Abstract
The freeze–drying behavior of three model proteins, namely, lysozyme, BSA, and IgG, has been studied using a variety of techniques under two different primary drying conditions (shelf temperatures of −25°C and +25°C, respectively) in an amorphous formulation. Manometric temperature measurements were used to characterize product temperature (Tpr), sublimation rates, and product resistance (Rp) during primary drying. Biophysical techniques such as circular dichroism, fluorescence, and Fourier transform infrared spectroscopy were used to study protein conformation. Size exclusion chromatography was used to monitor the formation of high-molecular-weight species (HMWS) over time on storage, and cake morphology was studied using scanning electron microscopy. The differences in the freeze–drying behavior of the three proteins were more evident at higher protein concentrations, where the protein significantly influences the behavior of the formulation matrix. However, these differences were minimized in the aggressive mode and were insignificant at lower protein concentrations where excipients dominated the freeze–drying behavior. Differences in cake morphology were observed between the two drying conditions employed as well as between the three proteins studied. The stability and the protein structure, however, were equivalent for the protein cakes generated using the two different primary drying conditions.
Key words: freeze drying, manometric temperature measurements, micro-collapse, product resistance, proteins
INTRODUCTION
Freeze–drying is a popular choice for the manufacture of protein drug products despite its higher cost because lyophilized proteins have excellent stability on storage. The higher cost is primarily due to the lyophilization process; hence, efforts are often invested in minimizing the freeze–drying cycle time without compromising product quality. Primary drying is the rate-limiting step in the freeze–drying cycle, and one way of shortening the cycle time is to raise the shelf temperature during primary drying while taking care to keep the product temperature below the collapse temperature (Tc) for amorphous formulations or below the eutectic point (Te) for crystalline formulations (1–4). The collapse temperature is typically determined by the use of a freeze–dry microscope and is usually a few degrees above the 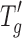 of the formulation, with
of the formulation, with 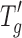 being the temperature above which the amorphous phase of the frozen solution changes from a glassy to a rubbery state and viscous flow becomes possible (1–4).
being the temperature above which the amorphous phase of the frozen solution changes from a glassy to a rubbery state and viscous flow becomes possible (1–4).
There has been recent interest, however, in lyophilizing proteins in amorphous formulations at or above their collapse temperatures (5–8). These aggressive freeze–drying cycles have been shown to produce stable drug products with little sign of visual or “macro” collapse, so the concept of a “micro-collapsed” state has been introduced (9,10). This gap between the visual “macro” collapse temperature (Tcmacro) and the onset of “micro” collapse (Tcmicro) seen in the freeze–dry microscope is usually only 2°C or 3°C for placebo (formulation containing excipients but no protein) or low protein concentrations, but can widen to 10°C or 15°C at moderate or high protein concentrations (5–8). In addition, freeze–drying in the “micro-collapsed” state using a model protein (bovine serum albumin, BSA) has been found to increase the size of the pores in the dry layer and reduce their tortuosity, resulting in a low resistance to the flow of water vapor, which does not increase with dry layer thickness (5).
This paper explores the freeze–drying behavior of three proteins, namely, lysozyme, BSA, and IgG, in an amorphous formulation at two different concentrations when subjected to different primary drying conditions (conservative drying with a low primary drying shelf temperature of −25°C and aggressive drying with a high primary drying shelf temperature of +25°C). These three model proteins differ substantially in shape, molecular weight, and the relative amounts of α-helix and β-sheet. In particular, manometric temperature measurements (MTM) measurements have been used to characterize product temperature (Tpr), sublimation rates, and product resistance (Rp) during primary drying. Stability of the different protein cakes has been evaluated by size exclusion chromatography (SEC), protein structure has been studied using a variety of biophysical techniques, and SEM has been used to study the morphology of the dried cakes. The aim of this paper was to understand whether differences or similarities exist in the freeze–drying behavior of the three proteins at different drying conditions.
MATERIALS AND METHODS
Materials
BSA, Fraction V (99% pure), was obtained from Sigma Chemical Company (St. Louis, MO). The monoclonal antibody (IgG) used in this study was obtained from Pfizer. All solutions contained 25 mg/mL sucrose and 6 mM sodium phosphate (pH 7.4). All formulation buffer components were multicompendial grade. Sodium phosphate dibasic, heptahydrate, and sodium phosphate monobasic dihydrate were obtained from J. T. Baker and sucrose from Ferro Pfanstiehl. Lyophilization was performed using 2-mL type 1 tubing vials (Wheaton Science Products, Millville, NJ) and 13-mm Daikyo 777-1 lyo stoppers with Flurotec coating (West Pharmaceutical Services Inc., Lionville, PA).
Modulated Differential Scanning Calorimetry
All thermograms were acquired on a DSC Q1000 series (TA Instruments, New Castle, Delaware) in the modulated mode (amplitude, 0.75°C; period, 30 s) with an autosampler and a refrigerated cooling system. Approximately 20 μL of the solution was weighed into an aluminum pan and hermetically sealed with an aluminum cover. Samples were cooled to −60°C at a rate of 2°C/min and then heated to 25°C at a rate of 2°C/min. Midpoint 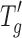 of the samples was determined using TA Instruments Universal Analysis software (version 4.5A). The solid cake samples were prepared in the glove box and heated to 150°C at a rate of 2°C/minute. Midpoint Tg of the samples was determined using TA Instruments Universal Analysis software (version 4.5A).
of the samples was determined using TA Instruments Universal Analysis software (version 4.5A). The solid cake samples were prepared in the glove box and heated to 150°C at a rate of 2°C/minute. Midpoint Tg of the samples was determined using TA Instruments Universal Analysis software (version 4.5A).
Freeze–Dry Microscopy
Liquid samples were tested using an Olympus BX51 microscope with a Linkam FDCS196 stage (Linkam Scientific Instruments, Surrey, UK). Images were recorded using a Pixelink PF-4662 camera attachment. A 0.5 μL sample was placed between a 10-mm glass coverslip and a 20-mm quartz slide which was placed on top of a silver block with a few microliters of silicon oil to ensure good thermal contact and then sealed within the vacuum stage. Samples were cooled to −50°C at a rate of 5°C/min, a vacuum (<10 mTorr) established within the stage, and then heated to 0°C at a rate of 1°C/min, with pictures taken at 1-s intervals.
