Abstract
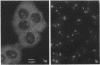
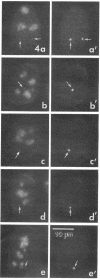
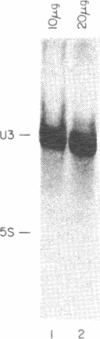
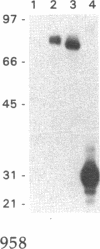
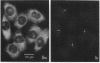
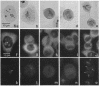
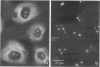
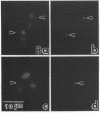
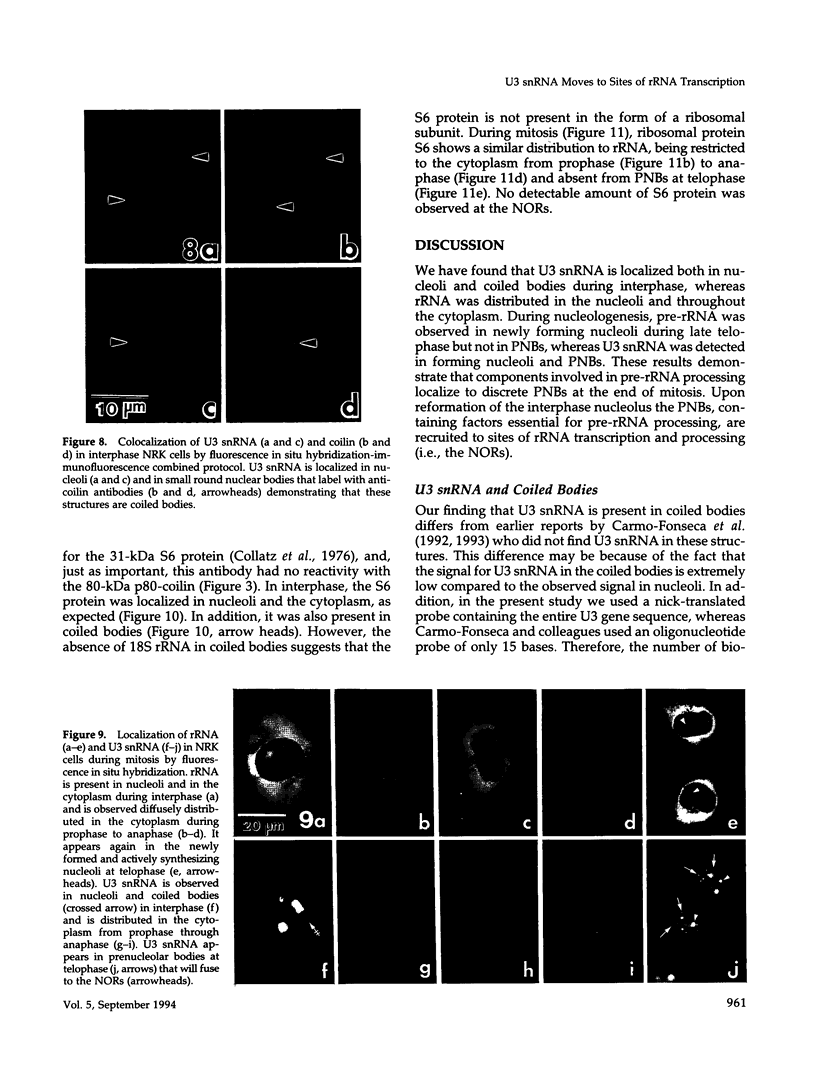
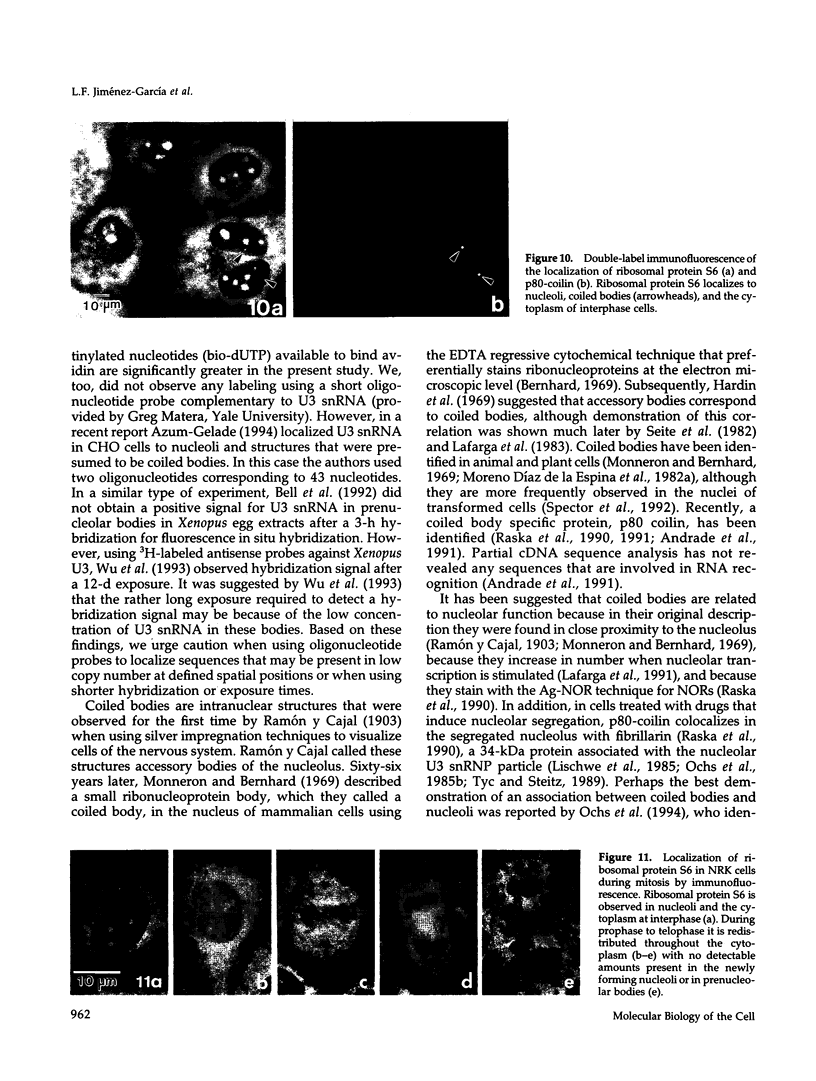
We have investigated the distribution of U3 snRNA and rRNA in HeLa cells and normal rat kidney cells during interphase and mitosis. U3 snRNA, known to be involved in pre-rRNA processing, was detected in nucleoli and coiled bodies during interphase, whereas rRNA was distributed in the nucleoli and throughout the cytoplasm. By comparison, ribosomal protein S6 was detected in nucleoli, coiled bodies, and in the cytoplasm. During nucleologenesis, pre-rRNA was observed in newly forming nucleoli during late telophase but not in prenucleolar bodies (PNBs), whereas U3 snRNA was detected in forming nucleoli and PNBs. Similar findings to those reported here for the localization of U3 snRNA have been reported previously for the U3 small nuclear ribonucleoprotein fibrillarin. These results suggest that components involved in pre-rRNA processing localize to discrete PNBs at the end of mitosis. The nucleolus is formed at specific telophase domains (nucleolar organizing regions) and the PNBs, containing factors essential for pre-rRNA processing, are recruited to these sites of rRNA transcription and processing.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade L. E., Chan E. K., Raska I., Peebles C. L., Roos G., Tan E. M. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J Exp Med. 1991 Jun 1;173(6):1407–1419. doi: 10.1084/jem.173.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade L. E., Tan E. M., Chan E. K. Immunocytochemical analysis of the coiled body in the cell cycle and during cell proliferation. