Abstract
The objective of this study was to evaluate the association between trace and toxic elements zinc (Zn), cadmium (Cd), nickel (Ni) and lead (Pb) in biological samples (scalp hair, blood and urine) of smoker and nonsmoker hypertensive patients (n=457), residents of Hyderabad, Pakistan. For the purpose of comparison, the biological samples of age-matched healthy controls were selected as referents. The concentrations of trace and toxic elements were measured by atomic absorption spectrophotometer prior to microwave-assisted acid digestion. The validity and accuracy of the methodology were checked using certified reference materials and by the conventional wet acid digestion method on the same certified reference materials and real samples. The recovery of all the studied elements was found to be in the range of 97.8–99.3% in certified reference materials. The results of this study showed that the mean values of Cd, Ni and Pb were significantly higher in scalp hair, blood and urine samples of both smoker and nonsmoker patients than in referents (P<0.001), whereas the concentration of Zn was lower in the scalp hair and blood, but higher in the urine samples of hypertensive patients. The deficiency of Zn and the high exposure of toxic metals as a result of tobacco smoking may be synergistic with risk factors associated with hypertension.
Keywords: biological samples, smokers, nonsmokers, toxic elements, zinc
Introduction
Hypertension (HT) is an increasingly important medical and public health issue. The prevalence of HT increases with advancing age (60–90 years).1 But nowadays, the age criteria have been changed and even people below 30 years of age have HT problems because of the lack of exercise, fast foods, smoking, coffee and alcohol consumption.2 Genetic effect may also be a factor.3 Smoking, however, is an important source of exposure to toxic elements (TEs) such as cadmium (Cd), nickel (Ni) and lead (Pb), which have been proposed as causative agents of cigarette smoke-induced physiological disorders.4, 5, 6 In fact, a study showed that serious symptoms (strong urges to smoke, feeling anxious or unsuccessful attempts at not smoking) appeared in youth within weeks or only days after the initial start of smoking.7
The essential trace element, zinc (Zn), is an important component of biomembranes and an essential cofactor in a variety of enzymes.8 Zn has antioxidant-like properties; thus, it can stabilise macromolecules against radical-induced oxidation in vitro as well as limit excess radical production.9 Zn deficiency is associated with an increase in Cd, as a results of the antagonistic relationships between these elements.10
Cigarette design has evolved considerably over the last few decades with the incorporation of new tobacco processes, papers, filters and several ingredients (flavour, humectants and casing materials), which either alone or in combination have the potential to modify the quantity and/or the quality of the smoke yielded.11 The tobacco plant absorbs TEs most probably from the soil, fertilizers or from pesticides.12 Other environmental factors that may influence the uptake of TEs by tobacco plants include the pH of soil, contaminated irrigated water and sewage sludge used as fertilizers.13 Tobacco smoking delivers 87 organic carcinogens to the lungs, in addition to TEs,14 which may partition into the smoke phase on combustion.15 Some of these (Cd, Ni and Pb) readily pass into the bloodstream and may accumulate in specific organs, such as the kidney and liver.16 There are a few studies that have reported on the large variations of heavy metal/TEs in the compositions of commercial tobacco products, which have tried to link smoking-related diseases with TEs derived from tobacco combustion.17, 18 The intake of TEs may promote hypertensive and atherosclerosis disorders by increasing oxidative stress (for example, by catalyzing the production of reactive oxygen species or inhibiting their degradation) due to the deficiency of an antioxidant element (Zn) and by increasing blood pressure levels.19 The deficiency of essential nutrients, lack of homeostatic control or an excess intake of some TEs cause chronic physiological disorders, such as HT and cardiovascular disease.20
In view of the above facts, it is important to determine the essential trace and TEs concentrations in biological samples of humans having physiological disorders such as HT.21 Among the various biopsy materials, serum, scalp hair, urine and other body fluids may be used as bio-indicators for these purposes.22 Atomic absorption spectrometric methods are frequently used for the specific determination of very low elemental concentrations in biological samples.23 At present, the mineralisation method frequently used for the analysis of biological samples is wet digestion with concentrated acids, using either convective systems or microwave ovens.24 The main advantage of microwave-assisted samples pretreatment is its requirement of a small amount of mineral acids and a reduction in the production of nitrous vapours.
The rate of mortality has increased in Pakistan during the last few decades because of HT and other related disorders. Although an extensive list of risk factors is present, tobacco smoke through active or passive smoking is also an important cause of HT. This study was aimed to assess the concentration of toxic elements, Cd, Ni, Pb and an essential trace element Zn in biological samples of smokers and nonsmokers hypertensive patients, to evaluate the possible influence of cigarette smoking and determine the potential harm to the general health of the people of Pakistan.
Although Pakistan is an agricultural country, but tobacco cultivation occupies a relatively small area: about 0.27% of the total irrigated land in Pakistan. The annual production of tobacco is 70–75 million kg, whereas the domestic requirement is 40–50 million kg; the remaining 30–35 million kg is exported. Although the consumption of cigarettes is falling in most countries of the world, while in Pakistan, both the production and consumption of this nonessential item are increasing at an alarming rate. It is surprising to note that as the world is fighting against smoking, an increasing number of Pakistani peoples are getting hooked, setting new records by manufacturing an additional five billion cigarettes each year. The rate of smoking among males is more than 50%, whereas it is 10% in females. This follow-up study of 3 years is aimed at evaluating the concentration of Cd, Ni, Pb and Zn in biological samples (blood, urine and scalp hair) of male smoker (SHP) and nonsmoker hypertensive patients (NSHPs). For a comparative study, 369 non-hypertensive individuals (smokers and nonsmokers) of the same age group (ranged 25–55 years), socioeconomic status, localities and dietary habits were selected as referents. The elements under study were analyzed by atomic absorption spectrometer, before microwave-assisted acid digestion.
