Abstract
BACKGROUND
Long-acting beta-agonist (LABA) therapy improves symptoms in patients whose asthma is poorly controlled by an inhaled glucocorticoid alone. Alternative treatments for adults with uncontrolled asthma are needed.
METHODS
In a three-way, double-blind, triple-dummy crossover trial involving 210 patients with asthma, we evaluated the addition of tiotropium bromide (a long-acting anticholinergic agent approved for the treatment of chronic obstructive pulmonary disease but not asthma) to an inhaled glucocorticoid, as compared with a doubling of the dose of the inhaled glucocorticoid (primary superiority comparison) or the addition of the LABA salmeterol (secondary noninferiority comparison).
RESULTS
The use of tiotropium resulted in a superior primary outcome, as compared with a doubling of the dose of an inhaled glucocorticoid, as assessed by measuring the morning peak expiratory flow (PEF), with a mean difference of 25.8 liters per minute (P<0.001) and superiority in most secondary outcomes, including evening PEF, with a difference of 35.3 liters per minute (P<0.001); the proportion of asthma-control days, with a difference of 0.079 (P = 0.01); the forced expiratory volume in 1 second (FEV1) before bronchodilation, with a difference of 0.10 liters (P = 0.004); and daily symptom scores, with a difference of −0.11 points (P<0.001). The addition of tiotropium was also noninferior to the addition of salmeterol for all assessed outcomes and increased the prebronchodilator FEV1 more than did salmeterol, with a difference of 0.11 liters (P = 0.003).
CONCLUSIONS
When added to an inhaled glucocorticoid, tiotropium improved symptoms and lung function in patients with inadequately controlled asthma. Its effects appeared to be equivalent to those with the addition of salmeterol. (Funded by the National Heart, Lung, and Blood Institute; ClinicalTrials.gov number, NCT00565266.)
Many adults with asthma have inadequate control of symptoms when receiving a low-to-medium dose of an inhaled glucocorticoid.1,2 Treatment options include the addition of a leukotriene modifier,2 the addition of a long-acting beta-agonist (LABA),2-4 or an increased dose of an inhaled glucocorticoid.2 Current guidelines of the National Asthma Education and Prevention Program favor the last two options.2 In recent communications, however, the Food and Drug Administration (FDA)5 and asthma experts6,7 have questioned the safety of LABA therapy and suggested strategies to minimize the use of these drugs. Because of such concerns and the heterogeneity of patients with asthma, alternative controller agents are needed.
Whether anticholinergic agents are useful for asthma management is not clear. A Cochrane Review reported that there is no justification for routinely introducing anticholinergic agents (the report focused on ipratropium bromide), while acknowledging that the role of long-acting anticholinergic agents such as tiotropium bromide has not been established.8 Tiotropium has a duration of action of more than 24 hours9,10 and was approved by the FDA for the treatment of chronic obstructive pulmonary disease (COPD) in January 2004. However, tiotropium has not been approved for the treatment of asthma.
In a double-blind, three-way, crossover trial, called the Tiotropium Bromide as an Alternative to Increased Inhaled Glucocorticoid in Patients Inadequately Controlled on a Lower Dose of Inhaled Corticosteroid (TALC) study, we tested two hypotheses. The primary hypothesis stated that in patients with asthma that is inadequately controlled by an inhaled glucocorticoid alone, the addition of tiotropium bromide would be superior to a doubling of the dose of an inhaled glucocorticoid. The secondary hypothesis stated that in such patients, the addition of tiotropium would not be inferior to the addition of a LABA. We evaluated the primary outcome, the morning peak expiratory flow (PEF), as well as additional outcomes, in 210 patients with asthma inadequately controlled by a low dose of an inhaled glucocorticoid.
METHODS
STUDY PATIENTS
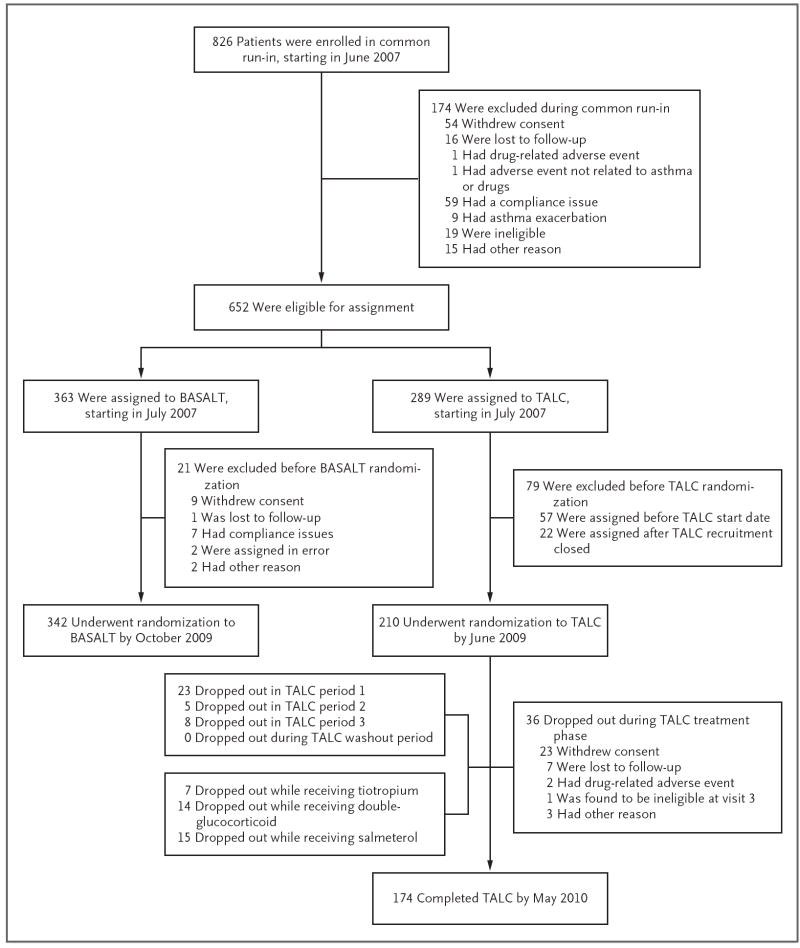
Beginning in June 2007, we enrolled 826 patients in a common run-in period for two asthma studies. One of the studies, called the Best Adjustment Strategy for Asthma over Long Term (BASALT) trial (ClinicalTrials.gov number, NCT00495157), involved patients with mild-to-moderate disease, and the results are not reported here. A total of 342 patients underwent randomization in the BASALT study, 210 patients underwent randomization in the TALC study (with the last patients completing the study on May 21, 2010), and 274 patients were excluded from both studies (Fig. 1). The inclusion criteria for enrollment in the common run-in period for both studies included an age of at least 18 years, a history of asthma confirmed by bronchodilator reversibility or bronchial hyperresponsiveness, a forced expiratory volume in 1 second (FEV1) of more than 40% of the predicted value, and nonsmoking status (<10 pack-years). Exclusion criteria are listed in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Figure 1. Enrollment and Outcomes.