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1947–1951. doi: 10.1073/pnas.90.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azum-Gélade M. C., Noaillac-Depeyre J., Caizergues-Ferrer M., Gas N. Cell cycle redistribution of U3 snRNA and fibrillarin. Presence in the cytoplasmic nucleolus remnant and in the prenucleolar bodies at telophase. J Cell Sci. 1994 Feb;107(Pt 2):463–475. doi: 10.1242/jcs.107.2.463. [DOI] [PubMed] [Google Scholar]
- Bell P., Dabauvalle M. C., Scheer U. In vitro assembly of prenucleolar bodies in Xenopus egg extract. J Cell Biol. 1992 Sep;118(6):1297–1304. doi: 10.1083/jcb.118.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente R., Rose K. M., Reimer G., Hügle-Dörr B., Scheer U. Inhibition of nucleolar reformation after microinjection of antibodies to RNA polymerase I into mitotic cells. J Cell Biol. 1987 Oct;105(4):1483–1491. doi: 10.1083/jcb.105.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard W. A new staining procedure for electron microscopical cytology. J Ultrastruct Res. 1969 May;27(3):250–265. doi: 10.1016/s0022-5320(69)80016-x. [DOI] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Ferreira J., Lamond A. I. Assembly of snRNP-containing coiled bodies is regulated in interphase and mitosis--evidence that the coiled body is a kinetic nuclear structure. J Cell Biol. 1993 Feb;120(4):841–852. doi: 10.1083/jcb.120.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Carvalho M. T., Lamond A. I. Transcription-dependent colocalization of the U1, U2, U4/U6, and U5 snRNPs in coiled bodies. J Cell Biol. 1992 Apr;117(1):1–14. doi: 10.1083/jcb.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Sproat B. S., Ansorge W., Swanson M. S., Lamond A. I. In vivo detection of snRNP-rich organelles in the nuclei of mammalian cells. EMBO J. 1991 Jul;10(7):1863–1873. doi: 10.1002/j.1460-2075.1991.tb07712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Tollervey D., Pepperkok R., Barabino