Materials and methods
Apparatus
The analysis of elements was carried out by means of a double beam Perkin-Elmer atomic absorption spectrometer model 700 (Perkin Elmer, Norwalk, CT, USA) equipped with a flame burner and graphite furnace HGA-400 (Perkin Elmer), a pyrocoated graphite tube with an integrated platform and an autosampler AS-800 (Perkin Elmer). The instrumental parameters are shown in Table 1. Zn was measured under optimised operating conditions using FAAS with an air–acetylene flame, whereas Cd, Ni and Pb were determined using ETAAS. Signals were measured as absorbance peaks in the flame absorption mode, whereas integrated absorbance values (peak area) were determined in the graphite furnace. A Pel (PMO23, Osaka, Japan) domestic microwave oven (maximum heating power of 900 W) was used for digestion of the biological samples. Acid-washed PTFE (polytetrafluoroethylene) vessels (Kartell, Milan, Italy) and flasks were used for preparing and storing solutions.
Table 1. Measurement conditions for electrothermal atomization AAS 700.
| Parameters | Cd | Ni | Pb |
|---|---|---|---|
| Lamp current (mA) | 6.0 | 3.5 | 8.0 |
| Wave length (nm) | 228.8 | 232.0 | 283.3 |
| Slit width (nm) | 0.7 | 0.2 | 0.7 |
| Drying temperature (°C)/ramp/hold (s) | 140/15/5 | 140/15/5 | 140/15/5 |
| Ashing temperature (°C)/ramp/hold (s) | 850/10/20 | 1000/10/20 | 700/10/20 |
| Atomization temperature (°C)/ramp/hold (s) | 1650/0/5.0 | 2300/0/5.0 | 1800/0/5.0 |
| Cleaning temperarture (°C)/ramp/hold (s) | 2600/1/3 | 2600/1/3 | 2600/1/3 |
| Chemical modifier | 5 μg Pd as Pd(NO3)2 | Mg(NO3)2 | 15 μg Mg(NO3)2 |
| Sample volume (10 μl), Cuvette=Cup, Carrier gas=(200 ml min−1) | |||
| Background correction (D2 Lamp) used for all elements | |||
| Measurement conditions for Zn | |||
| Wave length (nm) | 214 | ||
| Slit width (nm) | 0.7 | ||
| Lamp current (mA) | 7.5 | ||
| Burner height (mm) | 7.5 | ||
| Oxidant (Air) l min−1 | 17.0 | ||
| Fuel (Acetylene) l min−1 | 2.0 | ||
Reagents and glasswares
Ultrapure water obtained from an ELGA labwater system (ELGA, Bucks, UK) was used throughout the work. Concentrated nitric acid (65%) and hydrogen peroxide (30%) were obtained from Merck (Darmstadt, Germany), and checked for possible trace-metal contamination. Working standard solutions of Cd, Ni, Pb and Zn were prepared immediately before their use, by stepwise dilution of certified standard solution (1000 p.p.m.), Fluka Kamica (Buchs, Switzerland), with 0.2 HNO3. The stock standard solution of modifiers, Mg(NO3)2 (5.00 g l−1), was prepared from Mg(NO3)2 (Merck), whereas a Pd stock standard solution of 3.00 g l−1 was prepared from Pd 99.999% Sigma Aldrich (Milwaukee, WI, USA). All solutions were stored in polyethylene bottles at 4 °C. For the accuracy of methodology, certified reference materials, human hair BCR 397 (commission of European communities, Brussels, Belgium), Clincheck control-lyophilized human urine and human whole blood (Recipe, Munich, Germany) were used. All glassware and plastic materials used were earlier soaked for 24 h in 5 nitric acid, washed with distilled water and finally rinsed with ultrapure water (ELGA, Bucks, UK), dried, and stored in class 100 laminar flow hoods.
Sample collection and pretreatment
An epidemiological cross-sectional survey was conducted among 369 referent males, of whom 186 were nonsmokers (RNSs) and 183 were smokers (RSs). Out of 457 male hypertensive patients, 297 were smokers (SHP) and 160 were nonsmokers (NSHP), their age ranged from 25 to 55 years and they lived in urban areas of Hyderabad, Pakistan. For all patients, anthropometric parameters including weight, height and waist circumference were measured using the standard protocols. Blood pressure, glycohaemoglobin, fasting plasma glucose, fasting plasma insulin, serum total cholesterol, serum HDL cholesterol, serum LDL cholesterol, serum triglycerides and height and weight were measured using standard methods (Table 2). A questionnaire was also administered to them to collect details regarding physical data, ethnic origin, health, duration of smoking, frequency of smoking, dietary habits, age and consent. Physical examinations were carried out in a basic health unit of Hyderabad, Pakistan to measure participant's weight, height, blood pressure and biochemical data. There were no statistically significant differences between both groups of patients and referents with regard to height and weight. The 90% hypertensive patient used antihypertensive drugs.
Table 2. Characteristics of hypertensive and non-hypertensive patients.
| RNS | RS | NSHP | SHP | |
|---|---|---|---|---|
| Height (cm) | 176.6±1.5 | 175.4±2.2 | 172.5± 2.3 | 173.3± 1.8 |
| BMI (kg m−2) | 23.9±1.3 | 25.1± 0.8 | 26.7±2.8 | 26.6±2.4 |
| Weight (kg) | 74.7±1.8 | 77.3±2.4 | 79.5±5.5 | 79.9±4.5 |
| Hypertension duration (year) | — | — | 13 | 19 |
| Waist circumference (cm) | 75.9±6.5 | 76.6±6.2 | 83.4± 4.8 | 85.6±4.8 |
| Systolic blood pressure (mm Hg) | 119.2.1±4.2 | 126.9± 8.3 | 155.6±7.2 | 163.5±9.5 |
| Diastolic blood pressure (mm Hg) | 79.7± 6.2 | 84.6±6.9 | 91.5± 4.4 | 96.8± 8.3 |
| Glycohaemoglobin A1C (%) | 5.94±0.23 | 6.12±0.45 | 8.2±0.67 | 9.3±0.98 |
| Fasting plasma glucose (mmol l−1) | (90, 99) | (84, 97) | (112, 119) | (116, 128) |
| Fasting plasma insulin (mmol l−1) | 4.35 | 4.44 | 6.5 | 6.37 |
| Serum total cholesterol (mg per 100 ml) | 162±37 | 165±18 | 209±36 | 234±26 |
| Serum HDL cholesterol (mg per 100 ml) | 37±11.5 | 35±9.7 | 29±9.9 | 24.6±4.6 |
| Serum LDL cholesterol (mg per 100 ml) | 98±34 | 109±22 | 246±36 | 257±21.9 |
| Serum triglycerides (mg per 100 ml) | 155±88 | 158 ±62 | 244±52 | 258±39 |
Abbreviations: RNS, referent nonsmokers; RS, referent smokers; NSHP, nonsmoker hypertensive patients; SHP, smoker hypertensive patients.
