Abstract
Background
End organ damage in hypertension can be detected early, reflects accurately the hypertensive patient’s overall cardiovascular risk, and should be prevented and treated with antihypertensive treatment.
Method
We selectively review the relevant literature since 1995, including the German and European guidelines for the diagnosis and treatment of arterial hypertension.
Results
Measurement of the intima-media thickness in the common carotid artery and of the pulse-wave velocity is now recommended for the early diagnosis of hypertensive vasculopathy. Left ventricular hypertrophy, an important component of hypertensive heart disease, can be diagnosed by echocardiography and with the aid of new electrocardiographic indices. Early signs of hypertensive nephropathy, namely albuminuria and a decreased glomerular filtration rate, are prognostically valuable and easy to detect. Cerebrovascular damage, including early microangiopathic changes, is best diagnosed by magnetic resonance imaging. The treatment of end organ damage due to hypertension centers on blood pressure reduction. Blockade of the renin angiotensin-aldosterone system is an essential part of the treatment of early end organ damage.
Conclusion
Hypertensive end organ damage can now be diagnosed early and reversed with specific and aggressive treatment.
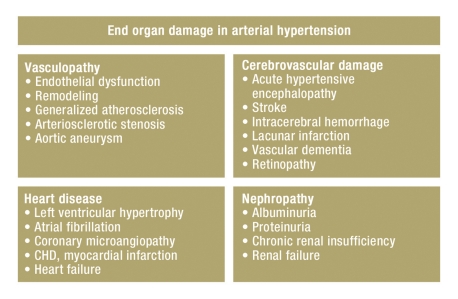
Hypertension is the leading risk factor for morbidity and mortality throughout the world (1). The early detection and severity of typical end organ damage and secondary diseases are key determinants of cardiovascular prognosis in patients suffering from arterial hypertension (2). The classic manifestations of hypertensive end organ damage include the following: vascular and hemorrhagic stroke, retinopathy, coronary heart disease/myocardial infarction and heart failure, proteinuria and renal failure and in the vasculature, atherosclerotic change including the development of stenoses and aneurysms (figure 1).
Figure 1.
Reversible and irreversible end organ damage in arterial hypertension;
CHD, coronary heart disease
The recommendations of medical societies specializing in hypertension have not only used blood pressure for risk stratification, but focus on additional cardiovascular risk factors, the detection of end organ damage, and clinically manifest cardiovascular diseases (2, 3). Hence, grade 1 hypertension can be associated with a slightly increased risk or with a very significantly increased risk depending on what additional end organ damage is present (table 1).
Table 1. Overall cardiovascular risk.
| Additional risk factors and comorbidities | Normal blood pressure SBP 120–129 mmHg or DBP 80–84 mmHg | High normal SBP 130–139 mmHg or DBP 85–89 mmHg | Grade 1 hypertension* SBP 140–159 mmHg or DBP 90–99 mmHg | Grade 2 hypertension SBP 160–179 mmHg or DBP 100–109 mmHg | Grade 3 hypertension SBP ≥180 mmHg or DBP ≥10 mmHg |
| No risk factors | Average risk | Average risk | Slightly elevated risk | Moderately elevated risk | Significantly elevated risk |
| 1 or 2 risk factors | Slightly elevated risk | Slightly elevated risk | Moderately elevated risk | Moderately elevated risk | Very significantly elevated risk |
| 3 or more risk factors or end organ damage** or DM or MS | Moderately elevated risk | Significantly elevated risk | Significantly elevated risk | Significantly elevated risk | Very significantly elevated risk |
| Clinically manifest cardiovascular or renal disease | Very significantly elevated risk | Very significantly elevated risk | Very significantly elevated risk | Very significantly elevated risk | Very significantly elevated risk |
| Framingham cardiac risk score | |||||
| Average risk | Slight risk | Moderate risk | Significant risk | Very signifcant risk | |
| < 10 % | 10–15% | 15–20% | 20–30% | >30% | Probability of a cardiovascular event within 10 years |
| <4% | 4–5 % | 5–8% | >8% | Risk of cardiovascular death per 10 years | |
SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; MS, metabolic syndrome; CHD, coronary heart disease;
*This risk group includes patients with, for example, a blood pressure of 145/85 mmHg, whose overall cardiovascular risk is slightly or significantly elevated, depending on whether or not early end organ damage is present;
**For a definition of end organ damage see Table 2
Early detection
The early detection of hypertensive end organ damage can slow or prevent damage, or allow disease regression with adequate therapy, where organ damage is still at a reversible stage. The diagnosis of hypertensive end organ damage is of decisive importance. This is reflected in European and German guidelines (2, 3). On the basis of these guidelines and a selective literature review of the past 15 years’ literature, this article will discuss early hypertensive end organ damage, its pathogenesis, diagnosis, and therapy (box).