The TALC and BASALT studies were companion trials that used a common run-in period: patients with better-controlled asthma were assigned to the BASALT trial, and those with poorer control were assigned to the TALC trial. Shown are the numbers of patients who enrolled in the common run-in period, those who underwent randomization to each study, and those who completed the TALC study. At the start of the recruitment period, TALC study drugs were not yet available, which accounted for the 57 patients who were assigned before the TALC start date, and randomization of patients to the TALC trial ended before all patients were assigned to the BASALT trial, which accounted for the 22 patients who were assigned after TALC recruitment closed.
The study was approved by the committee on human research at each institution. All patients provided written informed consent.
STUDY PROTOCOL
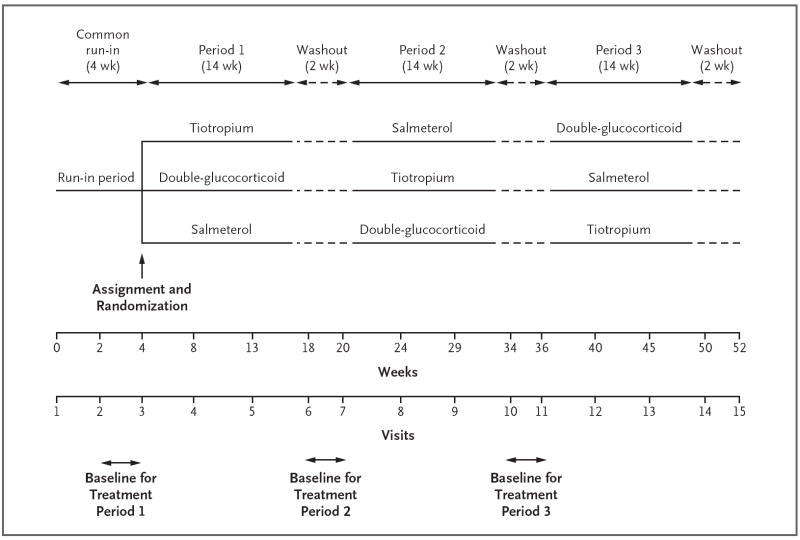
At the onset of the 4-week run-in period, all patients were treated with a hydrofluoroalkane metered-dose inhaler of beclomethasone (Qvar) at a dose of 80 μg (2 puffs of 40 μg) twice daily (Fig. 2, and Fig. S1 in the Supplementary Appendix). All other asthma medications were stopped. Patients were eligible for assignment to either the TALC study or the BASALT study if at week 4 they had at least 75% adherence to the run-in protocol (as shown by peak flow, diary card, and study medications), an FEV1 of more than 40% of the predicted value, and no need for additional asthma medications. Patients were assigned to the TALC study if at week 4 they had no medical contraindication to tiotropium and the FEV1 was 70% or less of the predicted value or if during the final 2 weeks of the run-in period they had symptoms 6 or more days per week or used a rescue inhaler 6 or more days per week or were awakened by symptoms of asthma two nights or more per week.
Figure 2. Outline of Study Protocol.
Shown are the durations of the common run-in, treatment, and washout periods, along with periods in which baseline data for variables that were collected daily were obtained before each treatment period. During the 4-week run-in period and the 2-week washout periods, all patients received beclomethasone at a dose of 80 μg (2 puffs of 40 μg) twice daily. Only three of the six possible treatment sequences are presented graphically.
Weeks 3 and 4 of the run-in period provided baseline data for the first treatment period and inflammatory biomarkers. Patients were treated for a 14-week period with the run-in dose of beclomethasone plus inhaled tiotropium bromide (Spiriva HandiHaler) at a dose of 18 μg every morning plus a salmeterol placebo inhaler; 160 μg (2 puffs of 80 μg) twice daily of beclomethasone (i.e., a doubling of the run-in dose) plus a tiotropium placebo inhaler and salmeterol placebo inhaler; or the run-in dose of beclomethasone plus salmeterol xinafoate (Serevent Diskus) at a dose of 50 μg twice daily plus a tiotropium placebo inhaler. Between each treatment, there was a 2-week washout period during which patients received only the run-in dose of beclomethasone to establish baseline data for the next period. The study was conducted in accordance with the protocol, which is available at NEJM.org.
