Abstract
The frontal eye field (FEF) is one of several cortical regions thought to modulate sensory inputs. Moreover, several hypotheses suggest that the FEF can only modulate early visual areas in the presence of a visual stimulus. To test for bottom-up gating of frontal signals, we microstimulated subregions in the FEF of two monkeys and measured the effects throughout the brain with functional magnetic resonance imaging. The activity of higher-order visual areas was strongly modulated by FEF stimulation, independent of visual stimulation. In contrast, FEF stimulation induced a topographically specific pattern of enhancement and suppression in early visual areas, but only in the presence of a visual stimulus. Modulation strength depended on stimulus contrast and on the presence of distractors. We conclude that bottom-up activation is needed to enable top-down modulation of early visual cortex and that stimulus saliency determines the strength of this modulation.
Contemporary hypotheses propose that feedback signals from areas in frontal and parietal cortex exert control over the processing of incoming visual information (1–5). Several models suggest that these signals are gated by bottom-up stimulation (6–9). In these models, feedback signals only influence neurons activated by visual input, just as has been observed for attentional effects, which are known to be strongest for neurons well driven by a visual stimulus (10–12). No causal evidence exists, however, to support these hypotheses, with the exception of area V4, where feedback effects evoked by stimulation of the FEF are most pronounced for neurons strongly activated by a visual stimulus (13). To (i) test these models of bottom-up dependent gating of frontal signals on a whole-brain scale, (ii) investigate the impact of increased FEF activity on visually driven responses throughout occipito-temporal cortex, (iii) examine the spatial organization of any observed modulations, and (iv) investigate the effects of visual saliency on such modulations, we developed a combination of functional magnetic resonance imaging (fMRI) and chronic electrical microstimulation (EM) in awake, behaving monkeys.
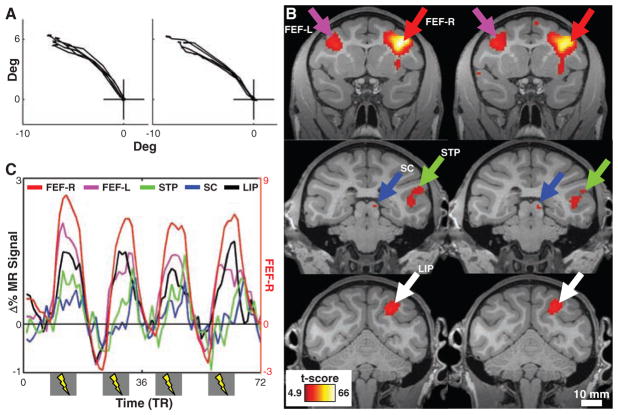
In our first experiment, the goal was to detect the functional consequences of EM of the FEF in the absence of a visual stimulus, using stimulation levels below those needed to evoke saccades. We first obtained anatomical (fig. S1B) and behavioral evidence (Fig. 1A and fig. S2) in two monkeys that several chronically implanted microelectrodes were positioned in the FEF. Before each fMRI experiment, we stimulated these electrodes inside the MR scanner to determine the threshold needed to evoke saccades and to identify the saccade end point, or movement field (MF), of each FEF stimulation site (Fig. 1A and fig. S2). During the actual fMRI experiment, the monkeys carried out a passive fixation task while we alternated between epochs of no-EM and epochs of EM, at a stimulation level of 50% of the saccade-inducing amplitude. The use of this method in awake animals allowed us to titrate the stimulation to functionally relevant levels (14), an advantage compared to a previous study in anesthetized animals (15).
Fig. 1.
fMRI activity induced by right FEF-EM. (A) Eye traces separated by 1 year showing saccades evoked by suprathreshold stimulation of one electrode. (B) Coronal slices showing activity (t-score maps) from the contrast EM versus no-EM (MM1, P < 0.05, corrected; R/L, right/left; see table S1 for anatomical abbreviations). Columns represent test-retest separated by 1 month. (C) Time courses of percentage change in MR signal in areas in (B). Note the secondary y axis for the FEF-R data. Gray bars (x axis) indicate stimulation epochs.
