Abstract
Despite a wealth of evidence for the involvement of the autonomic nervous system (ANS) in health and disease and the ability of music to affect ANS activity, few studies have systematically explored the therapeutic effects of music on ANS dysfunction. Furthermore, when ANS activity is quantified and analyzed, it is usually from a point of convenience rather than from an understanding of its physiological basis. After a review of the experimental and therapeutic literatures exploring music and the ANS, a “Neurovisceral Integration” perspective on the interplay between the central and autonomic nervous systems is introduced, and the associated implications for physiological, emotional, and cognitive health are explored. The construct of heart rate variability is discussed both as an example of this complex interplay and as a useful metric for exploring the sometimes subtle effect of music on autonomic response. Suggestions for future investigations using musical interventions are offered based on this integrative account.
Keywords: autonomic nervous system, entrainment, heart rate variability, neurovisceral integration, psychophysiological responses
Autonomic Imbalance and Disease
The autonomic nervous system (ANS) connects the central nervous system (CNS; brain and spinal cord) with the major peripheral organs and organ systems (targets in parenthesis): circulatory (heart, blood vessels), digestive (gastrointestinal tract glands and sphincters, kidney, liver, salivary glands), endocrine (adrenal glands), integumentary (sweat glands), reproductive (uterus, genitals), respiratory (bronchiole smooth muscles), urinary (sphincters), and visual (pupil dilator and ciliary muscles). The ANS is usually discussed as having two major branches—a sympathetic branch, associated with energy mobilization, and a parasympathetic branch, associated with vegetative and restorative functions.
Modern conceptions of organism function that are based on complexity theory hold that organism stability, adaptability, and health are maintained through a dynamic relationship among system elements; in this case, the sympathetic and parasympathetic branches of the ANS (Thayer & Lane, 2000). That is, patterns of organized variability, rather than static levels, are preserved in the face of constantly changing environmental demands. In contrast to homeostasis, this conception posits that the system has multiple points of stability, necessitating a dynamic organization of resources to match specific situational demands. These demands can be conceived in terms of energy regulation, with local energy minima serving as points of relative stability. For example, in healthy individuals, average heart rate is greater during the day, when energy demands are higher, than at night, when energy demands are lower. The system has a local energy minimum (attractor) for daytime and another for nighttime. Because the system operates “far from equilibrium,” it constantly searches for local energy minima to reduce the energy requirements of the organism. Consequentially, optimal system functioning is achieved via lability and variability in its component processes, whereas rigid regularity is associated with mortality, morbidity, and ill health (e.g., Peng et al., 1994).
Another corollary of this view is that autonomic imbalance, in which one branch of the ANS dominates over the other, is associated with a lack of dynamic flexibility and health. A large body of empirical evidence suggests that autonomic imbalance is associated with various pathological conditions. The broad label of “ANS dysfunction” is associated with a host of complex and heterogeneous disorders and diseases with distinct etiologies, such as diabetic autonomic neuropathy, hyperhidrosis, orthostatic intolerance/postural tachycardia syndrome, pure autonomic failure, and vasovagal syncope. More generally, autonomic dysfunction is present in conjunction with neurodegenerative diseases such as Alzheimer's disease, multiple system atrophy, and Parkinson's disease; neurodevelopmental disorders such as autism spectrum disorders; autoimmune diseases such as multiple sclerosis; mental disorders such as generalized anxiety, major depression, and schizophrenia; and following ischemic stroke or myocardial infarction. In particular, when the sympathetic branch is hyperactive and the parasympathetic branch is hypoactive over an extended duration, energy demands on the system become excessive and ultimately cannot be met, resulting in premature aging, disease, and ultimately death.
Music and the ANS: Experimental and Interventional Investigations
The literature on the effect of music on ANS activity in healthy subjects is quite large; the literature on how music affects individuals with ANS dysfunction (especially within the context of musical interventions) is less developed. In both literatures, however, changes in physiological activity (e.g., heart rate, blood pressure, electrodermal activity) are often investigated and discussed from one of two distinct (and tacit) perspectives: as either (1) the byproducts of arousal, mood, anxiety, and other psychological states that are the primary target of study; or (2) definitive barometers of those psychological states. The second perspective assumes that statistically significant changes in ANS activity reflect meaningful changes in the state of the organism (when in fact they may not). Conversely, the first perspective assumes that, since physiological changes are the downstream consequences of changes in “central” states, they have only limited diagnostic utility. Neither perspective addresses a fundamental issue: that the autonomic nervous system (and activity in its targets) is exquisitely linked, bidirectionally, with the central nervous system, endocrine system, and immune system. Given that the ANS is both associated with physiological health and responsive to music, the ANS may serve as one path by which music exerts its therapeutic effect. The implications of such an association have yet to be fully explored; the present paper may thus serve as a conceptual springboard for future study.