Venous blood (3–5 ml) was sampled using metal-free Safety Vacutainer blood collecting tubes (Becton Dickinson, Rutherford, NJ, USA) containing >1.5 mg K2EDTA per ml blood, and was stored at −20 °C until required for analysis. Morning urine samples were collected in acid-washed, decontaminated 100 ml polyethylene tubes (Kartell, Milan, Italy). In between sampling sessions, the container was wrapped in a clean polyethylene bag. Urine samples were acidified with ultrapure concentrated HNO3 (l% v/v) and kept at −4 °C. Before subsampling for analysis, the samples were shaken vigorously for 1 min to ensure a homogeneous suspension. The hair samples (∼1.0 g each) were taken from the nape of the neck. The scalp hair samples were washed and treated as reported in earlier study.25 After washing, the scalp hair samples were dried in an electrical oven at ∼75 °C and stored in pre-cleaned plastic bags with identity numbers. The hypertensive patients who had blood pressure exceeding 130/95 mm Hg (systolic/diastolic) were admitted for their uncontrolled HT and had earlier histories of high blood pressure.
Microwave-assisted acid digestion method
A microwave-assisted acid digestion procedure was carried out to achieve a shorter digestion time. For the digestion of biological samples, duplicate samples of scalp hair (200 mg), blood and urine (0.5 ml) of each subjects and five replicate samples of all three certified reference material samples were directly taken into Teflon PTFE flasks (Kartell). Added to each flask 2 ml of a freshly prepared mixture of concentrated HNO3–H2O2 (2:1, v/v). The flasks were kept for 10 min at room temperature and then placed in a covered PTFE container. This was then heated following a one-stage digestion programme at 80% of total power (900 W), for 2–4 min for blood and urine samples and for 5–8 min for hair samples. Thereafter, the digestion flasks were cooled and the resulting solution was evaporated to a semidried mass to remove excess acid, and then diluted upto 10.0 ml in volumetric flasks with 0.1 nitric acid.
Duplicate blanks (without sample) were carried through the complete procedure. The concentrations were obtained directly from calibration graphs after correction of absorbance for the signal from an appropriate reagent blank. The validity and efficiency of the microwave-assisted digestion method were also checked with a conventional wet acid digestion method on the same certified reference materials and real samples as reported elsewhere;26 results are given in Table 3.
Table 3. Determination of Cd, Ni, Pb and Zn in certified samples (CRM) by conventional (CDM) and microwave digestion method (MWD) (n=10).
| Elements | CDM | MWD | tvaluea | % Recoveryb | Certified values |
|---|---|---|---|---|---|
| CRM of whole blood (μg l−1) | |||||
| Cd | 1.21±0.07 | 1.187±0.09 | 0.0845 | 98.1 | 1.2± 0. 4 |
| (5.78)c | (7.58) | ||||
| Ni | 7.52±0.62 | 7.39±0.49 | 0.0412 | 98.3 | 7.5±1.8 |
| (8.24) | (6.63) | ||||
| Pb | 105±8.2 | 104±7.3 | 0.0523 | 99.2 | 105±24 |
| (7.76) | (6.96) | ||||
| Znd | 2.25± 0.09 | 2.20±0.08 | 0.0912 | 97.8 | 2.27±0.68 |
| (4.00) | (3.64) | ||||
| CRM of urine (μg l−1) | |||||
| Cd | 11.83±0.91 | 11.72±0.85 | 0.0340 | 99.0 | 11.8± 2.5 |
| (7.69) | (7.25 ) | ||||
| Ni | 11.9± 0.87 | 11.7± 0.69 | 0.0473 | 98.6 | 11.9±3.0 |
| (7.32) | (5.89) | ||||
| Pb | 41.2±1.96 | 40.77±2.86 | 0.0283 | 98.9 | 41.0±9.9 |
| (4.75) | (7.01) | ||||
| Zn | 205±6.0 | 201±7.0 | 0.0647 | 98.05 | 210±50 |
| (2.93) | (3.48) | ||||
| CRM of human hair (μg g−1) | |||||
| Cd | 0.53±0.025 | 0.524±0.024 | 0.0225 | 98.9 | 0.52±0.024e |
| (4.72) | (4.58) | ||||
| Ni | 46.1±1.41 | 45.7±1.38 | 0.0724 | 99.3 | 46.0±1.4f |
| (3.06) | (3.02) | ||||
| Pb | 33.3±1.21 | 32.6±1.18 | 0.096 | 97.8 | 33±1.2 |
| (3.63) | (3.62) | ||||
| Zn | 197±12.8 | 194±11.3 | 0.0345 | 98.6 | 199±5 |
| (6.2) | (5.7) | ||||
Abbreviations: Cd, cadmium; Ni, nickel; Pb, lead; Zn, zinc.
Paired t-test between CDM and MWD at d.f.=9, tcritical at 95% Cl=2.262.
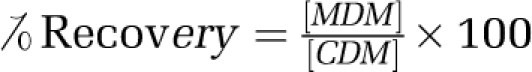 .
.
Values in parenthesis ( ) % of relative standard deviation.
Values indicates in mg l−1.
Informative value.
Indicative value.
Analytical results of the certified samples were in agreement with the certified values, confirming the reliability of our methods. The percentage recovery of all elements in the certified reference material samples obtained by both methods varied between 97.8–99.3%. The microwave-assisted digestion method was less time consuming, requiring <10 min to complete the digestion of samples and standards. The mean values for all elements differed <1–2% from the certified values. The coefficient of variation deviated <2% for all elements. Nonsignificant differences (P>0.05) were observed when comparing the values obtained by both methods (paired t-test) (Table 3).
Statistical evaluation
The total population of patients and referents was divided into two categories each: smoker and nonsmoker hypertensive patients and referents. Quantitative parameters were calculated using means and standard deviations, and qualitative parameters (ratio of smokers and nonsmokers) by number and percentage. Analysis of variance was used for quantitative parameter comparisons among the two hypertensive and referent groups. To assess the significance of the differences between concentrations of elements recorded in the biological samples of both male SHP and NSHP and referent individuals, calculated by the unpaired two-sample t-test.27 A P<0.05 was considered significant difference. Statistical analyses were performed using computer program Excel XL State (Microsoft Corp., Redmond, WA, USA) and Minitab 13.2 (Minitab Inc., State College, PA, USA).
