Abstract
AIM: To investigate whether deficiency of expression of cytochrome c oxidase I (CcOI) in colonic crypts is associated with colon cancer.
METHODS: The pattern and level of expression of CcOI in non-neoplastic colonic crypts, and in dysplastic tissues, was assessed using standard immunohistochemical methods. Biopsies were obtained from individuals undergoing colonoscopies for screening purposes or for a medically indicated reason. Tissue samples were also obtained from surgical colonic resections. Samples from resections were taken from colonic mucosa 1 and 10 cm from tumors and from the tumors themselves. Samples were evaluated for frequency of crypts with reduced or absent expression of CcOI. In most crypts the loss was apparent throughout the entire crypt, while in a small minority the loss was segmental. The strong immunoreactivity using this monoclonal antibody makes the scoring unambiguous. The percent of crypts with reduced or absent expression of CcOI or (infrequent) segmented loss of expression was then calculated. Data analyses were performed using SPSS statistical package 17.0.
RESULTS: The average frequency of CcOI deficient crypts (CcOI-DC) is low in individuals between 20 and 39 years of age, with 0.48% ± 0.40% CcOI-DC for women and 1.80% ± 0.35% for men. CcOI-DC increases after age 40 years, so that between the ages of 40 and 44 years the average frequency of CcOI-DC goes up to 5.89% ± 0.84% in women and 2.15% ± 1.27% in men. By 80-84 years of age, the average frequency of CcOI-DC goes up in women to 15.77% ± 0.97% and in men to 22.6% ± 0.65%. The increases in CcOI-DC from ages 40-44 years compared to 80-84 years in women and men are significantly different with P < 0.01. For women over age 60 years, deficiency of CcOI expression is greater in those women who have had a cancer in their colon. The frequency of CcOI-DC, measured in men, increased in tissues adjacent to colon cancer, being 4.03% ± 0.27% in individuals free of neoplasia in the age range 55-64 years and 14.13% ± 0.35% in resected histologically normal tissue of men with cancer in the same age range, P < 0.001. Similar significant differences were noted in older age ranges. The frequency of CcOI-DC crypts in the cecum and sigmoid colon of an individual are significantly correlated, with an R2 = 0.414 for women and R2 = 0.528 for men, P < 0.001. This suggests that the factors determining the level of CcOI deficiency act throughout the colon. Most defective crypts are in clusters of two or more, a likely consequence of crypt fission. In the non-neoplastic margins of cancers, crypts are frequently deficient for CcOI, and such crypts may appear in large clusters, some containing more than 100 deficient crypts. CcOI deficiency is also apparent in colon cancers and sometimes involves a large section of the tumor. Overall, CcOI deficient cells can be visualized in segments of crypts, in whole crypts that increase in frequency with age, in crypts undergoing fission, in clusters of crypts where the clusters increase in size with age, in increased frequency near tumors, in large clusters in the intimate margins of tumors, and in the tumors themselves. There is no clear dividing line between early stages that can be considered aspects of aging and later stages that can be considered aspects of the progression to cancer. This ambiguity may reflect a rather general situation leading to adult cancer where the early stages of cellular change appear to be relatively innocuous features of the aging process but over decades may evolve into malignancy.
CONCLUSION: CcOI deficient crypts increase in frequency with age, and clusters of deficient crypts are associated with, and may give rise to, colon cancer.
Keywords: Cytochrome c oxidase, Aging, Colon crypts, Colorectal cancer, Focal lesions
INTRODUCTION
In dividing cell populations, mutations (or epimutations) that provide a selective advantage may cause clonal expansion of the mutant cells to produce a focal lesion. The colon epithelium can give rise to a variety of focal lesions, such as aberrant crypt foci, adenomatous polyps and adenocarcinomas, whose incidence increases with age. Another example of a focal lesion in the colon epithelium is a cluster of crypts deficient in cytochrome c oxidase subunit I (CcOI).
Cytochrome c oxidase (CcO) (Complex IV of the electron transport chain) is a large trans-membrane protein complex found in the mitochondria of eukaryotic cells, and also in bacteria. In eukaryotes it is located in the mitochondrial membrane and is the last enzyme complex in the respiratory electron transport chain. In humans, CcO consists of 13 protein subunits, the largest of which is subunit I (CcOI). Of the 13 subunits, 3 subunits (I, II and III) are encoded by mitochondrial DNA (mtDNA) and the other 10 are encoded by nuclear DNA.
A human colonic crypt has approximately 2500-5000 cells[1], all arising from the stem cell region at the base of the crypt. There are about 85 to 106 cells along the crypt column length, and the great majority of cells of a crypt are replaced in 3 to 4 d[2]. We observe about 29-43 cells along the circumference of crypts. The number of stem cells in a crypt is small, approximately six[3]. There appears to be competition among stem cells. The descendents (daughter cells) of a mutant stem cell with a CcOI deficiency may take over a stem cell niche (niche succession). Thus, the progeny of a human colonic crypt stem cell that contains a mtDNA mutation in CcOI may then expand to occupy a whole crypt (crypt conversion). Greaves et al[4] have proposed that such a crypt may then further expand by fission to form a patch. This proposal was based on evidence that: (1) mutated crypts in the process of fission share the same mutated mitochondrial genotype, a genotype not present in neighboring CcOI-positive crypts; (2) neighboring mutated crypts have the same genotype, which is different from adjacent CcOI-positive crypts; (3) mutated crypts are clustered together throughout the colon; and (4) patches of CcOI-deficient crypts increase in size with age. Colonic crypts that are CcOI-deficient can be easily distinguished from crypts with normal CcOI expression using immunohistochemistry.
CcO-deficient cells tend to survive in the environment of the gastrointestinal tract much more frequently than CcO-deficient cells in the heart and brain. In mice with a mutation in polymerase gamma (the replicative polymerase of the mitochondria), where the polymerase has lost its proofreading function, almost all cells of the mouse duodenum show loss of CcO function, while considerably fewer cells of the heart or brain show such loss[5].
We previously reported a significantly increased frequency (27%) of CcOI-deficient crypts in histologically normal colonic mucosa in tissue from 8 patients with colon cancer and 3 patients with tubulovillous adenoma from the histologically normal mucosa in resections (near colon cancers). This was a notable increase compared to a frequency of about 3% of CcOI-deficient crypts in colonic mucosa of 5 patients without colonic neoplasia[6]. These results suggested a possible association of CcOI deficiency and cancer development. Subsequently, in a video-based “methods publication[7]” using tissues obtained from resections, we showed our method for evaluating CcOI deficiency, along with methods of assessing deficiencies in three other enzymes related to DNA repair and apoptosis. We found, in this study, an estimated frequency of CcOI deficient crypts near colon cancers of about 10%-20%. This further indicated a possible role of CcOI deficiency in progression to cancer. The current report presents a substantially larger assessment of the role of CcOI deficiency in progression to colon cancer.
Numerous studies, reviewed by Meissner[8] and by Hiona et al[9] in 2007 and 2008, have presented arguments on whether mutations of mitochondrial DNA are either the cause, or the consequence, of the aging process. Recent experimental data presented by Edgar et al[10] indicates that, in mtDNA mutator mice, mitochondrial mutations in CcO are a driving force in premature aging. However, it is not clear whether this mouse model is relevant to natural aging in humans.
Evidence is presented here that the frequency of crypts deficient for CcOI expression increases with age in women (n = 149) and men (n = 190). Within individual colons, the level of CcOI deficiency in the cecum correlates with the level in the sigmoid region, suggesting that the whole colon is affected by the factors causing CcOI deficiency. For women aged 60 years or older (n = 70), CcOI deficient crypts are significantly more frequent in individuals with a history of colon cancer. Furthermore, as determined in surgical resections, men aged 55 years or older with cancer or advanced colonic neoplasia have significantly more frequent CcOI deficient crypts in areas near the lesion, compared to age matched individuals without neoplasia. CcOI deficiency also occurs in apparently normal mucosa at the margins of tumors in large clusters of deficient crypts, and within areas of the tumor itself. Thus CcOI deficiency increases in the colon with age, and is associated with, and may give rise to, advanced colonic neoplasia including cancer.