The present review was based on a series of parallel searches with CINAHL, Google Scholar, ISI Web of Science, MEDLINE, and RILM during October 2009. The most relevant search terms are listed in Table 1; throughout the text, these terms will be references in braces. Three summary points may be made: (1) less research exists on ANS responses to music versus CNS responses to music, be it general discussions {A3-A5 vs. A1}, specific disorders {B5-B11 vs. B2-B4}, or measures of physiological activity {E3-E11 vs. E1-E2}; (2) specific conditions associated with ANS dysfunction {D1-D9} have received very little investigation in conjunction with music; and (3) when ANS activity is recorded during a musical intervention, it is most often in the context of reducing anxiety {C1} or pain {C3}.
TABLE 1. Search Terms Queried.
| Item | Category/Term | Item | Category/Term |
|---|---|---|---|
| General search | Physiological Measures/Tools | ||
| A1 | (Brain) or (Central Nervous System) | E1 | (Magnetic Resonance Imaging) or (Positron-Emission Tomography) or (Magnetoencephalography) |
| A2 | Cardiovascular System | E2 | Electroencephalography |
| A3 | Autonomic Nervous System | E3 | Blood Pressure |
| A4 | (Parasympathetic Nervous System) or (parasympathetic) | E4 | Chills |
| A5 | (Sympathetic Nervous System) or (sympathetic) | E5 | (Galvanic Skin Response) or (skin conductance) or (electrodermal activity) |
| Disorder Classes | E6 | gastric | |
| B1 | (Anxiety Disorders) or (Panic Disorder) | E7 | Heart Rate |
| B2 | Central Nervous System Diseases | E8 | (heart rate variability) or (hrv) |
| B3 | Multiple System Atrophy | E9 | (Respiration) or (breath*) |
| B4 | Neurodegenerative Diseases | E10 | Skin Temperature |
| B5 | (autonomic) and (disorder) | E11 | (Vasodilation) or (Vasoconstriction) |
| B6 | (autonomic) and (dysfunction) | Interventions/Therapies | |
| B7 | Autonomic Nervous System Diseases | F1 | (autonomic) or (parasympathetic) or (sympathetic) |
| B8 | Cardiovascular Diseases | F2 | “Biofeedback (Psychology)” or (biofeedback) |
| B9 | dysautonomia* or (Primary Dysautonomias) | F3 | (Breathing Exercises) |
| B10 | Dysautonomia, Familial | F4 | (dance) or (dancing) |
| B11 | Peripheral Nervous System Diseases | F5 | (drum) or (drumming) or (percussion) |
| General States | F6 | entrain* | |
| C1 | Anxiety | F7 | feedback |
| C2 | Arousal | F8 | (Gait) or (Walking) or (movement*) |
| C3 | Pain | F9 | (heart rate) or (respiration) or (breathing) or (blood pressure) |
| Specific Symptoms/Conditions | F10 | (heart rate variability) | |
| D1 | Arrhythmias, Cardiac | F11 | listen* |
| D2 | Bradycardia | F12 | Mind-Body Therapies |
| D3 | Hyperhidrosis | F13 | Music Therapy |
| D4 | (Hypertension) or (hypertensive) | F14 | (paced breath*) or (deep breath*) |
| D5 | (Hypotension) or (hypotensive) | F15 | music and (perform* or product* or making) |
| D6 | Hypotension, Orthostatic | F16 | (performance anxiety) |
| D7 | Syncope | F17 | “rhythmic auditory stimulation” |
| D8 | Tachycardia | F18 | (sing) or (singing) |
| D9 | Postural Orthostatic Tachycardia Syndrome | F19 | (Visceral Afferents) or (viscera* afferent*) or (interocept*) |
Note: Capitalized terms are MEDLINE MeSH terms.
In the following summary, distinctions will be made based on the study population (experimental studies on healthy subjects versus interventional studies on patients) and method of interaction with music (listening to versus entraining to versus making).
Listening to Music
Experimental
Physiological investigations of music date back over 125 years; in his review, Diserens (1926, pp. 129-154) cites some 24 investigations between 1880 and 1918 alone. Nearly every organ in the body with an electrical, chemical, or volumetric signature has at some point been investigated in conjunction with musical stimuli (for comprehensive reviews, see Bartlett, 1996; Hodges, 1980, 2010). Heart rate, electrodermal activity, blood pressure, and respiration rate are the most commonly measured responses; a MEDLINE search retrieved over 450 articles in English that recorded at least one of these measures while subjects listened to music. (Discussion of ANS activity in Music Perception has been limited to a handful of studies investigating musical chills.)
A number of studies have reported that listening to sedative music (i.e., slow tempo, legato phrasing, minimal dynamic contrasts) can lead to decreased heart rate, respiration rate, and blood pressure. Importantly, however, these effects are inconsistent. For example, of the 67 investigations reviewed by Hodges (2010) that measured heart rate changes to music, 32 reported significant effects, 15 reported non-significant effects, and 10 reported a mixture of significant and non-significant effects. Thus, despite the relative ease of recording the electrical signature of a beating heart, the utility of mean heart rate is not without question. This issue will be elaborated upon later.
Interventional
A number of randomized controlled trials have reported that music possesses anxiolytic and analgesic properties, and is associated with decreased heart rate, respiration rate, and blood pressure in perioperative patients (for reviews, see e.g., Dunn, 2004; Evans, 2002). Two caveats must be noted, however. First, the type of anxiety experienced would be considered “state” rather than “trait,” given the short-lived nature of the anxiogenic stimulus (the operative procedure). Second, and more relevant for the current topic, the primary target in these studies is usually a reduction of anxiety; physiological changes are considered secondary.