OUTCOME MEASURES
The predetermined primary outcome measure was the morning PEF. Predetermined secondary outcome measures included the FEV1 before bronchodilation, the number of asthma-control days (defined as days without symptoms and without the use of a rescue bronchodilator), asthma symptoms, rescue-bronchodilator use, asthma exacerbations (defined as increased asthma symptoms resulting in the use of oral glucocorticoids or the increased use of inhaled glucocorticoids or other asthma medications), use of health care services, biomarkers of airway inflammation, and results of validated questionnaires, including the Asthma Control Questionnaire,11,12 the Asthma Symptom Utility Index,13 and the Asthma Quality-of-Life Questionnaire.14 (For all questionnaires, the ranges, interpretations, and minimal clinically significant differences are presented in Table 1 and in the Supplementary Appendix.) Additional prespecified exploratory hypotheses are listed in the Supplementary Appendix.15 Also prespecified was an analysis of patients’ responses to the various study drugs, singly and in combination (a responder analysis).16,17
Table 1.
Baseline Characteristics of the 210 Patients.*
| Characteristic | Value |
|---|---|
| Male sex — no. (%) | 69 (32.9) |
| One or more positive skin tests for atopy — no./total no. (%) | 175/200 (87.5) |
| Age at visit 1 — yr | 42.2±12.3 |
| Duration of asthma — yr | 26.1±14.1 |
| Weight at visit 1 — kg | 88.3±25.3 |
| Body-mass index at visit 1† | 31.4±8.8 |
| FEV1 | |
| Value at visit 3 before bronchodilation — liters | 2.31±0.77 |
| Percent of predicted value at visit 3 before bronchodilation | 71.5±14.9 |
| Percent reversal of obstruction with albuterol (4 puffs) at visit 3 | 14.9±9.8 |
| Value after albuterol (4 puffs) at visit 3 — liters | 2.64±0.82 |
| Percent reversal of obstruction with ipratropium (4 puffs) at visit 2 | 12.4±9.5 |
| Value after ipratropium (4 puffs) at visit 2 — liters | 2.62±0.80 |
| PEF before visit 3 (2-wk mean) — liters/min | |
| Morning | 377.2±117.0 |
| Evening | 383.6±119.0 |
| Daily-symptom score before visit 3 (2-wk mean)‡ | 0.46±0.44 |
| Albuterol rescue use before visit 3 (2-wk mean) — puffs/day | 1.71±2.09 |
| Asthma-control days before visit 3 (2-week mean) | |
| Proportion of days | 0.212±0.331 |
| No. of days | 2.97±4.64 |
| Asthma Control Questionnaire score at visit 3§ | 1.64±0.73 |
| Asthma Quality-of-Life Questionnaire score at visit 3¶ | 5.43±1.05 |
| Asthma Symptom Utility Index score at visit 3 ∥ | 0.78±0.15 |
| Geometric mean exhaled nitric oxide at visit 3 — ppb (coefficient of variation) | 18.8 (0.7) |
Plus–minus values are means ±SD. FEV1 denotes forced expiratory volume in 1 second, and PEF peak expiratory flow.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Daily symptoms were evaluated on a scale from 0 to 3, with a higher score indicating a greater severity of symptoms.
Scores on the Asthma Control Questionnaire range from 0 to 6, with a higher score indicating worse asthma control; the minimal clinically important difference (MID) is 0.5.
Scores on the Asthma Quality-of-Life Questionnaire range from 1 to 7, with a higher score indicating a better quality of life; the MID is 0.5.
Scores on the Asthma Symptom Utility Index range from 0 to 1, with a higher score indicating better asthma control; the MID is unknown, but a difference of 0.3 is suggested to distinguish between mild-to-moderate and moderate-to-severe asthma.
STUDY OVERSIGHT
The study was funded by the National Heart, Lung, and Blood Institute. The protocol was approved by the protocol review committee of the institute’s Asthma Clinical Research Network and monitored by the network’s data and safety monitoring board. Tiotropium was used under the provisions of an approved application for an investigational new drug, submitted by the network’s data coordinating center. Beclomethasone canisters containing either 40 μg or 80 μg and rescue albuterol (Pro-Air) were supplied by Teva Specialty Pharmaceuticals. Tiotropium and matching placebo were supplied by Boehringer Ingelheim Pharmaceuticals, which had the opportunity to comment on the study design. This input resulted in an increase in the sample size to include more patients with the Arg/Arg polymorphism in the gene encoding the β2-adrenergic receptor. The company had no role in the performance of the trial, the analysis or interpretation of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication. Salmeterol and matching placebo were purchased from third-party vendors. Medication use was measured by means of an electronic device (for beclomethasone), a counter for dry-powder inhalers (for salmeterol), and assessment of used blister packs (for tiotropium), with mean (±SD) rates of compliance of 84.1±16.2%, 92.6±12.3%, and 93.0±12.2%, respectively.
The informed-consent document was amended in April 2008 to acknowledge the FDA’s Med-Watch alert regarding the association between tiotropium and the risk of stroke. No patient withdrew because of this modification.
STATISTICAL ANALYSIS
The primary hypothesis was that the addition of tiotropium to an inhaled glucocorticoid would be superior to a doubling of the dose of the inhaled glucocorticoid with respect to the morning PEF. The secondary hypothesis was that the addition of tiotropium to an inhaled glucocorticoid would not be inferior to the addition of salmeterol with respect to the morning PEF, the prebronchodilator FEV1, and the proportion of asthma-control days. All analyses were performed according to the intention-to-treat principle.
The original target sample size of 224 patients was reduced in May 2009 to 210 patients, which provided a power of 90% for detecting a between-treatment difference of 10.6 liters per minute in the morning PEF on the basis of a one-sided significance level of 0.025, allowing for a dropout rate of 10%. This effect size was chosen because of the comparison between the addition of tiotropium to an inhaled glucocorticoid and the doubling of the dose of an inhaled glucocorticoid, an active control. The study also had a power of 90% to detect a between-treatment difference in the proportion of asthma-control days of 0.07 and a power of 93% to detect a between-treatment difference in the prebronchodilator FEV1 of 0.09 liters.