MATERIALS AND METHODS
Tissue procurement
Before any biopsy tissue samples were obtained during colonoscopy, informed consent was given by the patient, using a form approved by the University of Arizona Institutional Review Board. Biopsied colonic mucosal samples were fixed in 10% buffered formalin for 4 h, then transferred to 70% alcohol, followed by paraffin embedment. Tissue samples from colonic resections were obtained after informed consent before surgery, and these larger tissue samples were fixed in 10% buffered formalin for 24 to 36 h, then transferred to 70% alcohol, followed by paraffin embedment.
Immunohistochemical procedure for evaluating cytochrome c oxidase subunit I
The pattern and level of expression of cytochrome c oxidase subunit I in non-neoplastic colonic crypts, and in dysplastic tissues, was assessed using standard immunohistochemical methods, as previously described [6,7,11-14] and shown in a video methods publication (starting at 3:30 min:s of the video)[7]. The evaluation method for CcOI is shown starting at minute 25 of the 28 min video[7]. Briefly, formalin-fixed and paraffin-embedded tissues were cut into 4 μm sections, deparaffinized, and then rehydrated. Antigen retrieval was performed by microwave exposure in 0.1 mol/L citrate buffer (pH 6.1). Endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxide in methanol for 30 min, and then the tissue sections were rinsed with distilled water and PBS. To prevent nonspecific binding, the slides were next incubated with normal rabbit serum (1.5%) for 60 min. The primary mouse monoclonal antibody against CcOI (obtained from Molecular Probes, Inc, Eugene, OR) was added at 2 μg/mL in 2% bovine serum albumin/PBS for 1 h. After rinsing with PBS, the slides were incubated with biotinylated rabbit anti-mouse secondary antibody IgG F(ab)2 (DAKO Corp., Carpinteria, CA) for 30 min at a 1:400 dilution in 2% bovine serum albumin/PBS. Immune control slides were prepared by replacing the primary antibody with mouse IgG2a at the same protein concentration used for the primary antibody. After rinsing in PBS, the Vectastain Elite avidin-biotin complex method kit (Vector, Burlingame, CA) was used according to the manufacturer’s instructions. Color development was carried out by applying diaminobenzidine tetrachloride (Sigma, St. Louis, MO) supplemented with 0.04% hydrogen peroxide. Sections were counterstained with hematoxylin (Sigma), dehydrated in a graded series of ethanols followed by xylene, and then mounted using Cytoseal XYL (Richard Allen Scientific, Kalamazoo, MI). Brown staining in the cytoplasm indicates CcOI expression, and blue staining from hematoxylin identifies nucleoproteins in the nucleus.
Quantitation of reduced or absent expression of CcOI
Biopsies were obtained from individuals undergoing colonoscopies for screening purposes or for a medically indicated reason. The biopsies were taken from six non-neoplastic sites (3 taken 2 cm distant from each other from the cecum, and 3 from the sigmoid colon at 40, 30 and 20 cm from the anal verge). Individuals were placed in one of two categories. The first group, considered a lower risk group, included individuals without a previous or current colon cancer. The second group, considered a higher risk group, included individuals with present or past colonic adenocarcinomas. Tissue samples from colonic resections were obtained from the colonic tumors and from colonic mucosa from 1 to 10 cm from the tumors. These samples were evaluated for frequency of crypts with reduced or absent expression of CcOI (substantial reduction or complete loss of immunostaining). An entire tissue section with 189 crypts, of which 45 crypts (23.8%) were deficient in CcOI, can be seen in a video presentation (starting at 25 min of the 28 min video)[7] for observing the method for scoring crypts with reduced or absent CcOI expression. In most crypts the deficiency was apparent throughout the entire crypt, while in a minority of crypts the deficiency was segmental. Segmental deficiency of immunostaining is one in which there is strong immunostaining of cells in a part of a crypt while the remaining cells of the crypt show reduced or absent expression of CcOI. The strong immunoreactivity using this monoclonal antibody makes the scoring unambiguous and easy to score. The percent of crypts with an aberrant immunostaining pattern showing reduced or absent expression of CcOI or (infrequent) segmental loss of expression was then calculated.
Statistical analysis
Data analyses were performed using SPSS statistical package 17.0. Linear regression was used, for men and women separately, to determine the associations between age of patients and CcOI-deficient crypts and the associations between CcOI deficiencies in the cecum and the sigmoid colon. Male patients were grouped in 10-year increments and analyzed by single-factor ANOVA with post-hoc Duncan’s multiple range to determine if the normal colonic mucosa from patients with no colonic neoplasia (biopsy samples) were significantly different in CcOI deficiency from non-neoplastic mucosa close to tumors from patients with advanced adenomas or colon cancers (surgical resection samples). Pearson correlations were used to determine if the percent CcOI defective crypts correlated with age, or intake of vitamins, non-steroidal anti-inflammatory drugs (NSAIDs), minerals or meat.
Photomicrographs
All micrographs were obtained with a Motic BA300 digital photomicroscope, using Motic Images Plus version 2.0 software. The software was set with Gain 0, Offset 0, Enhance disabled, Gamma disabled, R,G,B of gain 1, brightness 0, Edge Detection disabled, Sharpness 3, Resolution 1024/768, White Balance on, Auto Exposure on and then switched off with an increase in brightness sufficient to allow background (in areas of the slide without tissue present) to become white or near white. The images were then saved as tif images, and uniformly adjusted in Paint Shop Pro 5 with Color Adjustment settings of Brightness 0, Contrast 15, followed by Hue/Saturation/Lightness of 0/30/0, followed by Highlight/Midtone/Shadow of 100/50/30.
RESULTS
CcOI deficiency increases with age in colons of women and men
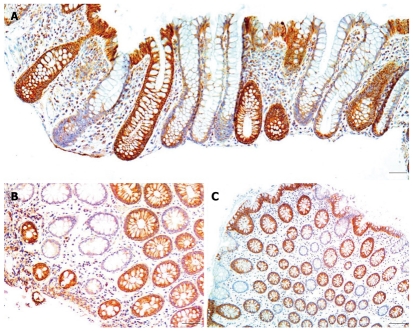
Biopsies were taken from the colons of 154 women and 196 men. For each woman and man 3 biopsies were taken from the cecum and 3 from the sigmoid colon. For each individual the mean percent CcOI deficient crypts for the 6 biopsies was plotted against age of the woman (Figure 1A) or the man (Figure 1B). This was possible because crypts tend to be either normal or defective, with minimal ambiguity (Figure 2 or, see reference[7] at 25 min of the 28 min video). The CcOI deficient crypts (CcOI-DC) appear morphologically normal. The average frequency of CcOI-DC is low between 20 to 39 years of age, with 0.48% ± 0.40% CcOI-DC for women and 1.80% ± 0.35% for men. CcOI-DC increases after age 40 years, so that between the ages of 40-44 years the average frequency of CcOI-DC goes up to 5.89% ± 0.84% in women and 2.15% ± 1.27% in men. By 80-84 years of age, the average frequency of CcOI-DC goes up in women to 15.77% ± 0.97% and in men to 22.6% ± 0.65%. The increases in CcOI-DC from ages 40-44 years compared to 80-84 years in women and men are significantly different with P < 0.01. For women aged sixty or over, those with a previous cancer (6 out of 70 women) have a significantly increased frequency of CcOI deficient crypts (P = 0.001) compared to those who never had colon cancer. For men 60 years or older, those with a previous or current cancer (14 of 125 men) exhibit no significant increase in percent CcOI deficient crypts.
Figure 1.
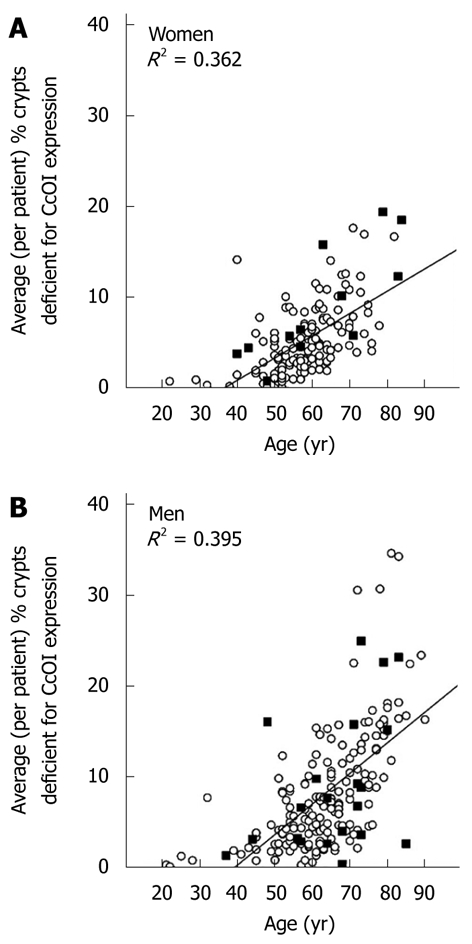
Average (per patient) percent of crypts deficient for cytochrome c oxidase subunit I expression plotted against patient age for women (A) and men (B). Solid squares represent patients with a prior or current colonic adenocarcinoma and circles represent patients without colonic adenocarcinoma. CcOI: Cytochrome c oxidase subunit I.