Results
In the study population, ∼65% of HT patients and ∼50% of referents were smokers. Blood pressure was measured in the population under study in the sitting position after a 5-min rest. A patient was diagnosed as having HT if systolic blood pressure was ⩾160 mm Hg and diastolic pressure was ⩾90 mm Hg, if the patient was receiving drug treatment for HT. The other physical parameters of both groups of patients and referents were obtained by a standard method as shown in Table 2. The weight, body mass index, LDL cholesterol and blood pressure (systolic and diastolic blood pressure) levels of patients were significantly higher than those in healthy referents (P<0.05). The smoker referents weighed more than nonsmoker referents (P=0.034).
The elemental contents in the biological samples, especially in those of blood and urine, varied widely among individuals; thus, a significantly large number of samples was required for statistical interpretation of the data to achieve a meaningful correlation between physiological disorders and concentrations of trace and TEs. The mean concentrations with standard deviations for each element in biological samples, as shown in Table 4, indicate that the concentrations of the essential trace element, Zn, and TEs (Cd, Ni and Pb) were altered in all three biological samples of smoker and nonsmoker hypertensive patients, whereas in the case of RSs, there is no significant difference for Zn, although the levels of TEs were high in their biological samples (Figures 1, 2, 3 and 4). The concentration of Zn, Cd, Ni and Pb in the biological samples of hypertensive patients and referents were also examined using a multiple logistic regression model.
Table 4. Trace element concentrations in biological samples (scalp hair, blood and urine samples) of smokers and nonsmokers referent and hypertensive patients.
| Scalp hair | Cadmium | Lead | Nickel | Zinc |
|---|---|---|---|---|
| RNS | 1.42±0.3 | 7.9±1.53 | 6.1±1.5 | 222.3±13.4 |
| RS | 1.99±0.54 | 12.9±1.6 | 7.85± 0.95 | 189.3±15.3 |
| NSHP | 2.86±0.43 | 15.5±2.6 | 12.2±1.48 | 153.2±12.8 |
| SHP | 3.70±0.69 | 22.4±2.2 | 15.7± 0.96 | 124.8±12.7 |
| Blood | ||||
| RNS | 4.24±1.27 | 197.9±25.1 | 1.95±0.56 | 11.4±1.88 |
| RS | 5.36±1.4 | 248.6±21.9 | 2.65± 0.83 | 7.9±1.8 |
| NSHP | 5.97±1.3 | 358.9± 46.8 | 3.4± 0.9 | 9.7±1.2 |
| SHP | 8.9±1.3 | 457.3±27.7 | 5.85± 1.2 | 6.12±1.1 |
| Urine | ||||
| RNS | 3.2±0.9 | 86.5±22.7 | 6.2±1.4 | 0.78±0.22 |
| RS | 3.98±1.22 | 126.3±18.4 | 8.5± 1.2 | 1.11±0.29 |
| NSHP | 4.69±0.46 | 128.8 ±12.2 | 8.9± 1.8 | 1.26±0.5 |
| SHP | 5.86±2.12 | 224.9±28.9 | 12.2± 1.5 | 1.75±0.25 |
Abbreviations: RNS, referent nonsmokers; RS, referent smokers; NSHP, nonsmokers hypertensive patients; SHP, smoker hypertensive patients.
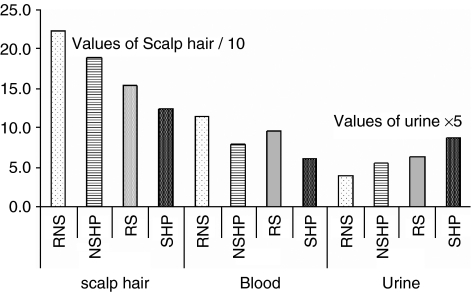
Figure 1.
Zn concentrations in the scalp hair, blood and urine samples of referent nonsmokers (RNSs), referent smokers (RSs), nonsmoker hypertensive patients (NSHPs) and smoker hypertensive patients (SHPs).
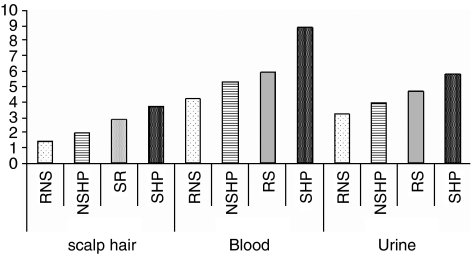
Figure 2.
Cd concentrations in the scalp hair, blood and urine samples of referent nonsmokers (RNSs), referent smokers (RSs), nonsmoker hypertensive patients (NSHPs) and smoker hypertensive patients (SHPs).
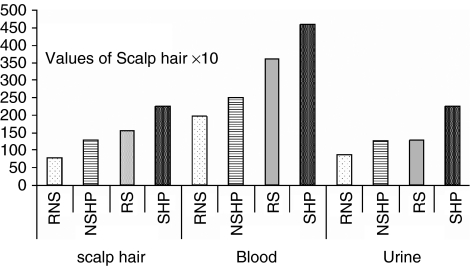
Figure 3.
Pb concentrations in the scalp hair, blood and urine samples of referent nonsmokers (RNSs), referent smokers (RSs), nonsmoker hypertensive patients (NSHPs) and smoker hypertensive patients (SHPs).
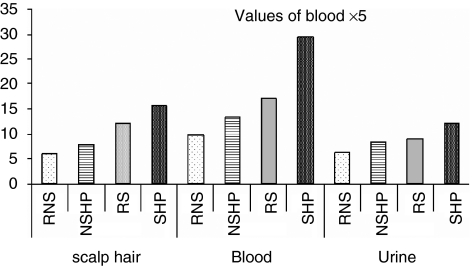
Figure 4.
Ni concentrations in the scalp hair, blood and urine samples of referent nonsmokers (RNSs), referent smokers (RSs), nonsmoker hypertensive patients (NSHPs) and smoker hypertensive patients (SHPs).