The left column of Fig. 1B shows t-score maps of regions with increased fMRI activity caused by FEF-EM overlaid on coronal, T1-weighted sections. Focal increases in fMRI activity were observed at the site of stimulation and across the brain, in regions known to be connected to the FEF (16–20) [Figs. S3 and S4; see (14) for a full list of areas]. To illustrate the amplitude of EM-induced fMRI effects in five representative regions, we plotted percentage change in MR signal relative to the no-EM condition (Fig. 1C). In comparison with classical tracer studies (17, 18), we obtained virtually identical results with EM-fMRI (figs. S3 and S4). This close correspondence demonstrates the precision of the technique and suggests that we primarily activated regions monosynaptically connected to the stimulation site. At the statistical threshold used (P < 0.05, corrected), we observed no negative EM-induced activations in visual cortex. The right column of Fig. 1B represents a replication of the experiment after 1 month, showing the reproducibility of the results even for small foci, such as those seen in the superior colliculus (14).
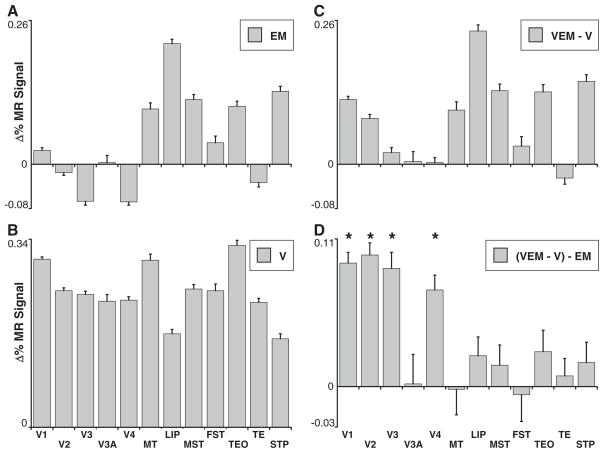
Our first experiment indicated that FEF-EM increased fMRI activity in higher-order visual areas known to be directly connected to the FEF. If feedback effects are gated by visual stimulation, however, one also predicts FEF-EM effects in visual areas separated from the FEF by multiple synapses, in the presence of a visual stimulus. In a second experiment, we therefore placed high-contrast, colored, moving gratings in the MFs of the FEF stimulation sites under passive viewing conditions (fig. S5A) (14) and measured the fMRI response to EM-only, visual-only (V), and combined visual-EM (VEM) stimulation, relative to a fixation-only (F) condition (i.e., a 2 by 2 factorial design with factors EM and visual stimulation). To investigate the net influence of visual stimulation on FEF-EM effects, we compared EM minus fixation (Fig. 2A) to VEM minus V (Fig. 2C) in all visually driven voxels for a number of cortical areas; visually driven activity without EM is shown in Fig. 2B. Visually driven voxels in areas directly connected to the FEF (16–20), such as the lateral intra-parietal area (LIP) and several areas within the superior temporal sulcus, showed an EM-driven increase in fMRI activity that was relatively independent of the presence of a visual stimulus (compare Figs. 2A and 2C). In contrast, visual stimulation unveiled a significant influence of FEF-EM on the activity of early visual areas (V1, V2, V3, and V4) (14). Figure 2D isolates the effect of the visual stimulus on FEF-EM by subtracting the activity evoked by EM in the absence of a visual stimulus (Fig. 2A) from the activity evoked in the presence of a visual stimulus (Fig. 2C). Visual stimulation enabled the effects of FEF microstimulation to reach early visual areas, including V1, which is not monosynaptically connected to the FEF.
Fig. 2.
