Abstract
The present work focuses on the preparation and evaluation of lecithin organogel system of thermoreversible polymer pluronic F127, which would enhance the stability and absorption of sumatriptan succinate across the skin. Formulations were developed with and without co-surfactant (pluronic F127). The prepared organogels were evaluated for its appearance, organoleptic characteristics, and feel upon application, homogeneity, occlusivenes, washability, pH, viscosity, spreadability, gel transition temperature of formulations. The formulations were also evaluated for drug content, in vitro drug diffusion properties and skin irritation testing. In vivo evaluation of formulations was carried out by hot plate and writhing test method, and finally the optimized formulation was subjected to stability studies. The developed formulations were easily washable, smooth in feel, and showed no clogging which indicate superior texture of system. Formulation, containing pluronic showed greater spreadability and higher drug diffusion rate as compared to pluronic free organogel. Drug content of organogel formulations was in the range of 94–97%. The pH of the formulations was 6.48 ± 0.5 and 6.98 ± 0.1, reflecting no risk of skin irritation. Pluronic not only enhances the stability of organogel by increasing the viscosity (from 6,541 ± 234.76 to 7,826 ± 155.65 poise) but also increases the release of drug from 67.39 ± 1.53% to 74.21 ± 1.7%. The sumatriptan exhibits higher and long lasting antinociceptive effect as indicated by the persistent increase in reaction time in hot plate and inhibited abdominal contraction in acetic acid-induced writhing test (p < 0.05). The prepared optimized formulation was found to be stable without any significant changes at room temperature.
KEY WORDS: organogel, pluronic f127, sumatriptan succinate, transdermal delivery, tubular micelles
INTRODUCTION
A gel is said to be a hydrogel or an organogel depending on the nature of the liquid component: water in hydrogels and an organic solvent in organogels. Gels can also be classified according to the bonds present in the gelator network: physical gels are held by weaker physical forces of attraction such as van der Waals interactions and hydrogen bonds, whereas chemical gels are held by covalent bonds (1–3). Organogels are bi-continuous colloidal systems that coexist as micro heterogeneous solid (i.e., gelator) and organic liquid phases (4). In general, organogels formation is based in the spontaneous self-assembly of individual gelator molecules into three-dimensional networks of randomly entangled fiber-like structures. This three-dimensional network holds micro domains of the liquid in a non-flowing state mainly through surface tension (5).
“Lecithin organogels, the jelly-like phases, consist of a 3-dimensional network of entangled reverse cylindrical (polymer-like) micelles, which immobilizes the continuous or macroscopic external organic phase, thus turning a liquid into a gel (6). The formation of a 3-dimentional network in the organogel is the result of transition at the micellar level in a low viscous Newtonian liquid, consisting of lecithin reverse micelles in non polar organic liquid” (7,8). Lecithin, when being dissolved in nonpolar media alone, self-assembles in to reverse spherical micelles at a concentration of ∼0.01 mM (9). Further, this spherical reverse micellar state of lipid aggregates turns on to form elongated tubular micelles with the addition of water (10). This system can also be called as polymer-like micelles or wormlike or threadlike micelles (11,12). Transition to polymer-like micelles is accompanied with a formation of hydrogen bonds between the phosphate group of a lecithin molecule and water (13,14).
In migraine, various stimuli may cause a serious of neurological and biochemical events, which affect the brain’s vascular system. In most people, a throbbing pain is felt only on one side of the head (15–17). Sumatriptan is a 5-hydroxy tryptamine 1D (5-HT1D)-receptor agonist, it was the first triptan available to use in the treatment of migraine. Sumatriptan is metabolized primarily by monoamine oxidase A and excreted in the urine and bile (18,19). Sumatriptan seems to act selectively on blood vessels located within the carotid circulation. Sumatriptan is generally given by oral or parental routes. Oral administration (as succinate) suffers from poor bioavailability problem due to pre-systemic metabolism and a substantial proportion of patients suffer from severe nausea or vomiting during their migraine attack, which may make oral treatment unsatisfactory (20). Transdermal delivery of sumatriptan may prove particularly helpful for migraineurs with gastric stasis (21–23). Whereas transdermal patches may have several limitations such as fluid leakage and increased skin irritation, likely from uneven contact to increased current density (24).
Transdermal vectorization of drug was made successful by using lipid-based colloidal systems, i.e., liposomal formulations (25–27), nanostructured lipid carrier (28,29), lipid microemulsions (30), solid lipid microparticles (31), elastic liposomes or transfersomes (32), ethosomes (33,34), and lipid cubic phases (35,36). Various other systems have also been developed for delivery of active principles through the skin such as acyclovir-loaded poly(lactic-co-glycolic acid) (PLGA) topical microparticles (37–39), microsponges for topical delivery mupirocin (40), biodegradable PLGA microparticles in o/w and w/o cream (41), block copolymer nanoparticles (42), biodegradable nanoparticles (43), and PLA nanoparticles (44). Further, the strategies for vectorization of drug across the skin have been extended to polymeric gels like pluronic lecithin organogels (45), bioadhesive gels, etc. (46).
Skin is a natural barrier against the penetration of most drugs (47), and the ability of drug to penetrate the skin epidermis, dermis, and subcutaneous fat layers depend on the properties of the drug and the carrier base (48). Traditional topical drug delivery systems like emulsion and suspension have various limitations such as instability and minimal systemic absorption of drug. Therefore in the present work, we selected topical formulation (organogel) of sumatriptan as alternative route to oral delivery in order to improve its penetration ability and overcome the problems related with oral route. In the present work, pluronic F127 was used in gel formulation, which is a thermo reversible polymer and is capable of making the bonds responsible for holding the gel network and act as a stabilizer due to gelling property at room temperature (49).