The concentrations of Zn in the scalp hair samples of RNS and RS were significantly higher at 95% confidence interval (CI) (214, 229) and (197, 205) μg g−1, respectively, compared with those in NSHP and SHP, (CI: 146, 159) and (CI: 116, 131) μg g−1, respectively, with P<0.01. The Zn levels in the blood of RNS and RS, (CI: 10.2, 11.9) and (CI: 9.63, 10.2) mg l−1, respectively, were found to be higher than those in NSHP and SHP, (CI: 7.3, 8.5) and (CI: 5.6, 6.7) mg l−1, respectively, (P=0.002–0.005). The excretion of Zn was higher in NSHP and SHP than in the referents (P=0.001–0.009). It was observed that the level of Zn did not vary significantly in all three biological samples of referent smokers and nonsmokers, indicating that the alteration of Zn in biological samples of NSHP and SHP was mainly because of the disease state of the patients.
The levels of Pb and Cd in blood were statistically significantly higher (P<0.01) in smoker and nonsmoker hypertensive patients, although RSs also had high levels of these TEs in their biological samples. An elevated level of Cd content was observed in the scalp hair of NSHP and SHP. The ranges of Cd in the blood samples of NSHP and SHP were (CI: 5.52–5.97) and (CI: 7.63–10.3) μg l−1, respectively, whereas those in RNS and RS were (CI: 3.95–4.47) μg l−1 and (CI: 5.07–5.58) μg l−1, respectively, (P<0.008). The excretion of Cd was higher in hypertensive patients than in referents, in both smokers and nonsmokers (P<0.001).
The Pb concentration in the scalp hair samples of RNS was found at 95% CI: (7.57, 8.14) μg g−1, whereas in the NSHP, the Pb level was in the range of (CI: 14.6–15.9) μg g−1 (Table 4). Similarly, a higher level of Pb was observed in RSs (CI: 12.6–13.2) μg g−1 than in RNS. The concentrations of Pb in the blood of RNS and RS were found to be in the range of (CI: 191–201) μg l−1 and (CI: 243–251) μg l−1, respectively, which is lower as compared with the concentrations of Pb in the blood of NSHP and SHP, (CI: 337–370) and (CI: 436–473) μg l−1, respectively, with P<0.003 and 0.005 (Table 4). The excretion of Pb was higher in SHP than in referents (Table 4).
The levels of Ni in the scalp hair samples of RNS and RS were found to be lower, (CI: 5.5–6.7) and (CI: 7.56–7.99) μg g−1, respectively, compared with those in NSHP and SHP, (CI: 11.8–12.5) μg g−1 and (CI: 15.4–15.9) μg g−1, respectively. The ranges of Ni concentration in the blood samples of RNS and RS were (CI: 1.83–1.95) and (CI: 2.55–2.74) μg l−1, respectively, compared with those of NSHP and SHP, [(CI: 3.26–3.42) and (CI: 5.6–6.3)] μg l−1, respectively. The excretion of Ni in hypertensive patients was found to be higher than that in referents (P<0.002).
The interelemental correlation (r) among Zn vs Cd, Ni and Pb in patients and referents, indicates that the values of Zn in scalp hair and urine have high correlation (0.954–0.994), whereas a low correlation level between Zn and TEs was observed in blood sample (0.569–0.822). The correlation of trace and TEs between referents and patients of both groups (smoker and nonsmoker) was statistically analyzed by a multiple linear regression equation and Pearson's correlation (Table 5).
Table 5. Linear regression and Pearson's coefficient for cadmium in smoker and nonsmoker controls vs hypertensive patients.
| Scalp hair | Cadmium | Lead | Nickel | Zinc |
|---|---|---|---|---|
| RNS vs NSHP | 0.175 × −3.10 | 0.179 × +14.1 | 0.065 × −12.6 | 0.533 × +33.9 |
| r=0.13 | r=0.11 | r=0.06 | r=0.53 | |
| RS vs SHP | 0.417 × +2.74 | 0.539 × +15.3 | 0.445 × −19 | 0.160 × −155 |
| r=0.54 | r=0.50 | r=0.56 | r=0.185 | |
| Blood | ||||
| RNS vs NSHP | 0.098 × +5.56 | 0.205 × +318 | 0.173 × −3.73 | 0.397 × +3.43 |
| r=0.097 | r=0.11 | r=0.099 | r=0.42 | |
| RS vs SHP | 0.81 × −13.5 | 0.735 × +262 | 0.61 × −7.47 | 0.198 × −8.01 |
| r=0.70 | r=0.50 | r=0.46 | r=0.22 | |
| Urine | ||||
| RNS vs NSHP | 0.058 × +4.51 | 0.062 × −134.2 | 0.17 × +7.8 | 0.35 × +0.953 |
| r=0.11 | r=0.12 | r=0.12 | r=0.15 | |
| RS vs SHP | 0.876 × +2.06 | 1.05 × +357 | 0.53 × +16.8 | 0.418 × +1.28 |
| r=0.51 | r=0.61 | r=0.52 | r=0.495 | |
Abbreviations: RNS, referent nonsmokers; RS, referent smokers; NSHP, nonsmoker hypertensive patients; SHP, smoker hypertensive patients.
Discussion
This study provides data on the essential trace element, Zn and TEs (Cd, Ni and Pb) in scalp hair, blood and urine samples obtained from smoker and nonsmoker hypertensive and non-hypertensive male referents.
There are many causes of high blood pressure, such as smoking, obesity, poor diet, lack of cold water fish, fresh fruits, vegetables, exercise, poor sleep, genetics, stress and insomnia. Cigarette smoking is a risk factor that alters LDL,28 reducing the endothelium-dependent relaxation induced by acetylcholine, in a manner similar to oxidised LDL, without altering non-endothelium-dependent relaxation. Both active and passive smoking29 are associated with the development of several clinical disorders.
