in pharmaceutical formulations and clinical administration. Hence, different kinds of solubilization techniques including cosolvency, complexation, micellization, and salt formation have been applied to increase the solubility (1,2). However, in some cases, it is required to reduce solubility in the medium, for example, in crystallization process (3). These methods not only influence the solubility of a compound, but can also alter its stability in the liquid medium (1). Cosolvency is the most feasible method for this purpose, and the most common pharmaceutical cosolvent is ethanol. Another useful and more employed method is salt formation, and an accountable proportion of the available medicinal compounds is in salt form. For developing liquid formulations of these compounds or crystallization process design, the use of cosolvents might be necessary to influence their solubility/stability.
To speed up the development processes in the pharmaceutical industry, calculative models for solubility prediction in mixture of solvents have been proposed in recent decades (1,2). Almost all of the proposed models were designed for solubility correlation/prediction of the non-electrolytes in the solvent mixtures (1,2). The main pattern of solubility behavior in water + ethanol mixture has a maximum of solubility in ethanol-rich area for most of the non-electrolyte solutes (1,2). This pattern is not the same for ionizable compounds in water such as sodium salts of medicines and amino acids where the solubility value in water is more than solubility value in neat ethanol (4).
Maybe changes in dielectric constant of the medium have a dominant effect on the solubility of the ionizable solute in which higher dielectric constant can cause more ionization of the solute and results in more solubilization (5). As an example, water (DW,298 = 78.5) has higher dissociation strength on ions in comparison with ethanol (DE,298 = 24.2) which is resulted in more solubilization power of ions in water. Born has proposed a theoretical model for solubility correlation in two different phases as following equation (6):
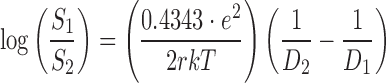 |
1 |
where S1 and S2 are the solubilities of the solute in media 1 and 2; e is the charge of an electron; r is the effective radius of the ion in the medium; k is the Boltzmann constant; T is the absolute temperature; and D1 and D2 are the dielectric constants of the media 1 and 2, respectively. Unfortunately, by using this equation, the predicted solubility values (when r values are known) or predicted r values (when solubility values are known) based on experimental data do not seem to be meaningful (7). However, one can consider the constant value of 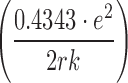 as AT for a specific solute and obtain:
as AT for a specific solute and obtain:
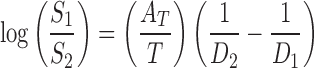 |
2 |
where AT is a slope which can be calculated using two experimental solubility data points (e.g., solubility values in water and ethanol).
In this technical note, the predictability of Eq. 2 for solubility data of ionizable solutes in water + ethanol mixtures with acceptable prediction error is reported.
DATA AND COMPUTATIONAL METHODS
Available solubility data for electrolytes and zwitterions in water + ethanol mixtures were extracted from the literature (1,4,8). The criterion for inclusion in selected data was the availability of solubility data in water and ethanol, where SW > SE.
Dielectric constants of water and ethanol at different temperatures were collected from the literature (9). For calculating dielectric constant of water and ethanol at different temperatures other than those reported by Akerlof, we interpolated the data with following equation described by Akerlof:
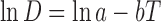 |
3 |
where lna and b are the intercept and coefficient of the regression, respectively. The calculated values of dielectric constant for water and ethanol at different temperatures were used in a previously trained version of the Jouyban-Acree model for prediction of dielectric constant of the water + ethanol at different temperatures (10):
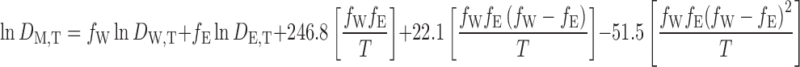 |
4 |
where DM,T, DW,T, and DE,T are dielectric constants of the solvent mixture, water, and ethanol at temperature T (Kelvin); fW and fE are the volume fractions of water and ethanol, respectively.