Entraining to Music
Entrainment is the process by which two oscillating systems assume the same period (or period ratio) when they interact. In experimental paradigms, entrainment usually refers to the synchronization of endogenous rhythms in the subject with an exogenous rhythm in the environment. Endogenous rhythms exist at many orders of magnitude in a number of physiological processes, such as reproduction and menstruation (∼30 days), sleep-wake (∼24 hours), rapid-eye-movement sleep (∼3 hours), blood pressure (∼0.1-0.15 Hz), breathing (∼0.15-0.4 Hz), cardiac pulse (∼1-2 Hz), and electroencephalographic activity (∼1-100 Hz).
In discussing this literature, it is useful to distinguish spontaneous entrainment (i.e., unconscious or passive) from volitional entrainment (i.e., conscious or active). Spontaneous entrainment has been reported in a number of experimental studies, while volitional entrainment is more often used in an interventional context.
Experimental
A few studies have reported spontaneous entrainment of blood pressure (Bernardi, Porta, Bernardi, & Sleight, 2009) and respiration rate (e.g., Etzel, Johnsen, Dickerson, Tranel, & Adolphs, 2006; Haas, Distenfeld, & Axen, 1986) to musical tempo. While all explicitly refer to entrainment, “correlated with” tempo rather than “entrained to” tempo seems a more accurate (and tenable) conclusion given: (1) the wide range of tempos frequently utilized (e.g., 42-124 bpm in Etzel et al.); (2) the lack of specificity of the period of entrainment (at the level of the beat? the measure? the phrase?); and in some cases (3) the lack of stimulus generalizability (Bernardi et al., 2009, explicitly examined Verdi arias that “contain phrases close to 6 cycles/min”). Furthermore, as Thaut, Kenyon, Schauer, and McIntosh (1999) hypothesize, there may be an evolutionarily protective mechanism that limits the entrainment of cyclic autonomic processes (as opposed to motor processes) to environmental stimuli.
Interventional
As an intervention, volitional entrainment has featured prominently in the recovery of speech function and gait. While some studies have recorded electromyographic activity in limb muscles (e.g., Thaut et al., 1996), none has measured ANS activity.
Breathing is one of the few physiological processes that can come under voluntary control (e.g., Feldman & Del Negro, 2006), making it a prime candidate for interventions. Several randomized controlled trials have reported significant decreases in both systolic and diastolic blood pressure in patients with chronic heart failure (Parati et al., 2008) or hypertension (e.g., Grossman, Grossman, Schein, Zimlichman, & Gavish, 2001; Schein et al., 2001) using a “device-guided breathing” paradigm. Subjects were instructed to synchronize their inhalations and exhalations to a high and a low tone, respectively. Tone duration was controlled by a device that monitored respiration via a chest strap and gradually lengthened the duration of the exhalation tone until the desired slow breathing rate was reached (10 breaths per minute). While such a paradigm has been termed “breathe with interactive music” (Grossman et al., 2001; Schein et al., 2001), music itself has not been used as a component of the experimental procedure. Future studies also should consider how device-guided breathing or entrained breathing to music differ from breathing exercises that do not utilize voluntary entrainment to an auditory signal, but that report similar changes in physiological activity (e.g., Brown & Gerbarg, 2009).
Making Music
Experimental
While singing can reduce tension, increase energy, and improve mood in healthy subjects (e.g., Clift & Hancox, 2001), its impact on physiological activity seems dependent on how the task is perceived by the subjects. For example, Grape, Sandgren, Hansson, Ericson, and Theorell (2003) found that professional singers showed greater heart rate variability (an index of parasympathetic activity; see below) after a singing lesson, whereas amateur singers showed less heart rate variability after the lesson. Valentine and Evans (2001) reported a slight (2.5 bpm) increase in heart rate after solo singing, but a comparable decrease after choral singing. Fechir et al. (2008) reported a more substantial (7 bpm) increase in heart rate after solo singing—a task that the authors considered to be stressful. Thus, how a task is perceived by the subjects should be considered when examining how that task affects physiological activity.
Interventional
Singing {F18} and drumming {F5} have been used in a variety of behavioral treatments, such as the recovery or improvement of language abilities and motor skills. Very few studies, however, have measured ANS changes associated with these activities. Wade (2002) found that singing improved expiratory flow rates more than relaxation therapy in children with asthma. Takahashi and Matsushita (2006) reported that elderly patients with dementia who participated in two years of weekly sessions of group singing and drumming activities did not experience a typical age-related increase in systolic blood pressure that was evident in a control group of subjects. In interpreting these results, and others like them, it is important to consider whether singing offers benefits over the non-musical breathing exercises discussed above.