Gating of FEF-EM effects by a visual stimulus. Mean percentage change in MR signal relative to fixation-only (MM1 and MM2) for all visually driven voxels (P < 0.05, uncorrected) in 12 visual areas for (A) EM and (B) V epochs, (C) the difference between VEM and V epochs, and (D) the interaction (VEM-V-EM). Error bars denote SEM; * indicates a significant difference between the VEM-V and EM distributions (P < 10−17, two-sample, two-tailed t test), revealing that on average voxels in early visual areas show a larger EM response in the presence of visual stimuli than in the absence. Higher-order visual areas show an EM response both with and without visual stimulation.
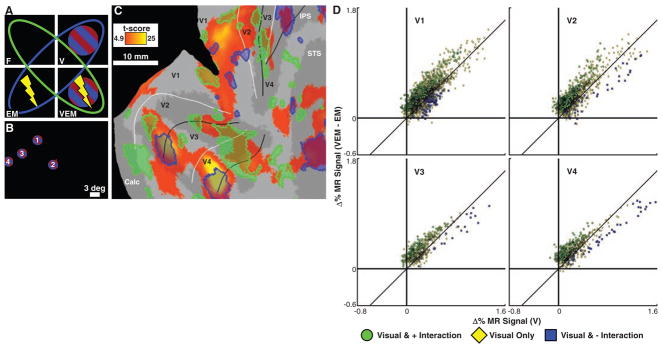
We next investigated the spatial patterns of increased or suppressed fMRI activity in each of the areas, because the absence of an overall net effect might nevertheless be associated with a pattern of balanced inhibitory and excitatory effects. Figure 3C shows the cortical regions where we observed positive and negative interactions between visual stimulation and FEF-EM in our 2 by 2 factorial design (Fig. 3A; see Fig. 3B for the stimulus positions used). Voxels shown in yellow-orange are visually driven; visually driven voxels with a positive or negative interaction are shaded green or blue, respectively (fig. S6; see fig. S7 for MM2). The spatial pattern of these interactions was heterogeneous in most visual areas: FEF-EM mostly amplified visual activity in subsets of voxels (green) adjacent to those maximally driven by the visual stimuli alone (yellow-orange), whereas it tended to have little influence or even suppressed the response (blue) in voxels strongly driven by the visual stimuli.
Fig. 3.
Interaction between FEF-EM and visually driven activity. (A) A 2 by 2 factorial design. A positive interaction, indicative of EM-induced enhancement of visually driven activity, occurs when voxels are more active in the green conditions than in the blue ones. (B) Location and sequence of visual stimuli presented (see also fig. S5A). The red dot close to stimulus 2 is the fixation point. (C) Flattened, right occipital cortex (MM1) showing voxels that are visually driven by the four gratings (yellow-orange; P < 0.05, corrected) and visually driven voxels with a positive (green) or negative (blue) interaction between visually and EM-driven activity (P < 0.05, conjunction). Sulci are dark gray (see table S1), and white and black lines indicate representations of the vertical and horizontal meridians, respectively. (D) Scatter plots (MM1 and MM2) of voxels in areas V1, V2, V3, and V4 (see also fig. S8) showing percentage change in MR signal for V epochs relative to fixation-only epochs (x axis) and the difference between VEM and EM epochs (y axis). Color code matches (C) (yellow now at P < 0.05, uncorrected). Points close to the y axis are weakly visually driven; voxels enhanced by FEF-EM (green) are mainly clustered near the y axis, whereas strongly visually driven voxels are either unaffected or suppressed (blue).
We further quantified these interactions between the visual stimuli and FEF-EM at the level of individual visually driven voxels by comparing the percentage change in MR signal in V epochs with the difference between VEM and EM epochs (Fig. 3D and fig. S8). The visual voxels boosted by FEF-EM were weakly driven by the visual stimuli alone, whereas the voxels that were strongly driven by the visual stimuli were unaffected (both subjects) or even suppressed (mostly MM1) by FEF-EM. We carried out several analyses to exclude the possibility that the modulation of visually driven activity by FEF-EM was due to differences in eye position or saccade rate (14) (table S2 and figs. S9 and S10).