Figure 2.
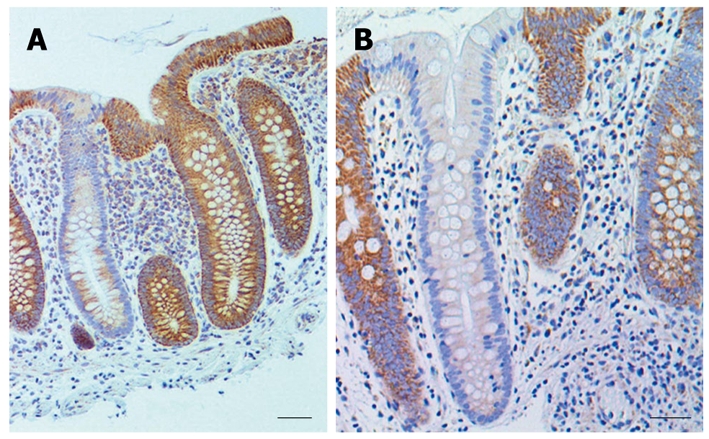
Examples of colon crypts immunohistochemically stained for cytochrome c oxidase I showing low expression next to highly expressing crypts. A: Bar shows 50 μm; B: Bar shows 40 μm. Brown staining indicates cytochrome c oxidase I expression and blue indicates nuclear hematoxylin staining.
CcOI deficiency within 1 to 10 cm of tumors, at the margins of tumors and within tumors
Thirty-four samples were taken within 1 to 10 cm of the tumor in resections from twelve males (ages 56 to 82 years) who had advanced adenoma or cancer for evaluation of CcOI deficiency. Comparison of CcOI deficiency level in these surgical resections was made with age-matched biopsy samples from all males in our patient population who were without a history of neoplasia. This comparison indicated that the samples from the resections had significantly elevated CcOI-DC, being 4.03% ± 0.27% in individuals free of neoplasia in the age range 55-64 years and 14.13% ± 0.35% in resected histologically normal tissue of men with cancer in the same age range, significantly different with P < 0.001. Similar significant differences were noted at older age ranges. The CcOI-DC in the age range 65-74 years was 7.22% ± 0.28% in individuals free of neoplasia and 23.80% ± 5.58% in resected histologically normal tissue of men with cancer, P < 0.001, and the CcOI-DC in the age range 75-84 years was 13.89% ± 0.34% in individuals free of neoplasia and 29.44% ± 3.86% in resected histologically normal tissue of men with cancer, P < 0.001 (Figure 3). In each of the three age groups, the results show a significantly increased frequency of CcOI deficient crypts in regions one to ten cm from an advanced colonic adenoma or cancer.
Figure 3.
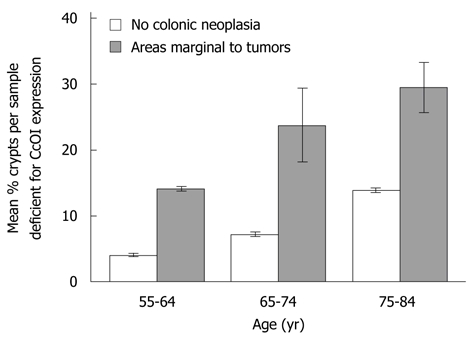
Comparison of mean percent cytochrome c oxidase I deficiency in areas marginal to tumors within surgical resections from males with aged-matched biopsy samples from males without a history of colonic neoplasia. S.E. indicated for each mean.
We also examined colon cancers from the resections described above and from archived colon cancers from additional patients. We found that crypts just at the margin of cancers are frequently deficient in CcOI (Table 1). All of these margins showed some observable CcOI deficient crypts. The percentage of CcOI-deficient crypts observed in tissue sections at the margins of tumors varied widely from 7% to 99%, with an overall average of 42.1% deficiency for the 35 margins evaluated (Table 1). Usually the deficient crypts were located in clusters.
Table 1.
Tumor marginal cytochrome c oxidase I deficiency, cluster size and tumor cytochrome c oxidase I deficiency
| Patient No. | Total No. of crypts at margin of tumor in tissue section | No. of CcOI deficient crypts at margin of tumor | % of crypts at margin of tumor that are CcOI deficient [100 × (CcOI-deficient crypts/total crypts)] | Maximum cluster size (No. of deficient crypts adjacent to each other) of deficient crypts | Approximate % of area in tumor with CcOI deficiency |
| 1 | 772 | 765 | 99 | 410 | 40 |
| 2 | 194 | 189 | 97 | 189 | 3 |
| 3 | 38 | 37 | 97 | 37 | 80 |
| 4 | 61 | 58 | 95 | 39 | 2 |
| 5 | 145 | 137 | 94 | 81 | 30 |
| 6 | 131 | 115 | 88 | 94 | N/A1 |
| 7 | 56 | 45 | 80 | 20 | 5 |
| 8 | 43 | 31 | 72 | 4 | 2 |
| 9 | 31 | 22 | 71 | 7 | 2 |
| 10 | 15 | 10 | 67 | 8 | 1 |
| 11 | 24 | 16 | 66 | 3 | 15 |
| 12 | 126 | 82 | 65 | 32 | 4 |
| 13 | 17 | 9 | 53 | 3 | 28 |
| 14 | 59 | 20 | 34 | 6 | 10 |
| 15 | 47 | 16 | 34 | 5 | 7 |
| 16 | 10 | 3 | 30 | 2 | 1 |
| 17 | 244 | 68 | 29 | 11 | 50 |
| 18 | 341 | 92 | 27 | 6 | 1 |
| 19 | 126 | 34 | 27 | 12 | 10 |
| 20 | 133 | 33 | 25 | 5 | 18 |
| 21 | 162 | 35 | 22 | 4 | 10 |
| 22 | 286 | 63 | 22 | 6 | N/A |
| 23 | 72 | 16 | 22 | 4 | 3 |
| 24 | 892 | 181 | 20 | 8 | N/A |
| 25 | 237 | 44 | 19 | 5 | 20 |
| 26 | 633 | 111 | 18 | 16 | 15 |
| 27 | 120 | 19 | 16 | 5 | N/A |
| 28 | 634 | 97 | 15 | 5 | 8 |
| 29 | 1813 | 262 | 14 | 14 | N/A |
| 30 | 61 | 7 | 11 | 2 | 2 |
| 31 | 239 | 26 | 11 | 4 | 35 |
| 32 | 396 | 40 | 10 | 4 | N/A |
| 33 | 79 | 7 | 9 | 1 | 5 |
| 34 | 80 | 6 | 8 | 3 | N/A |
| 35 | 140 | 10 | 7 | 3 | 50 |
| Overall average percent | 42.1 | 16.4 |
1N/A indicates that no tumor area was available in the tissue section as cut, and only the margin of the tumor was present. CcOI: Cytochrome c oxidase I.
The largest cluster of CcOI deficient crypts that we observed in tissue sections taken from biopsies (not associated with tumors) had 17 CcOI deficient crypts (Table 2). These clusters of CcOI deficient crypts in biopsies were among approximately 37 000 total crypts evaluated, of which 2476 crypts were deficient in CcOI (tissues from men and women, combined). However, at the margins of tumors, 7 of the 35 margins in tissue sections had CcOI deficient crypt clusters larger than the largest cluster (cluster of 17) that we had observed in tissue sections taken from biopsies. For the clusters seen in tumor-marginal tissue sections, the seven large clusters were of sizes 20, 37, 39, 81, 94, 189 and 410 countable CcOI deficient crypts per cluster seen (Table 1). Small areas from tissue sections through colon cancers with large clusters of CcOI deficient crypts along their margins are shown in Figure 4A and B.
Table 2.