Freeze–Drying Procedure
A volume of 1 mL of solution was filled into 2-mL glass vials (fill depth = 0.75 cm) and partially stoppered with 13-mm Lyo stoppers. Lyophilization cycles were performed in a LyoStar II freeze dryer (SP Industries, Stone Ridge, NY) in the auto-MTM mode. Calibrated 36-gauge thermocouples (OMEGA, Newport, CT) were placed bottom center (the tip touching the vial bottom) in both edge and center vials. Cycles were performed with the following initial freezing procedure: ramp from room temperature to −45°C (ramp rate, 1°C/min), hold for 2 h, ramp to −20°C (1°C/min), and hold for 1 h (annealing step), return to −45°C, and maintaining shelf temperature for another 2 h. Primary drying was conducted at chamber pressures (Pc) of 57 mTorr and shelf temperatures (Ts) of −25°C and +25°C. The Pc setting was maintained constant during primary and secondary drying. The primary drying duration for the conservative cycle was set at 4,000 min for BSA and 3,000 min for IgG and lysozyme. The primary drying duration for the aggressive cycle was set at 1,500 min for BSA and 1,000 min for IgG and lysozyme. The experiments were first conducted with BSA, and based on the duration it took to dry BSA, the programmed drying times were modified for lysozyme and IgG. Secondary drying was performed at a shelf temperature of 40°C for 10 h (1°C/min ramp rate). Auto-MTM runs were done with a row of empty vials around the edge of the tray and aluminum foil covering the door (11). Three thermocouple probes were used and all were placed in the center of the load. The probes (from LyoStar) are calibrated once a year.
Scanning Electron Microscopy
The cakes were extracted intact from the vial by carefully cutting the vial with a sharp diamond tip around the circumference about 2–3 mm above the cake. The cake was then freed on a clean cutting mat. A sharp blade was used to slice the cake along the cylinder axis; only the cake surface untouched by the blade was used for SEM examination. An ASPEX VP SEM instrument (ASPEX Corp., Delmont, PA) was used for investigating the cake morphology. All samples were scanned at an accelerated voltage of 17–20 kV and a working distance of 10–12 mm. In order to preserve the native morphology of the cake surface, no conductive coating was applied to the specimens. Consequently, all specimens were examined under variable pressure mode at 0.1–0.15 torr and by using exclusively the backscattered electron detector. Images for record were collected at a scanning rate of 128 ms per pixel.
Circular Dichroism
Far-UV circular dichroism (CD) analysis was performed on the reconstituted protein solutions to compare the secondary structural characteristics of the proteins. Data were collected on a Chirascan Applied Biophysics spectropolarimeter in the 260- to 190-nm range using a bandwidth of 1.0 nm and cell path length of 2 mm at 25°C. The reported spectra were corrected for buffer contribution and expressed as mean residue ellipticity (deg cm2 dmol−1) as a function of wavelength (nm).
Fluorescence Spectroscopy
Intrinsic fluorescence data were collected on a Varian Eclipse Spectroflurometer to compare the tertiary structure of the reconstituted protein solutions. Tryptophans were excited at 295 nm and emission spectra were recorded between 300 and 400 nm at 25°C using a 10-mm path length cell. All spectra were corrected for buffer contributions followed by concentration normalization.
Fourier Transform Infrared
FTIR spectra were collected for the solid protein cakes on a Bomem MB series equipped with a DTGS detector. A single-bounce diamond crystal ATR setup (Spectra-Tech) was used for running the lyophilized samples. The sample chamber was purged with dry nitrogen to eliminate uptake of water vapor. Solid samples were pressed down on top of the diamond crystal with the in-built apparatus to ensure sufficient contact between the solid and the crystal. A total of 64 scans were acquired at a resolution of 4 cm−1. Data analysis was performed using the GRAMS/AI 32 (version 7.0) software. The background spectrum was subtracted from the raw spectra to obtain the absorbance spectra. A second derivative spectrum was obtained using the Sav–Golay function followed by baseline and offset correction and was area normalized for direct comparison between proteins.
Size Exclusion Chromatography
Formation of high-molecular-weight species (HMWS) was measured by SEC-HPLC using an Agilent 1100 system equipped with G3000SWXL and G2000SWXL columns in series. The detection wavelength was 214 nm using a diode array detector and a nominal protein concentration of 1 mg/mL. The protein was eluted isocratically with a 200 mM sodium phosphate (pH 7.0) mobile phase at a flow rate of 0.7 mL/min.
Results
Properties Relevant to Freeze–Drying for Model Protein Formulations
Table I tabulates the relevant freeze–drying properties of the three model proteins, namely, lysozyme, BSA, and IgG, in sucrose formulations where the concentration of sucrose was maintained at 25 mg/mL in 6 mM phosphate buffer and the protein concentration was either fixed at 5 or 20 mg/mL. The 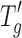 value for the 5 mg/mL protein cakes was −33°C and very similar to the value (−34°C) obtained for placebo (0 mg/mL protein, 25 mg/mL sucrose in 6 mM phosphate buffer) formulation. The addition of 20 mg/mL protein to the placebo increased the
value for the 5 mg/mL protein cakes was −33°C and very similar to the value (−34°C) obtained for placebo (0 mg/mL protein, 25 mg/mL sucrose in 6 mM phosphate buffer) formulation. The addition of 20 mg/mL protein to the placebo increased the 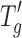 value from −34°C to approximately −29°C (about 4–5°C higher than the placebo). It should be noted that no difference in
value from −34°C to approximately −29°C (about 4–5°C higher than the placebo). It should be noted that no difference in 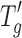 values was found between protein type at 5 mg/mL where the protein accounts for 16% of the cake composition and the freeze–drying properties are dominated by the excipients (mainly sucrose), which form 84% of the cake. In the case of cakes containing 20 mg/mL protein, the protein is 44% of the cake composition and influences the freeze–drying properties significantly. However, even at this concentration, no significant differences between
values was found between protein type at 5 mg/mL where the protein accounts for 16% of the cake composition and the freeze–drying properties are dominated by the excipients (mainly sucrose), which form 84% of the cake. In the case of cakes containing 20 mg/mL protein, the protein is 44% of the cake composition and influences the freeze–drying properties significantly. However, even at this concentration, no significant differences between 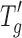 were observed between the three proteins.
were observed between the three proteins.
Table I.
Data on Formulation Properties Relevant to Freeze–Drying for the Three Model Proteins
| Protein |
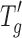 (°C) (°C) |
T cmicro (°C) | T cmacro (°C) | T g a (°C) | MCb (%) | MCc (%) |
|---|---|---|---|---|---|---|
| 0 mg/mL (placebo) | −34 | −32 | −29 | 65 | 0.7 | 0.59 |
| 5 mg/mL Lysozyme | −33 | −31.5 | −29 | 85 | 0.42 | 0.60 |
| 20 mg/mL Lysozyme | −30.3 | −27.4 | −17 | 96 | 0.30 | 0.18 |
| 5 mg/mL BSA | −33 | −31 | −28 | 87 | 0.31 | 0.43 |
| 20 mg/mL BSA | −29 | −27 | −14 | 102 | 0.15 | 0.21 |
| 5 mg/mL IgG | −33.5 | −32 | −29 | 84 | 0.31 | 0.11 |
| 20 mg/mL IgG | −29.3 | −30 | −21 | 107 | 0.27 | 0.13 |
All protein formulations are in 6 mM phosphate buffer containing 25 mg/mL sucrose
T g' glass transition temperature of the frozen matrix, T cmicro temperature of onset of collapse, T cmacro temperature at which complete collapse is observed under the freeze–drying microscope, T g glass transition temperature of the solid matrix, MC moisture content
aValue represents the average of the values obtained for cakes under both primary drying conditions
b T s = −25°C during primary drying
c T s = +25°C during primary drying
Freeze–drying microscopy was used to determine Tcmicro (the temperature at which the freeze–drying front begins to first exhibit holes and channels) and Tcmacro (the temperature at which the freeze–drying front completely collapses). There is a 3°C difference between Tcmicro and Tcmacro for all proteins at 5 mg/mL. This window, however, is wider at 20 mg/mL, with the difference between Tcmicro and Tcmacro approximating 10°C for lysozyme, 13°C for BSA, and 9°C for IgG (Table I). It has been shown before by us and others (5,6,8,12) that for any given protein, both the 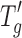 and the window between Tcmicro and Tcmacro increases with increasing protein concentration, and the same holds true in this study.
and the window between Tcmicro and Tcmacro increases with increasing protein concentration, and the same holds true in this study.