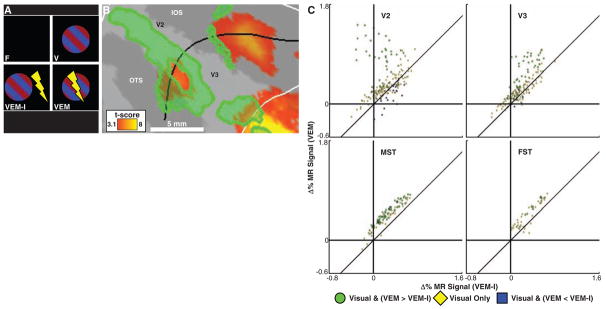
Modulatory effects from the FEF in visual cortex could be spatially nonspecific or could require the precise alignment of a visual stimulus with the FEF MF (13). Our third experiment compared the effects of incongruent epochs (VEM-I), in which EM was applied to a particular FEF MF while a visual stimulus was presented in another, nonstimulated MF (separated by 6.5 to 13.7 degrees), to congruent epochs that were identical to the VEM condition of experiment two (Fig. 4A and fig. S5B). Net EM and visual stimulation were exactly matched between congruent and incongruent epochs. In Fig. 4B, we show a portion of flattened right occipital cortex overlaid with a t-score map of visually driven fMRI activity. The spatial pattern supports that seen in experiment two—in general, voxels adjacent to those maximally driven by the visual stimulus alone showed more fMRI activity during congruent than during incongruent FEF-EM (green). An analysis at the level of individual voxels confirmed that most voxels were better activated during VEM than during VEM-I, especially in motion-sensitive areas such as MT, MST, and FST (Fig. 4C and fig. S11) (14, 21).
Fig. 4.
Retinotopic specificity of FEF-EM induced modulations. (A) The design used to test for retinotopic specificity of the interaction between EM and visual stimulation (VEM-I, incongruent visual stimulation and FEF-EM). (B) Flattened, right occipital cortex (MM1). Yellow-orange voxels show a visual-only response (P < 0.001, uncorrected); green voxels are visually driven and exhibit more activity during congruent than during incongruent visual stimulation (P < 0.05, conjunction). Sulci and white and black lines are as in Fig. 3C. (C) Responses of voxels (percentage change in MR signal, MM1) in areas V2, V3, MST, and FST (see also fig. S11) during the VEM-I (x axis) and VEM (y axis) epochs relative to the fixation epoch. Color code matches (A) (yellow now at P < 0.05, uncorrected; blue at P < 0.05, conjunction).
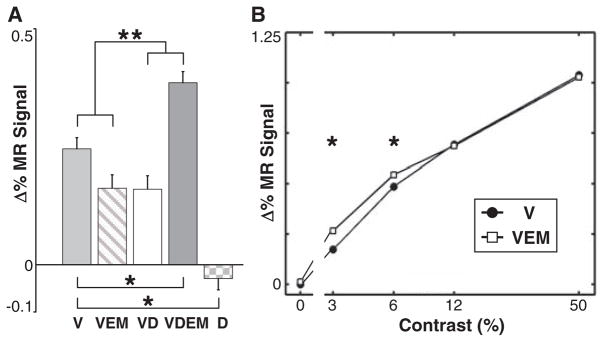
To assess more directly whether FEF-EM mainly influences the activity of nonoptimally driven voxels, our next two experiments manipulated the saliency or strength of the visual response. In experiment four, we placed one stimulus in the FEF-MF and added three distractors (D) in the opposite hemifield (fig. S12). For comparative purposes (13), we focus here on visually driven regions in V4 only. As expected, the distractors did not evoke a response in V4 representing the opposite visual field (Fig. 5A). Microstimulation of the FEF in the presence of distractors, however, produced the largest response for the stimulus in the FEF MF, in agreement with a previous study (13). The interaction between distractors and FEF-EM was significant (P < 1.4 × 10−5). In a fifth experiment, we varied the luminance contrast of a single stimulus placed in the FEF MFs and observed significant enhancement effects of FEF-EM for low-contrast stimuli only. These results are in accordance with experiment two, where we obtained the strongest modulation in voxels with a weaker visual response.