For solubility prediction using Eq. 2, the AT values were calculated using only two solubility data points in mono-solvents, i.e., SW,T and SE,T, as:
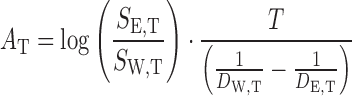 |
5 |
After calculation of the AT values for each solute at the temperature of interest and calculation of the dielectric constants of water + ethanol mixture at related temperature, the solubility amount in mixed solvents were calculated using:
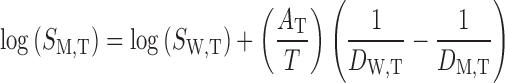 |
6 |
The mean relative deviation (MRD) is used as an error criterion:
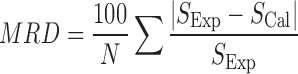 |
7 |
where N is the number of data in each set, and SExp and SCal are experimental and calculated solubility values.
RESULTS
The resulted equations for dielectric constant prediction of water and ethanol at different temperatures are as follows, respectively:
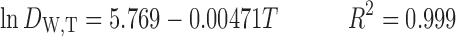 |
8 |
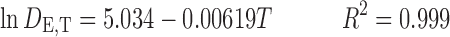 |
9 |
List of extracted data sets, their related AT values calculated by Eq. 5, and produced MRD values are tabulated in Table I. For total 400 data points of 45 sets of 14 compounds, the overall MRD value is 23.8 ± 25.4%, with the highest and the lowest MRD values of 46.3% and 7.0% for clindamycin phosphate (at 283 K) and sodium sulfamethazine (at 283 K), respectively. By excluding clindamycin phosphate data sets which produce high errors in prediction, for 302 data points of 31 sets of 13 compounds, the overall MRD value is 18.8 ± 18.8%, with the highest MRD value of 40.2% for glycyl glycine. The expected prediction error (23.8% or 18.8%) is within an acceptable range, where 30% error is accepted for correlating the solubility data in the pharmaceutical area (11,12). The prediction error for solubility of drugs in water + ethanol mixtures employing experimental data in mono-solvents using previously trained versions of the cosolvency models are 52.9% (13) and 40.7% (14), and the produced prediction error for the method proposed in this work is less than these errors.
Table I.
List of Studied Data Sets in Calculations and Related Properties
| Set | Solute | T | A T | MRD | N | logS W | logS E | logS W–logS E | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Alanine (Beta) | 298 | 32882.36 | 38.2 | 7 | −0.816 | −3.955 | 3.139 | 8 |
| 2 | Alanine (DL) | 298 | 29935.18 | 20.3 | 7 | −1.490 | −4.347 | 2.858 | 8 |
| 3 | Asparagine (L) | 298 | 35637.41 | 22.6 | 5 | −2.468 | −5.870 | 3.402 | 8 |
| 4 | Aspartic acid (L) | 298 | 31415.81 | 35.2 | 7 | −3.168 | −6.167 | 2.999 | 8 |