MATERIAL AND METHODS
Material
Sumatriptan succinate was procured as a gift sample from Aurobindo Pharmaceutical Ltd. Hyderabad, India. Soyalecithin (Phosphatidylcholine) was purchased from Across Organics, New Jersey, NJ, USA. Pluronic F 127 was purchased from Sigma Chemical Co. St. Louis, MO, USA, the isopropyl myristate was a product of CDH (P) Ltd., New Delhi, India, and the sorbic acid was purchased from Himedia Lab. (P) Ltd. Mumbai, India. All other reagents and solvents used were of reagent grade.
Methods
Preparation of Organogel System
Organogel was prepared by method describe by Sudaxshima Murdan (50) with slight modification and optimization of organogel was done by preparing the system with and without the pluronic F 127.
In brief, the system was prepared by mixing the lecithin, oil phase, and aqueous phase in a ratio of 20:80 v/v. The oil phase was prepared by mixing lecithin, sorbic acid, and isopropyl myristate and allowing the mixture to stand overnight at room temperature to ensure complete dissolution. The aqueous phase was prepared by adding pluronic F 127 to ice-cold water, the mixture was placed in a refrigerator and agitated to ensure complete dissolution. Sorbic acid was added as a preservative in the formulation. To prepare organogel, oil phase was mixed in an aqueous phase using high-speed mechanical stirrer (Remi, India). Incorporation of drug within organogels was done by dispersing the appropriate quantity of drug into aqueous phase before mixing two phases. Same procedure was followed for the preparation of pluronic-free organogel.
Visualization of Organogel System
The knowledge of molecular packing within the organogel network can be obtained using scanning electron microscope (Leo 430 England) using gold sputter technique. The system was vacuum dried, coated with gold palladium, and observed microscopically (51).
Organoleptic Characteristics
Each formulation was tested for color, odor, texture, and phase separation as well as feels upon application (stiffness, grittiness, greasiness, and tackiness).
Homogeneity Test
Hundred milligram of gel was pressed between the thumb and the index finger in order to notice the consistency of gel that any coarse particles being attached or detached on finger.
Washability
A small quantity (100 mg) of gel was rubbed on the skin of the back of the hand, than patch was washed with water and observed weather it is washable or not.
pH Determination
A solution containing 1 g of gel in 30 ml of neutralized distilled water (pH 7) was prepared and subjected to pH measurement by using a pH meter (Systronic μ pH system 361).
Rheological Studies
Rheological studies were performed with a thermostatically controlled Brookfield programmable rheometer (Model DV-1+ Brookfield viscometer) by using concentric cylinder spindle LV-4 at 100 rpm and at temperature 25°C (52).
Spreadability
Spreadability was determined by modified wooden block and glass slide apparatus. For determination of spreadability, a measured amount of gel was placed on fixed glass slide, the movable glass slide with a pan attached to it and was placed over the fixed glass slide, such that gel was sandwiched between the two slides for 5 min. The weight was continuously removed. Spreadability was determined using the following formula: S = M/T, where S is the spreadability in g/s, M is the mass in grams, and T is the time in seconds (53).
Drug Content
The content of sumatriptan succinate in the formulations was determined as per the method described by Willimann et al. (54). For estimation of sumatriptan content 1 g of accurately weighed gel was diluted to 100 ml with phosphate buffer pH 7.4 and analyzed spectrophotometrically at 227 nm.
Gel Transition Temperature
The gel transition temperature was determined in a 10-ml transparent vial containing a magnetic bar and each formulation was placed in a water bath. The vials were heated at a constant rate while stirring. The gelation temperature was measured when the magnetic bar stopped moving due to gelation. (55).
In vitro Drug Release Studies
Abdominal skin of male Wistar rats (250–280 g) was used for in vitro drug release study; the dialysis system was consisted of donor and receptor compartments separated by an abdominal skin of rat. The donor phase contained formulation equivalent to 10 mg of drug incorporated into vehicle components whereas the receptor phase contained phosphate buffer pH 7.4. The medium was agitated at 100 rpm with the help of magnetic stirrer at room temperature. Sink condition was also maintained during drug release study. After making proper dilution, the samples were withdrawn at specified time intervals (1 h) and assayed spectrophotometrically at λmax 227 nm (Shimadzu 1700 UV/visible spectrophotometer). The study was continuous for 24 h. Each test was carried out in triplicate and the mean of three observations was reported. The cumulative% drug release was plotted against time.
Skin Irritation Study
Skin irritation study was performed as per the method reported by Kulkarni and Jain (51). The hairs were depilated from the back of the mice with the help of hair removing cream; an area of 4 cm2 was marked on both the sides. One side served as control while the other as test and animals were used after 24 h of depilation. The formulation was applied (100 mg/mice) once a day for 7 days and sight was covered with cotton bandage. The mice were observed for sensitivity and the reaction if any and were graded as:
-
0
No reaction
-
0.5
Slight, patchy erythema
-
1
Slight but confluent or moderate but patchy erythema
-
2
Moderate erythema
-
3
Severe erythema with or without edema.