Fig. 5.
Effects of distractor stimuli and stimulus contrast. (A) Mean percentage change in MR signal relative to fixation-only in visually driven voxels in V4 (MM1; P < 0.05, uncorrected) during V, VEM, visual + distractor (VD), visual + distractor + EM (VDEM), and distractor-only (D) epochs. Two FEF MFs were stimulated (fig. S12). Error bars denote SEM. * denotes a significant difference between the pair of conditions indicated (P < 0.05, two-sample, two-tailed t test); ** indicates that the interaction between distractors and EM is significant (P < 1.4 × 10−5). (B) Mean percentage change in MR signal relative to the fixation epoch in visually driven voxels in V4 (MM1 and MM2) as a function of stimulus luminance contrast. Only one visual stimulus was used per session (fig. S5A), with the data combined across all four MF locations from each subject. The highest contrast stimulus was used as a localizer (P < 0.05, uncorrected) (14). Error bars, smaller than the plot symbols, denote SEM. * denotes a significant difference between the VEM and V conditions at a given contrast (P < 0.05, two-sample, two-tailed t test).
We have demonstrated spatially specific, causal interactions between activity in area FEF and many areas of the visual cortex. Signals from frontal cortex activated higher-order areas directly connected to the FEF irrespective of visual stimulation, mimicking attention-driven baseline shifts in activity (14, 22). In the presence of visual stimulation, modulations were observed at even the earliest levels of visual cortex, including area V1, which may account for previous findings that the effects of FEF-EM are retinotopically highly specific (23). These effects are likely trans-synaptic, because these early areas do not receive direct connections from the FEF and were only observed when the neurons were congruently activated by a visual stimulus. One interpretation is that visual stimulation opens feedback pathways closed in the absence of stimuli (6–9), allowing these frontal signals to propagate from higher to earlier visual cortical areas. The effect of FEF-EM thus resembles spatial attention, which interacts with visual stimuli in a comparable, multiplicative manner (10–12).
An unexpected finding was that the voxels most strongly enhanced by FEF-EM were adjacent to the voxels with the strongest visual response, which were themselves unaffected or even suppressed by FEF-EM. These results support a recent model for the effects of feedback connections proposing that feedback and horizontal connections mediate a contrast-dependent inhibition of a central zone in the next lower area while exciting the near surround (24). Strong effects of FEF-EM in weakly driven voxels also dovetails with previous findings that attentional effects and the effects of FEF microstimulation on neuronal activity in area V4 are most pronounced in the presence of competitive distractors that reduce the strength of neuronal responses (13, 25). As another means to reduce the activity of V4 neurons, we lowered the luminance contrast of the visual stimulus. We observed that FEF-EM evoked stronger effects in low-contrast rather than high-contrast stimuli. This result is reminiscent of a nonproportional scaling of the contrast response function in area V4, with stronger positive modulations for low-contrast stimuli, as seen in previous spatial attention studies [(26), but see (27)]. All these results taken together make it tempting to speculate that FEF-EM engages similar neuronal circuits as spatial attention. Speculation aside, the present results clearly strengthen past observations that structures involved in generating eye movements (1, 13, 28–30) are well suited to modulate sensory-driven activity in a topographically specific manner.
Supplementary Material
Acknowledgments
We thank H. Deng for animal training and care; M. Khachaturian, H. Kolster, J. Mandeville, G. Madan, T. van Kerkoerle, and L. Wald for technical assistance; R. Tootell and B. Rosen for advice and support; and R. Buckner, G. Orban, and J. Sharma for valuable comments. This work received support from a Natural Sciences and Engineering Research Council of Canada Postgraduate Scholarship, Human Frontier Science Program Organization, Geneeskundige Stichting Koningin Elisabeth, Inter University Attraction Pole 5/04, Excellence Financing/05/014, Geconcerteerde Onderzoeks Actie 2005/18, Fonds Wetenschappelijk Onderzoek–Vlaanderen G.0.622.08, European Union grant FP7/2007-2013 # F2-2008-200728, R01-EB000790. The Martinos Center is supported by National Center for Research Resources grant P41RR14075 and the Mind Research Network Institute.