Distribution of cytochrome c oxidase I defective crypts according to cluster size
| Gender | (a) size of cluster | (b) number of defective clusters of each cluster size | (a) × (b) number of defective crypts for each cluster size | % of total crypts in each size class |
| Female | 1 | 309 | 309 | 43.5 |
| 2 | 82 | 164 | 23.1 | |
| 3 | 38 | 114 | 16 | |
| 4 | 15 | 60 | 8.4 | |
| 5 | 9 | 45 | 6.3 | |
| 6 | 2 | 12 | 1.7 | |
| 7 | 1 | 7 | 1 | |
| Total 456 | Total 711 | Total 100.0 | ||
| Male | 1 | 622 | 622 | 35.2 |
| 2 | 223 | 446 | 25.3 | |
| 3 | 95 | 285 | 16.1 | |
| 4 | 31 | 124 | 7 | |
| 5 | 20 | 100 | 5.7 | |
| 6 | 7 | 42 | 2.4 | |
| 7 | 5 | 35 | 2 | |
| 8 | 5 | 40 | 2.3 | |
| 9 | 1 | 9 | 0.5 | |
| 10 | 2 | 20 | 1.1 | |
| 12 | 1 | 12 | 0.7 | |
| 13 | 1 | 13 | 0.7 | |
| 17 | 1 | 17 | 1 | |
| Total 1014 | Total 1765 | Total 100.0 |
Figure 4.
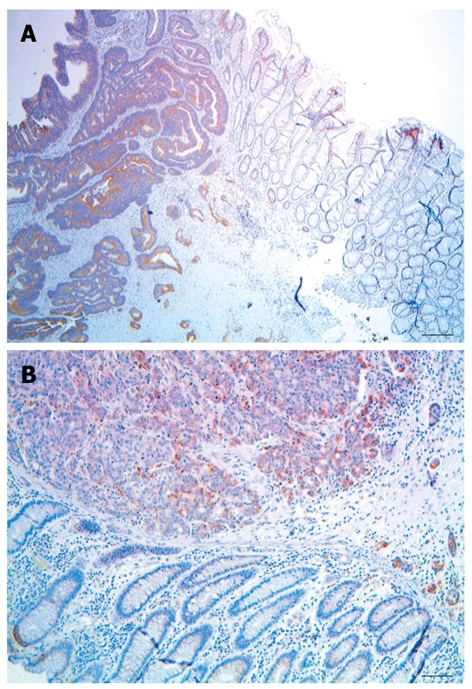
Large clusters of cytochrome c oxidase I deficient crypts along the margins of colon adenocarcinomas. A: Bar shows 100 μm; B: Bar shows 50 μm.
As indicated in Table 1, all tumors evaluated also showed at least some areas of deficiency in CcOI expression. The areas defective within the tumors varied widely from 1% to 80% with a mean of 16.4% deficiency (for the 28 tumors indicated in Table 1, and 17.4% for all 57 tumors evaluated, including those where the margins were not available). A small area of tumor is shown in Figure 5,
Figure 5.
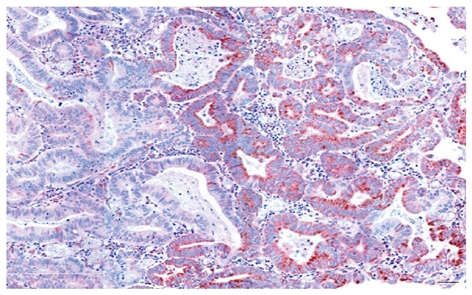
Image of colonic adenocarcinoma showing an area of high cytochrome c oxidase I expression (brown) adjacent to an area of low cytochrome c oxidase I expression. Bar shows 50 μm.
where approximately half the epithelial cells show high expression of CcOI and the other half shows reduced expression of CcOI.
CcOI deficiency in the cecum correlates with CcOI deficiency in the sigmoid colon both for men and women
Within individual colons the level of CcOI deficiency in the cecum correlates with the level in the sigmoid region. That is, a relatively high deficiency of CcOI in the sigmoid almost always occurs along with a relatively high CcOI deficiency in the cecum of the same patient. Likewise, a relatively low deficiency in the sigmoid is almost always associated with a relatively low deficiency in the cecum of the same patient. For women, CcOI deficiency in the cecum correlates with CcOI deficiency in the sigmoid, with an R2 = 0.414, and this correlation is highly significant (P < 0.001) (Figure 6A). For men, CcOI deficiency in the cecum correlates with CcOI deficiency in the sigmoid, with an R2 = 0.528, and this correlation is highly significant (P < 0.001) (Figure 6B). Since the percent of defective crypts in the cecum and sigmoid show a highly significant correlation in both men and women, and since these regions are at opposite ends of the colon, it is likely that the factors that determined the level of the CcOI defect in the sigmoid and cecum acted similarly throughout the whole colon.
Figure 6.
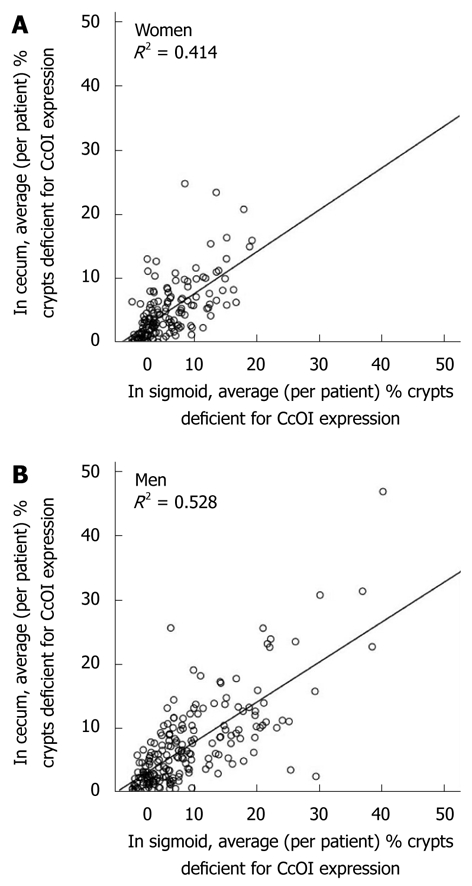
Correlation, within individuals, of average cytochrome c oxidase I deficiency in the cecum with average cytochrome c oxidase I deficiency in their sigmoid colon for women (A) and men (B). CcOI: Cytochrome c oxidase I.
However, there is a small but significant systematic bias, so that CcOI deficiencies in the sigmoid are usually slightly higher than CcOI deficiencies in the cecum. Among 154 women, the average % deficient crypts in the cecum (459 total biopsies) is 4.81% ± 0.25% and in the sigmoid (464 total biopsies) is 5.81% ± 0.25%. The % deficiency in the cecum for women is significantly different from the % deficiency in the sigmoid colon (P < 0.001). The ratio of CcOI deficiency for women in the sigmoid compared to the cecum is 5.81/4.81 = 1.21. Among 196 men, the average % deficient crypts in the cecum (583 total biopsies) is 7.15% ± 0.47% and in the sigmoid (585 total biopsies) is 8.88% ± 0.56%. The % deficiency for men in the cecum is significantly different from the % deficiency in the sigmoid colon (P < 0.001). The ratio of CcOI deficiency for men in the sigmoid compared to the cecum is 8.88/7.15 = 1.24. Thus, on average, for men and women, the percent defective crypts in the sigmoid is 21%-24% higher than in the cecum.
Defective crypts in biopsies tend to occur in clusters
In tissue samples (biopsies) obtained during colonoscopies, crypts with CcOI deficiency tend to be either single crypts (cluster size one) occurring among crypts with normal high levels of CcOI expression, or in clusters of varying sizes (Figure 7). The distribution of CcOI-deficient crypts (among about 37 000 crypts evaluated in biopsies) according to cluster size was determined for 711 deficient crypts for women and 1765 for men. This distribution of CcOI defective crypts according to cluster size is shown in Table 2. For the 2476 total CcOI-deficient crypts observed, 402 of them in women and 1143 in men (total of 1545 or 62% of CcOI-deficient crypts) occurred within clusters larger than a single crypt. On the other hand, the majority of “clusters” of CcOI-deficient crypts (309/456 or 67.8% for women and 622/1014 or 61.3% for men) were single deficient crypts (cluster size one). Thus, although the most common cluster size was one, most deficient crypts were within clusters of size two or greater.
Figure 7.