S. M., Merdes A., Brunner C., Zamore P. D., Green M. R., Hurt E., Lamond A. I. Mammalian nuclei contain foci which are highly enriched in components of the pre-mRNA splicing machinery. EMBO J. 1991 Jan;10(1):195–206. doi: 10.1002/j.1460-2075.1991.tb07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E. K., Imai H., Hamel J. C., Tan E. M. Human autoantibody to RNA polymerase I transcription factor hUBF. Molecular identity of nucleolus organizer region autoantigen NOR-90 and ribosomal RNA transcription upstream binding factor. J Exp Med. 1991 Nov 1;174(5):1239–1244. doi: 10.1084/jem.174.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. L., Wool I. G. The primary structure of rat ribosomal protein S6. J Biol Chem. 1988 Feb 25;263(6):2891–2896. [PubMed] [Google Scholar]
- Collatz E., Wool I. G., Lin A., Stöffler G. The isolation of eukaryotic ribosomal proteins. The purification and characterization of the 40 S ribosomal subunit proteins S2, S3, S4, S5, S6, S7, S8, S9, S13, S23/S24, S27, and S28. J Biol Chem. 1976 Aug 10;251(15):4666–4672. [PubMed] [Google Scholar]
- Fakan S., Bernhard W. Localisation of rapidly and slowly labelled nuclear RNA as visualized by high resolution autoradiography. Exp Cell Res. 1971 Jul;67(1):129–141. doi: 10.1016/0014-4827(71)90628-8. [DOI] [PubMed] [Google Scholar]
- Fakan S., Leser G., Martin T. E. Ultrastructural distribution of nuclear ribonucleoproteins as visualized by immunocytochemistry on thin sections. J Cell Biol. 1984 Jan;98(1):358–363. doi: 10.1083/jcb.98.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakan S., Puvion E., Sphor G. Localization and characterization of newly synthesized nuclear RNA in isolate rat hepatocytes. Exp Cell Res. 1976 Apr;99(1):155–164. doi: 10.1016/0014-4827(76)90690-x. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of synthesis and processing of nucleolar components in metaphase-arrested cells. J Mol Biol. 1971 Jul 14;59(1):27–42. doi: 10.1016/0022-2836(71)90411-6. [DOI] [PubMed] [Google Scholar]