ANS changes associated with dancing {F4} as part of an intervention have been reported for patients with chronic heart failure (Belardinelli, Lacalaprice, Ventrella, Volpe, & Faccenda, 2008), diabetes (Murrock, Higgins, & Killion, 2009), who are overweight (Gillett & Eisenman, 1987), or who are elderly (Hui, Chui, & Woo, 2009). Although dancing can be considered a musical behavior, it is also clearly a form of exercise. From an empirical perspective, therefore, it is important to consider: (1) whether dancing offers additional physiological benefits that other forms of exercise to music (e.g., Schwartz, Fernhall, & Plowman, 1990) do not, and (2) whether exercise to music results in different outcomes than exercise without music (e.g., Jolliffe et al., 2000).
Neurovisceral Integration: Linking the Central and Autonomic Nervous Systems
Much of the literature cited above examines changes in ANS activity de facto rather than de jure; that is, measured and analyzed from a point of convenience rather than from an understanding of their physiological basis. The next section will probe deeper into the basis of heart rate (the most commonly investigated measure of ANS activity) as an illustrative example of the complex machinery driving “front-end” physiological measures.
Heart Rate Variability: An Index of Neurovisceral Integration
Most studies that measure heart rate (HR) report it as an average value in beats per minute. In reality, however, there is no such thing as an “average” HR. Heart rate variability (HRV) refers to a phenomenon first noted in the mid 1700s in the work of Hales and von Haller (Parati, Saul, Di Rienzo, & Mancia, 1995): the acceleration of HR during inhalation and the deceleration of HR during exhalation, now termed the respiratory sinus arrhythmia.
While the concept of “variability” intuitively raises red flags (just as it did for early psychophysiologists; see Porges, 1992), it is the lack of variability in beat-to-beat HR that has come to reflect general autonomic dysfunction in individuals with cardiovascular disease, diabetes, hypertension, high cholesterol, multiple sclerosis, who have had an ischemic stroke or myocardial infarction, who are obese, or who smoke, and evidence from several sources suggests that HRV is an independent predictor of all-cause mortality (for discussions, see e.g., Berntson et al., 1997; Thayer & Lane, 2007).
Sympathetic and Parasympathetic Control of Heart Rate
Chronotropic (i.e., the timing of heart beats) control of the heart is achieved via the complex interplay of the sympathetic (SNS) and parasympathetic (PNS) branches of the autonomic nervous system. Medical physiology texts (e.g., Guyton & Hall, 2005, chapters 9-10) often discuss ANS control over the heart as a push-pull system: the SNS increases the force and rate of contractions and the PNS decreases the force and rate of contractions. This, however, is an oversimplification.
Under resting conditions, the PNS dominates cardiovascular physiology (e.g., Levy, 1997). PNS governance of the heart is accomplished through direct enervation of the heart via the vagus nerve (cranial nerve X) at the sinoatrial node (a small muscle strip in the upper part of the right atrium, and the location of cardiac pacemaker cells). While the intrinsic firing rate of pacemaker cells is around 105 beats per minute, healthy adult resting HRs are only 60-80 beats per minute. That is, the PNS exerts a tonic inhibition over the heart via the vagus, and the removal of that inhibition (without any change in SNS activity) can lead to an increase in heart rate. Furthermore, pacemaker cells respond rapidly (150 ms latency) to changes in PNS input but more slowly to changes in SNS input (30-60 s until maximum effect) due to neurotransmitter differences (acetylcholine for PNS, norepinephrine for SNS). Furthermore, an “accentuated antagonism” has been reported in the interaction between SNS and PNS inputs: the deceleratory chronotropic effects of PNS activation are increased as the level of background SNS activity increases (e.g., Uijtdehaage & Thayer, 2000).
The complexity of this dual innervation is redoubled, however, by its connection to an intricate neuroarchitecture with descending, ascending, and bidirectional links between cortical, midbrain, and brainstem structures (Figure 1; for reviews, see Benarroch, 1993; Berntson, Sarter, & Cacioppo, 1998; Berthoud & Neuhuber, 2000; Loewy, 1990; Thayer & Lane, 2009). These structures include the orbitofrontal, ventromedial prefrontal, anterior cingulate, and insular cortices, basal ganglia, central nucleus of the amygdala, nucleus of the solitary tract, nucleus ambiguus, and periaqueductal gray matter, among others. Thayer and Lane (2000) have noted that subsets of these structures have been given various labels: central autonomic network (Benarroch, 1993), anterior executive region (Devinsky, Morrell, & Vogt, 1995), and emotion circuits (LeDoux, 2000). This suggests a shared “neural wetware” driving cognitive, affective, and physiological regulation, formalized by Thayer and Lane (2000, 2007) as the Neurovisceral Integration model.
FIGURE 1. Neural structures involved in the control of heart rate.