Box. Diagnosis of early hypertensive end organ damage (2, 3).
Left ventricular hypertrophy (LVM) (ECG: Sokolow-Lyon ≥ 38 mm, Cornell QRS > 244 mV*msec)
ECG: left ventricular hypertrophy (≥ 125 g/m2 for men and ≥ 110 g/m2 for women)
Ultrasound examination for arterial wall thickening, (intima-media thickness [IMT] > 0.9 mm or arteriosclerotic plaque)
Pulse wave velocity > 10 to 12 m/sec, depending on the device used
Ankle-Brachial Index < 0.9
Serum creatinine elevated men 1.3–1.5 mg/dL (115–133 µmol/L) women 1.2–1.4 mg/dL (107–124 µmol/L)
Elevated albumin excretion (microalbuminuria 30–300 mg/24 hours, albumin-creatinine ratio: men ≥ 22, women ≥ 31 mg/g creatinine; men ≥ 2.5, women ≥ 3.5 mg/mm ol); normal up to a value of 10 mg/g creatinine
Calculated glomerular filtration rate (<60 mL/min/1.73 m2) or creatinine clearance <60 mL/min
Pathogenesis
Increasing the arterial blood pressure leads to organ damage via hemodynamic load. Currently, 24-hour ambulatory blood pressure measurement is the chosen method of measuring cardiovascular load. Several studies have found that hypertensive end organ damage and its modification with treatment correlate more closely with ambulatory 24-hour blood pressure measurement than with office based blood pressure readings (e1). Ambulatory 24-hour blood pressure measurement is not associated with a white coat effect, except for the first two measurements when attaching the device in the doctor’s office. This technique is also used to diagnose masked hypertension (normal values in the doctor’s office, but not in daily life) (e1).
In addition to this elevated pressure load, a multitude of pathogenetically relevant factors have been identified that affect the severity of hypertensive end organ damage and are independent of the pressure load (blood pressure level) (4, e2). This especially applies to stage 1 and 2 arterial hypertension. Noteworthy factors that can be influenced include the sympathetic nervous system and the renin-angiotensin system as well as metabolic and inflammatory factors. For example, the author has been able to show that the degree of left ventricular hypertrophy is modified by the activity of the renin-angiotensin system (measured via the angiotensin II level). This was observed independently of other pathogenetically relevant factors, including the blood pressure (e2). Overweight and high salt consumption are hypertension-independent determinants of the severity of hypertensive end organ damage (e3). Thus high salt consumption was found to affect the severity of left ventricular hypertrophy, albuminuria and vascular change as well as the incidence of strokes, independently of blood pressure (e3). The clinical implication of this is that blood pressure alone is an inadequate predictor of end organ damage, whose accurate detection requires specific tests to detect early end organ damage. The importance of non-hemodynamic factors also can be seen in the fact that a given reduction in blood pressure results in variable amounts of early hypertensive end organ damage, depending on the mechanism of action of antihypertensive agent used (2, 3).
Hypertensive vasculopathy
Hypertensive vasculopathy is characterized by endothelial dysfunction and remodeling of the small and large arteries. This leads to a reduced dilation capability of the high resistance vasculature which manifests clinically as, among other things, angina resulting from reduced coronary reserve, plaque formation and stenoses and aneurysms, especially in the aorta. Ultrasound can allow plaques or stenoses to be imaged by measuring the increased intima-media wall thickness in the carotid artery (2).