Examples of clusters of cytochrome c oxidase I deficient crypts. A: Cut along the long axes of crypts, bar shows 50 μm; B: Cut along the short axes of crypts, bar shows 50 μm; C: Cut along the short axes of crypts, bar shows 100 μm.
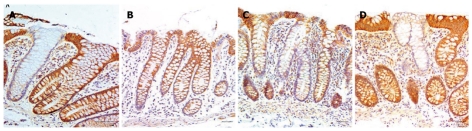
Mean cluster size also increased significantly (P < 0.001) with patient age for both men and women (Figure 8). Clusters of CcOI deficient crypts probably arise by the process of crypt fission[4] which occurs both in crypts with normal CcOI expression (Figure 9A and B) and in crypts with defective CcOI expression (Figure 9C and D).
Figure 8.
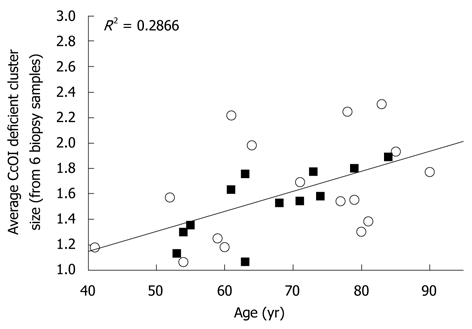
Mean cluster size of cytochrome c oxidase I deficient crypts (data averaged from 6 biopsies from each of 12 female patients (black squares) and 16 male patients (white circles) plotted against age. CcOI: Cytochrome c oxidase I.
Figure 9.
Examples of crypts undergoing fission. (A) and (B) are examples of crypts undergoing fission where the crypts have high levels of cytochrome c oxidase I (CcOI) expression; (C) and (D) are examples of crypts undergoing fission where the crypts have reduced or absent levels of CcOI expression. Bars show 50 μm.
Crypts with segmental CcOI deficiency
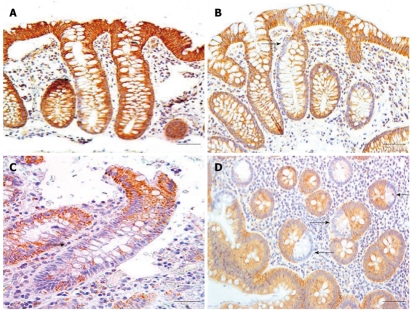
Crypts with deficient CcOI expression in only a segment of the crypt were observed. The smallest segmental deficiencies appear near the base of the crypt in the stem cell region, suggesting that the CcOI defective progeny of an originally mutated stem cell are starting to populate the crypt. Larger segment patterns are ordinarily continuous (i.e without interruption by normal cells) and include cells on one side near the base of the crypt (Figure 10A), cells on one entire side of the crypt (Figure 10B), or cells on both sides of a crypt most of the way up a crypt (Figure 10C). A cross-section through several segmented crypts is shown (arrows) in Figure 10D.
Figure 10.
Examples of crypts with segmental cytochrome c oxidase I deficiency, panels A, B and C cut along the long axes of crypts, panel D cut along the short axes of crypts. Panel A shows one crypt with deficient cytochrome c oxidase I (CcOI) expression halfway up crypt from stem cell region and only on one side (arrow); panel B has one entire side of crypt deficient for CcOI (arrow); panel C has deficiency for CcOI on both sides but only about ¾ of the way up the crypt (arrow); panel D shows three crypts in cross-section with one side deficient for CcOI expression (arrows). Bars show 50 μm.
Relationship of CcOI deficiency with intake of vitamins, minerals, NSAIDs or meat
When corrected for age, no significant correlation of % defective crypts with vitamins or minerals was found in men or women. When not corrected for age, in women, average CcOI deficiency (% CcOI defective crypts, averaged over six biopsies for each patient) correlates positively with age (P < 0.001) (Table 3). However, it also correlates positively with vitamin intake (P = 0.019), but not with intake of NSAIDs (P = 0.111), minerals (P = 0.177) or meat (P = 0.588). When not corrected for age, in males, average CcOI deficiency (% CcOI defective crypts, averaged over six biopsies for each patient) correlates positively with age (P < 0.001) (Table 3). However, it also correlates positively with vitamin intake (P = 0.010) and mineral intake (P = 0.021), but not with intake of NSAIDs (P = 0.099) or meat (P = 0.228). The correlations of average CcOI deficiency with vitamin intake and/or mineral intake appear to reflect the increase in these intakes with age rather than a causal relationship.
Table 3.
Correlations among non-steroidal anti-inflammatory drugs, vitamins, minerals, meat, age, cytochrome c oxidase I deficiency
| Gender | NSAIDs | Vitamins | Minerals | Meat | Age | Average CcOI deficiency | ||
| Female | NSAIDs | Pearson correlation | 1 | 0.242b | 0.160a | 0.068 | 0.220b | 0.13 |
| Sig. (2-tailed) | 0.002 | 0.046 | 0.402 | 0.006 | 0.111 | |||
| N | 155 | 155 | 155 | 155 | 155 | 151 | ||
| Vitamins | Pearson correlation | 0.242b | 1 | 0.682b | -0.1 | 0.223b | 0.191a | |
| Sig. (2-tailed) | 0.002 | 0 | 0.215 | 0.005 | 0.019 | |||
| N | 155 | 155 | 155 | 155 | 155 | 151 | ||
| Minerals | Pearson correlation | 0.160a | 0.682b | 1 | -0.127 | 0.191a | 0.111 | |
| Sig. (2-tailed) | 0.046 | 0 | 0.114 | 0.017 | 0.177 | |||
| N | 155 | 155 | 155 | 155 | 155 | 151 | ||
| Meat | Pearson correlation | 0.068 | -0.1 | -0.127 | 1 | -0.036 | -0.044 | |
| Sig. (2-tailed) | 0.402 | 0.215 | 0.114 | 0.656 | 0.588 | |||
| N | 155 | 155 | 155 | 155 | 155 | 151 | ||
| Age | Pearson correlation | 0.220b | 0.223b | 0.191a | -0.036 | 1 | 0.582b | |
| Sig. (2-tailed) | 0.006 | 0.005 | 0.017 | 0.656 | 0 | |||
| N | 155 | 155 | 155 | 155 | 158 | 153 | ||
| Average CcOI | Pearson correlation | 0.13 | 0.191a | 0.111 | -0.044 | 0.582b | 1 | |
| deficiency | Sig. (2-tailed) | 0.111 | 0.019 | 0.177 | 0.588 | 0 | ||
| N | 151 | 151 | 151 | 151 | 153 | 153 | ||
| Male | NSAIDs | Pearson correlation | 1 | 0.173a | 0.112 | 0.1 | 0.184 | 0.119 |
| Sig. (2-tailed) | 0.014 | 0.117 | 0.16 | 0.009 | 0.099 | |||
| N | 200 | 199 | 199 | 200 | 200 | 192 | ||
| Vitamins | Pearson correlation | 0.173a | 1 | 0.576b | -0.062 | 0.195b | 0.184a | |
| Sig. (2-tailed) | 0.014 | 0 | 0.384 | 0.006 | 0.01 | |||
| N | 199 | 201 | 201 | 200 | 201 | 193 | ||
| Minerals | Pearson correlation | 0.112 | 0.576b | 1 | -0.111 | 0.198b | 0.166a | |
| Sig. (2-tailed) | 0.117 | 0 | 0.118 | 0.005 | 0.021 | |||
| N | 199 | 201 | 201 | 200 | 201 | 193 | ||
| Meat | Pearson correlation | 0.1 | -0.062 | -0.111 | 1 | -0.105 | -0.087 | |
| Sig. (2-tailed) | 0.16 | 0.384 | 0.118 | 0.138 | 0.228 | |||
| N | 200 | 200 | 200 | 201 | 201 | 193 | ||
| Age | Pearson correlation | 0.184 | 0.195b | 0.198b | -0.105 | 1 | 0.663b | |
| Sig. (2-tailed) | 0.009 | 0.006 | 0.005 | 0.138 | 0 | |||
| N | 200 | 201 | 201 | 201 | 203 | 195 | ||
| Average | Pearson correlation | 0.119 | 0.184a | 0.166a | -0.087 | 0.663b | 1 | |
| CcOI | Sig. (2-tailed) | 0.099 | 0.01 | 0.021 | 0.228 | 0 | ||
| deficiency | N | 192 | 193 | 193 | 193 | 195 | 195 |
Correlation is significant at the 0.05 level (2-tailed);
Correlation is significant at the 0.01 level (2-tailed). NSAIDs: Non-steroidal anti-inflammatory drugs; CcOI: Cytochrome c oxidase I.