Smoking was the most important risk factor, considering that 65% of all the patients were smokers (Table 2). Some evidence indicated that the influence of smoking is independent but also synergistic with other risk factors such as HT, a high blood concentration of cholesterol and other physiological disorders.4, 5 Cigarette smokers and people living in contaminated areas have a higher level of Cd in their blood and urine, with smokers having Cd levels more than twice as that of nonsmokers.30, 31 Toxic elements (Cd, Pb and Ni) may deplete glutathione and protein-bound sulfhydryl groups, resulting in the production of reactive oxygen species, such as superoxide anion, hydrogen peroxide and hydroxyl radical.32
Zinc plays an important role in normal metabolism and assists more than 200 enzymatic reactions.33 Another biological function of Zn is the maintenance of the integrity of proteins required for the stability of membrane structures.34
The concentrations of Zn in the scalp hair and blood samples of the male hypertensive patients were low, whereas a higher level was observed in urine (Table 4). Our results are consistent with other investigations, which showed that the concentration of Zn in blood, hair and fingernail was significantly lower in the aged patients with HT and cardiovascular diseases than in the aged healthy controls.35 The low Zn levels may correlate with the intake of antihypertensive medication and also with reduced essential intake of macro and micronutrients.36 An epidemiological study reported that low concentrations of Zn in serum and high concentrations of Zn in urine were found in patients of cardiovascular diseases, possibly because of diuretic medicines.37
Our results indicated a high level of all three TEs in hypertensive patients, with smokers being more prone to accumulate these metals than were referents. The Cd oxides generated during the burning of cigarettes are highly bioavailable, ∼10% of the inhaled Cd is deposited in lung tissues and ∼40% is absorbed into the systemic blood circulation of smokers.38 It was reported in a study that Thai men who on an average smoked nine cigarettes per day for 9 years had an approximately twofold greater body Cd load than did nonsmoking men of the same age.39 In another study on men older than 50 years of age in northern Taiwan, smokers were 2.5 times more likely to excrete higher urinary Cd levels than were nonsmokers.40 The uptake of Cd following environmental or occupational exposure results in a gradual accumulation in the liver and kidney, eventually resulting in kidney dysfunction and HT; its half-life in the body is not known exactly, but it may be as long as 30 years.41
The antagonistic effect of Cd and Zn was investigated for the fact that the accumulation of Cd in the human body may replace Zn in the arteries, which contributes to arteries becoming brittle and inflexible. Once the arteries become inflamed and brittle, the body may coat them with Ca and fatty plaques to prevent their rupture.42 This plaque unfortunately reduces the interior diameter of the arteries, resulting in more pressure being required to force the blood through the smaller diameter arteries, which in turn raises blood pressure.42
Lead may also be present in high concentrations in tobacco smoke. Smokers have considerably higher levels of Pb in their blood than do nonsmokers.43 Children are more sensitive to the toxic effects of Pb compared with adults and passive smoking plays an important role in exposure of children to Pb.43 Other possible health consequences of Pb accumulation are HT and peripheral arterial diseases.44 Pb may also replace Zn and Ca, contributing to the severity of HT problems. Toxic substances can accumulate in kidneys, which damage their ability to regulate the water balance in the body. This can lead to water retention, salt retention and high blood pressure.45
It was observed in our study that the level of Ni was significantly higher in hypertensive patients than in normotensive age-matched referents (Table 4). Significant Ni levels in control smokers compared with those in nonsmokers have also been reported at P<0.005. Besides this, the inhalation of vapours of Ni carbonyl obtained from burning of tobacco and from certain occupations (welding, fitting and so on) may also cause elevated Ni levels in biological samples.46 As is the case with Cd, tobacco plants absorb Ni from the soil and concentrate it in the leaves.42 Ni has long been known to produce nasal and lung cancers.47 The amount of Ni in tobacco plant lies between 0.640 and 1.15 mg g−1, and varies greatly in cigarettes of different brands.48
The possibility of TEs contamination of various medications and its effects on the metabolism of TEs are still unknown. Experimental studies show that both metals (Cd and Pb) contribute to oxidative stress by catalyzing the formation of reactive oxygen species,49, 50 increasing lipid peroxidation51, 52 and depleting the glutathione and protein-bound sulfhydryl groups.51 The lipid peroxidation in patients, such as HT and cardiovascular disease, may be because of a disturbance in essential trace element metabolism and antioxidant levels.53 In patients, disturbances in the enzymatic mechanisms of free-radicals detoxification lead to an alteration in the antioxidant system and reactive oxygen species. An attack on cell membranes also results in the formation of lipid peroxidation products.51 In vitro and in vivo studies suggested that Pb-induced oxidation contributes to red blood cell damage.52 Pb and Cd may also stimulate the production of inflammatory cytokines and may induce endothelial damage by downregulating the production of nitric oxide.54, 55
The results of this study revealed that hypertensive patients have a different pattern of essential trace and TEs in their biological samples than controls/referents, with the prevalence being more in smoker patients. However, higher levels of Cd, Pb and Ni, as well as a lower level of Zn, correlated well with the consequences of HT. The deficiency of the essential element, Zn, which is replaced by TEs (Cd, Pb, Ni), may result in abnormal physiology disorders, and, in addition to other factors, this may have a role in HT disease. This study provides some support for the hypothesis that dietary intake and inhalation of TEs (Cd, Pb and Ni), most probably through smoking, may increase the risk of HT and related disorders, which indicates that the causal link may be stronger among smokers. We propose that TE measurements may be performed on patients presenting to the emergency department, to test whether TE levels serve not only as markers of high blood pressure and its remedies (heart problems) but also as predictors of adverse outcomes. 
Acknowledgments
We thank the Higher Education Commission, Islamabad, Pakistan for sponsoring this project.