| 5 | Clindamycin phosphate | 278 | 50080.77 | 45.6 | 7 | −0.754 | −5.222 | 4.468 | 1 |
| 6 | Clindamycin phosphate | 283 | 36393.46 | 46.3 | 7 | −0.745 | −4.046 | 3.301 | 1 |
| 7 | Clindamycin phosphate | 288 | 35941.97 | 37.7 | 7 | −0.731 | −4.046 | 3.315 | 1 |
| 8 | Clindamycin phosphate | 293 | 34733.16 | 35.8 | 7 | −0.700 | −3.959 | 3.259 | 1 |
| 9 | Clindamycin phosphate | 298 | 33114.89 | 34.0 | 7 | −0.693 | −3.854 | 3.161 | 1 |
| 10 | Clindamycin phosphate | 303 | 30269.38 | 32.2 | 7 | −0.679 | −3.620 | 2.941 | 1 |
| 11 | Clindamycin phosphate | 308 | 28760.03 | 32.6 | 7 | −0.664 | −3.509 | 2.844 | 1 |
| 12 | Clindamycin phosphate | 313 | 27969.18 | 33.8 | 7 | −0.652 | −3.469 | 2.817 | 1 |
| 13 | Clindamycin phosphate | 318 | 26662.22 | 36.2 | 7 | −0.642 | −3.377 | 2.734 | 1 |
| 14 | Clindamycin phosphate | 323 | 25957.73 | 41.7 | 7 | −0.635 | −3.347 | 2.712 | 1 |
| 15 | Clindamycin phosphate | 328 | 25355.32 | 43.7 | 7 | −0.629 | −3.328 | 2.699 | 1 |
| 16 | Clindamycin phosphate | 333 | 24276.41 | 43.1 | 7 | −0.619 | −3.252 | 2.633 | 1 |
| 17 | Clindamycin phosphate | 338 | 22900.79 | 42.1 | 7 | −0.599 | −3.131 | 2.532 | 1 |
| 18 | Clindamycin phosphate | 343 | 21441.68 | 39.5 | 7 | −0.534 | −2.951 | 2.417 | 1 |
| 19 | Dexamethasone sodium phosphate | 298 | 22617.99 | 31.0 | 10 | −1.598 | −3.757 | 2.159 | 1 |
| 20 | Glycine | 298 | 35530.97 | 27.9 | 7 | −1.246 | −4.638 | 3.392 | 8 |
| 21 | Glycyl glycine | 298 | 45746.19 | 40.2 | 7 | −1.522 | −5.889 | 4.367 | 8 |
| 22 | Leucine (L) | 298 | 16991.14 | 25.2 | 7 | −2.503 | −4.125 | 1.622 | 8 |
| 23 | Norleucine (DL) | 298 | 14812.25 | 24.8 | 7 | −2.801 | −4.215 | 1.414 | 8 |
| 24 | Sodium sulfadiazine | 278 | 30661.88 | 17.8 | 11 | −1.389 | −4.124 | 2.735 | 4 |
| 25 | Sodium sulfadiazine | 283 | 30178.20 | 16.9 | 11 | −1.371 | −4.108 | 2.737 | 4 |
| 26 | Sodium sulfadiazine | 288 | 29687.43 | 17.0 | 11 | −1.354 | −4.092 | 2.738 | 4 |
| 27 | Sodium sulfadiazine | 293 | 29200.49 | 17.2 | 11 | −1.337 | −4.076 | 2.740 | 4 |
| 28 | Sodium sulfadiazine | 298 | 28714.22 | 18.1 | 11 | −1.321 | −4.062 | 2.741 | 4 |
| 29 | Sodium sulfadiazine | 303 | 28244.33 | 19.0 | 11 | −1.304 | −4.048 | 2.744 | 4 |
| 30 | Sodium sulfadiazine | 308 | 27752.49 | 20.6 | 11 | −1.288 | −4.033 | 2.745 | 4 |
| 31 | Sodium sulfamerazine | 278 | 22493.65 | 10.1 | 11 | −1.936 | −3.943 | 2.007 | 4 |
| 32 | Sodium sulfamerazine | 283 | 22293.56 | 10.9 | 11 | −1.884 | −3.906 | 2.022 | 4 |
| 33 | Sodium sulfamerazine | 288 | 21972.89 | 11.7 | 11 | −1.831 | −3.858 | 2.027 | 4 |