Descriptive statistics were counts and percentages for categorical variables, means and standard deviations for normally distributed variables, geometric means and coefficients of variation for normally distributed log-transformed variables, and medians and first and third quartiles for variables that were not normally distributed on the original or log-transformed scales.
A linear mixed-effects model was applied to crossover data for each continuous outcome variable.18-20 Fixed-effects terms included clinical center (stratifying variable), treatment regimen, treatment sequence, treatment period, and homogeneous carryover effects. Evaluation of clinical outcomes was performed at weeks 0, 4, 9, and 14 during each 14-week treatment period. Outcomes that were recorded on daily diary cards were averaged between visits, so that the week 0 measurement represented the mean during the last 2 weeks of the run-in period or the 2 weeks of the washout period between treatments, the week 4 measurement represented the mean between week 0 and week 4, and so forth. Restricted maximum-likelihood estimates were determined for the treatment effects (the model-based change between week 0 and week 14) with the use of PROC MIXED of the SAS/STAT statistical-analysis software, version 9.2 (SAS Institute). The null hypothesis of inferiority for the secondary hypothesis was rejected in favor of noninferiority if the upper 97.5% confidence limit for the difference between salmeterol treatment and tiotropium treatment was less than the prespecified bound (10.6 liters per minute for the morning PEF, 0.07 for the proportion of asthma-control days, and 0.09 liters for the prebronchodilator FEV1).
The statistical analysis plans included an exploratory analysis to identify patients, among those who completed the trial, with certain prespecified responses with respect to the morning PEF, prebronchodilator FEV1, and asthma-control days.16,17 We defined a lung-function response as a relative increase in the morning PEF or FEV1 of at least 7.5% and an asthma-control-day response as a proportional increase of at least 0.10. Data regarding the morning PEF and asthma-control days were collected daily; therefore, 2-week averages before baseline and at the end of the treatment period were used to characterize the response. In addition, using information from Lemanske and colleagues,21 we defined a three-dimensional response as a positive response with respect to both lung function (either morning PEF or FEV1) and the number of asthma-control days, with no exacerbations of asthma. We defined a two-dimensional response as a positive response with respect to either lung function or the number of asthma-control days, with no asthma exacerbations. These definitions were not prespecified but were established before the data were examined.
RESULTS
STUDY PATIENTS
Of the 210 study patients, 141 (67.1%) were women; 59 (28.1%) were black, and 24 (11.4%) were Hispanic (Table 1, and Table S1 in the Supplementary Appendix). The mean baseline FEV1 before bronchodilation was 2.31±0.77 liters (71.5±14.9% of the predicted value), and the mean score on the Asthma Control Questionnaire was 1.64±0.73. The mean percentages of reversibility of airway obstruction after four puffs of albuterol and ipratropium bromide were 14.9±9.8% and 12.4±9.5%, respectively. The mean morning PEF was 377.2±117.0 liters per minute, and the proportion of asthma-control days was 0.21±0.33 (2.97±4.64 days) during the 2 weeks before randomization. Baseline values before each of the three active treatment periods were similar for the morning PEF (377.2±117.0, 383.9±117.6, and 383.0±115.0 liters per minute, respectively) and FEV1 (2.31±0.77, 2.36±0.77, and 2.36±0.75 liters, respectively), whereas the proportion of asthma-control days increased from 0.21±0.33 before treatment period 1 to 0.34±0.40 and 0.34±0.41 before treatment periods 2 and 3, respectively. Although minimal carryover effects between periods were observed for measures of lung function, an effect was seen for asthma-control days (Table S2 in the Supplementary Appendix). Markers of inflammation at randomization (exhaled nitric oxide and sputum eosinophils) were low at baseline and thereafter.
PRIMARY OUTCOME
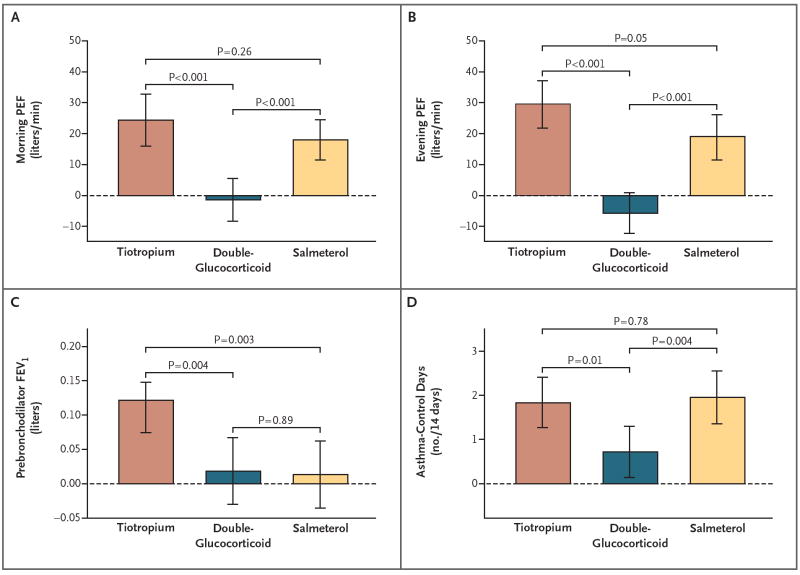
Patients receiving tiotropium had a morning PEF that was 25.8 liters per minute higher than that of patients receiving a double dose of glucocorticoid (95% confidence interval [CI], 14.4 to 37.1; P<0.001) (Table 2, and Table S3 in the Supplementary Appendix). Similar results favoring tiotropium over a double glucocorticoid dose were obtained for the evening PEF, with a difference of 35.3 liters per minute (95% CI, 24.6 to 46.0; P<0.001); the prebronchodilator FEV1, with a difference of 0.10 liters (95% CI, 0.03 to 0.17; P = 0.004); the proportion of asthma-control days, with a difference of 0.079 (95% CI, 0.019 to 0.140; P = 0.01); score for daily symptoms, with a difference of −0.11 points (95% CI, −0.16 to −0.06; P<0.001); the score on the Asthma Control Questionnaire, with a difference of −0.18 points (95% CI, −0.34 to −0.03; P = 0.02); and the FEV1 after four puffs of albuterol, with a difference of 0.04 liters (95% CI, 0.01 to 0.08; P = 0.01).