References
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- Privitera JR, Stang A. Silent Clots—Life's Biggest Killers. The Catacombs Press: Covina, CA, USA; 1996. pp. 1–55. [Google Scholar]
- American Academy of Pediatrics October 1998 Child Health Month Report: The Risks of Tobacco Use: A Message to Parents and Teens;Milam JE.Perceived invulnerability and cigarette smoking among adolescents Addict Behav 20002571–80. [DOI] [PubMed] [Google Scholar]
- Kazi TG, Jalbani N, Kazi N, Jamali MK, Arain MB, Afridi HI, et al. Evaluation of toxic metals in blood and urine samples of chronic renal failure patients, before and after dialysis. Ren Fail. 2008;30:737–745. doi: 10.1080/08860220802212999. [DOI] [PubMed] [Google Scholar]
- Kazi TG, Memon AR, Afridi HI, Jamali MK, Arain MB, Jalbani N, et al. Determination of cadmium in whole blood and scalp hair samples of Pakistani male lung cancer patients by electro thermal atomic absorption spectrometer. Sci Total Environ. 2008;389:270–276. doi: 10.1016/j.scitotenv.2007.08.055. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick TM, Blair EA. Smoking and pulmonary and cardiovascular disease: upper airway complications of smoking. Clin Chest Med. 2000;21:147–157. doi: 10.1016/s0272-5231(05)70014-9. [DOI] [PubMed] [Google Scholar]
- Russell MA. The nicotine addiction trap: a 40 year sentence for four cigarettes. Br J Addict. 1990;85:293–300. doi: 10.1111/j.1360-0443.1990.tb03085.x. [DOI] [PubMed] [Google Scholar]
- Soylak M, Kidnap M. Serum copper and zinc concentrations of patients with rheumatoid arthritis from Kayseri-Turkey. Fresenius Environ Bull. 2001;10:409–410. [Google Scholar]
- Hennig B, Meerarani P, Toborek M, Mc-Clain C. Antioxidant-like properties of zinc in activated endothelial cells. J Am Coll Nutr. 1999;18:152–158. doi: 10.1080/07315724.1999.10718843. [DOI] [PubMed] [Google Scholar]
- Memon AR, Kazi TG, Afridi HI, Jamali MK, Arain MB, Jalbani N, et al. Evaluation of zinc status in whole blood and scalp hair of female cancer patients. Clin Chim Acta. 2007;379:66–70. doi: 10.1016/j.cca.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Connor RJ. Cigarette filter ventilation is a defective design because of misleading taste, bigger puffs, and blocked vents. Tob Control. 2002;11:140–150. doi: 10.1136/tc.11.suppl_1.i40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GJ. Accumulation of cadmium in crop plants and its consequences to human health. Adv Agron. 1993;51:173–212. [Google Scholar]
- Kazi TG, Arain MB, Baig JA, Jamali MK, Afridi HI, et al. The correlation of arsenic levels in drinking water with the biological samples of skin disorders. Sci Total Environ. 2009;407:1019–1026. doi: 10.1016/j.scitotenv.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Chiba M, Masironi R. Toxic and trace-elements in tobacco and tobacco-smoke. Bull WHO. 1992;70:269–275. [PMC free article] [PubMed] [Google Scholar]
- Reilly C. Metal Contamination of Food its Significance for Food Quality and Human Health. Blackwell Science Ltd: Oxford, UK; 2002. [Google Scholar]
- Csalari J, Szantai K. Transfer rate of cadmium, lead, zinc and iron from the tobacco-cut of the most popular Hungarian cigarette brands to the combustion products. Acta Aliment. 2002;31:279–288. [Google Scholar]
- Kazi TG, Jalbani N, Arain MB, Jamali MK, Afridi HI, Sarfraz RA, et al. Toxic metals distribution in different components of Pakistani and imported cigarettes by electrothermal atomic absorption spectrometer. J Hazard Mater. 2009;163:302–307. doi: 10.1016/j.jhazmat.2008.06.088. [DOI] [PubMed] [Google Scholar]
- Klaus KA, Witte MB, Andrew L, Clark MA, John GF. Chronic heart failure and micronutrients. J Am Coll Cardiol. 2001;37:1765–1774. doi: 10.1016/s0735-1097(01)01227-x. [DOI] [PubMed] [Google Scholar]
- Nawrot TS, Thijs L, Den Hond EM, Roels HA, Staessen JA. An epidemiological reappraisal of the association between blood pressure and blood lead: a meta-analysis. J Hum Hypertens. 2002;16:123–131. doi: 10.1038/sj.jhh.1001300. [DOI] [PubMed] [Google Scholar]
- Witte KKA, Nikitin NP, Parker AC, von Haehling S, Volk HD, Anker SD, et al. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J. 2005;26:2238–2244. doi: 10.1093/eurheartj/ehi442. [DOI] [PubMed] [Google Scholar]
- Zakrgynska-Fontaine V, Dore JC, Ojasoo T, Poirier-Duchene F, Viel C. Study of the age and sex dependence of trace elements in hair by correspondence analysis. Biol Trace Elem Res. 1998;61:151–168. doi: 10.1007/BF02784027. [DOI] [PubMed] [Google Scholar]
- Soylak M, Saraymen R, Dogan M. Investigation of lead, chromium, cobalt and molybdenum concentrations in hair samples collected from diabetic patients. Fresenius Environ Bull. 1995;4:485–490. [Google Scholar]
- Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- Afridi HI, Kazi TG, Kazi GH, Jamali MK, Shar GQ. Essential trace and toxic element distribution in the scalp hair of Pakistani myocardial infarction patients and controls. Biol Trace Elem Res. 2006;113:19–34. doi: 10.1385/BTER:113:3. [DOI] [PubMed] [Google Scholar]
- Afridi HI, Kazi TG, Kazi GH, Jamali MK, Arain MB, Jalbani N. Determination of Cd and Pb in biological samples by three ultrasonic-based samples treatment procedures followed by electrothermal atomic absorption spectrophotometer. JAOAC International. 2007;90:470–478. [PubMed] [Google Scholar]
- Afridi HI, Kazi TG, Kazi GH, Jamali MK, Arain MB, Jalbani N. Evaluation of essential and toxic metals by ultrasound-assisted acid leaching from scalp hair samples of children with macular degeneration patients. Clin Chim Acta. 2006;369:52–60. doi: 10.1016/j.cca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Armitage P, Berry G.Statistical Methods in Medical Research3rd edn.Blackwell, Oxford, UK; 1994 [Google Scholar]