| 34 | Sodium sulfamerazine | 293 | 21728.39 | 13.6 | 11 | −1.790 | −3.829 | 2.039 | 4 |
| 35 | Sodium sulfamerazine | 298 | 21551.10 | 16.0 | 11 | −1.736 | −3.793 | 2.057 | 4 |
| 36 | Sodium sulfamerazine | 303 | 21371.01 | 18.9 | 11 | −1.687 | −3.763 | 2.076 | 4 |
| 37 | Sodium sulfamerazine | 308 | 21064.28 | 21.5 | 11 | −1.642 | −3.725 | 2.083 | 4 |
| 38 | Sodium sulfamethazine | 278 | 14728.76 | 8.5 | 11 | −1.699 | −3.013 | 1.314 | 4 |
| 39 | Sodium sulfamethazine | 283 | 14311.38 | 7.0 | 11 | −1.656 | −2.954 | 1.298 | 4 |
| 40 | Sodium sulfamethazine | 288 | 13810.28 | 9.3 | 11 | −1.611 | −2.885 | 1.274 | 4 |
| 41 | Sodium sulfamethazine | 293 | 13488.69 | 13.9 | 11 | −1.568 | −2.833 | 1.266 | 4 |
| 42 | Sodium sulfamethazine | 298 | 13211.81 | 17.7 | 11 | −1.524 | −2.785 | 1.261 | 4 |
| 43 | Sodium sulfamethazine | 303 | 12942.27 | 21.1 | 11 | −1.479 | −2.737 | 1.257 | 4 |
| 44 | Sodium sulfamethazine | 308 | 12938.35 | 23.7 | 11 | −1.430 | −2.709 | 1.280 | 4 |
| 45 | Valine (DL) | 298 | 22610.62 | 21.2 | 7 | −1.967 | −4.126 | 2.158 | 8 |
MRD mean relative deviation, DL mean racemic mixture of the solute, L mean L-enantiomer of the solute
DISCUSSION
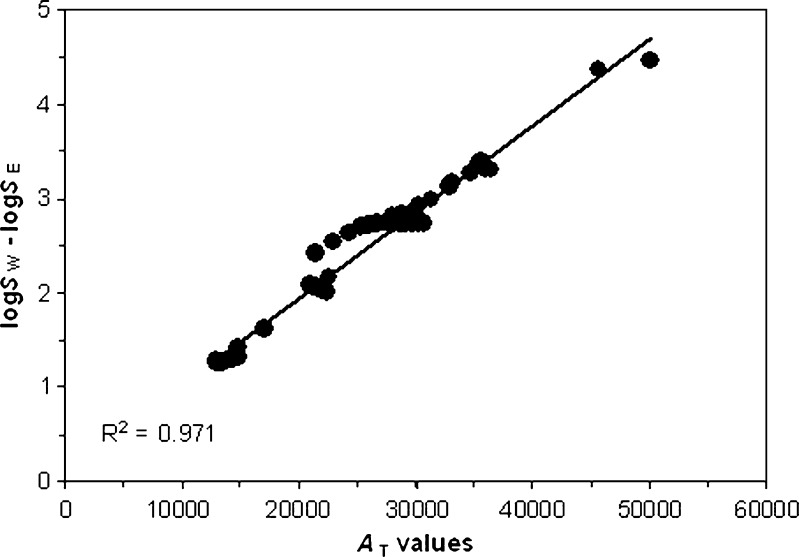
The resulted AT values show indirect relation with temperature. This is expected, as it has indirect correlation with r of Eq. 1 which has direct correlation with temperature. Also, it seems that AT values are not mainly affected by the structure of the solutes under study. For example, the structural difference between three studied sulfonamides is just on two methyl groups. But, their AT values are rather different values. On the other hand, there is a good correlation between AT values and logSW–logSE of the solutes (Fig. 1). This might suggest the effect of the difference among solubilities in water and ethanol on the solubility behavior of drugs in their mixture.
Fig. 1.
The correlation between A T values and logS W–logS E of the solutes
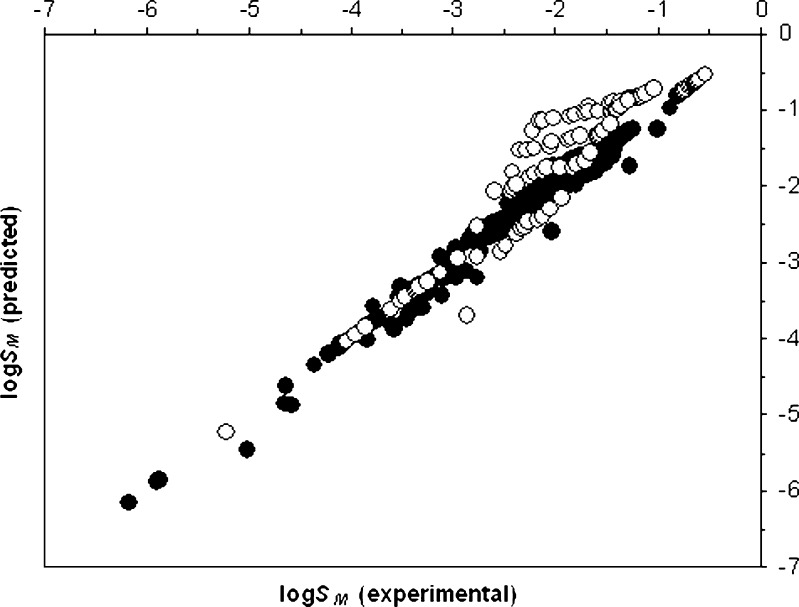
The main advantage of the proposed model is that it does not require any experimental solubility data in mixed solvents. Just two experimental solubility data points in mono-solvents and dielectric constants of solvent systems under consideration are employed in the prediction process. It almost provides good results which might show its applicability in solubility prediction (Fig. 2). These characteristics make it a suitable model for solubility prediction of electrolyte and zwitterionic solutes in water + ethanol mixtures.