Table 2.
Outcome Variables.*
| Variable | Mean Change from Baseline | Mean Difference in Change from Baseline | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tiotropium | P Value | Double-Glucocorticoid | P Value | Salmeterol | P Value | Tiotropium vs. Double-Glucocorticoid | P Value | Tiotropium vs. Salmeterol | P Value | Salmeterol vs. Double-Glucocorticoid | P Value | |
| Morning PEF — liters/min | 24.4 (16.0 to 32.7) | <0.001 | −1.4 (−8.4 to 5.6) | 0.69 | 18.0 (11.5 to 24.5) | <0.001 | 25.8 (14.4 to 37.1) | <0.001 | 6.4 (−4.8 to 17.5) | 0.26 | 19.4 (9.4 to 29.4) | <0.001 |
| Evening PEF — liters/min | 29.6 (21.9 to 37.3) | <0.001 | −5.7 (−12.3 to 0.9) | 0.09 | 19.0 (11.7 to 26.3) | <0.001 | 35.3 (24.6 to 46.0) | <0.001 | 10.6 (−0.1 to 21.3) | 0.05 | 24.7 (15.2 to 34.3) | <0.001 |
| Albuterol rescue use — puffs/day | −0.11 (−0.26 to 0.03) | 0.12 | −0.07 (−0.19 to 0.06) | 0.30 | −0.16 (−0.28 to −0.03) | 0.01 | −0.05 (−0.24 to 0.14) | 0.63 | 0.04 (−0.13 to 0.22) | 0.63 | −0.09 (−0.27 to 0.09) | 0.33 |
| Mean daily-symptom score | −0.09 (−0.12 to −0.05) | <0.001 | 0.03 (−0.01 to 0.06) | 0.11 | −0.04 (−0.08 to −0.01) | 0.02 | −0.11 (−0.16 to −0.06) | <0.001 | −0.04 (−0.09 to 0.01) | 0.10 | −0.07 (−0.12 to −0.02) | 0.005 |
| Proportion of asthma-control days | 0.131 (0.090 to 0.171) | <0.001 | 0.051 (0.010 to 0.093) | 0.02 | 0.139 (0.096 to 0.183) | <0.001 | 0.079 (0.019 to 0.140) | 0.01 | −0.009 (−0.070 to 0.053) | 0.78 | 0.088 (0.028 to 0.148) | 0.004 |
| Prebronchodilator FEV1 — liters | 0.12 (0.07 to 0.17) | <0.001 | 0.02 (−0.03 to 0.07) | 0.47 | 0.01 (−0.04 to 0.06) | 0.60 | 0.10 (0.03 to 0.17) | 0.004 | 0.11 (0.04 to 0.18) | 0.003 | 0.00 (−0.08 to 0.07) | 0.89 |
| Asthma Symptom Utility Index score | 0.03 (0.01 to 0.05) | 0.004 | 0.00 (−0.02 to 0.02) | 0.77 | 0.04 (0.03 to 0.06) | <0.001 | 0.03 (0.00 to 0.06) | 0.09 | −0.01 (−0.04 to 0.02) | 0.38 | 0.04 (0.01 to 0.07) | 0.005 |
| Asthma Control Questionnaire score | −0.22 (−0.33 to −0.11) | <0.001 | −0.03 (−0.13 to 0.06) | 0.49 | −0.31 (−0.40 to −0.22) | <0.001 | −0.18 (−0.34 to −0.03) | 0.02 | 0.09 (−0.04 to 0.23) | 0.18 | −0.28 (−0.41 to −0.15) | <0.001 |
| Asthma Quality-of-Life Questionnaire score | 0.15 (0.03 to 0.26) | 0.01 | 0.05 (−0.06 to 0.15) | 0.38 | 0.28 (0.18 to 0.38) | <0.001 | 0.10 (−0.07 to 0.27) | 0.24 | −0.13 (−0.28 to 0.02) | 0.09 | 0.23 (0.09 to 0.37) | 0.002 |
| FEV1 after 4 puffs of albuterol — liters | 0.02 (−0.01 to 0.05) | 0.16 | −0.02 (−0.05 to 0.01) | 0.11 | −0.05 (−0.08 to −0.03) | <0.001 | 0.04 (0.01 to 0.08) | 0.01 | 0.07 (0.05 to 0.10) | <0.001 | −0.03 (−0.06 to 0.00) | 0.06 |
Values in parentheses are 95% confidence intervals. Restricted maximum-likelihood estimates were determined for the treatment effects (the model-based change between the beginning and the end of each of the three treatment periods). Patients received tiotropium or salmeterol in addition to a low dose of beclomethasone or received a double dose of beclomethasone. The primary comparison was between tiotropium and double-glucocorticoid. The secondary comparison was between tiotropium and salmeterol. The comparison between salmeterol and double-glucocorticoid was performed to determine whether the patients in the TALC study were similar to those in previous trials comparing long-acting beta-agonists with inhaled glucocorticoids. FEV1 denotes forced expiratory volume in 1 second, and PEF peak expiratory flow.