The Tg values of the solid cakes for any given protein were similar irrespective of the primary drying temperature employed (data not shown) and show a significant increase upon the addition of protein to the sucrose-containing cake. The Tg values in Table I are an average of the Tg values of the cakes obtained under the two primary drying conditions employed in this study (namely, aggressive with Ts = +25°C and conservative with Ts = −25°C). The Tg values were similar irrespective of the protein type for the 5 mg/mL protein cakes and increased by at least 20°C from the placebo cakes (from 65°C for the placebo to approximately 85°C for the protein-containing cakes). The Tg values for the cakes containing 20 mg/mL protein also increased significantly from the placebo cake, with the highest increase being observed for IgG (42°C) and the lowest for lysozyme (31°C). The moisture content of all the cakes was well below 1.0% for both the aggressively and conservatively dried cakes.
Application of MTM to Characterize the Drying Behavior of Model Proteins
Manometric temperature measurements in the auto-MTM mode in the Lyostar II lyophilizer enabled the estimation of product temperature at the bottom of the vial along with the sublimation rates and product resistance (Rp) for the two cycle conditions used in this study. Tables II and III summarize the product temperature (Tpr) obtained using thermocouples (TC), the MTM estimated temperature at the vial bottom (TbMTM), the estimated sublimation rate, and the time taken to complete primary drying for the 5 and 20 mg/mL protein formulations, respectively. There is a good agreement in the steady-state primary drying product temperatures obtained by TC and TbMTM, with the estimated temperatures being slightly lower than the actual temperatures for any given protein under both drying conditions.
Table II.
Product Temperature, Sublimation Rates, and Time Taken to Complete Primary Drying for the Three Model Proteins at 5 mg/mL in the Conservative and Aggressive Cycles
| Protein | T pr (TC)a | T pr (TC)a | T pr (T bMTM)a | T pr (T bMTM)a | dm/dt a | dm/dt a | Time to complete PD | Time to complete PD |
|---|---|---|---|---|---|---|---|---|
| T s = −25°C | T s = +25°C | T s = −25°C | T s = +25°C | T s = −25°C | T s = +25°C | T s = −25°C | T s = +25°C | |
| 0 mg/mL (placebo) | −37.5 | −32.4 | −38.1 | −33.4 | 0.040 | 0.17 | 22.5 (TC) | 8.6 (TC) |
| 27.4 (PG) | 10.2 (PG) | |||||||
| 5 mg/mL Lysozyme | −35.7 | −31.03 | −36.3 | −31.1 | 0.038 | 0.17 | 25.6 (TC) | 9.5 (TC) |
| 32.3 (PG) | 9.8 (PG) | |||||||
| 5 mg/mL BSA | −35.9 | −30.5 | −36.5 | −30.7 | 0.036 | 0.16 | 26.2 (TC) | 9.8 (TC) |
| 29.9 (PG) | 9.9 (PG) | |||||||
| 5 mg/mL IgG | −37.1 | −29.9 | −37.5 | −30.5 | 0.033 | 0.15 | 27.9 (TC) | 9.6 (TC) |
| 29.0 (PG) | 9.2 (PG) |
Runs were carried out in the AutoMTM mode, in Lyostar II, and all formulations are in 6 mM phosphate buffer containing 25 mg/mL sucrose. Shelf temperatures during PD were maintained constant at −25°C and +25°C. The time to complete primary drying (in hours) was determined both by average TC data placed bottom center in the center of the batch as well as PG data. Measured T cmicro values for 5 mg/mL lysozyme, BSA, and IgG are −31.5°C, −31°C, and −32°C, respectively
T s shelf inlet temperature, dm/dt sublimation rate (g/h per vial), T bMTM product temperature at the vial bottom (°C), T pr product temperature (°C), TC thermocouple, PD primary drying
aAverage value obtained during steady-state primary drying but limited to the region where MTM data are valid
Table III.
Product Temperature, Sublimation Rates, and Time Taken to Complete Primary Drying for the Three Model Proteins at 20 mg/mL in the Conservative and Aggressive Cycles
| Protein | T pr (TC)a | T pr (TC)a | T pr (T bMTM)a | T pr (T bMTM)a | dm/dt a | dm/dt a | Time to complete PD | Time to complete PD |
|---|---|---|---|---|---|---|---|---|
| T s = −25°C | T s = +25°C | T s = −25°C | T s = +25°C | T s = −25°C | T s = +25°C | T s = −25°C | T s = +25°C | |
| 0 mg/mL (placebo) | −37.5 | −32.4 | −38.1 | −33.4 | 0.040 | 0.17 | 22.5 (TC) | 8.6 (TC) |
| 27.4 (PG) | 10.2 (PG) | |||||||
| 20 mg/mL Lysozyme | −36.2 | −26.2 | −36.9 | −27.6 | 0.034 | 0.14 | 28.2 (TC) | 9.6 (TC) |
| 32.4 (PG) | 9.4 (PG) | |||||||
| 20 mg/mL BSA | −36.0 | −25.2 | −36.7 | −26.5 | 0.033 | 0.13 | 34 (TC) | 10.3 (TC) |
| 43.8 (PG) | 10.8 (PG) | |||||||
| 20 mg/mL IgG | −37 | −25.3 | −37.3 | −26.6 | 0.031 | 0.12 | 35.8 (TC) | 12.3 (TC) |
| 44 (PG) | 12.6 (PG) |
Runs were carried out in the auto-MTM mode, in Lyostar II, and all formulations are in 6 mM phosphate buffer containing 25 mg/mL sucrose. Shelf temperatures during PD were maintained constant at −25°C and +25°C. The time to complete primary drying (in hours) was determined both by average TC data placed bottom center in the center of the batch as well as PG data. Measured T cmicro values for 20 mg/mL lysozyme, BSA, and IgG are −27.4°C, −27°C, and −30°C, respectively
T s shelf inlet temperature, dm/dt sublimation rate (g/h per vial), T bMTM product temperature at the vial bottom (°C), T pr product temperature (°C), TC thermocouple, PD primary drying
aAverage value obtained during steady state primary drying but limited to region where MTM data are valid
As seen in Table II, the product temperature (as measured by TC and TbMTM) during steady-state primary drying in the aggressive cycle for the 5-mg/mL formulations is higher than the product temperature in the conservative cycles. This difference is at least 5°C for the placebo, lysozyme, and BSA and around 7°C for IgG between the two cycles. In the conservative cycle, the Tpr is 4°C below Tcmicro, and in the aggressive mode, it is at or 2–3°C above Tcmicro (Tables I and II). The sublimation rates in the aggressive cycles are also four to five times higher when compared to the conservative cycles for all the protein formulations (at 5 mg/mL) including the placebo. The time taken to complete primary drying for the different proteins in the aggressive cycle ranges between 9 and 10 h, and in the conservative cycle, it ranges between 26 and 28 h, as measured by the time it take thermocouples to reach shelf temperature. The time to complete primary drying based on the Pirani gauge measurements is similar to the TC estimates in the aggressive cycle and differs by a few hours in the conservative cycle.