- Gall J. G., Stephenson E. C., Erba H. P., Diaz M. O., Barsacchi-Pilone G. Histone genes are located at the sphere loci of newt lampbrush chromosomes. Chromosoma. 1981;84(2):159–171. doi: 10.1007/BF00399128. [DOI] [PubMed] [Google Scholar]
- Goessens G. Nucleolar structure. Int Rev Cytol. 1984;87:107–158. doi: 10.1016/s0074-7696(08)62441-9. [DOI] [PubMed] [Google Scholar]
- Goodpasture C., Bloom S. E. Visualization of nucleolar organizer regions im mammalian chromosomes using silver staining. Chromosoma. 1975 Nov 20;53(1):37–50. doi: 10.1007/BF00329389. [DOI] [PubMed] [Google Scholar]
- Hadjiolov A. A., Georgiev O. I., Nosikov V. V., Yavachev L. P. Primary and secondary structure of rat 28 S ribosomal RNA. Nucleic Acids Res. 1984 Apr 25;12(8):3677–3693. doi: 10.1093/nar/12.8.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. H., Spicer S. S., Greene W. B. The paranucleolar structure, accessory body of Cajal, sex chromatin, and related structures in nuclei of rat trigeminal neurons: a cytochemical and ultrastructural study. Anat Rec. 1969 Aug;164(4):403–431. doi: 10.1002/ar.1091640403. [DOI] [PubMed] [Google Scholar]
- Huang S., Spector D. L. U1 and U2 small nuclear RNAs are present in nuclear speckles. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):305–308. doi: 10.1073/pnas.89.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. M., Ares M., Jr Depletion of U3 small nucleolar RNA inhibits cleavage in the 5' external transcribed spacer of yeast pre-ribosomal RNA and impairs formation of 18S ribosomal RNA. EMBO J. 1991 Dec;10(13):4231–4239. doi: 10.1002/j.1460-2075.1991.tb05001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
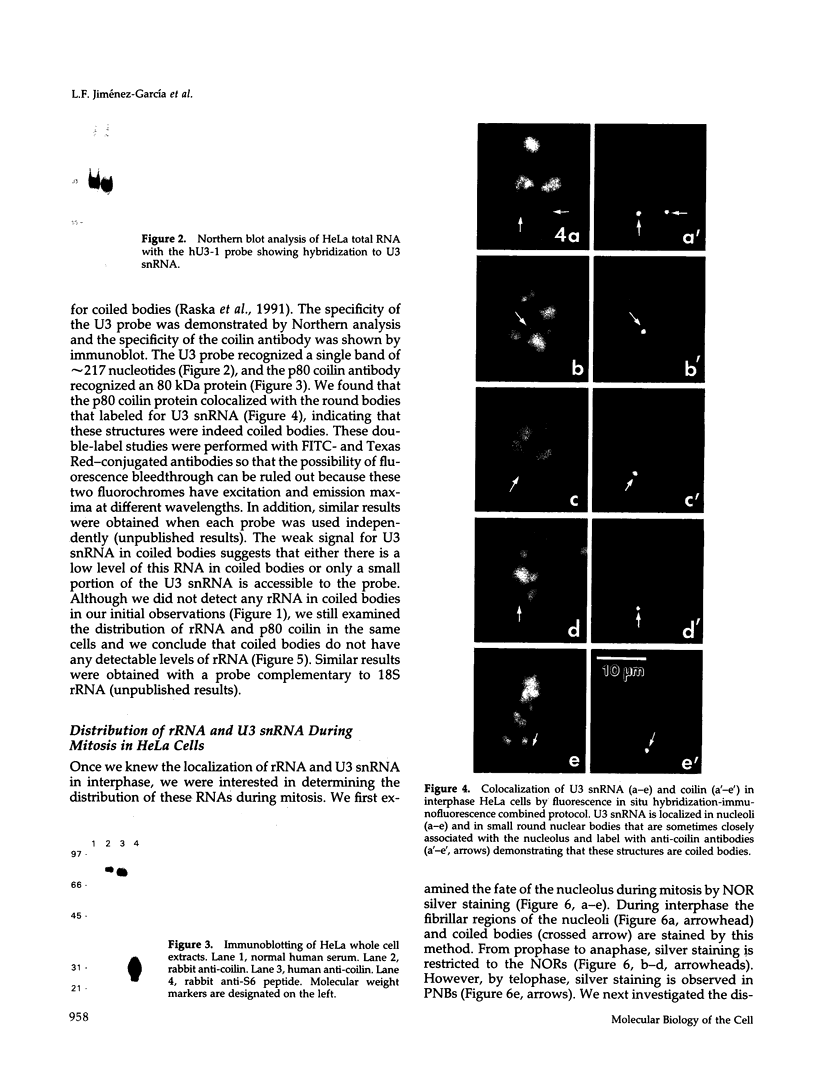