In vivo Studies
The purpose of this study was to examine the efficacy of prepared optimized organogel formulation as a topical drug delivery system, which enhances drug diffusion inside the deeper layer of skin and reduces systemic side effects of drug. The effect of optimized sumatriptan organogel formulation (organogel with pluronic) on basal reaction time (hot plate method) and on 0.6% acetic acid induced abdominal writhing in mice was depicted. The in vivo experimental protocol was approved by the Institutional Animal Ethics Committee of IPS Academy (Indore, India). Inbred albino mice (Swiss albino mice) weighting between 25 and 30 g of either sex were used for the study. They were housed in clean polypropylene cages at room temperature 27–31°C with standard laboratory feed and water ad libitum (56). The drug sumatriptan was applied topically in the form of organogel. Animals were divided in six groups, each consisting of three animals. The hairs of animals were shaved in 2 cm2 areas with the help of hair removing cream in interscapular region. The treatment was provided topically on shaved area. The treated areas of animals were protected by using nylon mesh, which was supported by plastic squares having small pores. Treated animals were kept in separate cages and housed in standard laboratory conditions. Group (I) was kept as a control (without treatment); group (II) was administered with solution of 1 mg/kg pure sumatriptan drug in phosphate buffer (pH 7.4), group III, IV, V, and VI was administered with 0.5, 1, 1.5, and 2 mg/kg sumatriptan organogel formulation, respectively.
Hot Plate Method
In this method, heat was used as a source of pain. All animals were placed on Eddy’s hot plate (Techno Instrument India) maintained at constant temperature 55 ± 1°C. Time taken by animal for the reaction either by licking the paw or jumping or raising the limbs which ever will observed first taken as end point. Reaction time was noted before and after 15, 30, 60, and 120 min after administration of the drug (57).
Writhing Test
Appropriate volume of acetic acid was administered to the first group (which serves as control) and was placed aside for observation. To the second group, drug formulation of different doses was administered 15 min later; freshly prepared 0.6% acetic acid solution was given i.p. to each animal. The number of abdominal contraction in 15 min was noted down.
Stability Studies
The prepared optimized organogel preparation was subjected to stability studies in amber colored glass vials at three different temperatures (4°C, RT, and 60°C) and evaluated periodically (every 15th day) for percent drug content, cumulative% drug released, pH, color change, phase separation, and viscosity for a period of 60 days.
RESULTS AND DISCUSSION
Formulation and Characterization of Pluronic Organogel Formulations
In the present work, pluronic lecithin organogel was prepared for topical delivery of sumatriptan, in which pluronic F 127 was added in order to stabilize the gel formulation. For this purpose, the formulations (with pluronic and without pluronic) were prepared and evaluated.
Surface Morphology
The surface morphology of pluronic organogel was examined by scanning electron microscopy (Fig. 1). Some cylindrical structures were visualized in SEM photographs. These cylindrical aggregates formed instead of the initial spherical ones, after reaching threshold length, after that the extended micelles begin overlapping and forming a temporal three-dimensional network as shown in Fig. 1.
Fig. 1.
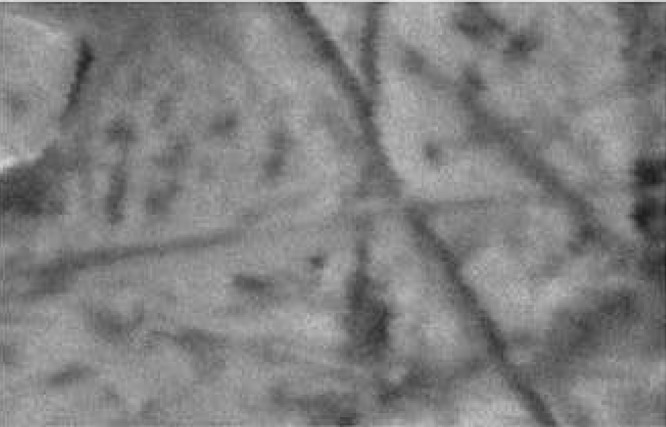
Visualization of lecithin organogel of sumatriptan by scanning electron microscope at 200× magnification
Effect on Physical Characteristics
The developed formulations were easily washable, off-white in color, smooth in feel, and did not show any phase separation. The system also showed no clogging, which indicated excellent texture of system. Freedom from grittiness reflects the degree of acceptability of formulation by the patients. The pH of all the formulations (Table I) was compatible with the skin pH (slightly acidic) reflecting no risk of skin irritation.
Table I.
Effect of Co-surfactant Addition in Lecithin Organogel System
| S. No | Test | Observation | |
|---|---|---|---|
| Organogel with pluronic | Pluronic free organogel | ||
| 1 | Washability | Not easy to wash | Washable |
| 2 | Occlusiveness | Yes | Yes |
| 3 | Homogeneity | Yes | Yes |
| 4 | Organoleptic characteristics | ||
| 4.1 | Color | Off-white | White |
| 4.2 | Odor | No | No |
| 4.3 | Phase separation | No | Yes |
| 4.4 | Feel upon application | Smooth | Gritty |
| 5 | Spreadability (gcm/s, mean ± SDa, n = 3) | 20.24 ± 0.323 | 17.52 ± 0.432 |
| 6 | Viscosity (poise) | 7,826 ± 155.65 | 6,541 ± 234.76 |
| 7 | Gelation temperature | 32°C | 36°C |
| 8 | pH (mean ± SDa, n = 3) | 6.48 ± 0.5 | 6.98 ± 0.1 |
aDenotes standard deviation
Rheological Measurements
It was observed that in the presence of pluronic, viscosity increases from 6,541 ± 234.76 to 7,826 ± 155.65 poise (Table I). The increase in viscosity might be due to formation of complex network, as in the case of gel, the consistency depends on percentage of solids in relation to liquid. In case of pluronic lecithin organogel, consistency was increased, which could due to combination of pluronic and lecithin present in formulation.
Effect on Spreadability
The spreadability formulations with pluronic and pluronic-free system was found to be 20.24 ± 0.323 and 17.52 ± 0.432 gcm/s (Table I), respectively, which revealed that the presence of pluronic increases the spreadability of formulation.