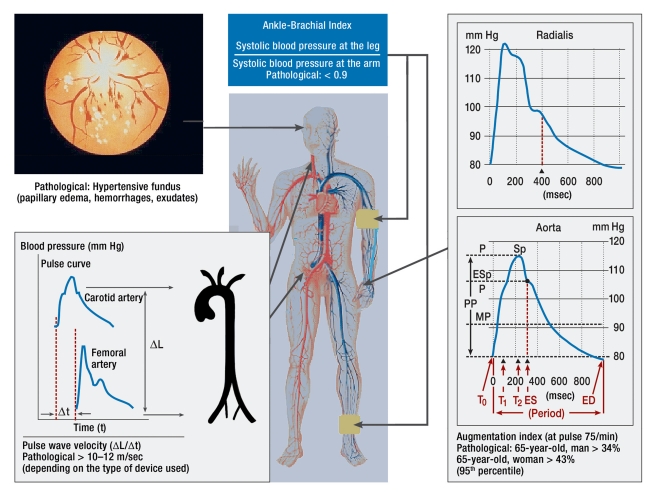
Hypertensive, generalized disease of the large vessels can be detected noninvasively by determining the ankle-brachial index, the pulse wave velocity, and by pulse wave analysis (2) (figure 2). Although measurement of the ankle-brachial index is indicative of hemodynamically relevant stenoses and is simple to perform, it is infrequently used. Measurement of the pulse wave velocity between the carotid and femoral arteries is also noninvasive and values above 10 to 12 m/sec (depending on the device used) are classified as pathological (2). An elevated pulse wave velocity signifies an increased risk of cardiovascular mortality (according to a study of the general population, the risk is four-fold) irrespective of the blood pressure level, other cardiovascular risk factors and ECG abnormalities (5, e4).
Figure 2.
Measurement of generalized vasculopathy (adapted according to [e25]); P, intra-arterial pressure; Esp, augmentation pressure; Sp, systolic blood pressure; PP, pulse pressure (blood pressure amplitude); MP, mean arterial pressure; ED, enddiastolic; ES, endsystolic
By analyzing the central pulse wave either in the carotid or with the assistance of a transfer function in the radial artery, it is possible to measure the central systolic blood pressure, the central pulse pressure (blood pressure amplitude, and the augmentation index (a measure of vascular wall damage). The central pulse pressure and the augmentation index have additive predictive value (6, e5). Measuring the ankle-brachial index, the pulse wave velocity and augmentation index (figure 2) more precisely specify cardiovascular risk and prevent hypertensive patients from being incorrectly classified as low risk (e5).
Treatment of hypertensive vasculopathy should focus primarily on significantly lowering the blood pressure. A positive effect on vascular resistance in large arteries has been confirmed for many antihypertensive agents, but only to a slight degree for beta blockers. Thus, at the same peripheral blood pressure measured on the upper arm, the central pulse pressure was significantly less reduced if cardioselective beta blockers were used (CAFÉ study) (6). This finding explains why cardioselective beta blockers despite (peripheral) blood pressure reduction (ASCOT) could not improve the prognosis, especially the incidence of strokes in clinical studies (e6, e7).
Hypertensive heart disease
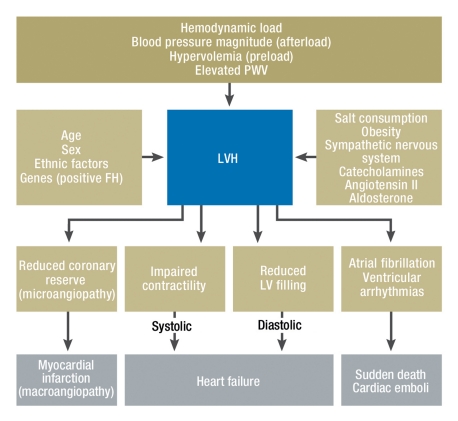
The term hypertensive heart disease includes a multitude of functional and structural cardiac changes (7, e8). Of central importance is left ventricular hypertrophy (figure 3). Hypertensive heart disease is usually clinically asymptomatic, but manifests at a more advanced stage as angina pectoris, dyspnea and arrhythmia. These symptoms can be attributed to reduced coronary reserve, impaired systolic and diastolic left ventricular function, atrial fibrillation, and ventricular arrhythmia (8). Hence as well as coronary heart disease, angina should also raise the possibility of hypertensive heart disease. Even at an early stage, a diastolic filling disorder can exist as a result of delayed left ventricular relaxation (9). In the late stages reduced left ventricular compliance ensues leading to diastolic heart failure.