- Jiménez-García L. F., Rothblum L. I., Busch H., Ochs R. L. Nucleologenesis: use of non-isotopic in situ hybridization and immunocytochemistry to compare the localization of rDNA and nucleolar proteins during mitosis. Biol Cell. 1989;65(3):239–246. doi: 10.1111/j.1768-322x.1989.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Jiménez-García L. F., Spector D. L. In vivo evidence that transcription and splicing are coordinated by a recruiting mechanism. Cell. 1993 Apr 9;73(1):47–59. doi: 10.1016/0092-8674(93)90159-n. [DOI] [PubMed] [Google Scholar]
- Kass S., Tyc K., Steitz J. A., Sollner-Webb B. The U3 small nucleolar ribonucleoprotein functions in the first step of preribosomal RNA processing. Cell. 1990 Mar 23;60(6):897–908. doi: 10.1016/0092-8674(90)90338-f. [DOI] [PubMed] [Google Scholar]
- LAFONTAINE J. G., CHOUINARD L. A. A correlated light and electron microscope study of the nucleolar material during mitosis in Vicia faba. J Cell Biol. 1963 Apr;17:167–201. doi: 10.1083/jcb.17.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAFONTAINE J. G. Structure and mode of formation of the nucleolus in meristematic cells of Vicia faba and Allium cepa. J Biophys Biochem Cytol. 1958 Nov 25;4(6):777–784. doi: 10.1083/jcb.4.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafarga M., Andres M. A., Berciano M. T., Maquiera E. Organization of nucleoli and nuclear bodies in osmotically stimulated supraoptic neurons of the rat. J Comp Neurol. 1991 Jun 15;308(3):329–339. doi: 10.1002/cne.903080302. [DOI] [PubMed] [Google Scholar]
- Lafarga M., Hervás J. P., Santa-Cruz M. C., Villegas J., Crespo D. The "accessory body" of Cajal in the neuronal nucleus. A light and electron microscopic approach. Anat Embryol (Berl) 1983;166(1):19–30. doi: 10.1007/BF00317942. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs R. L., Reddy R., Cook R. G., Yeoman L. C., Tan E. M., Reichlin M., Busch H. Purification and partial characterization of a nucleolar scleroderma antigen (Mr = 34,000; pI, 8.5) rich in NG,NG-dimethylarginine. J Biol Chem. 1985 Nov 15;260(26):14304–14310. [PubMed] [Google Scholar]