Footnotes
References and Notes
- 1.Rizzolatti G, Riggio L, Dascola I, Umilta C. Neuropsychologia. 1987;25:31. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- 2.Kastner S, Ungerleider LG. Annu Rev Neurosci. 2000;23:315. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 3.Corbetta M, Shulman GL. Nat Rev Neurosci. 2002;3:201. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 4.Moore T, Armstrong KM, Fallah M. Neuron. 2003;40:671. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 5.Hamker FH. Cereb Cortex. 2005;15:431. doi: 10.1093/cercor/bhh146. [DOI] [PubMed] [Google Scholar]
- 6.Fukushima K. IEEE Computer. 1988;21:65. [Google Scholar]
- 7.Grossberg S. Spat Vis. 1999;12:163. doi: 10.1163/156856899x00102. [DOI] [PubMed] [Google Scholar]
- 8.van der Velde F, de Kamps M. J Cogn Neurosci. 2001;13:479. doi: 10.1162/08989290152001907. [DOI] [PubMed] [Google Scholar]
- 9.Roelfsema PR. Annu Rev Neurosci. 2006;29:203. doi: 10.1146/annurev.neuro.29.051605.112939. [DOI] [PubMed] [Google Scholar]
- 10.Treue S, Martinez Trujillo JC. Nature. 1999;399:575. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- 11.McAdams CJ, Maunsell JH. J Neurosci. 1999;19:431. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Trujillo JC, Treue S. Curr Biol. 2004;14:744. doi: 10.1016/j.cub.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Moore T, Armstrong KM. Nature. 2003;421:370. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- 14.Materials and methods are available as supporting material on Science Online.
- 15.Tolias AS, et al. Neuron. 2005;48:901. doi: 10.1016/j.neuron.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 16.Huerta MF, Krubitzer LA, Kaas JH. J Comp Neurol. 1986;253:415. doi: 10.1002/cne.902530402. [DOI] [PubMed] [Google Scholar]
- 17.Huerta MF, Krubitzer LA, Kaas JH. J Comp Neurol. 1987;265:332. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- 18.Schall JD, Morel A, King DJ, Bullier J. J Neurosci. 1995;15:4464. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanton GB, Goldberg ME, Bruce CJ. J Comp Neurol. 1988;271:473. doi: 10.1002/cne.902710402. [DOI] [PubMed] [Google Scholar]
- 20.Stanton GB, Bruce CJ, Goldberg ME. J Comp Neurol. 1995;353:291. doi: 10.1002/cne.903530210. [DOI] [PubMed] [Google Scholar]
- 21.Vanduffel W, et al. Neuron. 2001;32:565. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 22.Colby CL, Duhamel JR, Goldberg ME. J Neurophysiol. 1996;76:2841. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong KM, Fitzgerald JK, Moore T. Neuron. 2006;50:791. doi: 10.1016/j.neuron.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Schwabe L, Obermayer K, Angelucci A, Bressloff PC. J Neurosci. 2006;26:9117. doi: 10.1523/JNEUROSCI.1253-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds JH, Chelazzi L, Desimone R. J Neurosci. 1999;19:1736. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds JH, Pasternak T, Desimone R. Neuron. 2000;26:703. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- 27.Williford T, Maunsell JH. J Neurophysiol. 2006;96:40. doi: 10.1152/jn.01207.2005. [DOI] [PubMed] [Google Scholar]
- 28.Corbetta M, et al. Neuron. 1998;21:761. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 29.Cavanaugh J, Wurtz RH. J Neurosci. 2004;24:11236. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruff CC, et al. Curr Biol. 2006;16:1479. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.