Figure 3.
Hypertensive heart disease: pathogenetic factors and clinical presentation. PWV, pulse wave velocity; FH, family history; LVH, left ventricular hypertrophy; LV, left ventricle
Echocardiography is the gold standard for diagnosing hypertensive heart disease. It not only is able to determine the dimensions of the left ventricle, but also its systolic and diastolic function, as well as the size of the left atrium. Electrocardiography is more commonly used in everyday practice; it is more cost-effective and has a high specificity, but a lower sensitivity. The introduction of new ECG criteria for left ventricular hypertrophy has somewhat increased the sensitivity especially in obese hypertensive patients (LIFE) (10, e9), but it does not approach that of echocardiography (figure 4). Left ventricular hypertrophy increases the risk of myocardial infarction, heart failure, and sudden cardiac death by a factor of three to five-fold. Furthermore, concentric hypertrophy (relative wall thickness ≥ 0.42) results in a worse cardiovascular prognosis (10). Both the left ventricular mass and the size of the left atrium are independent risk factors for the incidence of atrial fibrillation in hypertensive patients and, thereby, increase the cardioembolic risk (11).
Figure 4.
ECG for the diagnostic evaluation of left ventricular hypertrophy
Treatment of left ventricular hypertrophy has shown that inhibitors of the renin-angiotensin system and calcium antagonists effect a reduction of left ventricular mass that goes beyond that of merely reducing the blood pressure (2, 12). A reduction of left ventricular mass reduces the risk of cardiovascular events by more than one half (13). Because of the variability of echocardiographic measurements, a change in the left ventricular mass between two measurements is not considered significant until it is at least 35 g (e11). Agents of first choice in the primary prevention of atrial fibrillation are ACE inhibitors and angiotensin receptor blockers. This is so even when compared to beta blockers, which in contrast are preferred in absolute tachyarrhythmia to control the heart rate (2, e12).
Hypertensive cerebrovascular damage
Arterial hypertension is the most important risk factor for stroke, which in 80% of cases is due to an underlying ischemic infarction (e13). Lacunar infarctions, microhemorrhages and focal or diffuse white matter lesions are early hypertensive, microangiopathic complications (14). The development of vascular dementia often is also attributable to untreated or inadequately treated hypertension (14).
Fundoscopy is today no longer recommended as a method for detecting early hypertensive retinopathy and as an indirect indicator of cerebral vasculopathy (2, e14). The reason for this is that several studies have been unable to find any reproducibility for grades 1 and 2 according to Wagner and Barker (15, e15). This does not apply to fundus hypertension III and IV (papilledema, hemorrhages, exudates (figure 2). Therefore, in a hypertensive emergency fundoscopy continues to be important (for example, in diagnosing hypertensive encephalopathy) (2). In the search for methods to detect hypertensive retinopathy early, the arterio-venous ratio has been identified as a useful parameter (e16). Newer methods of automatically analyzing the fundus of the eye by means of scanning laser Doppler flowmetry are presently still being investigated (16, e16).
A number of questions relating to antihypertensive therapy in cerebral microangiopathy and macroangiopathy remain unanswered. For example, the question of what level of blood pressure during the acute phase of a stroke is associated with the lowest neurological deficit has still not been clarified. As a preventative measure and to prevent the progression of cerebrovascular damage, the systolic blood pressure should be reduced to at least <150 mm Hg, better <140 mm Hg (generally recommended if tolerated by the patient <130 mm Hg) (2). It also has been shown repeatedly that a dose-response relationship exists between blood pressure reduction and a reduction in stroke incidence (e17). A recent study suggests that even intracerebral arterial stenosis is not a contraindication to blood pressure reduction, because higher rates of blood pressure reduction were not associated with an increase in ischemic strokes in the poststenotic region (e18). The guidelines of the European Hypertension/Cardiology Association do not recommend any specific group of medications for primary and secondary prevention and the British guidelines no longer recommend beta blockers for hypertensive patients with cerebrovascular damage. The reason for this are studies which have shown that beta blockers effect a reduction of the central blood pressure and pulse pressure to a lesser degree than other antihypertensive substances (6, e7). However, studies have shown that diuretics (ALLHAT) (e19), calcium antagonists (SYSTEUR), and angiotensin receptor blockers (LIFE) reduce the stroke rate (17).