SECONDARY OUTCOMES
There were no significant differences between tiotropium treatment and salmeterol treatment with respect to the morning PEF, which was 6.4 liters per minute higher among patients receiving tiotropium (95% CI, −4.8 to 17.5; P = 0.26); the evening PEF, with a difference of 10.6 liters per minute (95% CI, −0.1 to 21.3; P = 0.05); the proportion of asthma control days, with a difference of −0.009 (95% CI, −0.070 to 0.053; P = 0.78); the score for daily symptoms, with a difference of −0.04 points (95% CI, −0.09 to 0.01; P = 0.10); the score on the Asthma Control Questionnaire, with a difference of 0.09 (95% CI, −0.04 to 0.23; P = 0.18); and a difference in the proportion of sputum eosinophils of 0.20% (95% CI, −0.36 to 0.76; P = 0.49) (Table S3 in the Supplementary Appendix). The null hypothesis of inferiority was rejected in favor of the alternative hypothesis of noninferiority at the 0.025 significance level for the morning PEF, the prebronchodilator FEV1, and the proportion of asthma-control days. The prebronchodilator FEV1 favored tiotropium, with an increase of 0.11 liters (95% CI, 0.04 to 0.18; P = 0.003), as did the FEV1 after four puffs of albuterol, with an increase of 0.07 liters (95% CI, 0.05 to 0.10; P<0.001).
COMPARISON OF SALMETEROL AND DOUBLE-DOSE GLUCOCORTICOID
This comparison was performed to determine whether the patients in the TALC study were similar to those in previous trials comparing LABA with an inhaled glucocorticoid.3,4 Salmeterol was superior to the double dose of beclomethasone with respect to the morning PEF, with a between-group difference of 19.4 liters per minute (95% CI, 9.4 to 29.4; P<0.001); the evening PEF, with a difference of 24.7 liters per minute (95% CI, 15.2 to 34.3; P<0.001); the proportion of asthma-control days, with a difference of 0.088 (95% CI, 0.028 to 0.148; P = 0.004); the daily-symptom score, with a difference of −0.07 units (95% CI, −0.12 to −0.02; P = 0.005); the score on the Asthma Control Questionnaire, with a difference of −0.28 (95% CI, −0.41 to −0.15; P<0.001); the score on the Asthma Symptom Utility Index, with a difference of 0.04 units (95% CI, 0.01 to 0.07; P = 0.005); and the score on the Asthma Quality-of-Life Questionnaire, with a difference of 0.23 units (95% CI, 0.09 to 0.37; P = 0.002). A summary of changes in the morning and evening PEF, the prebronchodilator FEV1, and the proportion of asthma-control days (per 2-week period) according to treatment period is shown in Figure 3.
Figure 3. Primary and Secondary Outcomes.
Shown are the mean differences among patients receiving tiotropium, those receiving double-glucocorticoid, and those receiving salmeterol with respect to the morning peak expiratory flow (PEF) (Panel A), the evening PEF (Panel B), the prebronchodilator forced expiratory volume in 1 second (FEV1) (Panel C), and the proportion of asthma-control days per 14-day period (Panel D). The I bars indicate 95% confidence intervals.
EXPLORATORY RESPONSE ANALYSES
The proportions of patients with a two-dimensional response or a three-dimensional response to the various treatments are shown in Table 3. A total of 31.3% of patients had a two-dimensional response to all three treatment regimens, whereas 9.4% had no such response to any of them. A small proportion of patients (5.6 to 8.8%) had a response to only one treatment. Two-dimensional responses occurred in 66.3% of patients receiving tiotropium, 53.1% of those receiving double-glucocorticoid, and 70.6% of those receiving salmeterol.
Table 3.
Response to Treatment.*
| Variable | No Response | Tiotropium Only | Double-Glucocorticoid Only | Salmeterol Only | Tiotropium plus Double-Glucocorticoid | Tiotropium plus Salmeterol | Salmeterol plus Double-Glucocorticoid | Tiotropium plus Salmeterol plus Double-Glucocorticoid | All Patients |
|---|---|---|---|---|---|---|---|---|---|
| number of patients (percent) | |||||||||
| FEV1 | 73 (44.0) | 32 (19.3) | 12 (7.2) | 17 (10.2) | 11 (6.6) | 14 (8.4) | 6 (3.6) | 1 (0.6) | 166 (100.0) |
| Morning PEF | 57 (33.9) | 30 (17.9) | 10 (6.0) | 28 (16.7) | 4 (2.4) | 32 (19.0) | 5 (3.0) | 2 (1.2) | 168 (100.0) |
| Asthma-control days† | 63 (38.0) | 9 (5.4) | 8 (4.8) | 17 (10.2) | 12 (7.2) | 19 (11.4) | 9 (5.4) | 29 (17.5) | 166 (100.0) |
| Two-dimensional response‡ | 15 (9.4) | 14 (8.8) | 9 (5.6) | 13 (8.1) | 9 (5.6) | 33 (20.6) | 17 (10.6) | 50 (31.3) | 160 (100.0) |
| Three-dimensional response§ | 93 (58.1) | 21 (13.1) | 9 (5.6) | 19 (11.9) | 3 (1.9) | 13 (8.1) | 1 (0.6) | 1 (0.6) | 160 (100.0) |
Patients received tiotropium or salmeterol in addition to a low dose of beclomethasone or received a double dose of beclomethasone. FEV1 denotes forced expiratory volume in 1 second, and PEF peak expiratory flow.
An asthma-control-day response was defined as a proportional increase of at least 0.10.