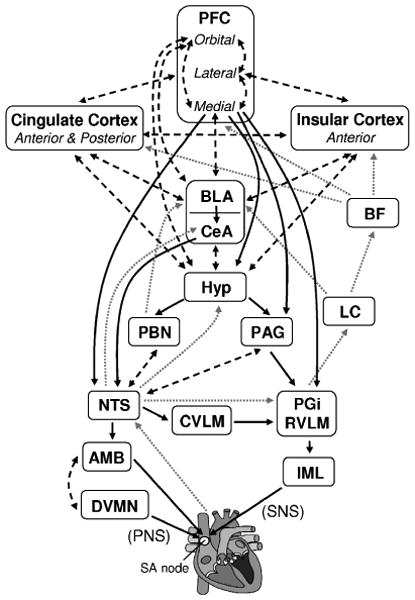
Note: Adapted from Benarroch (1993), Berntson et al. (1998), Berthoud and Neuhuber (2000), Gianaros (2008), Loewy (1990), Thayer and Lane (2009), and Verberne and Owens (1998). Solid black arrows indicate efferent pathways to the heart, including right vagus nerve (PNS) and stellate ganglion (SNS) inputs to the SA node. Dotted gray arrows indicate afferent pathways to medullary structures via aortic baroreceptor signals carried through the vagus. Dashed black arrows indicate bidirectional connections. AMB: nucleus ambiguus; BF: basal forebrain; BLA: basolateral amygdala; CeA: central nucleus of the amygdala; CVLM: caudal ventrolateral medullary neurons; DVMN: dorsal vagal motor nuclei; Hyp: hypothalamus (lateral and paraventricular); IML: intermediolateral cell column of the spinal cord LC: locus coeruleus; NTS: nucleus of the solitary tract; PAG: periaqueductal gray; PBN: parabrachial nucluei; PFC: prefrontal cortex; PGi: nucleus paragigantocellularis; RVLM: rostral ventrolateral medullary neurons. Heart graphic (http://en.wikipedia. org/wiki/File:Heartgraphic.svg) used under the GNU Free Documentation License.
Empirical findings support this hypothesis. Decreased HRV at rest is found in individuals with: (1) depression, generalized anxiety disorder, and post-traumatic stress disorder (e.g., Thayer, Friedman, & Borkovec, 1996); (2) poorer emotion regulation abilities (e.g., Thayer & Brosschot, 2005), and (3) poorer performance on tasks of executive function (e.g., Thayer, Hansen, Saus-Rose, & Johnsen, 2009).
The Importance of Inhibition
Central to the Neurovisceral Integration model is the role of hierarchical inhibition. This concept is not new. In his second Croonian Lecture in 1884, Hughlings Jackson (1884) proposed that autonomic activity (and HR in particular) is tonically inhibited by the brain, and that removal of that inhibition “permits” rather than “causes” an increase in physiological activity: “In other words, the lower level of evolution is not ‘goaded into activity,’ but is ‘let go’” (p. 662).
The Neurovisceral Integration model discusses inhibition from the perspective of a self-organizing, nonlinear dynamical system. As outlined by Thayer and Lane (2000), an organism is seen as “a complex set of reverberating circuits or sub-systems working together in a coordinated fashion (a set of loosely coupled bio-oscillators)” (p. 203). These bio-oscillators organize via a small number of control parameters into “preferred configurations and trajectories” that help define the “behavioral repertoire” of the organism. Within this perspective, dysfunction (physiological, emotional, cognitive) reflects a “stuck” configuration: an inability to select an appropriate response, or more often, an inability to inhibit an inappropriate response. A related concept is that of “situationally appropriate responding”; mounting an appropriate cognitive/behavioral/physiological response to meet a challenge presented by the environment, and terminating that response when the challenge has passed. Physiological responses to music also have been discussed from a dynamical systems perspective, with musically induced patterns of physiological activity viewed as attractors in a state-space defined by the dimensions of valence and arousal (Thayer & Faith, 2001). As such, music may be able to move an organism through the behavioral repertoire of its state-space and thus induce a flexibility that may have positive health benefits.
Implications for Psychophysiological Research
Based on the above discussion, it becomes clear that the concept of “mean HR,” although easy to obtain, is only partially grounded physiologically. Rather, it is the beat-to-beat changes in HR rather than the average HR that more accurately reflects the underlying dynamics and complex interplay of the sympathetic and parasympathetic branches of the ANS.
HR and interbeat interval are significantly correlated with both time-domain and frequency-domain measures of HRV at rest, and all respond significantly to standard physical stressors such as the orthostatic challenge (i.e., successive 5-minute periods of sitting, standing, and sitting; Ellis, 2009, Appendix A). Crucially, however, they do not all change in response to more subtle manipulations. Ellis (2009, Exp. 1) compared changes in mean HR and HRV while subjects listened to 2.5-minute excerpts of music at three different tempos (60, 90, 120 bpm). HRV decreased as tempo increased, indicating that the “challenge” of arousing music prompted a withdrawal of PNS activity. No significant change was observed in mean HR, however, despite significant correlations between mean HR and measures of HRV at baseline (all r(28) > |.69|, all p < .001). Not only does this finding help explain previous mixed findings on the effect of music on mean HR, but illustrates an important point: that not all physiological measures—and relatedly, not all analysis measures—are created equal.
HRV and Music
HRV is a physiologically grounded (e.g., Levy, 1997), theoretically explicated (e.g., Thayer & Lane, 2000), empirically supported (e.g., Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996; Thayer & Lane, 2007), computationally tractable (e.g., Berntson et al., 1997) measure of autonomic (dys)function. HRV can be recorded noninvasively, inexpensively, and with high fidelity via commercially available fitness watches (e.g., Polar RS800; www.polar.fi/en/) and analyzed with freeware (e.g., Kubios; http://kubios.uku.fi/).