Hypertensive nephropathy
After 15 to 20 years, hypertensive nephropathy often results in chronic renal failure; this occurs mostly unnoticed and without clinical symptoms. Hypertensive nephropathy can be detected by means of early symptoms such as the occurrence of mild albuminuria and a reduced (calculated) glomerular filtration rate (eGFR), both of which are easily measured parameters. A more recent Italian study demonstrated that measuring the albuminuria and the eGFR as renal parameters for hypertensive end organ damage resulted in a significant change in the estimation of the overall cardiovascular risk (18).
Microalbuminuria can be traced to structural and functional transformational processes in the glomeruli (endothelium, glomerular basal membrane, podocytes) that are associated with increased permeability. Interestingly, this permeability disorder is not limited to the renal vessels, but can be observed in the entire vascular system. This explains why albuminuria is not only a predictor for developing chronic renal insufficiency, but is also predictive of cardiovascular complications (19). In the HOPE study the confirmation of microalbuminuria was of comparable value to the diagnosis of coronary heart disease (e20). Detecting an eGFR <60 mL/min/1.73m2 is likewise a potent predictor of terminal renal failure and cardiovascular complications. Hence it is best to measure the albuminuria and the eGFR simultaneously to better be able to estimate the prognosis (e21, 20). The cardiovascular fatality rate in patients with hypertensive type 2 diabetes increased from 0.54 to 1.71% (2.8-fold risk) if the eGFR was <60 mL/min/1.73m2 and further to 2.77% per year (4.3-fold risk) if the albuminuria was >30 mg/g (20).
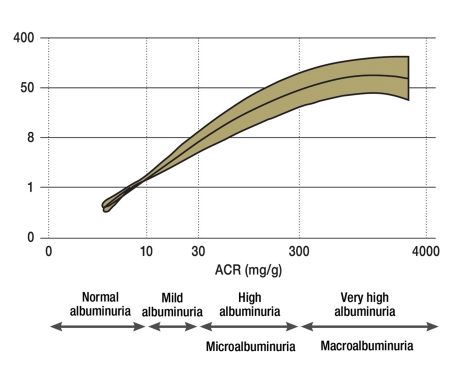
Based on a new very detailed study, albuminuria should be measured using the first spontaneous urine collected in the morning, or if this is not possible, a spontaneous urine obtained during the course of the day (e22). Albuminuria is best expressed in terms of the creatinine clearance in the urine. Caution is required since prior physical activity, urinary tract infections, but also other infections can deliver incorrectly high values. A new classification of albuminuria avoids the term microalbuminuria, because it suggests that the organ damage is minimal (“micro”) (21). The new classification defines an albuminuria of up to 10 mg/day, respectively an albumin-creatinine ratio of <10 mg/g as normal (21). Starting with this value the risk of developing chronic renal failure (figure 5) and cardiovascular complications increases exponentially (e23).
Figure 5.
Albuminuria as a prognostic marker for terminal chronic renal failure; ACR, albumin-creatinine ratio; NKF, National Kidney Foundation; FDA, Food and Drug Administration; ESRD, end-stage renal disease; eGFR, calculated glomerular filtration rate
Rigorous antihypertensive therapy, mostly in multiple combinations, can prevent the progression of chronic renal failure and proteinuria and thereby improve both the renal and cardiovascular prognosis (2). Hence the LIFE study showed that a reduction in albuminuria in hypertensive patients with left ventricular hypertrophy (22) was associated with fewer cardiovascular complications; in the RENAAL study in patients with hypertension, diabetes mellitus, and proteinuria, reduced albuminuria also resulted in fewer cardiovascular complications (23).
In hypertensive nephropathy a target blood pressure of <130/80 mm Hg is recommended and in the presence of proteinuria the blood pressure should be even lower (2, 3). Nephroprotective effects have been confirmed for ACE inhibitors, angiotensin receptor blockers and direct renin inhibitors in large prospective studies (2, 3, e24). Diuretics (in hypervolemia, elevated sodium chloride sensitivity or increased salt consumption) and calcium antagonists (ideally of the 3rd generation) are available for combination therapy. Since calcium antagonists cause the preglomerular resistance vessels to dilate and there is a risk of transmitting the elevated blood pressure in the aorta into the glomerular capillary bed, calcium antagonists should not be used initially, but preferably later on to optimize the antihypertensive therapy (24).