DISCUSSION
CcOI deficiency increases with age
Based on biopsied tissues obtained during colonoscopies, from 154 women and 196 men, we found that the frequency of CcOI deficient crypts increases with age in both men and women. This finding is consistent with the results of Taylor et al[15] based on their samples from 28 individual patients. While we saw deficient crypts in individuals as young as 21 years of age (Figure 1), in both women and men CcOI deficiency seems to increase largely after age of 40 years.
CcOI deficiency is associated with advanced neoplasia in older individuals
Women over sixty or older who had a previous or current cancer (6 out of 70 women) had a significantly increased frequency of CcOI deficient crypts (P < 0.001) compared to women in this age range who never had colon cancer. Also men with cancer or advanced colonic neoplasia had a significantly increased frequency of CcOI defective crypts specifically in areas 1 to 10 cm from the lesion, as determined in surgical resections. Consistent with these results, we reported in a previous study (Payne et al[6]), also involving surgical resections, that patients who had neoplasia [tubulovillous adenoma (n = 3) or adenocarcinoma (n = 8)] also had a significantly higher mean incidence of CcOI defective crypts in regions 1 to 10 cm from the tumor, than patients without colonic neoplasia (n = 5). In that study, however, age of the patients was not taken into account because of the small number of patients evaluated.
In our previous study (Payne et al[6]) we proposed that increased frequency of CcOI deficient crypts in biopsies of colonic mucosa of patients undergoing colonoscopy might be a potential hypothesis-driven biomarker of colon cancer risk. Our current extensive data, however, do not support CcOI deficiency in biopsies as a good biomarker of risk. In Figure 1, patients with a previous or current colon cancer are indicated as filled squares. Patients with a recently resected colon cancer have a 35% risk (a relatively high risk) of having a subsequent advanced colonic neoplasia (advancing towards colon cancer) in the subsequent 5 years[16]. However, for women under 60 years of age, and for all men, CcOI deficiency in colon biopsies does not correlate with the high risk patients with a prior colon cancer.
We have shown a strong association of CcOI deficiency with colon cancer as indicated by the frequent finding of large clusters of CcOI deficient crypts in the non-dysplastic regions immediately adjacent (marginal) to the cancer, indicating that tumors may tend to arise in such clusters. Finally the observation that the cancers themselves often contain regions of CcOI deficient cells is another indication of an association of CcOI deficiency and cancer.
A hypothetical sequence of events in progression from a defect in CcOI expression to cancer
We previously proposed a sequence of events by which a mutation or epimutation causing a CcOI deficiency might lead to colon cancer[6]. Based on that proposal and more recent evidence, including that presented here, we now outline how a CcOI deficiency might initiate a chain of events leading to colon cancer. Evidence that CcOI deficient crypts can arise by mutation in the CcOI gene in mitochondrial DNA was presented by Greaves et al[4]. Of the 13 protein subunits in the CcO complex, 3 proteins including CcOI are encoded by mitochondrial DNA and 10 proteins are encoded by nuclear DNA. Although reduced expression of CcOI in a stem cell of a colonic crypt can arise from a mutation in the CcOI gene itself, in principal, a mutation or epimutation in another protein component of the CcO complex may lead to failure of assembly of the CcO complex, in which case the CcOI protein will rapidly degrade[17]. However, here we will confine our discussion to the consequences of mutations in the mitochondrial CcOI gene, since so far this is the only type of mutation demonstrated to give rise to a CcOI deficiency.
In mammalian cells, each mitochondrion can contain several DNA molecules, estimated to be 1 to 3 DNA molecules per mitochondrion by Wiesner et al[18] and an average of 2.6 DNA molecules per mitochondrion by Robin et al[19]. Mammalian cells typically have hundreds of mitochondria per cell[19]. Mitochondria with a CcO deficiency likely produce lower levels of damaging reactive oxygen species (ROS) and thus probably turn over less frequently [mitochondria turn over on average in about 2 d[20]. If a deficiency of CcOI in a mitochondrion produces lower ROS and this provides a selective advantage in competition with other mitochondria within the same cell, this selective advantage need not depend on a CcOI mutation being present in all 1 to 3 copies of the mitochondrial genome in the mitochondrion. Rather, mutations in some of the CcOI gene copies in a mitochondrion may be sufficient to drive selection of progeny of that mitochondrion in competition among the hundreds of mitochondria within a cell. In turn, cells, and their descendents, with a significant fraction of CcOI deficient mitochondria may be selected within the stem cell niche of a crypt, causing niche succession. Alternatively niche succession by CcOI deficient cells may be a stochastic process, not involving positive selection, which is possible because the number of cells in the stem cell niche is small (estimated to be six[3]). In either case, niche succession can lead to subsequent monoclonal conversion whereby all of the cells of a crypt are deficient for CcOI[21]. In a small proportion of crypts, we have found deficient CcOI expression in only a segment of the crypt. The pattern of segmentation probably represents intermediate stages of monoclonal conversion of the crypt as proposed by Humphries et al[21]. After crypt conversion, crypt fission may then occur.
CcOI deficient cells may be defective in apoptosis. When cells of human colonic origin (HCT116) were treated with the bile acid deoxycholate (0.5 mmol/L) for 4 h, the majority of cells entered apoptosis. However pretreatment of the cells with sodium azide, which binds to CcO and inhibits the protein complex from functioning, significantly reduced the fraction of cells undergoing apoptosis upon further treatment with deoxycholate[22], indicating that CcO deficient cells are resistant to induction of apoptosis. We previously showed that reduction in CcOI expression correlated with a reduction in apoptotic competence in nearby tissue samples from the same colonic epithelium[6]. Linkage of CcOI expression to apoptosis competence is also suggested by the finding that active CcO oxidizes cytochrome c that can then activate pro-caspase 9 leading to apoptosis[23]. Thus, cells with a deficiency in CcO may also have a deficiency in apoptosis capability. Reduced ability of a cell to undergo apoptosis could provide a selective advantage under conditions of cytotoxic stress, particularly stress caused by high bile acid exposure.
Among the 10-20 million crypts in the human colon, some colonic crypts may be removed due to localized damage. Since CcOI-deficient cells probably resist apoptosis under damaging conditions, CcOI-deficient cells may compete successfully to reconstitute crypts under stress conditions. As the new crypts compete for space in the re-constituting colon epithelium, the CcOI deficient crypts may form a patch, or focal region, of the colon epithelium.
The largest cluster of CcOI deficient crypts that we observed in biopsies had 17 CcOI deficient crypts. These clusters of CcOI deficient crypts were among approximately 37 000 total crypts evaluated, of which 2476 crypts were deficient in CcOI (tissues from men and women, combined). However, among the 35 margins of tumors that were visualized in tissue sections, the non-neoplastic margins were often associated with clusters of CcOI deficient crypts, and larger clusters of 20, 32, 37, 39, 81, 94, 189 and 410 CcOI deficient crypts were observed. This observation suggests that colonic tumors may often arise in a cluster of CcOI deficient crypts.
A field of CcOI defective crypts may be stimulated to progress further to colon cancer by continuing exposure of the colon to dietary factors associated with increased cancer risk. Colon cancer incidence is associated with a high fat Western-style diet leading to increased secretion of bile acids into the intestine to emulsify the dietary fat. Exposure of colon cells to high physiological levels of bile acids induces ROS, DNA damage, and apoptosis. Population studies have found that fecal bile acid concentrations are increased in populations with a high incidence of colon cancer (reviewed in Bernstein et al[24]). Polyak et al[25] described a colorectal tumor in which a G to C somatic mutation in the CcOI gene appeared to have arisen as a result of oxidative DNA damage and apparently was present in every mitochondrial genome within the tumor.
Crypts with CcOI defective cells may be favored when bile acid exposure is elevated, and survive despite increased DNA damage because of their reduced ability to undergo apoptosis. Unrepaired DNA damage in these surviving cells would tend to cause replication errors leading to mutations. Some of these mutations may confer a further proliferative advantage, enhancing spread of the CcOI defective field. Ultimately, mutations may occur in tumor suppressor genes or oncogenes. By this reasoning, fields of CcOI deficient cells may be predisposed to progress further to advanced neoplasia and colon cancer.