As seen in Table III, the product temperature (as measured by TC and TbMTM) in the aggressive cycle for the 20-mg/mL formulations is higher than the product temperature in the conservative cycles. This difference in Tpr is 10–11°C for lysozyme and BSA, respectively, and around 12°C for IgG between the two cycles. In the conservative cycle, the Tpr is 6–7°C below Tcmicro and in the aggressive mode is at the edge of Tcmicro, except IgG which is 5°C above Tcmicro (Tables I and III). The sublimation rates in the aggressive cycles are also four times higher when compared to the conservative cycles for all the protein formulations including the placebo. The time taken to complete primary drying (as measured by the time it takes the thermocouples to reach shelf temperature) for the different proteins in the aggressive cycle ranges between 8 and 12 h and in the conservative cycle ranges between 28 and 36 h depending on the type of protein. The time to complete primary drying based on the Pirani gauge (PG) measurement is very similar to the TC estimates in the aggressive cycle, but deviates significantly in the conservative cycle. Deviations between the TC and Prani gauge readings are likely due to the presence of insulated ice slabs in the product (13). This deviation or difference in PD time estimates between TC and PG is smaller for lysozyme (4.2-h difference between TC and PG) and about 8–9 h for BSA and IgG. Between the three proteins, lysozyme formulations dried the fastest at 20 mg/mL in both cycles.
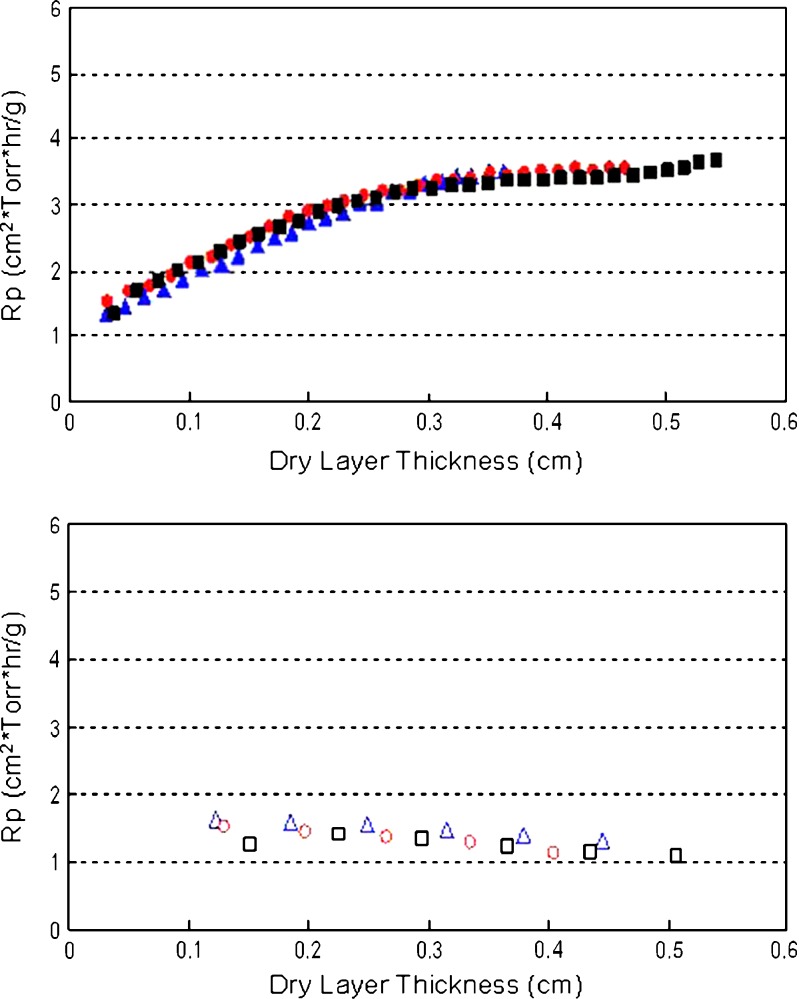
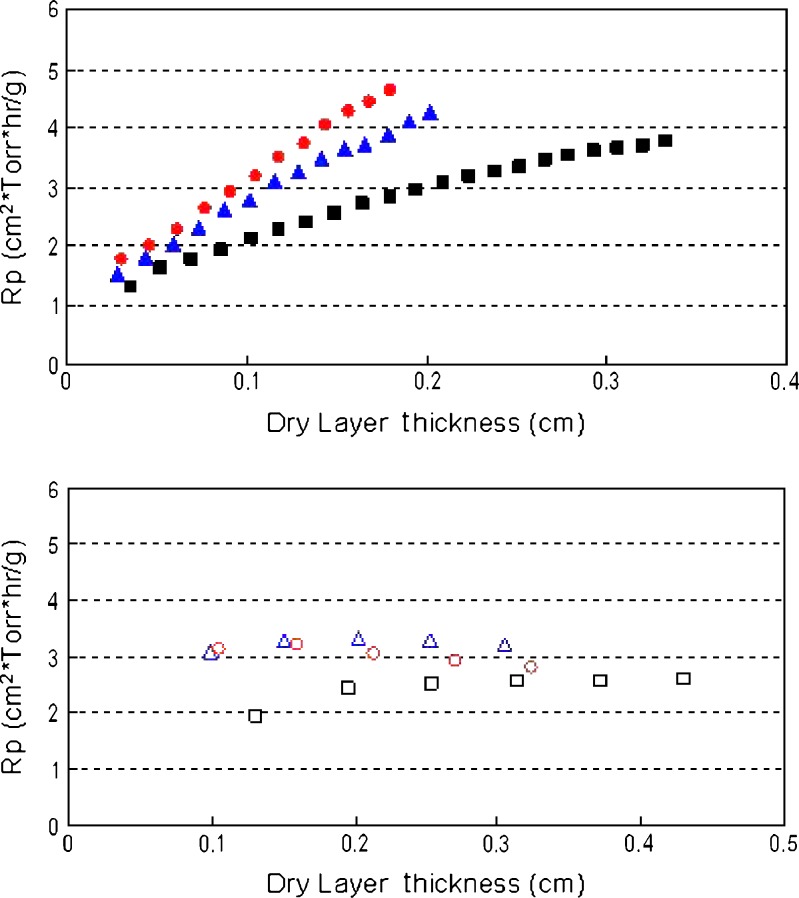
MTM measurements allow the estimation of product resistance (Rp) as a function of dry layer thickness (DLT). As seen in Fig. 1, for the 5 mg/mL protein formulations, the Rp increases as a function of dry layer thickness in the conservative cycle, but is independent of DLT in the aggressive mode, as observed by Overcashier et al. (10) and Milton et al. (9). It is also interesting to note that at 5 mg/mL (where excipients dominate the drying behavior), the three proteins exhibit similar product resistance irrespective of the primary drying conditions employed. However, differences between proteins become more evident for the 20-mg/mL protein formulations where the protein is now 44% of the cake composition (Fig. 2). Lysozyme cakes exhibit the lowest Rp values when compared to BSA and IgG, which appear to have similar Rp values. The difference in Rp values are more significant between the three proteins in the conservative mode at 20 mg/mL compared to the aggressive mode (Fig. 2). It should be noted that even for the 20 mg/mL formulations, the estimated Rp values in the conservative mode increase monotonically as a function of DLT, but are independent of DLT in the aggressive mode as observed for the 5-mg/mL formulations (Figs. 1 and 2).