Effect on Gel Transition Temperature
The phase behavior of organogels varies with changing the temperature conditions (58). The phase transition temperature (i.e., sol-to-gel, TSG, or gel-to-sol, TGS) gives an insight into the nature of microstructures that form the gelling cross-linked network. The phase transition temperatures also help in optimizing the organogel composition. Gelation temperature, defined as the temperature at which the liquid phase makes a transition to gel (59). The gel transition temperature for pluronic organogel was found to be 32°C; however it was 36°C for simple organogel system, which is attributed to increase in viscosity of the system which decreases gel transition temperature and improves the adhesive properties of formulations (Table I).
Drug Content
Sumatriptan succinate content in organogel was determined by UV visible spectrophotometer. Drug content in the system with pluronic and pluronic-free system was found to be 97.52 ± 1.062% and 94.83 ± 2.567% (mean ± SD, n = 3), respectively. Drug content in the pluronic organogel system was found to be higher than pluronic-free system, which could be due the presence of pluronic which causes uniform distribution of drug throughout the system and also prevent its interaction with the components of base present in system.
In vitro Drug Release Studies
Franz diffusion cells (Erweka, Germany) was used for in vitro release studies of sumatriptan succinate. The formulations were subjected to in vitro drug release in phosphate buffer pH 7.4 medium using the abdominal skin of male Wistar rats.
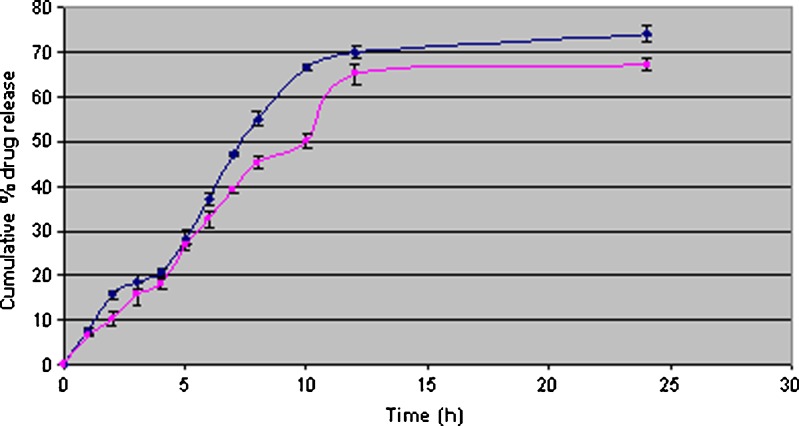
Pluronic not only enhances the stability of organogel by increasing the viscosity but also increase the release of drug from the formulation. As shown in Fig. 2, the organogel formulation, where pluronic was added, showed greater spreadability and high release as compared to formulation where pluronic was not added. Pluronic would facilitate the permeation of comparatively large molecules across lipid bilayer whereas permeation of small solute remains unaffected.
Fig. 2.
In vitro release of sumatriptan succinate from organogel formulations [pluronic (black diamond suit) and pluronic free organogel (black square)] in phosphate buffer pH 7.4 at 37 ± 2°C (λmax 227 nm). Each point represents mean ± SD of three different determinations
The Skin Irritation Study
When the gel was applied on mice skin for 7 days, the mice were observed for its sensitivity and reaction if any was observed; the optimized formulations showed no detectable level of skin irritancy in mice indicating the compatibility of formulations with skin.
In vivo Studies
Antinociception means reducing sensitivity to painful stimuli. The physiology of nociception involves a complex interaction of peripheral and central nervous system structures, extending from the skin, the viscera to the musculoskeletal tissues to the cerebral cortex.
Hot Plate Method
In hot plate method, heat was used as a source of pain. Animals were individually placed on hot plate; and reaction of animals, such as paw licking or jumping response was first taken as end point, the analgesics are generally increasing the reaction time. Normally, animals showed such response in 6–8 s, but due to the antinociceptive effect of sumatriptan, reaction time increases and it produced dose-dependent increase in basal reaction time at all doses tested (0.5, 1, 1.5, 2 mg/kg) throughout the period of observation (till 120 min) when compared with control group (Table II; p < 0.05). The drug sumatriptan had induced dose-dependent analgesia with peak effect observed between 60 and 120 min after treatment. As the reaction time increases with sumatriptan, 15 s was taken as maximum analgesia and animals were removed from the hot plate to avoid injury to the paws.
Table II.
Effect of Sumatriptan Formulations on Basal Reaction Time of Mice in Hot Plate Method
| S. No. | Basal reaction time | Reaction time after drug administration (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Paw licking (s, mean ± SD, n = 3) | Jump response (s, mean ± SD, n = 3) | Paw licking (s, mean ± SD, n = 3) | Jump response (s, mean ± SD, n = 3) | |||||||
| 15 | 30 | 60 | 120 | 15 | 30 | 60 | 120 | |||
| 1 | 7.51 ± 0.31 | 6.56 ± 0.56 | 6.83 ± 0.10 | 7.41 ± 0.61 | 7.66 ± 0.42 | |||||
| 2 | 6.82 ± 0.42 | 6.15 ± 0.24 | 6.73 ± 0.41 | 6.88 ± 0.35 | 7.06 ± 0.36 | |||||
| 3 | 5.61 ± 0.15 | 7.14 ± 0.42 | 5.42 ± 0.17 | 7.66 ± 0.22 | 7.22 ± 0.16 | |||||
| 4 | 4.35 ± 1.17 | 5.57 ± 0.72 | 5.12 ± 0.37 | 6.86 ± 0.47 | 7.19 ± 1.36 | |||||
| 5 | 7.54 ± 0.19 | 8.82 ± 0.56 | 9.76 ± 0.57 | 10.3 ± 0.41 | 10.8 ± 0.17 | |||||
| 6 | 9.76 ± 0.37 | 8.91 ± 1.24 | 7.63 ± 0.55 | 10.82 ± 0.75 | 15a | |||||
SD standard deviation
aA cut off- time of 15 s was taken as maximum antinociception response to avoid injury to the paws
Writhing Test
Painful reactions in animals may be produced by chemicals also; the intraperitonial injection of acetic acid produces pain reaction which was characterized by writhing response. Constriction of abdomen, turning of trunk (twist), and extension of hind legs were taken as reaction to chemically induced pain. In comparison to control, a dose-dependent increase in time for onset of writhing induced by intraperitonial administration of 0.6% acetic acid was observed at all the doses tested in the mice pretreated with sumatriptan. The abdomen contraction induced by acetic acid was inhibited by sumatriptan administration throughout the period of observation (15 min, p < 0.05; Table III).