- Kagota S, Yamaguchi Y, Shinozuka K, Kwon YM, Kunitomo M. Cigarette smoke-modified low density lipoprotein impairs endothelium-dependent relaxation in isolated rabbit arteries. Gen Pharmacol. 1996;27:477–481. doi: 10.1016/0306-3623(95)02074-8. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Colditz GA, Speizer FE, Manson JE, Stampfer MJ, Willett WC, et al. A prospective study of passive smoking and coronary heart disease. Circulation. 1997;95:2374–2379. doi: 10.1161/01.cir.95.10.2374. [DOI] [PubMed] [Google Scholar]
- Landsberger S, Wu D. The impact of heavy metals from environmental tobacco smoke on indoor air quality as determined by Compton suppression neutron activation analysis. Sci Total Environ. 1995;173:323–337. doi: 10.1016/0048-9697(95)04755-7. [DOI] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D, Bagchi M. Toxicity of trace elements in tobacco smoke. Inhal Toxicol. 1997;9:867–890. [Google Scholar]
- Goyer RA.Toxic effects of metalsIn: Klaassen CD (ed). Casarett and Doull's toxicology: The basic science of poisons 1996. vol. 5,McGraw-Hill: New York; 691–736. [Google Scholar]
- Fabris N, Mocchegiani F. Zinc, human diseases and aging. Aging Clin Exp Res. 1995;7:77–93. doi: 10.1007/BF03324297. [DOI] [PubMed] [Google Scholar]
- Henning B, Meerarani P, Ramadass P, Toborek M, Malecki A, Slim R, et al. Zinc nutrition and apoptosis of vascular endothelial cells: implications in atherosclerosis. Nutrition. 1999;15:744–748. doi: 10.1016/s0899-9007(99)00148-3. [DOI] [PubMed] [Google Scholar]
- Tang YR, Zhang SQ, Xiong Y, Zhao Y, Fu H, Zhang HP, et al. Studies of five microelement contents in human serum, hair, and fingernails correlated with aged hypertension and coronary heart disease. Biol Trace Elem Res. 2003;92:97–104. doi: 10.1385/BTER:92:2:97. [DOI] [PubMed] [Google Scholar]
- Eltayeb MA, Van Grieken RE. Iron, copper, zinc and lead in hair from Sudanese populations of different age groups. Total Environ. 1990;95:157–165. doi: 10.1016/0048-9697(90)90061-x. [DOI] [PubMed] [Google Scholar]
- Wastney ME, Ahmed S, Henkin RI. Changes in regulation of human zinc metabolism with age. Am J Physiol. 1992;263:R1 162–R1 168. doi: 10.1152/ajpregu.1992.263.5.R1162. [DOI] [PubMed] [Google Scholar]
- Wagner KA, McDaniel R, Self D. Collection and preparation of side stream cigarette smoke for trace elemental determinations by graphite furnace atomic absorption spectrometry and inductively coupled plasma mass spectrometry. JAOAC International. 2001;84:1934–1940. [PubMed] [Google Scholar]
- Satarug S, Vanavanitkun Y, Baker JR, Reilly PE, Moore MR. Influence of body iron store status and cigarette smoking on Cd body burden of healthy Thi man and women. Toxicol Lett. 2004;148:177–185. doi: 10.1016/j.toxlet.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Chen YC, Pu YS, Lin RS, Yang CY, Lai MK, Liu SH, Sung FC. Blood and urine cadmium levels in relation to demographic and life style in middle aged and elderly men. bull environ. Contam Toxicol. 2001;66:287–294. doi: 10.1007/s001280003. [DOI] [PubMed] [Google Scholar]
- Dickel H, Kuss O, Schmidt A, Diepgen TL. Occupational relevance of positive standard patch-test results in employed persons with an initial report of an occupational disease. Int Arch Occup Environ Health. 2002;75:423–434. doi: 10.1007/s00420-002-0328-2. [DOI] [PubMed] [Google Scholar]
- Cohen N, Golik A. Zinc balance and medications commonly used in the management of heart failure. Heart Fail Rev. 2006;11:19–24. doi: 10.1007/s10741-006-9189-1. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Albalak R, Grosse S, Repace J. Second-hand smoke exposure and blood lead levels in US children. Epidemiology. 2003;14:719–727. doi: 10.1097/01.EDE.0000081998.02432.53. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109:3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Roels H, Fagard R. Lead exposure and conventional and ambulatory blood pressure: a prospective population study, Phee Cad Investigators. JAMA. 1996;275:1604–1606. [PubMed] [Google Scholar]
- Doll R. Report of the international committee on nickel carcinogenesis in man. Scand J Work Environ Health. 1990;16:1–84. doi: 10.5271/sjweh.1813. [DOI] [PubMed] [Google Scholar]
- Tian L, Lawrence DA. Metal-induced modulation of nitric oxide production in vitro by murine macrophages: Lead, nickel and cobalt utilize different mechanisms. Toxicol Appl Pharmacol. 1996;141:540–547. doi: 10.1006/taap.1996.0320. [DOI] [PubMed] [Google Scholar]
- Torjussen W, Zachariasen H, Andersen I. Cigarette smoking and nickel exposure. J Environ Monit. 2003;5:198–201. doi: 10.1039/b209065c. [DOI] [PubMed] [Google Scholar]
- Richard MJ, Arnoud J, Jurkovitz C, Hachache T, Meftabi H, Laporte F. Trace elements and lipid peroxidation abnormalities in patients with chronic renal failure. Nephron. 1991;57:10–15. doi: 10.1159/000186208. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Ding Y, Ni Z. Compensatory up-regulation of nitric-oxide synthase isoforms in lead-induced hypertension: reversal by a superoxide dismutase-mimetic drug. J Pharmacol Exp Ther. 2001;298:679–685. [PubMed] [Google Scholar]
- Ding Y, Gonick HC, Vaziri ND. Lead promotes hydroxyl radical generation and lipid peroxidation in cultured aortic endothelial cells. Am J Hypertens. 2000;13:552–555. doi: 10.1016/s0895-7061(99)00226-5. [DOI] [PubMed] [Google Scholar]
- Yiin SJ, Chern CL, Sheu JY, Tseng WC, Lin TH. Cadmium-induced renal lipid peroxidation in rats and protection by selenium. J Toxicol Environ Health A. 1999;57:403–413. doi: 10.1080/009841099157601. [DOI] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Demontis MP, Varoni MV, Volpe AR, Emanueli C, Madeddu P. Role of nitric oxide synthase inhibition in the acute hypertensive response to intracerebroventricular cadmium. Br J Pharmacol. 1998;123:129–135. doi: 10.1038/sj.bjp.0701573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham J, Meltzer A, Ashkenazi R, Ribak J. Biological monitoring of exposure to cadmium, a human carcinogen, as a result of active and passive smoking. J Occup Environ Med. 1996;38:1220–1228. doi: 10.1097/00043764-199612000-00007. [DOI] [PubMed] [Google Scholar]