Nevertheless, there have been relatively few empirical investigations of HRV and music compared to mean HR and music {E8 vs. E7}. HRV is not mentioned in either of the major literature reviews of music and physiological response (Bartlett, 1996; Hodges, 2010). A majority of studies have been experimental rather than interventional, reporting significant changes in HRV as a function of musical mood (e.g., Etzel et al., 2006; Nyklíček, Thayer, & Van Doornen, 1997), genre (Bernardi, Porta, & Sleight, 2006), familiarity (Iwanaga, Kobayashi, & Kawasaki, 2005), or tempo (Ellis, 2009). Only a few reports exist of musical interventions that have included HRV as an index of autonomic function: in pediatric oncology patients (Kemper, Hamilton, McLean, & Lovato, 2008), myocardial infarction patients (White, 1999), and geriatric patients (Okada et al., 2009).
The Importance of Afferent Feedback
Writing about emotion, William James (1884) famously theorized that “bodily changes follow directly the PERCEPTION of the exciting fact, and that our feeling of the same changes as they occur IS the emotion” (pp. 189-190). If we encounter a bear while on a nature hike, we don't flee from it because we are afraid, but are rather afraid because we flee. Similarly, we feel sorry because we cry, feel angry because we tremble, and feel happy because we smile. “Whistling to keep up courage is no mere figure of speech,” asserted James (neatly prefiguring Oscar Hammerstein's “I whistle a happy tune” from the 1951 musical The King and I). While James' view of emotion quickly came under fire, his recognition of the existence and significance of visceral feedback was ahead of its time. For example, the vagus nerve, which innervates the entirety of the viscera except the adrenal gland, is composed of three times more afferent fibers than efferent fibers (Berthoud & Neuhuber, 2000). Vagal afferents from the heart arise from cardiac baroreceptors, arterial baroreceptors, and arterial chemoreceptors, terminating at the nucleus of the solitary tract (e.g., Loewy, 1990; cf. Figure 1).
While the anatomical architecture is relatively clear, its implications are only beginning to be understood. Visceral afferent information is relayed to limbic, forebrain, and cortical regions, implicating its role in attention, emotion, and anxiety (e.g., Sarter & Bruno, 2000). Damasio's somatic marker hypothesis similarly posits a role for afferent feedback influencing decision making (e.g., Damasio, 1996). Other work has explored individual differences in the neural underpinnings of conscious internal sensitivity to visceral activity, termed interoceptive awareness (e.g., Craig, 2003).
The above work is distinct from more controversial biofeedback techniques, in which physiological signals are quantified and conveyed to subjects using visual or auditory signals. While music has been used in the context of biofeedback paradigms {F2}, no systematic work has been performed linking music with visceral afferent feedback or interoceptive awareness {F19}.
Conclusions
Humans interact with music, both consciously and unconsciously, at behavioral, emotional, and physiological levels. James (1884) mused that the ANS “forms a sort of sounding-board, which every change of our consciousness, however slight, may make reverberate” (p. 191). While that sounding-board certainly reverberates to music, it is hoped that the present review begins to illustrate just how complex that interaction may be, and the associated implications for future research. With respect to experimental studies, it is important to explore how specific features of music (e.g., its beat, tempo, or pitch level) trigger neurophysiological, psychophysiological, emotional, and behavioral responses. With respect to interventions with physiological targets (e.g., hypertension, tachycardia), it is important to consider that ANS dysfunction is mediated by the CNS, and that treatment of the former should be sensitive to the state of the latter. With respect to interventions with psychological targets (e.g., depression, anxiety), it is important to understand that ANS processes are not merely the downstream flotsam of activity in the CNS, but function as part of a sensitive feedback and feed-forward mechanism. Continued work within these different paradigms may reveal a common finding: that the ANS serves as the final common pathway by which music exerts a therapeutic effect on health and disease.