Differential treatment of end organ damage
Generally and irrespective of the hypertensive patient’s age, a blood pressure reduction to <140/90 mm Hg is recommended. If organ damage is present, a reduction to values of about 130/80 mm Hg should be the objective. This especially applies to patients with diabetes mellitus, hypertensive nephropathy, and after a stroke or myocardial infarction (2, 3). Also according to more recent studies a potential increased risk due to a blood pressure reduction that is too low should not be a concern unless it drops to <120/75 mm Hg (17, 25, e17). This was shown in the INVEST study (hypertensive patients after myocardial infarction) and in the ONTARGET study (high-risk patients of which 70% had hypertension) (25, e17). Whether the increased incidence of cardiovascular death was the result of an excessive blood pressure reduction or only the expression of the poor prognosis of the patients is unclear.
Various studies of individual antihypertensive agents have found effects on hypertensive end organ damage that were independent of the blood pressure (table 2). Differential treatment is based on the understanding that not only the blood pressure but also additional factors are of significance in the pathogenesis of organ damage. Table 2 lists the current differential treatment recommendations (2).
Table 2. Differential treatment considerations for the selection of antihypertensive agents (2, 3).
| Subclinical end organ damage | |
| Left-ventricular hypertrophy | ACEI, ARB, CA |
| Elevated albuminuria | ACEI, ARB |
| Renal dysfunction | ACEI, ARB |
| Irreversible hypertensive end organ damage | |
| Prior stroke | Any antihypertensive |
| Prior myocardial infarction | BB, ACEI, ARB |
| Angina pectoris, CHD | BB, CA |
| Heart failure | Diuretics, BB, ACEI, ARB, MR antagonists |
| Left-ventricular dysfunction | ACEI, ARB |
| Atrial Fibrillation | |
|
ARB, ACEI |
|
BB, non-dihydropyridine calcium antagonists |
| Tachyarrhythmia | BB |
| Chronic renal insufficieny, proteinuria | ACEI, ARB, loop diuretics |
| Peripheral arterial occlusive disease | CA |
ACEI, ACE inhibitor; ARB, angiotensin receptor blocker; CA, calcium antagonist; BB, beta blocker; MR-antagonist, mineralcorticoid antagonist; CHD, coronary heart disease; MI, myocardial infarction
Acknowledgments
Translated from the original German by mt-g.
Footnotes
Conflict of interest statement
Prof. Schmieder, a Bavarian civil servant, has received financial support from DFG, BMBF, PHRI (Canada), as well as professional fees for presentations, outside funding for projects, and/or travel reimbursement from AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Novartis, Servier, and Takeda.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757 . doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, Laurent S, Agabiti-Rosei E, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Foerce document. J Hypertens. 2009;27:2121–2158. doi: 10.1097/HJH.0b013e328333146d. [DOI] [PubMed] [Google Scholar]
- 3.Deutsche Hochdruckliga eV. Leitlinien zur Behandlung der arteriellen Hypertonie. Nieren- und Hochdruckkrankheiten. 2009;38:137–188. [Google Scholar]
- 4.Schmieder RE. The role of non-haemodynamic factors of the genesis of LVH. Nephrol Dial Transplant. 2005;20:2610–2612. doi: 10.1093/ndt/gfi190. [DOI] [PubMed] [Google Scholar]
- 5.Laurent S, Boutouyrie P. Arterial stiffness: a new surrogate end point for cardiovascular disease? J Nephrol. 2007;20(suppl 12):45–50. [PubMed] [Google Scholar]
- 6.Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 7.Schmieder RE, Messerli FH. Hypertension and the heart. J Hum Hypertens. 2000;14:597–604. doi: 10.1038/sj.jhh.1001044. [DOI] [PubMed] [Google Scholar]
- 8.Motz W, Scheler S, Schwartzkopff B, Strauer BE. Evaluation of cardiac damage in hypertension. J Cardiovasc Risk. 1995;2:16–26. [PubMed] [Google Scholar]