Table III.
Effect of Sumatriptan Formulations on 0.6% Acetic Acid Induced Abdominal Writhing in Mice
| S. No. | Treatment | Number of writhing (15 min) |
|---|---|---|
| 1 | Control (acetic acid) | 43.45 ± 1.92 |
| 2 | Solution of 1 mg/kg pure sumatriptan drug in phosphate buffer (pH 7.4) | 39.83 ± 1.86 |
| 3 | Organogel containing Sumatriptan 0.5 mg/kg + acetic acid | 37.61 ± 2.47 |
| 4 | Organogel containing Sumatriptan 1 mg/kg + acetic acid | 34.77 ± 2.56 |
| 5 | Organogel containing Sumatriptan 1.5 mg/kg + acetic acid | 30.53 ± 2.33 |
| 6 | Organogel containing Sumatriptan 2 mg/kg + acetic acid | 28.68 ± 3.2 |
The data revealed that the solution of 1 mg/kg pure sumatriptan in phosphate buffer (pH 7.4) produces a slight change in response due to less diffusion of drug, as compared to drug-containing formulations. The present study suggests that sumatriptan at different doses studied have antinociceptive effect in both the experimental models (thermal- and chemical-induced pain in mice). Sumatriptan exhibits higher and long-lasting antinociceptive effect as indicated by the persistent increase in reaction time in hot plate and inhibited abdominal contraction in acetic acid induced writhing test (p < 0.05).
Stability Studies
The optimized formulation (organogel with pluronic) was subjected to stability studies at three different temperatures (4°C, RT, and 60°C). It was found that more than 98% of drug was present in all the formulations after 60 days at RT, whereas at 4°C, there was about 2% and at 60°C, there was about 4% decline in the drug content observed in the tested formulations. The degradation observed at 60°C temperature was attributed to the degradation of drug at higher temperature condition. Other macroscopic characters like transparency, feel, and pH were also observed as given in Table IV and no significant change was found in these characteristics, which showed that during stability studies, the prepared optimized formulation was found to be stable without any significant changes at room temperature.
Table IV.
Stability of Optimized Organogel Formulation (Organogel with Pluronic)
| Time (days) | Temperature | Surface characteristics | Drug content | Viscosity (poise) | pH | Spreadability (gcm/s, mean ± SD, n = 3) |
|---|---|---|---|---|---|---|
| 0 | RT | – | 95.81 ± 1.32 | 8,959 | 6.48 ±0.5 | 18.21 ± 0.53 |
| 15 | 4°C | – | 95.66 ± 0.52 | 9,263 | 6.96 ± 0.3 | 18.11 ± 0.35 |
| 30 | 4°C | – | 95.52 ± 0.25 | 8,751 | 7.10 ± 0.4 | 19.50 ± 0.65 |
| 45 | 4°C | – | 95.45 ± 1.12 | 9,026 | 6.87 ± 0.52 | 17.92 ± 0.69 |
| 60 | 4°C | + | 93.58 ± 0.4 | 9,172 | 6.95 ± 0.2 | 17.70 ± 0.5 |
| 15 | RT | – | 95.75 ± 1.86 | 7,741 | 7.10 ± 0.2 | 18.06 ± 0.65 |
| 30 | RT | – | 95.43 ± 0.86 | 7,652 | 6.77 ± 0.4 | 18.11 ± 0.42 |
| 45 | RT | + | 94.84 ± 1.87 | 7,800 | 6.52 ± 0.3 | 18.66 ± 1.2 |
| 60 | RT | + | 93.42 ± 1.19 | 7,720 | 7.21 ± 0.6 | 17.97 ± 0.82 |
| 15 | 60°C | + | 94.45 ± 0.38 | 9,041 | 7.51 ± 0.1 | 17.97 ± 0.78 |
| 30 | 60°C | + | 93.82 ± 0.63 | 7,660 | 6.91 ± 0.3 | 18.65 ± 0.71 |
| 45 | 60°C | ++ | 92.51 ± 0.24 | 8,972 | 6.85 ± 0.5 | 17.35 ± 1.67 |
| 60 | 60°C | ++ | 91.85 ± 1.54 | 9,010 | 6.71 ± 0.3 | 15.70 ± 0.69 |
– no change, + slight change, ++ considerable change
SD standard deviation
CONCLUSION
Lecithin and pluronic (from the organogel) was thought to penetrate into the skin, interact and disorganize the lipid layers of the stratum corneum. However, improved topical drug delivery has been attributed to the biphasic drug solubility, the desired drug partitioning, and the modification of skin barrier function by the organogel components. The partition coefficient of sumatriptan succinate was found to be 0.93 and it was readily soluble in aqueous phase. This would result in increased drug diffusion through the skin. Organogels offers improved microbial resistance as compared to hydrogels. The findings of the present study suggest that the prepared sumatriptan organogels containing lecithin and pluronic were observed to be safe, stable, and cost-effective drug delivery system. The topical organogel formulation of sumatriptan provides significant antinociceptive activity when applied topically. The migraine patients that may find this delivery system particularly helpful are those with gastrointestinal symptoms (e.g., nausea and vomiting) frequently associated with their migraine attacks.