Fig. 1.
Calculated product resistance as a function of dry layer thickness for the three proteins at 5 mg/mL in the conservative (T s = −25°C; top panel, solid symbols) and aggressive (T s = +25°C; bottom panel, open symbols) cycles. Triangles represent IgG, circles represent BSA, and squares represent lysozyme
Fig. 2.
Calculated product resistance as a function of dry layer thickness for the three proteins at 20 mg/mL in the conservative (T s = −25°C; top panel, solid symbols) and aggressive (T s = +25°C; bottom panel, open symbols) cycles. Triangles represent IgG, circles represent BSA, and squares represent lysozyme
Effect of Drying on Protein Conformation and Stability
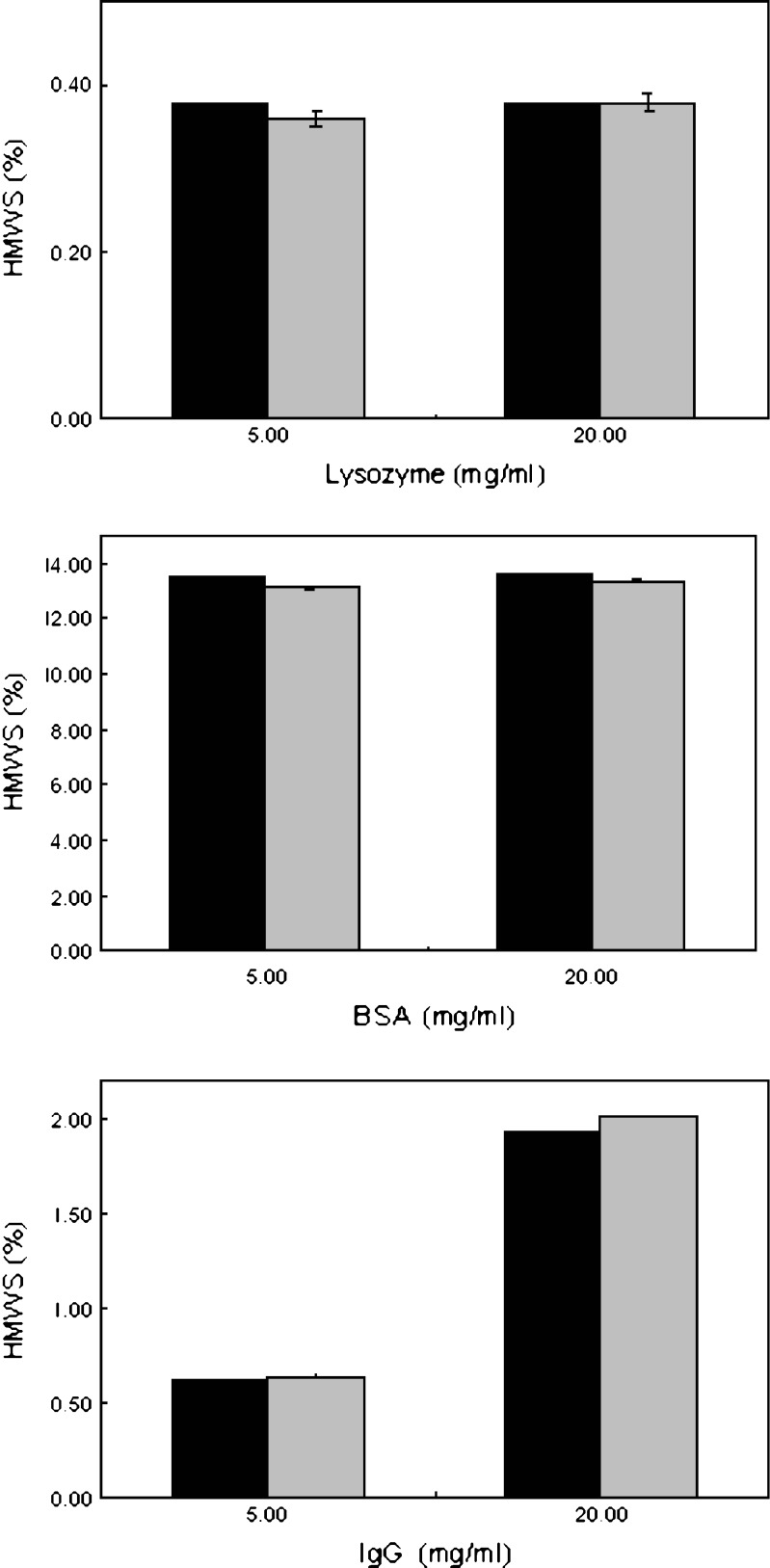
SEC was used to monitor the formation of HMWS as a function of time after storage at three different storage temperatures, namely, 5°C, 25°C, and 40°C. No differences were observed in the formation of high-molecular-weight species for the cakes dried aggressively and conservatively after 13 weeks. This was true for all storage temperatures for the three proteins at both concentrations from time 0 to 13 weeks (data not shown). Figure 3 shows the formation of HMWS for the three proteins at different concentrations after 13 weeks of storage at 40°C. It is interesting to note that the %HMWS is different for IgG at 5 and 20 mg/mL, but is similar for lysozyme and BSA. This probably implies that different proteins need different sucrose-to-protein ratios to achieve the desired level of cryoprotection (14,15). The sucrose-to-protein ratio employed in this study was 4 for the lower protein loading and 1.25 for the higher protein loading. There are different opinions in the literature regarding the stability of proteins in the micro-collapsed state. Some studies have shown that freeze–drying above the collapse temperature does not impact protein stability (16–18). However, Passot et al. (19) presented data that toxins can lose antigenic activity after 6 months of storage when primary drying was performed at a product temperature higher than 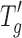 . This was observed for both partially crystalline and completely amorphous formulations.