A two-dimensional response was defined as a positive response in lung function or in the number of asthma-control days, with no asthma exacerbations.
A three-dimensional response was defined as a positive response in both lung function (either morning PEF or FEV1) and the number of asthma-control days, with no asthma exacerbations.
ASTHMA EXACERBATIONS AND ADVERSE EVENTS
An asthma exacerbation occurred in 9 patients receiving tiotropium, 16 receiving double-glucocorticoid, and 5 receiving salmeterol; the respective numbers of patients with asthma exacerbations for which oral or intravenous glucocorticoids were administered were 7, 13, and 5. Patients receiving the double dose of beclomethasone had the highest numbers of unscheduled visits for asthma symptoms (2 for tiotropium, 6 for double-glucocorticoid, and 2 for salmeterol), emergency room visits (2, 4, and 1, respectively), and events for which urgent care was needed (4, 9, and 3, respectively). Two hospitalizations for asthma occurred, 1 among patients receiving tiotropium and 1 among those receiving double-glucocorticoid. Reasons for withdrawal from the trial (7 for tiotropium, 14 for double-glucocorticoid, and 15 for salmeterol) are provided in Table S4 in the Supplementary Appendix.
A total of 12 serious adverse events involving hospitalization or an emergency room visit occurred: 3 among patients receiving tiotropium (2 hospitalizations for pneumonia and 1 for a fractured radius), 4 among those receiving double-glucocorticoid (1 hospitalization for spinal stenosis surgery, 1 for atypical chest pain, 1 for transient global amnesia, and 1 for pneumonia), 4 among those receiving salmeterol (1 hospitalization and subsequent death from sepsis after hysterectomy for endometrial carcinoma, 1 hospitalization for hysterectomy to remove fibroids, 1 hospitalization for knee-replacement surgery, and 1 emergency room visit for stridor after ingestion of orange juice), and 1 during the single-dose-glucocorticoid run-out period (hospitalization for tonsillitis).
DISCUSSION
We report two findings with implications for the treatment of asthma in adults. First, our study shows that the use of tiotropium was superior to a doubling of the dose of an inhaled glucocorticoid for patients whose symptoms were inadequately controlled while they were receiving inhaled beclomethasone alone at a dose of 80 μg twice a day. Second, among patients in our study who were similar to those in trials showing the clinical efficacy of LABA therapy,3,4 tiotropium was noninferior to salmeterol on the basis of predefined criteria, a finding that meets the standards established in the FDA’s draft guidance for industry on noninferiority clinical trials.22
Our selection of the morning PEF as the primary outcome might attract criticism, even though the trial was adequately powered and analyzed for another key patient-centric outcome, the proportion of asthma-control days. Our rationale was that pulmonary function remains an important element of asthma control, improvements in the PEF were similar to those in previous Asthma Clinical Research Network trials comparing an active treatment with placebo,23,24 and improvements in pulmonary function that were induced by tiotropium were accompanied by improvements in both asthma symptoms and the proportion of asthma-control days.
We did not evaluate whether increasing the dose of an inhaled glucocorticoid by more than a factor of two would provide an increased benefit. Although an increase in the dose of an inhaled glucocorticoid by a factor of four has been reported to reduce asthma exacerbations,25 low doses of an inhaled glucocorticoid have been reported to provide a benefit equivalent to that of a high dose with respect to measures of asthma control,26 the outcomes that we studied. In addition, combinations of inhaled glucocorticoids and LABA therapy have been reported to provide superior asthma control, as compared with an increased dose of an inhaled glucocorticoid, even when the dose was more than doubled.27
Although the effects of tiotropium and salmeterol were similar in general, measures of the prebronchodilator FEV1 favored tiotropium. The small decrease in FEV1 after four puffs of albuterol among patients receiving salmeterol (0.05 liters) suggests possible tachyphylaxis to the effect of an additional dose of a beta-agonist, a finding not observed in the tiotropium group (with an increase of 0.02 liters). At baseline, the short-term response to four puffs of albuterol (reversibility of airway obstruction of 14.9%) was similar to the response to four puffs of ipratropium (reversibility of 12.4%), which suggests that ipratropium could be considered as an acute bronchodilator for patients with asthma, as was shown in the Asthma Clinical Research Network’s Long-Acting Beta Agonist Response by Genotype (LARGE) trial (NCT00200967).28
The exploratory response analysis provides several insights. In evaluating the response to treatment on the basis of the very stringent three-dimensional measurement, we found that only 36% of patients receiving a bronchodilator and an inhaled glucocorticoid had such a response, as compared with less than 10% of patients receiving a double dose of an inhaled glucocorticoid (Table 3). These data could be used to examine how treatment responses are distributed in a population of patients with asthma. For example, if the less stringent two-dimensional criteria were applied and if the goal were to treat the greatest number of patients with a drug to which they had a response and to maximize the use of inhaled glucocorticoids, 53.1% of patients would be treated with a double dose of an inhaled glucocorticoid, 8.8% with tiotropium plus an inhaled glucocorticoid, and 8.1% with salmeterol plus an inhaled glucocorticoid, leaving 20.6% to be treated with either one of the bronchodilators combined with a low-dose inhaled glucocorticoid and 9.4% who had no response to any treatment.
On the basis of our study’s design, we evaluated only a small number of patients, with no treatment lasting longer than 14 weeks. Since we could not examine either the rate of asthma exacerbations or long-term safety issues, our findings cannot be considered clinically directive. Additional studies that have sufficient statistical power to evaluate exacerbations and safety events are required to further establish the clinical efficacy of tiotropium. However, our data establish clinical equipoise to study larger cohorts of adults for longer periods of time with tiotropium as a therapy for asthma control.