- 9.Schwab J, Schneider MP, Pauschinger M, Schmieder RE. Hypertension and diastolic dysfunction. MMW Fortschr Med. 2009;151:41–43. doi: 10.1007/BF03365781. [DOI] [PubMed] [Google Scholar]
- 10.Okin PM, Jern S, Devereux RB, Kjeldsen SE, Dahlof B. Effect of obesity on electrocardiographic left ventricular hypertrophy in hypertensive patients : the losartan intervention for endpoint (LIFE) reduction in hypertension study. Hypertension. 2000;35:13–18. doi: 10.1161/01.hyp.35.1.13. [DOI] [PubMed] [Google Scholar]
- 11.Verdecchia P, Reboldi G, Gattobigio R, et al. Atrial fibrillation in hypertension: predictors and outcome. Hypertension. 2003;41:218–223. doi: 10.1161/01.hyp.0000052830.02773.e4. [DOI] [PubMed] [Google Scholar]
- 12.Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. 2003;115:41–46. doi: 10.1016/s0002-9343(03)00158-x. [DOI] [PubMed] [Google Scholar]
- 13.Verdecchia P, Angeli F, Borgioni C, et al. Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: a meta-analysis. Am J Hypertens. 2003;16:895–899. doi: 10.1016/s0895-7061(03)01018-5. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Wen W, Anstey KJ, Sachdev PS. Prevalence, incidence, and risk factors of lacunar infarcts in a community sample. Neurology. 2009;73:266–272. doi: 10.1212/WNL.0b013e3181aa52ea. [DOI] [PubMed] [Google Scholar]
- 15.van den Born BJ, Hulsman CA, Hoekstra JB, Schlingemann RO, van Montfrans GA. Value of routine funduscopy in patients with hypertension: systematic review. BMJ. 2005:331–373. doi: 10.1136/bmj.331.7508.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michelson G, Welzenbach J, Pal I, Harazny J. Automatic full field analysis of perfusion images gained by scanning laser Doppler flowmetry. Br J Ophthalmol. 1998;82:1294–1300. doi: 10.1136/bjo.82.11.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanchetti A, Mancia G, Black HR, et al. Facts and fallacies of blood pressure control in recent trials: implications in the management of patients with hypertension. J Hypertens. 2009;27:673–679. doi: 10.1097/HJH.0b013e3283298ea2. [DOI] [PubMed] [Google Scholar]
- 18.Leoncini G, Ratto E, Viazzi F, et al. Global risk stratification in primary hypertension: the role of the kidney. J Hypertens. 2008;26:427–432. doi: 10.1097/HJH.0b013e3282f35c79. [DOI] [PubMed] [Google Scholar]
- 19.Schmieder RE, Schrader J, Zidek W, et al. Subclinical albuminuria, microalbuminuria and proteinuria-accepted cardiovascular risk markers? Dtsch Med Wochenschr. 2006;131:2665–2671. doi: 10.1055/s-2006-956273. [DOI] [PubMed] [Google Scholar]
- 20.Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54:205–226. doi: 10.1053/j.ajkd.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: Losartan intervention for endpoint reduction in hypertension study. Hypertension. 2005;45:198–202. doi: 10.1161/01.HYP.0000154082.72286.2a. [DOI] [PubMed] [Google Scholar]
- 23.de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110:921–927. doi: 10.1161/01.CIR.0000139860.33974.28. [DOI] [PubMed] [Google Scholar]
- 24.Ruggenenti P, Perna A, Benini R, Remuzzi G. Effects of dihydropyridine calcium channel blockers, angiotensin-converting enzyme inhibition, and blood pressure control on chronic, nondiabetic nephropathies. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN) J Am Soc Nephrol. 1998;9:2096–2101. doi: 10.1681/ASN.V9112096. [DOI] [PubMed] [Google Scholar]
- 25.Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med. 2006;144:884–893. doi: 10.7326/0003-4819-144-12-200606200-00005. [DOI] [PubMed] [Google Scholar]
- e1.Mancia G, Bombelli M, Facchetti R, et al. Long-term risk of sustained hypertension in white-coat or masked hypertension. Hypertension. 2009;54:226–232. doi: 10.1161/HYPERTENSIONAHA.109.129882. [DOI] [PubMed] [Google Scholar]