ACKNOWLEDGMENTS
The authors would like to acknowledge Aurobindo Pharmaceutical Ltd. Hyderabad (India), for providing free gift sample of sumatriptan succinate. The authors are very thankful to the management of School of Pharmaceutical Sciences, Rajiv Gandhi Technical University, for providing necessary facilities and encouragement. Authors are also thankful to Head, Department of Pharmaceutical Sciences, IPS Academy, Indore (India) for providing facilities for in vivo animal studies.
REFERENCES
- 1.Hermans PH. Gels. In: Kruyt HR, editor. Colloid science (volume 2) Amsterdam, The Nederlands: Elsevier; 1949. pp. 483–651. [Google Scholar]
- 2.Flory PJ. Introductory lecture. Disc Faraday Soc. 1974;57:7–18. doi: 10.1039/dc9745700007. [DOI] [Google Scholar]
- 3.Almdal K. Towards a phenomenological definition of the term gel. Polym Gels Netw. 1993;1:5–17. doi: 10.1016/0966-7822(93)90020-I. [DOI] [Google Scholar]
- 4.Abdallah DJ, Lu L, Weiss RG. Thermoreversible organogels from alkane gelators with one heteroatom. Chem Mater. 1999;11:2907–2911. doi: 10.1021/cm9902826. [DOI] [Google Scholar]
- 5.Abdallah DJ, Weiss RG. n-Alkanes gel n-alkanes (an many other organic liquids) Langmuir. 2000;16:352–355. doi: 10.1021/la990795r. [DOI] [Google Scholar]
- 6.Capitani D, Segre AL, Dreher F, Walde P, Luisi PL. Multinuclear NMR investigation of phosphatidylcholine organogels. J Phys Chem. 1996;100:15211Y15217. doi: 10.1021/jp960811i. [DOI] [Google Scholar]
- 7.Walde P, Giuliani AM, Boicelli CA, Luisi PL. Phospholipid-based reverse micelles. Chem Phys Lipids. 1990;53:265Y288. doi: 10.1016/0009-3084(90)90026-N. [DOI] [PubMed] [Google Scholar]
- 8.Shumilina EV, Khromova Y, Shchipunov YA. A study of the structure of lecithin organic gels by Fourier-transform IR spectroscopy. Zh Fiz Khim. 2000;74:1210Y1219. [Google Scholar]
- 9.Shchipunov YA, Duressschmidt T, Hoffmann H. Electrorheological effects in lecithin organogels with water and glycerol. J Colloid Interface Sci. 1990;212:390–401. doi: 10.1006/jcis.1998.6046. [DOI] [PubMed] [Google Scholar]
- 10.Shchipunov YA. Lecithin organogel: a micellar system with unique properties. Colloids surf A Physicochem Eng Asp. 2001;183–185:541–554. doi: 10.1016/S0927-7757(01)00511-8. [DOI] [Google Scholar]
- 11.Shchipunov YA. Lecithin organogel: rheological properties of polymer-like micelles formed in the presence of water. Colloid J. 1995;57:556–560. [Google Scholar]
- 12.Shchipunov YA, Duressschmidt T, Hoffmann H. End to end fusion of polymer-like micelles in the lecithin organogel under the action of an electric field. Langmuir. 2000;16:297–299. doi: 10.1021/la990810s. [DOI] [Google Scholar]
- 13.Shchipunov YA, Shumilina EV. Lecithin bridging by hydrogen bonds in the organogel. Mater Sci Eng C. 1995;3 Suppl 1:43. doi: 10.1016/0928-4931(95)00102-6. [DOI] [Google Scholar]
- 14.Shchipunov YA, Shumilina EV. Molecular model for the lecithin self-organization into polymer-like micelles. Prog Colloid Polym Sci. 1997;106 Suppl 1:228. doi: 10.1007/BF01189526. [DOI] [Google Scholar]
- 15.Wessman M, Gisela M. Migraine: a complex genetic disorder. Lancet Neurol. 2007;6:521–532. doi: 10.1016/S1474-4422(07)70126-6. [DOI] [PubMed] [Google Scholar]
- 16.The Headache Classification Committee of the International Headache Society The international classification of headache disorders: 2nd edition. Cephalalgia. 2004;24(suppl. 1):160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 17.Villalon CM, Centurion D, Valdivia LF, De Varis P, Saxena PR. Migraine: pathophysiology, pharmacology treatment and future trends. Curr Vasc Pharmacol. 2003;1:71–84. doi: 10.2174/1570161033386826. [DOI] [PubMed] [Google Scholar]
- 18.Humphrey PP, Feniuk W, Marriott AS, Tanner RJ, Jackson MR, Tucker ML. Preclinical studies on antimigrine drugs. Eur Neurol. 1991;31:282–290. doi: 10.1159/000116755. [DOI] [PubMed] [Google Scholar]
- 19.Jhee SS, Shiovitz T, Crawford AW, Cutler NR. Pharmacokinetics and pharmacodynamics of the triptan antimigraine agents: a comparative review. Clin Pharmacokinet. 2001;40:189–205. doi: 10.2165/00003088-200140030-00004. [DOI] [PubMed] [Google Scholar]
- 20.Upadhyay KK, Tiwari C, Khopade AJ, Bohidar HB, Jain SK. Sorbitan Ester organogels for transdermal delivery of sumatriptan. Drug Dev Ind Pharm. 2007;33:617–625. doi: 10.1080/03639040701199266. [DOI] [PubMed] [Google Scholar]