Supplementary Material
Acknowledgments
Supported by grants from the National Heart, Lung, and Blood Institute (U10 HL074225, U10 HL074227, U10 HL074231, U10 HL074204, U10 HL074212, U10 HL074073, U10 HL074206, U10 HL074208, and U10 HL074218).
We thank all the patients who took part in this trial; the members of the data and safety monitoring board, Andrea Apter, Serpil Erzurum, Barbara Layman, Yancy Phillips, Bruce Psaty, and James Sheller; and our study coordinators, Denise Beaver, Kelly Bixler, Jennifer Brandorff, Terry Britton, Peggy Cadbury, Alyson Clayborn, Vanessa Curtis, Mary Gill, Robert Hmieleski, Donna Jinwright, Christena Kolakowski, Jeffrey Krings, Lauren Leshak, Aimee Merchlinski, Barbara Miller, Surinder Narula, Brenda Patterson, Melanie Payton, Jean Schenkkan, Ann Sexton, Kerrie Sheaffer, Allen Stevens, Melissa Thrasher, Suzanne Vogt, Rhonda Webb, Lynda Weichel-Williams, Cheryl Wilmoth, Tiffany Wirth, Muhammad Zahid, Ronald Zimmerman, and Kathy Zheng.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Global Initiative for Asthma (GINA) home page. http://www.ginasthma.com.
- 2.Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. National Asthma Education and Prevention Program. Expert panel report III: guidelines for the diagnosis and management of asthma. (NIH publication no. 08-4051). http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. [Google Scholar]
- 3.Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med. 1996;153:1481–8. doi: 10.1164/ajrccm.153.5.8630590. [DOI] [PubMed] [Google Scholar]
- 4.Gibson PG, Powell H, Ducharme FM. Differential effects of maintenance long-acting beta-agonist and inhaled corticosteroid on asthma control and asthma exacerbations. J Allergy Clin Immunol. 2007;119:344–50. doi: 10.1016/j.jaci.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 5.Rockville, MD: Food and Drug Administration; Long-acting beta agonist (LABA) information. http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm199565.htm. [Google Scholar]
- 6.Drazen JM, O’Byrne PM. Risks of long-acting beta-agonists in achieving asthma control. N Engl J Med. 2009;360:1671–2. doi: 10.1056/NEJMe0902057. [DOI] [PubMed] [Google Scholar]
- 7.von Mutius E, Drazen JM. Choosing asthma step-up care. N Engl J Med. 2010;362:1042–3. doi: 10.1056/NEJMe1002058. [DOI] [PubMed] [Google Scholar]
- 8.Westby M, Benson M, Gibson P. Anticholinergic agents for chronic asthma in adults. Cochrane Database Syst Rev. 2004;3:CD003269. doi: 10.1002/14651858.CD003269.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes PJ. The pharmacological properties of tiotropium. Chest. 2000;117(Suppl):63S–66S. doi: 10.1378/chest.117.2_suppl.63s. [DOI] [PubMed] [Google Scholar]
- 10.Idem. Tiotropium bromide. Expert Opin Investig Drugs. 2001;10:733–40. doi: 10.1517/13543784.10.4.733. [DOI] [PubMed] [Google Scholar]
- 11.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–7. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 12.Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the Asthma Control Questionnaire. Respir Med. 2005;99:553–8. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment. Chest. 1998;114:998–1007. doi: 10.1378/chest.114.4.998. [DOI] [PubMed] [Google Scholar]
- 14.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32–8. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 15.Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836–44. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 16.Szefler SJ, Phillips BR, Martinez FD, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–42. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Zeiger RS, Szefler SJ, Phillips BR, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Laird NM, Ware JH. Random effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 19.Jennrich RI, Schluchter MD. Unbalanced repeated measures models with structured covariance matrices. Biometrics. 1986;42:805–20. [PubMed] [Google Scholar]
- 20.Vonesh EF, Chinchilli VM. Linear and nonlinear models for the analysis of repeated measurements. New York: Marcel Dekker; 1997. [Google Scholar]
- 21.Lemanske RF, Jr, Mauger DT, Sorkness CA, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362:975–85. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rockville, MD: Food and Drug Administration; 2010. Guidance for industry: non-inferiority clinical trials: draft guidance. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf. [Google Scholar]
- 23.Lazarus SC, Boushey HA, Fahy JV, et al. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA. 2001;285:2583–93. doi: 10.1001/jama.285.20.2583. [DOI] [PubMed] [Google Scholar]
- 24.Lemanske RF, Jr, Sorkness CA, Mauger EA, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA. 2001;285:2594–603. doi: 10.1001/jama.285.20.2594. [DOI] [PubMed] [Google Scholar]
- 25.Pauwels RA, Löfdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997;337:1405–11. doi: 10.1056/NEJM199711133372001. Erratum, N Engl J Med 1998; 338: 139. [DOI] [PubMed] [Google Scholar]
- 26.Powell H, Gibson PG. High dose versus low dose inhaled corticosteroid as initial starting dose for asthma in adults and children. Cochrane Database Syst Rev. 2004;2:CD004109. doi: 10.1002/14651858.CD004109.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenstone IR, Ni Chroinin MN, Masse V, et al. Combination of inhaled long-acting beta2-agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma. Cochrane Database Syst Rev. 2005;4:CD005533. doi: 10.1002/14651858.CD005533. [DOI] [PubMed] [Google Scholar]
- 28.Wechsler ME, Kunselman SJ, Chinchilli VM, et al. Effect of beta2-adrenergic receptor polymorphism on response to longacting beta2 agonist in asthma (LARGE trial): a genotype-stratified, randomised, placebo-controlled, crossover trial. Lancet. 2009;374:1754–64. doi: 10.1016/S0140-6736(09)61492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.