Cancers are likely to arise from a CcOI deficient crypt
If CcOI deficient areas in a malignant mass were to ordinarily arise from a new mutation in the malignant mass, then an association of cancers with large clusters of CcOI deficient crypts at the margins of the cancer would not be expected. However, crypts just at the margin of cancers are frequently deficient in CcOI (Table 1), suggesting that these cancers arise from a CcOI deficient crypt. Large clusters of crypts with CcOI deficiency outside the focal lesions of two cancers are illustrated for two cancers in Figure 4.
Patches of CcOI deficiency within tumors
We observed that within tumors there are regions with normal CcOI expression and usually smaller regions of CcOI deficiency (an example is shown in Figure 5, and areas deficient in CcOI within cancers are tabulated in Table 1). If a tumor arises from a CcOI deficient crypt it may contain replicative cells (e.g. stem cells) with a large fraction of the several hundred mitochondria in the cell being deficient for CcOI, but also a significant fraction of mitochondria without such deficiency (heteroplasmy). Further, in those mitochondria deficient for CcOI, either all copies of the several chromosomes present may have a CcOI mutation, or there may be a mixture of chromosomes, some with a CcOI mutation and some without such a mutation. In the new environment of tumor growth, some lines of descent with a majority of mitochondria defective for CcOI expression may retain their selective advantage and give rise to regions within the tumor with deficient CcOI expression. However, in other lines of descent, cells having larger fractions of mitochondria with wild-type CcOI may have a selective advantage, giving rise to sub-clones within the tumor with high levels of CcOI expression. For instance, under tumor growth conditions, there may be selection in regions of the tumor for cells with increased CcOI expression since that would enhance electron transport efficiency.
CcOI deficient patches in cancers are less likely to be from newly arising mutations, but more likely reflect segregation of heteroplasmic mitochondria
In a diploid cell nucleus, genes occur in just two copies. About 80 percent of cancers are aneuploid and have genes in just one copy. Thus, newly arising mutations in nuclear DNA of cells can form clearly distinguished subclones of cells, requiring just a single dominant change in the nuclear DNA of a diploid cell or just a single change in an aneuploid cell.
However, CcOI is coded for by mitochondrial DNA. There are several chromosomes per mitochondrion and several hundred mitochondria per cell. It is not likely that a newly arising mitochondrial mutation in CcOI in one of the chromosomes of a single mitochondrion within a single cancer cell (containing several hundred mitochondrial genomes) could generate a large CcOI deficient patch in a cancer as seen in our observations (e.g. Figure 5). However, as pointed out above, a CcOI deficient stem cell of a CcOI deficient colonic crypt, when initiating a cancer, is likely to be heteroplasmic, with a large fraction of several hundred mitochondrial chromosomes deficient for CcOI and a smaller but significant fraction of mitochondrial chromosomes with wild-type CcOI. In the environment of an expanding cancer, as mitochondria segregate into daughter cells, some daughter cells may have more mitochondria with wild-type CcOI, and some have mostly mitochondria with mutated CcOI. In the cancer environment, different from the environment of a colonic crypt, the daughter cells with a larger proportion of mitochondria with wild-type CcOI may be selected for, and generate patches with mostly wild-type CcOI, while remaining daughter cells with mitochondria carrying CcOI deficient chromosomes, would generate other patches.
Aging and colon cancer
As noted above, CcOI deficient crypts occur infrequently in individuals in their twenties, start to increase in frequency at about the age of 40 years, and show an association with colon cancer in individuals aged 60 years and above. Since an increased frequency of CcOI deficient crypts in the cecum correlates with an increase in the sigmoid colon, progressive increases in aberrant crypt frequency probably occur throughout the colon. Although we have suggested a causal relationship between CcOI deficiency and cancer progression, and outlined a specific sequence of events that may occur in such a progression, it remains unclear whether CcOI deficiency is an underlying cause of progression, or whether the association has some other significance.
Because CcOI deficiency can be visualized by immunohistochemistry, it can be followed at successive stages during aging and progression to cancer. Starting as a small segment of defective cells within a crypt, the CcOI deficiency can then be visualized in larger segments within crypts, in whole crypts that increase in frequency with age, in crypts undergoing fission, in clusters of crypts that increase in size with age, in increased frequency near tumors, in large clusters in the intimate margins of tumors, and in the tumors themselves. In this sequence there is no clear dividing line between early stages that can be considered aspects of aging and later stages that can be considered aspects of progression to cancer. This ambiguity may reflect a rather general situation leading to cancer where the early stages of cellular change appear to be relatively innocuous features of the aging process but over decades may evolve to malignancy.
COMMENTS
Background
Some cells of the body divide frequently, such as the cells that give rise to the inner lining of the colon, and some very infrequently or not at all, such as muscle and nerve cells. The entire lining of the colon contains about 10 to 20 million cave-like structures, called crypts. Each crypt is shaped like a tube with a hole or “lumen” down the center of the structure. Each crypt is comprised of about 2500-5000 cells, being about 85-106 cells long and about 29-43 cells around the circumference. At the base of each crypt there are a few stem cells that remain at the base, are long-lived, and which can divide and produce daughter cells (that can also divide). These daughter cells are pushed up towards the top of the crypt. When the cells reach the top of the crypt after about 3 or 4 d they undergo programmed cell death. If one stem cell acquires a mutation that lets it and its first daughter cells out-compete the other stem cells, the mutant type of stem cell can take over the stem cell region, and then all the cells that occur in the crypt will have that mutation. Some types of mutant cells which take over the stem cell region tend to become more common with age. A second mutation may occur in a stem cell with the first mutation, and if this new mutation helps the stem cell and its daughters compete against the other stem cells, this double mutant can take over the crypt from the first mutant cells. This process may be repeated multiple times, leading to stem cells with multiple mutations, each providing a proliferative advantage. Such a stem cell can give rise to a cancer.
Research frontiers
It was recently shown, by the Vogelstein laboratory, that colon cancers ordinarily have about 15 “driver” mutations, which together cause the cancer to grow, and about 65 “passenger” mutations that happen to occur and get carried along with the driver mutations when they occur. The high number of 15 critical mutations can be achieved if the overall mutation rate is increased early in a cell’s progression to cancer, as pointed out by the Loeb laboratory. It is possible to imagine several types of genetic defects that could cause the mutation rate to increase (i.e. cause a mutator effect). However, there has been little evidence of just how such an increase in overall mutation rate (mutator effect) may actually occur.
Innovations and breakthroughs
High levels of bile acids are present in the colon after high fat meals. Bile acids act as detergents to help in digestion of fats. Both high fat diets and high bile acids are associated with increased incidence of colon cancer. Cytochrome c oxidase, the 4th protein complex in the electron transport chain of the mitochondria, was recently shown, by us, to be important in apoptosis (programmed cell death) that occurs when the DNA of cells is damaged by a bile acid. If cytochrome c oxidase is not active (mutated) in cells encountering bile acids, then cells with DNA damage do not undergo apoptosis efficiently. Such surviving cells with DNA damage acquire further mutations when their DNA is replicated using a damaged template. Thus a colonic area with cells deficient in cytochrome c oxidase would have an elevated mutation rate. A key protein in cytochrome c oxidase (the first and largest subunit of this 13 member protein complex) is called cytochrome c oxidase subunit one (CcOI). CcOI deficient crypts accumulate with age, and large clusters of CcOI-deficient crypts tend to occur in areas that gave rise to a cancer. The defect in CcOI appears to contribute to a mutator phenotype in the area that gave rise to a colon cancer, and was probably central in progression to colon cancer. Previously, isolated small clusters with 2 or 3 CcOI defective crypts were reported to increase with age. We report here the occurrence of large clusters of CcOI deficient crypts (some clusters with over 100 crypts) immediately adjacent to cancers. This finding is dramatic evidence indicating the likely role of CcOI in progression to colon cancer.
Applications
Individuals who have had previous large polyps or cancers removed from their colons are known to be at much increased risk of a subsequent large polyp or colon cancer. If a treatment could be found which targets cells with CcOI deficiency for cell death, this could protect patients at increased risk of colon cancer from progression towards colon cancer.