- Monneron A., Bernhard W. Fine structural organization of the interphase nucleus in some mammalian cells. J Ultrastruct Res. 1969 May;27(3):266–288. doi: 10.1016/s0022-5320(69)80017-1. [DOI] [PubMed] [Google Scholar]
- Morcillo G., De la Torre C., Giménez-Martín G. Nucleolar transcription during plant mitosis. In situ assay for RNA polymerase activity. Exp Cell Res. 1976 Oct 15;102(2):311–316. doi: 10.1016/0014-4827(76)90046-x. [DOI] [PubMed] [Google Scholar]
- Mowry K. L., Steitz J. A. Identification of the human U7 snRNP as one of several factors involved in the 3' end maturation of histone premessenger RNA's. Science. 1987 Dec 18;238(4834):1682–1687. doi: 10.1126/science.2825355. [DOI] [PubMed] [Google Scholar]
- Ochs R. L., Lischwe M. A., Shen E., Carroll R. E., Busch H. Nucleologenesis: composition and fate of prenucleolar bodies. Chromosoma. 1985;92(5):330–336. doi: 10.1007/BF00327463. [DOI] [PubMed] [Google Scholar]
- Ochs R. L., Lischwe M. A., Spohn W. H., Busch H. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol Cell. 1985;54(2):123–133. doi: 10.1111/j.1768-322x.1985.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Ochs R. L., Stein T. W., Jr, Tan E. M. Coiled bodies in the nucleolus of breast cancer cells. J Cell Sci. 1994 Feb;107(Pt 2):385–399. doi: 10.1242/jcs.107.2.385. [DOI] [PubMed] [Google Scholar]
- Ochs R., Lischwe M., O'Leary P., Busch H. Localization of nucleolar phosphoproteins B23 and C23 during mitosis. Exp Cell Res. 1983 Jun;146(1):139–149. doi: 10.1016/0014-4827(83)90332-4. [DOI] [PubMed] [Google Scholar]
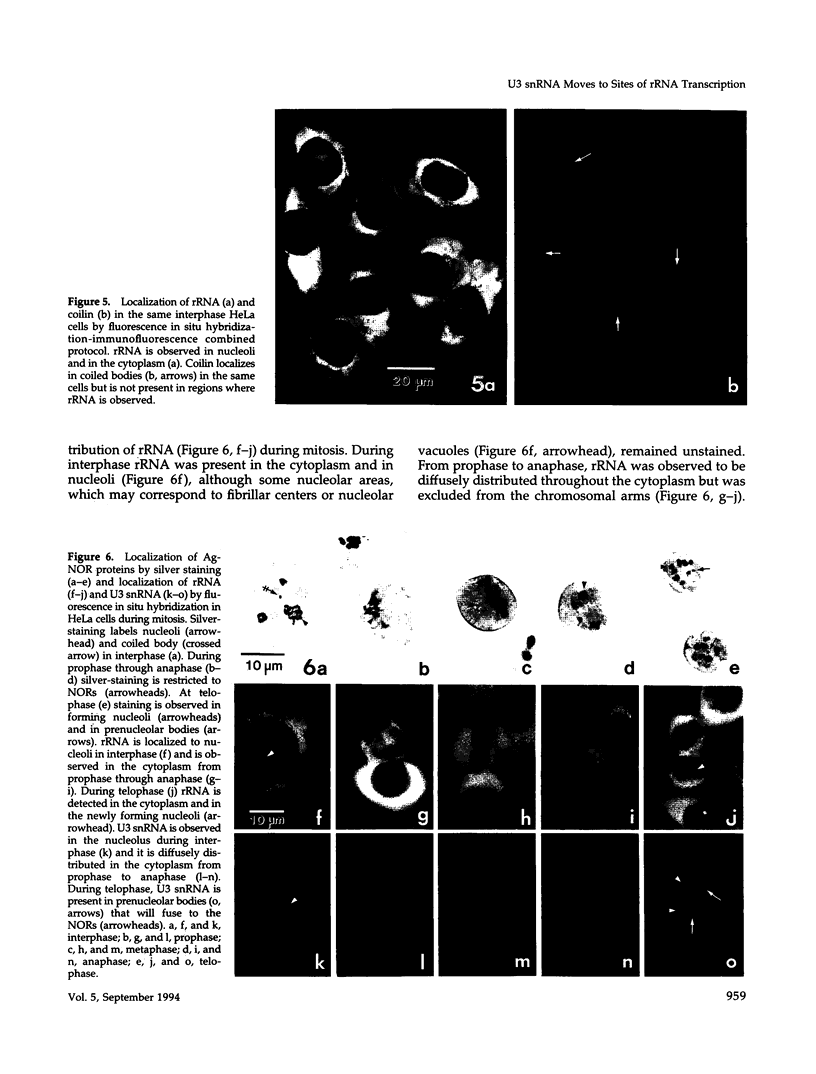
- Peculis B. A., Steitz J. A. Disruption of U8 nucleolar snRNA inhibits 5.8S and 28S rRNA processing in the Xenopus oocyte. Cell. 1993 Jun 18;73(6):1233–1245. doi: 10.1016/0092-8674(93)90651-6. [DOI] [PubMed] [Google Scholar]
- Perry R. P. Processing of RNA. Annu Rev Biochem. 1976;45:605–629. doi: 10.1146/annurev.bi.45.070176.003133. [DOI] [PubMed] [Google Scholar]