- e2.Schmieder RE, Langenfeld MR, Friedrich A, Schobel HP, Gatzka CD, Weihprecht H. Angiotensin II related to sodium excretion modulates left ventricular structure in human essential hypertension. Circulation. 1996;94:1304–1309. doi: 10.1161/01.cir.94.6.1304. [DOI] [PubMed] [Google Scholar]
- e3.Messerli FH, Schmieder RE, Weir MR. Salt. A perpetrator of hypertensive target organ disease? Arch Intern Med. 1997;157:2449–2452. doi: 10.1001/archinte.157.21.2449. [DOI] [PubMed] [Google Scholar]
- e4.Shokawa T, Imazu M, Yamamoto H, et al. Pulse wave velocity predicts cardiovascular mortality: findings from the Hawaii-Los Angeles-Hiroshima study. Circ J. 2005;69:259–264. doi: 10.1253/circj.69.259. [DOI] [PubMed] [Google Scholar]
- e5.Laurent S. Hypertension and macrovascular disease. European Society of Hypertension Scientific Newsletter. www.eshonline.de (12. 8. 2010) 2007;8(31):1–2. [Google Scholar]
- e6.Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- e7.Messerli FH, Grossman E, Goldbourt U. Are beta-blockers efficacious as first-line therapy for hypertension in the elderly? A systematic review. JAMA. 1998;279:1903–1907. doi: 10.1001/jama.279.23.1903. [DOI] [PubMed] [Google Scholar]
- e8.Agabiti-Rosei E, Muiesan ML, Salvetti M. Evaluation of subclinical target organ damage for risk assessment and treatment in the hypertensive patients: left ventricular hypertrophy. J Am Soc Nephrol. 2006;17:104–108. doi: 10.1681/ASN.2005121336. [DOI] [PubMed] [Google Scholar]
- e9.Alfakih K, Walters K, Jones T, Ridgway J, Hall AS, Sivananthan M. New gender-specific partition values for ECG criteria of left ventricular hypertrophy: recalibration against cardiac MRI. Hypertension. 2004;44:175–179. doi: 10.1161/01.HYP.0000135249.66192.30. [DOI] [PubMed] [Google Scholar]
- e10.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- e11.Palmieri V, Dahlof B, DeQuattro V, et al. Reliability of echocardiographic assessment of left ventricular structure and function: the PRESERVE study. Prospective Randomized Study Evaluating Regression of Ventricular Enlargement. J Am Coll Cardiol. 1999;34:1625–1632. doi: 10.1016/s0735-1097(99)00396-4. [DOI] [PubMed] [Google Scholar]
- e12.Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis. 2001;12:171–180. doi: 10.1159/000047700. [DOI] [PubMed] [Google Scholar]
- e13.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- e14.Schmieder RE. Hypertensive retinopathy. European Society of Hypertension Scientific Newsletter. www.eshonline.de (12. 8. 2010) 2009;10(14):1–2. [Google Scholar]
- e15.Dimmitt SB, West JN, Eames SM, Gibson JM, Gosling P, Littler WA. Usefulness of ophthalmoscopy in mild to moderate hypertension. Lancet. 1989;1:1103–1106. doi: 10.1016/s0140-6736(89)92384-2. [DOI] [PubMed] [Google Scholar]
- e16.Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004;351:2310–2317. doi: 10.1056/NEJMra032865. [DOI] [PubMed] [Google Scholar]
- e17.Sleight P, Redon J, Verdecchia P, et al. Prognostic value of blood pressure in patients with high vascular risk in the ongoing telmi-sartan alone and in combination with ramipril global endpoint trial study. J Hypertens. 2009;27:1360–1369. doi: 10.1097/HJH.0b013e32832d7370. [DOI] [PubMed] [Google Scholar]
- e18.Turan TN, Cotsonis G, Lynn MJ, Chaturvedi S, Chimowitz M. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation. 2007;115:2969–2975. doi: 10.1161/CIRCULATIONAHA.106.622464. [DOI] [PubMed] [Google Scholar]
- e19.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- e20.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- e21.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e22.Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol. 2009;20:436–443. doi: 10.1681/ASN.2008030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e23.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- e24.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- e25.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]