. This was observed for both partially crystalline and completely amorphous formulations.
Fig. 3.
High-molecular-weight species (HMWS) formation for the different protein cakes after storage at 40°C for 3 months. Black bars represent cakes dried aggressively (T s = +25°C) and gray bars represent cakes dried conservatively (T s = −25°C)
Biophysical analyses were performed to evaluate the effect of lyophilization on protein structure. Circular dichroism and fluorescence were performed on the reconstituted solution while FTIR was performed on the solid cake. No differences were observed in the reconstitution time between the aggressively and conservatively dried cakes for any given protein. All three protein cakes, irrespective of the drying condition employed, reconstituted within 10 s.
Typical CD and FTIR spectra were obtained for lysozyme, BSA, and IgG as observed in the literature (20–22). Figure 4 shows six panels representing the CD spectra of reconstituted solutions at 5 and 20 mg/mL for each protein type. Each panel has two overlaid CD spectra representing aggressive and conservative primary drying. The CD spectra (Fig. 4a, b) for lysozyme show both α-helical (strong negative band at 208 nm and weaker band at 222 nm) and β-sheet structures (weak band at 218 nm). Strong negative bands at 222 and 208 nm in the CD spectra for BSA (Fig. 4c, d) show predominantly α-helical structure. The strong negative band at 218 nm in the CD spectra observed for IgG (Fig. 4e, f) show predominantly β-sheet characteristics. Fluorescence data indicated no change in tertiary structure for the three proteins irrespective of the primary drying condition employed. The emission maxima obtained for lysozyme, BSA, and IgG under both drying conditions were 341, 348, and 335 nm, respectively (data not shown). The FTIR data on the solid cakes for the different proteins are shown in Fig. 5, with the six panels representing the absorbance spectra of reconstituted solutions at 5 and 20 mg/mL for each protein type. Each panel has two overlaid FTIR spectra representing aggressive and conservative primary drying. A typical solid-state amide I absorbance spectrum was observed for lysozyme (Fig. 5a, b) with a strong absorbance band at ∼1,650 cm−1 indicating a predominantly α-helical protein. For BSA (Fig. 5c, d), a typical solid-state amide I spectrum was observed (Fig. 5c, d) with strong absorbance bands at 1,652–1,654 cm−1 indicating a predominantly α-helical protein. For IgG (Fig. 5e, f), a typical solid-state amide I spectrum was observed for IgG (Fig. 5e, f) with strong absorbance band at ∼1,640 cm−1 indicating a predominantly β-sheet protein. A shoulder was also observed at ∼1,680 cm−1 which indicates the presence of β-sheet.
Fig. 4.
Secondary structure by CD of the three proteins subjected to conservative (T s = −25°C, solid line in each panel) and aggressive drying (T s = +25°C, dashed line in each panel). a, b Lysozyme 5 and 20 mg/mL, respectively. c, d BSA 5 and 20 mg/mL, respectively. e, f IgG 5 and 20 mg/mL, respectively
Fig. 5.
Secondary structure by FTIR of the solid cakes of the three proteins subjected to conservative (T s = −25°C, solid line in each panel) and aggressive drying (T s = +25°C, dashed line in each panel). a, b Lysozyme 5 and 20 mg/mL, respectively. c, d BSA 5 and 20 mg/mL, respectively. e, f IgG 5 and 20 mg/mL, respectively
Effect of Drying on Cake Morphology
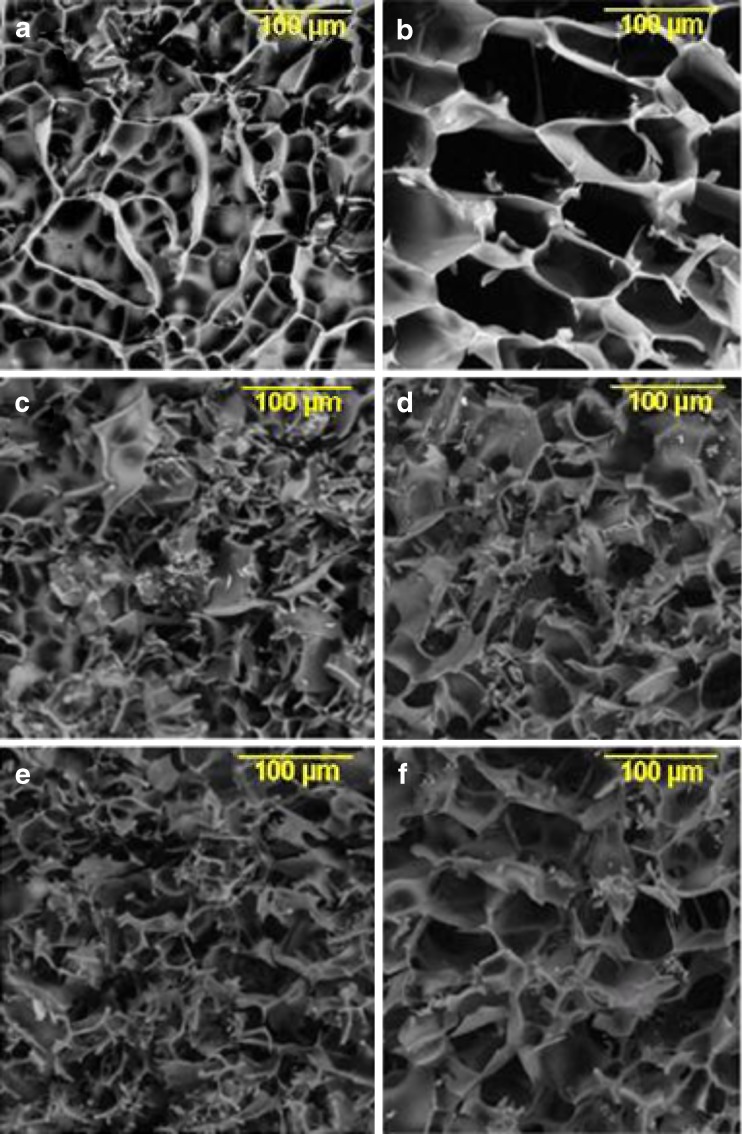
Representative examples of the cake morphology by SEM for the different protein cakes at 20 mg/mL are shown in Fig. 6. The morphology of the cakes in the aggressive cycle is more open and porous than that observed in the conservative cycle, in line with previous observations made for cakes dried in the micro-collapse region (5,10,23). This is more evident for the lysozyme and IgG cakes compared to BSA. Between the three proteins, the lysozyme cake morphology appears to be distinctly different compared to BSA and IgG.