Fig. 2.
Logarithm of experimental solubility data versus logarithm of predicted solubility data for 400 studied solubility data points (closed circle: solubility data for drugs except clindamycin phosphate; open circle: data for clindamycin phosphate)
The proposed model is tested on the solubility of electrolytes or zwitterions in water + ethanol mixtures in which the ionization is a dominant parameter in the solubility phenomenon. We expect more solubility in higher dielectric constants (water-rich area) and considered SW > SE as an inclusion criteria.
The main disadvantage of the proposed prediction method is that it is applicable only for the solubility prediction of electrolytes or zwitterions in which the ionization is the dominant parameter and the phenomenon could be represented using Born model.
SUMMARY AND CONCLUSIONS
Improving solubility prediction methods or making them easy to use is highly demanded in the pharmaceutical industries. It can be used in formulation design and pharmaceutically related processes such as crystallization. In this work, we have showed that dielectric constants of solvents and their mixtures might be helpful in solubility prediction of electrolytes and zwitterions with acceptable error in water + ethanol mixtures.
REFERENCES
- 1.Jouyban A. Handbook of solubility data for pharmaceuticals. Boca Raton: CRC Press; 2009. [Google Scholar]
- 2.Jouyban A. Review of the cosolvency models for predicting solubility of drugs in water-cosolvent mixtures. J Pharm Pharm Sci. 2008;11:32–58. doi: 10.18433/j3pp4k. [DOI] [PubMed] [Google Scholar]
- 3.Widenski DJ, Abbas A, Romagnoli JA. Effect of the solubility model on antisolvent crystallization predicted volume mean size. Chem Eng Trans. 2009;17:639–44. [Google Scholar]
- 4.Delgado DR, Martínez F, Fakhree MAA, Jouyban A. Study of the solubility of some sodium sulfonamides in ethanol + water cosolvent mixtures. Biomed Int. 2010 (in press).
- 5.Paruta AN, Sciarrone BJ, Lordi NG. Correlation between solubility parameters and dielectric constants. J Pharm Sci. 1962;51:704–5. doi: 10.1002/jps.2600510726. [DOI] [PubMed] [Google Scholar]
- 6.Born M. Volumen und Hydratationswärme der Ionen. Zeitschrift für Physik. 1920;1:45–48. doi:10.1007/BF01881023.
- 7.Larson RG, Hunt H. Molecular forces and solvent power. J Phys Chem. 1939;43:417–23. doi: 10.1021/j150391a003. [DOI] [Google Scholar]
- 8.Greenstein JP, Winitz M. Chemistry of amino acids. New York: Wiley; 1961. [Google Scholar]
- 9.Akerlof G. Dielectric constants of some organic solvent-water mixtures at various temperatures. J Am Chem Soc. 1932;54:4125–39. doi: 10.1021/ja01350a001. [DOI] [Google Scholar]
- 10.Jouyban A, Soltanpour Sh, Chan H-K. A simple relationship between dielectric constant of mixed solvents with solvent composition and temperature. Int J Pharm. 2004;269:353–60. doi: 10.1016/j.ijpharm.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Reillo A, Cordoba M, Escalera B, Selles E, Cordoba M., Jr Prediction of sulfamethiazole solubility in dioxane-water mixtures. Pharmazie. 1995;50:472–5. [Google Scholar]
- 12.Beerbower A, Wu PL, Martin A. Expanded solubility parameter approach 1. Naphthalene and benzoic acid in individual solvents. J Pharm Sci. 1984;73:179–88. doi: 10.1002/jps.2600730210. [DOI] [PubMed] [Google Scholar]
- 13.Jouyban A, Acree WE., Jr In silico prediction of drug solubility in water–ethanol mixtures using Jouyban-Acree model. J Pharm Pharm Sci. 2006;9:262–9. [PubMed] [Google Scholar]
- 14.Jouyban A, Soltanpour Sh, Acree WE., Jr Improved prediction of drug solubility in ethanol + water mixtures at various temperatures. Biomed Int. 2010;1:19–24. [Google Scholar]