Terminology
“Electron transport chain” is the sequence of protein complexes, in the membrane of the mitochondria, which generates ATP in order to provide a source of useful energy for the cell. “Dysplastic tissues” are disorganized tissue areas seen in the microscope, which indicate abnormal development and possible progression towards cancer or the actual presence of cancer.“Nonneoplastic colonic crypts” are crypts that look “normal.” Their appearance contrasts with neoplastic crypts, which are ones that show a new (neo) plastic form(bent, stretched or other odd shape) that indicates progression to colon cancer. “Immunohistochemical method” uses a thin piece of tissue (4 micro-meters thick) that is allowed to interact with an antibody that targets a protein of interest. This first “primary” antibody attaches to the protein of interest. Then another antibody, a “secondary” antibody that attaches to the first type of antibody and carries a molecule that can show a brown color when “stained,” is allowed to interact with the primary antibody on the thin piece of tissue. In the microscope, after staining, the presence of brown color indicates the location and relative amount of the protein of interest.
Peer review
It is an interesting study and data presented is relatively new. The authors describe the correlation between CcOI expression and colon cancer progression. This report provides solid data that indicate the deficiency of CcOI in some crypts and its correlation with aging. The discussion of the selective advantage of CcOI deficient (stem) cells is interesting.
Footnotes
Supported by Grants from the National Institutes of Health (5 R01 CA119087), Arizona Biomedical Research Commission Grant #0803, Veterans Affairs Merit Review Grant 0142 administered by the Southern Arizona Veterans Affairs Health Care System and from Biomedical Diagnostics and Research, Inc., Tucson, AZ 85719
Peer reviewers: De-Liang Cao, MD, PhD, Associate Professor, Department of Medical Microbiology, Immunology, and Cell Biology Simmons Cooper Cancer Institute, Southern Illinois University School of Medicine 913 N. Rutledge Street, Springfield, IL 62794-9626, United States; Joseph T Tseng, Assistant Professor, Institute of Bioinformatics, College of Bioscience and Biotechnology, National Cheng-Kung University, No.1 University Rd, Tainan, 701, Taiwan, China
S- Editor Wang JL L- Editor Hughes D E- Editor Ma WH
References
- 1.Nooteboom M, Johnson R, Taylor RW, Wright NA, Lightowlers RN, Kirkwood TB, Mathers JC, Turnbull DM, Greaves LC. Age-associated mitochondrial DNA mutations lead to small but significant changes in cell proliferation and apoptosis in human colonic crypts. Aging Cell. 2010;9:96–99. doi: 10.1111/j.1474-9726.2009.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipkin M, Bell B, Sherlock P. Cell proliferation kinetics in the gastrointestinal tract of man. I. cell renewal in colon and rectum. J Clin Invest. 1963;42:767–776. doi: 10.1172/JCI104769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 4.Greaves LC, Preston SL, Tadrous PJ, Taylor RW, Barron MJ, Oukrif D, Leedham SJ, Deheragoda M, Sasieni P, Novelli MR, et al. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proc Natl Acad Sci USA. 2006;103:714–719. doi: 10.1073/pnas.0505903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, Loeb LA. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40:392–394. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- 6.Payne CM, Holubec H, Bernstein C, Bernstein H, Dvorak K, Green SB, Wilson M, Dall’Agnol M, Dvorakova B, Warneke J, et al. Crypt-restricted loss and decreased protein expression of cytochrome C oxidase subunit I as potential hypothesis-driven biomarkers of colon cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2066–2075. doi: 10.1158/1055-9965.EPI-05-0180. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen H, Loustaunau C, Facista A, Ramsey L, Hassounah N, Taylor H, Krouse R, Payne CM, Tsikitis VL, Goldschmid S, et al. Deficient Pms2, ERCC1, Ku86, CcOI in field defects during progression to colon cancer. J Vis Exp. 2010:Jul 28, (41). doi: 10.3791/1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meissner C. Mutations of mitochondrial DNA - cause or consequence of the ageing process? Z Gerontol Geriatr. 2007;40:325–333. doi: 10.1007/s00391-007-0481-z. [DOI] [PubMed] [Google Scholar]
- 9.Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol. 2008;43:24–33. doi: 10.1016/j.exger.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edgar D, Shabalina I, Camara Y, Wredenberg A, Calvaruso MA, Nijtmans L, Nedergaard J, Cannon B, Larsson NG, Trifunovic A. Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell Metab. 2009;10:131–138. doi: 10.1016/j.cmet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Payne CM, Crowley C, Washo-Stultz D, Briehl M, Bernstein H, Bernstein C, Beard S, Holubec H, Warneke J. The stress-response proteins poly(ADP-ribose) polymerase and NF-kappaB protect against bile salt-induced apoptosis. Cell Death Differ. 1998;5:623–636. doi: 10.1038/sj.cdd.4400395. [DOI] [PubMed] [Google Scholar]
- 12.Garewal H, Ramsey L, Fass R, Hart NK, Payne CM, Bernstein H, Bernstein C. Perils of immunohistochemistry: variability in staining specificity of commercially available COX-2 antibodies on human colon tissue. Dig Dis Sci. 2003;48:197–202. doi: 10.1023/a:1021871423154. [DOI] [PubMed] [Google Scholar]
- 13.Romagnolo DF, Chirnomas RB, Ku J, Jeffy BD, Payne CM, Holubec H, Ramsey L, Bernstein H, Bernstein C, Kunke K, et al. Deoxycholate, an endogenous tumor promoter and DNA damaging agent, modulates BRCA-1 expression in apoptosis-sensitive epithelial cells: loss of BRCA-1 expression in colonic adenocarcinomas. Nutr Cancer. 2003;46:82–92. doi: 10.1207/S15327914NC4601_11. [DOI] [PubMed] [Google Scholar]
- 14.Holubec H, Payne CM, Bernstein H, Dvorakova K, Bernstein C, Waltmire CN, Warneke JA, Garewal H. Assessment of apoptosis by immunohistochemical markers compared to cellular morphology in ex vivo-stressed colonic mucosa. J Histochem Cytochem. 2005;53:229–235. doi: 10.1369/jhc.4A6386.2005. [DOI] [PubMed] [Google Scholar]
- 15.Taylor RW, Barron MJ, Borthwick GM, Gospel A, Chinnery PF, Samuels DC, Taylor GA, Plusa SM, Needham SJ, Greaves LC, et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J Clin Invest. 2003;112:1351–1360. doi: 10.1172/JCI19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieberman DA, Weiss DG, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, Schnell TG, Chejfec G, Campbell DR, Kidao J, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–1085. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Krummeck G, Rödel G. Yeast SCO1 protein is required for a post-translational step in the accumulation of mitochondrial cytochrome c oxidase subunits I and II. Curr Genet. 1990;18:13–15. doi: 10.1007/BF00321109. [DOI] [PubMed] [Google Scholar]
- 18.Wiesner RJ, Rüegg JC, Morano I. Counting target molecules by exponential polymerase chain reaction: copy number of mitochondrial DNA in rat tissues. Biochem Biophys Res Commun. 1992;183:553–559. doi: 10.1016/0006-291x(92)90517-o. [DOI] [PubMed] [Google Scholar]
- 19.Robin ED, Wong R. Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J Cell Physiol. 1988;136:507–513. doi: 10.1002/jcp.1041360316. [DOI] [PubMed] [Google Scholar]
- 20.Miwa S, Lawless C, von Zglinicki T. Mitochondrial turnover in liver is fast in vivo and is accelerated by dietary restriction: application of a simple dynamic model. Aging Cell. 2008;7:920–923. doi: 10.1111/j.1474-9726.2008.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat Rev Cancer. 2008;8:415–424. doi: 10.1038/nrc2392. [DOI] [PubMed] [Google Scholar]
- 22.Payne CM, Crowley-Weber CL, Dvorak K, Bernstein C, Bernstein H, Holubec H, Crowley C, Garewal H. Mitochondrial perturbation attenuates bile acid-induced cytotoxicity. Cell Biol Toxicol. 2005;21:215–231. doi: 10.1007/s10565-005-0166-6. [DOI] [PubMed] [Google Scholar]
- 23.Brown GC, Borutaite V. Regulation of apoptosis by the redox state of cytochrome c. Biochim Biophys Acta. 2008;1777:877–881. doi: 10.1016/j.bbabio.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol. 2009;15:3329–3340. doi: 10.3748/wjg.15.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polyak K, Li Y, Zhu H, Lengauer C, Willson JK, Markowitz SD, Trush MA, Kinzler KW, Vogelstein B. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat Genet. 1998;20:291–293. doi: 10.1038/3108. [DOI] [PubMed] [Google Scholar]