Fig. 6.
Scanning electron micrographs of the three model protein cakes subjected to conservative (T s = −25°C, left panels) (a, c, e) and aggressive drying (T s = +25°C, right panels) (b, d, f). a, b Lysozyme. c, d BSA. e, f IgG. Micrographs represent cross-sectional view
Discussion
The objective of this paper was to characterize the freeze–drying behavior of three model proteins when subjected to two different primary drying conditions (aggressive, Ts = +25°C; conservative, Ts = −25°C) at two different concentrations (5 and 20 mg/mL) in amorphous formulations. The three proteins used in this study differed in molecular weights and secondary structural attributes, namely, IgG (150 kDa and predominantly β-sheet), BSA (66 kDa and predominantly α-helical), and lysozyme (15 KDa and a mix of α-helical and β-sheet) (22,24).
Impact of Aggressive and Conservative Drying
The two primary drying shelf temperatures employed in this study (namely, −25°C and +25°C) resulted in Tpr being either below the collapse temperature at Ts = −25°C or at the edge of Tcmicro in the micro-collapse regime at Ts = +25°C. Based on the MTM measurements, the estimated Tpr and sublimation rates were higher in the aggressive mode compared to the conservative mode, and therefore, the cakes dried faster at the higher shelf temperature. The Rp values increased with increasing protein concentration, and in the conservative cycle, Rp increased monotonically as a function of DLT, but in the aggressive mode was independent of DLT.
The different biophysical analyses (CD, fluorescence, and FTIR) showed no significant difference in secondary and tertiary structure for all three proteins subjected to the two primary drying conditions, at both concentrations of 5 and 20 mg/mL. No differences were observed in the formation of HMWS between the two cycles for the three proteins at both concentrations. This indicates that the aggressive and conservative primary drying conditions did not affect the structure or the stability of the proteins.
The morphology of the cakes dried aggressively had a more open and porous structure compared to the cakes dried conservatively (10). The open and fragmented structure of the cake in the aggressive cycle decreases the tortuosity of the path that the water molecules have to travel through the dry layer thickness (5,23), resulting in shorter drying times. The change in pore structure while in the micro-collapsed state has been attributed to the viscous flow of the newly freeze-dried material while it is still in the “rubbery” state (8–10).
Comparison of the Freeze–Drying Behavior of the Three Proteins
Based on the data in Table I, increasing the protein concentration (from 5 to 20 mg/mL) significantly increases the 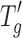 of the formulations as well as the window between Tcmicro and Tcmacro. The Tg of the solid cake also increases significantly with increasing protein concentration. Similar observations have been reported in the literature (8,25). The differences between proteins in
of the formulations as well as the window between Tcmicro and Tcmacro. The Tg of the solid cake also increases significantly with increasing protein concentration. Similar observations have been reported in the literature (8,25). The differences between proteins in 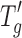 , Tcmicro, and Tcmacro are less evident at 5 mg/mL where the excipients (sucrose in this case) dominate the freeze–drying behavior. At the higher protein concentration of 20 mg/mL, the differences between the protein formulations become more evident. For example, the window between Tcmicro and Tcmacro is narrower for IgG, but is the widest for BSA (Table I). Similarly, IgG-containing cakes have the highest Tg at 20 mg/mL, while lysozyme-containing cakes at the same concentration have the lowest. The aim of this paper was to highlight these differences and/or similarities but not necessarily explain them since no obvious or straightforward explanation can be garnered from the data collected in this study.
, Tcmicro, and Tcmacro are less evident at 5 mg/mL where the excipients (sucrose in this case) dominate the freeze–drying behavior. At the higher protein concentration of 20 mg/mL, the differences between the protein formulations become more evident. For example, the window between Tcmicro and Tcmacro is narrower for IgG, but is the widest for BSA (Table I). Similarly, IgG-containing cakes have the highest Tg at 20 mg/mL, while lysozyme-containing cakes at the same concentration have the lowest. The aim of this paper was to highlight these differences and/or similarities but not necessarily explain them since no obvious or straightforward explanation can be garnered from the data collected in this study.
MTM measurements (using the SMART™ freeze-dryer technology) have been used by researchers to study the formulation-dependent mass transfer resistance of the dried layer (26). One of the limitations of the SMART™ freeze-dryer technology is the significant deviation from the model at high protein concentrations of approximately 50 mg/mL (5,13) because of reabsorption of water by the dry layer. This study therefore limited itself to protein concentrations below 50 mg/mL where deviations from the SMART model are less significant. At 5 mg/mL (where excipients dominate the freeze–drying behavior), the estimated Tpr, Rp, and sublimation rates from MTM measurements are similar for all three proteins at both primary drying conditions. However, at 20 mg/mL (where the protein begins to significantly influence the freeze–drying behavior), differences in product resistance between the three proteins become evident especially in the conservative mode, but these differences are less significant in the aggressive mode. This indicates that at higher protein concentrations, depending on the protein type, there may exist differences in the freeze–drying behavior especially in the conservative mode (i.e. below Tcmicro), but drying in the aggressive mode can significantly decrease these differences.
The cake morphology between BSA and IgG is similar, but the lysozyme cakes have a different cake morphology. These differences are difficult to explain, but interesting to note.
Conclusions
The differences in the freeze–drying behavior of the three proteins using MTM measurements were more evident at higher protein concentrations where the protein significantly influences the behavior of the formulation matrix. However, these differences were minimized in the aggressive mode and at lower protein concentrations where excipients dominated the freeze–drying behavior. Even though no visual cake collapse was observed after freeze–drying in aggressive mode, the cake morphology is more open and porous in the aggressively dried cakes when compared to their conservatively dried counterparts. The stability and the protein structure are equivalent for all the protein cakes generated using the two primary drying conditions employed in this study. Significantly shorter primary drying times could be achieved by employing aggressive primary drying shelf temperatures with no detrimental effect to the proteins studied.