Contributor Information
Robert J. Ellis, Beth Israel Deaconess Medical Center and Harvard Medical School
Julian F. Thayer, The Ohio State University
References
- Bartlett DL. Physiological responses to music and sound stimuli. In: Hodges DA, editor. Handbook of music psychology. 2nd. San Antonio, TX: IMR Press; 1996. pp. 343–385. [Google Scholar]
- Belardinelli R, Lacalaprice F, Ventrella C, Volpe L, Faccenda E. Waltz dancing in patients with chronic heart failure: New form of exercise training. Circulation: Heart Failure. 2008;1:107–114. doi: 10.1161/CIRCHEARTFAILURE.108.765727. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clinic Proceedings. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Porta C, Bernardi NF, Sleight P. Music and the autonomic nervous system: Dynamic interactions between musical, cardiovascular and cerebral rhythms in man. Autonomic Neuroscience: Basic and Clinical. 2009;149:42–43. [Google Scholar]
- Bernardi L, Porta C, Sleight P. Cardiovascular, cerebrovascular, and respiratory changes induced by different types of music in musicians and non-musicians: The importance of silence. Heart. 2006;92:445–452. doi: 10.1136/hrt.2005.064600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: The basal forebrain cholinergic link. Behavioural Brain Research. 1998;94:225–248. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Autonomic Neuroscience: Basic and Clinical. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Brown RP, Gerbarg PL. Yoga breathing, meditation, and longevity. Annals of the New York Academy of Sciences. 2009;1172:54–62. doi: 10.1111/j.1749-6632.2009.04394.x. [DOI] [PubMed] [Google Scholar]
- Clift S, Hancox G. The perceived benefits of singing: Findings from preliminary surveys of a university college choral society. The Journal of the Royal Society for the Promotion of Health. 2001;121:248–256. doi: 10.1177/146642400112100409. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain: A Journal of Neurology. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Diserens CM. The influence of music on behavior. Princeton, NJ: Princeton University Press; 1926. [Google Scholar]
- Dunn K. Music and the reduction of post-operative pain. Nursing Standard. 2004;18:33–39. doi: 10.7748/ns2004.05.18.36.33.c3612. [DOI] [PubMed] [Google Scholar]
- Ellis RJ. Unpublished doctoral dissertation. The Ohio State University; 2009. The effect of musical tempo on subjective and physiological indices of affective response. [Google Scholar]
- Etzel JA, Johnsen EL, Dickerson J, Tranel D, Adolphs R. Cardiovascular and respiratory responses during musical mood induction. International Journal of Psychophysiology. 2006;61:57–69. doi: 10.1016/j.ijpsycho.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Evans D. The effectiveness of music as an intervention for hospital patients: A systematic review. Journal of Advanced Nursing. 2002;37:8–18. doi: 10.1046/j.1365-2648.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- Fechir M, Schlereth T, Purat T, Kritzmann S, Geber C, Eberle T, et al. Patterns of sympathetic responses induced by different stress tasks. The Open Neurology Journal. 2008;2:25–31. doi: 10.2174/1874205X00802010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: New perspectives on respiratory rhythm. Nature Reviews Neuroscience. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ. Brain-body pathways to cardiovascular disease risk; Herbert Weiner Early Career Award Lecture presented at the 66th Annual Meeting of the American Psychosomatic Society; Baltimore, MD. 2008. Mar, [Google Scholar]
- Gillett PA, Eisenman PA. The effect of intensity controlled aerobic dance exercise on aerobic capacity of middle-aged, overweight women. Research in Nursing and Health. 1987;10:383–390. doi: 10.1002/nur.4770100606. [DOI] [PubMed] [Google Scholar]
- Grape C, Sandgren M, Hansson L, Ericson M, Theorell T. Does singing promote well-being? An empirical study of professional and amateur singers during a singing lesson. Integrative Physiological And Behavioral Science: The Official Journal Of The Pavlovian Society. 2003;38:65–74. doi: 10.1007/BF02734261. [DOI] [PubMed] [Google Scholar]
- Grossman E, Grossman A, Schein M, Zimlichman R, Gavish B. Breathing-control lowers blood pressure. Journal of Human Hypertension. 2001;15:263–269. doi: 10.1038/sj.jhh.1001147. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Hall JE. Textbook of medical physiology. 11th. Philadelphia, PA: Elsivier Saunders; 2005. [Google Scholar]
- Haas F, Distenfeld S, Axen K. Effects of perceived musical rhythm on respiratory pattern. Journal of Applied Physiology. 1986;61:1185–1191. doi: 10.1152/jappl.1986.61.3.1185. [DOI] [PubMed] [Google Scholar]
- Hodges DA. Physiological responses to music. In: Hodges DA, editor. Handbook of music psychology. Lawrence, KS: National Association for Music Therapy; 1980. pp. 393–400. [Google Scholar]
- Hodges DA. Psychophysiological responses to music. In: Juslin PN, Sloboda JA, editors. Handbook of music and emotion: Theory, research, applications. New York: Oxford University Press; 2010. pp. 279–311. [Google Scholar]
- Hughlings Jackson JH. The Croonian lectures on evolution and dissolution of the nervous system. British Medical Journal. 1884;1214:660–663. doi: 10.1136/bmj.1.1214.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E, Chui BT, Woo J. Effects of dance on physical and psychological well-being in older persons. Archives of Gerontology and Geriatrics. 2009;49:e45–e50. doi: 10.1016/j.archger.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Iwanaga M, Kobayashi A, Kawasaki C. Heart rate variability with repetitive exposure to music. Biological Psychology. 2005;70:61–66. doi: 10.1016/j.biopsycho.2004.11.015. [DOI] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database of Systematic Reviews. 2000 doi: 10.1002/14651858.CD001800. Art. No.: CD001800. [DOI] [PubMed] [Google Scholar]