- 21.Goadsby PJ, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 22.Aurora SK, Kori SH, Barrodale P, McDonald SA, Haseley D. Gastric stasis in migraine: more than just a paroxysmal abnormality during a migraine attack. Headache. 2006;46:57–63. doi: 10.1111/j.1526-4610.2006.00311.x. [DOI] [PubMed] [Google Scholar]
- 23.Pierce M, Marbury T, Neill CO, Siegel S, Du W, Sebree T. Zelrix™: a novel transdermal formulation of sumatriptan. Headache. 2009;49:817–825. doi: 10.1111/j.1526-4610.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 24.Siegel SJ, Neill CO, Dube LM, Kaldeway P, Morris R, Jackson D, Sebree T. Aunique iontophoretic patch for optimal transdermal delivery of sumatriptan. Pharm Res. 2007;24:1919–1926. doi: 10.1007/s11095-007-9317-1. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia A, Singh B, Amarji B, Katare OP. Tamoxifen-Loaded liposomal topical formulation arrests hair growth in mice. Br J Dermatol Nurs. 2009 doi: 10.1111/j.1365-2133.2010.09772.x. [DOI] [PubMed] [Google Scholar]
- 26.Gupta M, Goyal AK, Paliwal SR, Paliwal R, Mishra N, Vaidya B, Dubey D, Jain SK, Vyas SP. Development and characterization of effective topical liposomal system for localized treatment of cutaneous candiasis. J Liposome Res. 2010;20(4):341–350. doi: 10.3109/08982101003596125. [DOI] [PubMed] [Google Scholar]
- 27.Dragicevic-Curic N, Winter S, Krajisnik D, Stupar M, Milic J, Graefe S, Fahr A. Stability evaluation of temoporfin-loaded liposomal gels for topical application. J Liposome Res. 2010;20(1):38–48. doi: 10.3109/08982100903030263. [DOI] [PubMed] [Google Scholar]
- 28.Puglia C, Filosa R, Peduto A, de Caprariis P, Rizza L, Bonina F, Blasi P. Evaluation of alternative strategies to optimize ketorolac transdermal delivery. AAPS PharmSciTech. 2006;7(3):E1–E9. doi: 10.1208/pt070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferderber K, Hook S, Rades T. Phosphatidyl choline-based colloidal systems for dermal and transdermal drug delivery. J Liposome Res. 2009;19(4):267–277. doi: 10.3109/08982100902814006. [DOI] [PubMed] [Google Scholar]
- 30.Luciana BL, Hillary VDW, Hsin TL, Vijay V, Hsin KL, Stan N, Jaclyn H, Mark L, Haian Z, Vitória M, Bentley LB, Robert L, Martha AH. Topical delivery of lycopene using microemulsions: enhanced skin penetration and tissue antioxidant activity. J Pharm Sci. 2009;99(3):1346–1357. doi: 10.1002/jps.21929. [DOI] [PubMed] [Google Scholar]
- 31.Scalia S, Mezzena M, Iannuccelli V. Influence of solid lipid microparticle carriers on skin penetration of the sunscreen agent, 4-methylbenzylidene camphor. J Pharm Pharmacol. 2007;59:1621–1627. doi: 10.1211/jpp.59.12.0003. [DOI] [PubMed] [Google Scholar]
- 32.Jain SK, Gupta Y, Jain A, Rai K. Enhanced transdermal delivery of acyclovir sodium via elastic liposomes. Drug Deliv. 2006;15:141–147. doi: 10.1080/10717540801952407. [DOI] [PubMed] [Google Scholar]
- 33.Maestrelli F, Capasso G, González-Rodríguez ML, Antonio M, Rabasco CG, Mura P. Effect of preparation technique on the properties and in vivo efficacy of benzocaine-loaded ethosomes. J Liposome Res. 2009;19(4):253–260. doi: 10.3109/08982100902788408. [DOI] [PubMed] [Google Scholar]
- 34.Bendas ER, Tadros MI. Enhanced Transdermal Delivery of Salbutamol Sulfate via Ethosomes. AAPS PharmSciTech 2007; 8 (4) Article 107 [DOI] [PMC free article] [PubMed]
- 35.Bender J, Simonsson C, Smedh M, Engström S, Ericson MB. Lipid cubic phases in topical drug delivery: visualization of skin distribution using two-photon microscopy. J Control Release. 2008;129(3):163–169. doi: 10.1016/j.jconrel.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 36.Bender J, Ericson MB, Merclin N, Iani V, Rosen A, Engstrom S, Moan J. Lipid cubic phases for improved topical drug delivery in photodynamic therapy. J Control Release. 2005;106:350–360. doi: 10.1016/j.jconrel.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 37.de Jalon EG, Blanco-Prıeto MJ, Ygartua P, Santoyo S. Topical application of acyclovir-loaded microparticles: quantification of the drug in porcine skin layers. J Control Release. 2001;75:191–197. doi: 10.1016/S0168-3659(01)00395-9. [DOI] [PubMed] [Google Scholar]
- 38.de Jalon EG, Blanco-Prıeto MJ, Ygartua P, Santoyo S. PLGA microparticles: possible vehicles for topical drug delivery. I J Pharm. 2001;226:181–184. doi: 10.1016/s0378-5173(01)00811-0. [DOI] [PubMed] [Google Scholar]