References
- 1.Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. 2000;203:1–60. doi: 10.1016/S0378-5173(00)00423-3. [DOI] [PubMed] [Google Scholar]
- 2.Tang X, Pikal MJ. Design of freeze–drying processes for pharmaceuticals: practical advice. Pharm Res. 2004;21:191–200. doi: 10.1023/B:PHAM.0000016234.73023.75. [DOI] [PubMed] [Google Scholar]
- 3.Schwegman JJ, Hardwick LM, Ackers MJ. Practical formulation and process development of freeze-dried products. Pharm Dev Technol. 2005;10:151–73. doi: 10.1081/pdt-56308. [DOI] [PubMed] [Google Scholar]
- 4.Manning MC, Patel K, Borchardt RT. Stability of protein pharmaceuticals. Pharm Res. 1989;6:903–18. doi: 10.1023/A:1015929109894. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RE, Oldroyd ME, Ahmed SS, Gieseler H, Lewis LM. Use of manometric temperature measurements (MTM) to characterize the freeze–drying behavior of amorphous protein formulations. J Pharm Sci. 2010;99:2863–73. doi: 10.1002/jps.22031. [DOI] [PubMed] [Google Scholar]
- 6.Meister E, Gieseler H. Freeze–dry microscopy of protein/sugar mixtures: drying behavior, interpretation of collapse temperatures and a comparison to corresponding glass transition data. J Pharm Sci. 2009;98:3072–87. doi: 10.1002/jps.21586. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca F, Passot S, Cunin O, Marin M. Collapse temperature of freeze-dried Lactobacillus bulgaricus suspensions and protective media. Biotechnol Prog. 2004;20:229–38. doi: 10.1021/bp034136n. [DOI] [PubMed] [Google Scholar]
- 8.Colandene JD, Maldonado LM, Creagh AT, Vrettos JS, Goad KG, Spitznagel TM. Lyophilization cycle development for a high-concentration monoclonal antibody formulation lacking a crystalline bulking agent. J Pharm Sci. 2007;96:1598–1608. doi: 10.1002/jps.20812. [DOI] [PubMed] [Google Scholar]
- 9.Milton N, Pikal MJ, Roy ML, Nail SL. Evaluation of manometric temperature measurement as a method of monitoring product temperature during lyophilization. J Parent Sci Technol. 1997;51:7–15. [PubMed] [Google Scholar]
- 10.Overcashier DE, Patapoff TW, Hsu CC. Lyophilization of protein formulations in vials: Investigation of the relationship between resistance to vapor flow during primary drying and small-scale product collapse. J Pharm Sci. 1999;88:688–95. doi: 10.1021/js980445+. [DOI] [PubMed] [Google Scholar]
- 11.Tang X, Nail SL, Pikal MJ. Freeze–drying process design by manometric temperature measurement: design of a smart freeze-dryer. Pharm Res. 2005;22:685–700. doi: 10.1007/s11095-005-2501-2. [DOI] [PubMed] [Google Scholar]
- 12.Lewis LM, Teagarden DL, Johnson RE, Ahmed SS. Rational design of a freeze-dried formulation for a biologic. Amer Pharm Rev. 2008;11(3):91–6. [Google Scholar]
- 13.Gieseler H, Kramer T, Pikal MJ. Use of manometric temperature measurement (MTM) and SMART™ freeze dryer technology for development of an optimized freeze–drying cycle. J Pharm Sci. 2007;96:3402–18. doi: 10.1002/jps.20982. [DOI] [PubMed] [Google Scholar]
- 14.Cleland JL, Lam X, Kendrick B, Yang J, Yang T-H, Overcashier D, Brooks D, Hsu C, Carpenter JF. A specific molar ratio of stabilizer to protein is required for storage stability of a lyophilized monoclonal antibody. J Pharm Sci. 2001;90:310–21. doi: 10.1002/1520-6017(200103)90:3<310::AID-JPS6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 15.Han Y, Jin B-S, Lee S-B, Sohn Y, Joung J-W, Lee J-H. Effects of sugar additives on protein stability of recombinant human serum albumin during lyophilization and storage. Arch Pharm Res. 2007;30:1124–31. doi: 10.1007/BF02980247. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Hey J, Nail S. Effect of collapse on stability of freeze-dried recombinant factor VIII and α-amylase. J Pharm Sci. 2004;93:1253–63. doi: 10.1002/jps.20065. [DOI] [PubMed] [Google Scholar]
- 17.Luthra S, Obert J-P, Kalonia D, Pikal M. Investigation of drying stresses on proteins during lyophilization: differentiation between primary and secondary-drying stresses on lactate dehydrogenase using a humidity controlled mini freeze-dryer. J Pharm Sci. 2007;96:61–70. doi: 10.1002/jps.20758. [DOI] [PubMed] [Google Scholar]
- 18.Schersch K, Betz O, Garidel P, Muehlau S, Bassarab S, Winter G. Systematic investigation of the effect of lyophilizate collapse on pharmaceutically relevant proteins I: stability after freeze–drying. J Pharm Sci. 2010;99:2256–78. doi: 10.1002/jps.22000. [DOI] [PubMed] [Google Scholar]
- 19.Passot S, Fonseca F, Barbouche N, Marin M, Alarcon-Lorca M, Rolland D, Rapaud M. Effect of product temperature during primary drying on the long-term stability of lyophilized proteins. Pharm Dev Technol. 2007;12:543–53. doi: 10.1080/10837450701563459. [DOI] [PubMed] [Google Scholar]
- 20.Venyaminov SY, Yang JT. In: Circular dichroism and the conformational analysis of biomolecules. Gasman GD, editor. New York: Plenum; 1996. pp. 69–104. [Google Scholar]
- 21.Takeda K, Miura M, Takagi T. Stepwise formation of complexes between sodium dodecyl sulfate and bovine serum albumin detected by measurements of electric conductivity, binding isotherm, and circular dichroism. J Coll Interface Sci. 1981;82:38–44. doi: 10.1016/0021-9797(81)90121-1. [DOI] [Google Scholar]
- 22.K-y N, Zhao L, Meyer JD, Rittmann-Grauer L, Manning MC. Use of circular dichroism spectroscopy in determining the conformation of a monoclonal antibody prior to its incorporation in an immunoliposome. J Pharm Biomed Analysis. 1997;16:507–13. doi: 10.1016/S0731-7085(97)00101-5. [DOI] [PubMed] [Google Scholar]
- 23.Parker A, Rigby-Singleton S, Perkins M, Bates D, Le Roux D, Roberts CJ, Madden-Smith C, Lewis L, Teagarden DL, Johnson RE, Ahmed SS. Determination of the influence of primary drying rates on the microscale structural attributes and physicochemical properties of protein containing lyophilized products. J Pharm Sci. 2010;99:4616–29. doi: 10.1002/jps.22185. [DOI] [PubMed] [Google Scholar]
- 24.Costantino HR, Griebenow K, Mishra P, Langer R, Klibanov AM. Fourier-transform infrared spectroscopic investigation of protein stability in the lyophilized form. Biochim Biophys Acta. 1995;1253:69–74. doi: 10.1016/0167-4838(95)00156-o. [DOI] [PubMed] [Google Scholar]
- 25.Liao X, Krishnamurthy R, Suryanarayanan R. Influence of the active pharmaceutical ingredient concentration on the physical state of mannitol—implication in freeze–drying. Pharm Res. 2005;22:1978–84. doi: 10.1007/s11095-005-7625-x. [DOI] [PubMed] [Google Scholar]
- 26.Kramer T, Kremer DM, Pikal MJ, Petre WJ, Shalaev EY, Gatlin LA. A procedure to optimize scale-up for the primary drying phase of lyophilization. J Pharm Sci. 2009;98:307–18. doi: 10.1002/jps.21430. [DOI] [PubMed] [Google Scholar]