- Kemper KJ, Hamilton CA, McLean TW, Lovato J. Impact of music on pediatric oncology outpatients. Pediatric Research. 2008;64:105–109. doi: 10.1203/PDR.0b013e318174e6fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Levy MN. Neural control of cardiac function. Baillière's Clinical Neurology. 1997;6:227–244. [PubMed] [Google Scholar]
- Loewy AD. Central autonomic pathways. In: Loewy AD, Spyer KM, editors. Central regulation of autonomic functions. New York: Oxford University Press; 1990. pp. 88–103. [Google Scholar]
- Murrock CJ, Higgins PA, Killion C. Dance and peer support to improve diabetes outcomes in African American women. The Diabetes Educator. 2009;35:995–1003. doi: 10.1177/0145721709343322. [DOI] [PubMed] [Google Scholar]
- Nyklíček I, Thayer JF, Van Doornen LJ. Cardiorespiratory differentiation of musically-induced emotions. Journal of Psychophysiology. 1997;11:304–321. [Google Scholar]
- Okada K, Kurita A, Takase B, Otsuka T, Kodani E, Kusama Y, et al. Effects of music therapy on autonomic nervous system activity, incidence of heart failure events, and plasma cytokine and catecholamine levels in elderly patients with cerebrovascular disease and dementia. International Heart Journal. 2009;50:95–110. doi: 10.1536/ihj.50.95. [DOI] [PubMed] [Google Scholar]
- Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation: A critical appraisal. Hypertension. 1995;25:1276–1286. doi: 10.1161/01.hyp.25.6.1276. [DOI] [PubMed] [Google Scholar]
- Parati G, Malfatto G, Boarin S, Branzi G, Caldara G, Giglio A, et al. Device-guided paced breathing in the home setting: Effects on exercise capacity, pulmonary and ventricular function in patients with chronic heart failure: A pilot study. Circulation: Heart Failure. 2008;1:178–183. doi: 10.1161/CIRCHEARTFAILURE.108.772640. [DOI] [PubMed] [Google Scholar]
- Peng CK, Buldyrev SV, Hausdorff JM, Havlin S, Mietus JE, Simons M, et al. Non-equilibrium dynamics as an indispensable characteristic of a healthy biological system. Integrative Physiological and Behavioral Science: The Official Journal of the Pavlovian Society. 1994;29:283–293. doi: 10.1007/BF02691332. [DOI] [PubMed] [Google Scholar]
- Porges SW. Autonomic regulation and attention. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults. Hillsdale, NJ: Erlbaum; 1992. pp. 201–226. [Google Scholar]
- Sarter M, Bruno JP. Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: Differential afferent regulation of the basal forebrain by telencephalic and brainstem afferents. Neuroscience. 2000;95:933–952. doi: 10.1016/s0306-4522(99)00487-x. [DOI] [PubMed] [Google Scholar]
- Schein MH, Gavish B, Herz M, Rosner-Kahana D, Naveh P, Knishkowy B, et al. Treating hypertension with a device that slows and regularises breathing: A randomised, double-blind controlled study. Journal of Human Hypertension. 2001;15:271–278. doi: 10.1038/sj.jhh.1001148. [DOI] [PubMed] [Google Scholar]
- Schwartz SE, Fernhall B, Plowman SA. Effects of music on exercise performance. Journal of Cardiopulmonary Rehabilitation and Prevention. 1990;10:312–316. [Google Scholar]
- Takahashi T, Matsushita H. Long-term effects of music therapy on elderly with moderate/severe dementia. Journal of Music Therapy. 2006;43:317. doi: 10.1093/jmt/43.4.317. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Thaut MH, Kenyon GP, Schauer ML, McIntosh GC. The connection between rhythmicity and brain function. IEEE Engineering in Medicine and Biology Magazine. 1999;18:101–108. doi: 10.1109/51.752991. [DOI] [PubMed] [Google Scholar]
- Thaut MH, McIntosh GC, Rice RR, Miller RA, Rathbun J, Brault JM. Rhythmic auditory stimulation in gait training for Parkinson's disease patients. Movement Disorders. 1996;11:193–200. doi: 10.1002/mds.870110213. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology. 2005;30:1050–1058. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Faith ML. A dynamic systems model of musically induced emotions. Physiological and self-report evidence. Annals of the New York Academy of Sciences. 2001;930:452–456. doi: 10.1111/j.1749-6632.2001.tb05768.x. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman B, Borkovec T. Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biological Psychology. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Uijtdehaage SH, Thayer JF. Accentuated antagonism in the control of human heart rate. Clinical Autonomic Research. 2000;10:107–110. doi: 10.1007/BF02278013. [DOI] [PubMed] [Google Scholar]
- Valentine E, Evans C. The effects of solo singing, choral singing and swimming on mood and physiological indices. British Journal of Medical Psychology. 2001;74:115–120. [PubMed] [Google Scholar]
- Verberne AJ, Owens NC. Cortical modulation of the cardiovascular system. Progress in Neurobiology. 1998;54:149–68. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- Wade LM. A comparison of the effects of vocal exercises/singing versus music-assisted relaxation on peak expiratory flow rates of children with asthma. Music Therapy Perspectives. 2002;20:31–37. [Google Scholar]
- White JM. Effects of relaxing music on cardiac autonomic balance and anxiety after acute myocardial infarction. American Journal of Critical Care. 1999;8:220–230. [PubMed] [Google Scholar]