- 39.Santoyo S, de Jalon EG, Ygartua P, Renedo MJ, Blanco-Prıeto MJ. Optimization of topical cidofovir penetration using microparticles. I J Pharm. 2002;242:107–113. doi: 10.1016/s0378-5173(02)00178-3. [DOI] [PubMed] [Google Scholar]
- 40.Amrutiya N, Bajaj A, Madan M. Development of microsponges for topical delivery of mupirocin. AAPS PharmSciTech. 2009;10(2):402–409. doi: 10.1208/s12249-009-9220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haddadi A, Aboofazeli R, Erfan M, Farboud ES. Topical delivery of urea encapsulated in biodegradable PLGA microparticles: O/W and W/O creams. J Microencapsul. 2008;25(6):379–386. doi: 10.1080/02652040802000714. [DOI] [PubMed] [Google Scholar]
- 42.Shim J, Kang HS, Won-Seok P, Sang-Hun H, Kim J, Ih-Seop C. Transdermal delivery of mixnoxidil with block copolymer nanoparticles. J Control Release. 2004;97:477–484. doi: 10.1016/j.jconrel.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 43.Alvarez-Roman R, Naik A, Kalia YN, Guy RH, Fessi H. Enhancement of topical delivery from biodegradable nanoparticles. Pharm Res. 2004;21(10):1818–1825. doi: 10.1023/B:PHAM.0000045235.86197.ef. [DOI] [PubMed] [Google Scholar]
- 44.Rancan F, Papakostas D, Hadam S, Hackbarth S, Delair T, Primard C, Verrier B, Sterry W, Blume-Peytavi U, Vogt A. Investigation of polylactic acid (PLA) nanoparticles as drug delivery systems for local dermatotherapy. Pharm Res. 2009;26(8):2027–2036. doi: 10.1007/s11095-009-9919-x. [DOI] [PubMed] [Google Scholar]
- 45.Pandey M, Belgamwar V, Gattani S, Surana S, Tekade A. Pluronic lecithin organogel as a topical drug delivery system. Drug Deliv. 2010;17(1):38–47. doi: 10.3109/10717540903508961. [DOI] [PubMed] [Google Scholar]
- 46.Dhiman M, Yedurkar P, Sawant KK. Formulation, characterization, and in vitro evaluation of bioadhesive gels containing 5-fluorouracil. Pharm Dev Technol. 2008;13(1):15–25. doi: 10.1080/10837450701702438. [DOI] [PubMed] [Google Scholar]
- 47.Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin’s barrier function. Pharm Sci Technol Today. 2000;3:318–326. doi: 10.1016/S1461-5347(00)00295-9. [DOI] [PubMed] [Google Scholar]
- 48.Idson B, Lazarus J. The theory and practice of industrial pharmacy. Bombay, India: Vargehese Publishing House; 1991. Semisolids; pp. 534–563. [Google Scholar]
- 49.Escobar-Chávez JJ, López-Cervantes M, Naïk A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A. Applications of thermoreversible pluronic f-127 gels in pharmaceutical formulations. J Pharm Pharmaceut Sci. 2006;9(3):339–358. [PubMed] [Google Scholar]
- 50.Murdan S. A review of pluronic lecithin organogel as a topical and transdermal drug delivery system. Hosp Pharm. 2005;12:267–270. [Google Scholar]
- 51.Kumar R, Katare OP. Lecithin organogels as a potential phospholipid-structured system for topical drug delivery: a review. AAPS PharmSciTech. 2005;06 Suppl 02:E298–E310. doi: 10.1208/pt060240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nasseri AA, Aboofazeli R, Zia H, Needham TE. Lecithin stabilized microemulsion: an organogel for topical application of ketorolac tromethamine I: phase behavior studies. Iran J Pharm Res. 2003;2:59–61. [Google Scholar]
- 53.Shchipunov YA, Shumilina EV. Lecithin organogels: role of polar solvent and nature of intermolecular interactions. Colloid J. 1996;58:117–1125. [Google Scholar]
- 54.Willimann H, Walde P, Luisi PL, Gazzaniga A, Stroppolo F. Lecithin organogel as matrix for transdermal transport of drugs. J Pharm Sci. 1992;81:871–874. doi: 10.1002/jps.2600810906. [DOI] [PubMed] [Google Scholar]
- 55.Anand B. Applications of organogels in pharmaceuticals. J Sci Ind Res. 2001;60(Suppl 4):311–318. [Google Scholar]
- 56.Suh H, Jun HW. Physicochemical and release studies of naproxen in poloxamer gels. Int J Pharm. 1996;129:13–20. doi: 10.1016/0378-5173(95)04210-5. [DOI] [Google Scholar]
- 57.Ghelardini C, Galeotti N, Figini M, Imperato A, Nicolodi M, Sicuteri F, Gessa GL, Bartolini A. The central cholinergic system has a role in the Antinociception induced in rodents by anti migraine drug sumatriptan. J Pharmacol Ther. 1996;279:884–890. [PubMed] [Google Scholar]
- 58.Rohit, Rao C, Krishna G. Effect of captopril and losartan on thermal and chemical induced pain in mice. Indian J Physiol Pharmacol. 2006;50 Suppl 2:169–174. [PubMed] [Google Scholar]
- 59.Kulkarni SK, Jain NK. Pharmacological and pharmacokinetics studies on marketed gel formulations of nimesulide. Indian Drugs. 2001;38(Suppl 2):63–66. [Google Scholar]