- Pfeifle J., Boller K., Anderer F. A. Phosphoprotein pp135 is an essential component of the nucleolus organizer region (NOR). Exp Cell Res. 1986 Jan;162(1):11–22. doi: 10.1016/0014-4827(86)90422-2. [DOI] [PubMed] [Google Scholar]
- Ploton D., Thiry M., Menager M., Lepoint A., Adnet J. J., Goessens G. Behaviour of nucleolus during mitosis. A comparative ultrastructural study of various cancerous cell lines using the Ag-NOR staining procedure. Chromosoma. 1987;95(2):95–107. doi: 10.1007/BF00332182. [DOI] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Mazan S., Nicoloso M., Christensen M. E., Bachellerie J. P. Localization of U3 RNA molecules in nucleoli of HeLa and mouse 3T3 cells by high resolution in situ hybridization. Eur J Cell Biol. 1991 Dec;56(2):178–186. [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Mazan S., Nicoloso M., Pichard E., Bachellerie J. P., Puvion E. Alterations of nucleolar ultrastructure and ribosome biogenesis by actinomycin D. Implications for U3 snRNP function. Eur J Cell Biol. 1992 Jun;58(1):149–162. [PubMed] [Google Scholar]
- RITOSSA F. M., SPIEGELMAN S. LOCALIZATION OF DNA COMPLEMENTARY TO RIBOSOMAL RNA IN THE NUCLEOLUS ORGANIZER REGION OF DROSOPHILA MELANOGASTER. Proc Natl Acad Sci U S A. 1965 Apr;53:737–745. doi: 10.1073/pnas.53.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raska I., Andrade L. E., Ochs R. L., Chan E. K., Chang C. M., Roos G., Tan E. M. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res. 1991 Jul;195(1):27–37. doi: 10.1016/0014-4827(91)90496-h. [DOI] [PubMed] [Google Scholar]
- Raska I., Ochs R. L., Andrade L. E., Chan E. K., Burlingame R., Peebles C., Gruol D., Tan E. M. Association between the nucleolus and the coiled body. J Struct Biol. 1990 Jul-Sep;104(1-3):120–127. doi: 10.1016/1047-8477(90)90066-l. [DOI] [PubMed] [Google Scholar]
- Rothblum L. I., Parker D. L., Cassidy B. Isolation and characterization of rat ribosomal DNA clones. Gene. 1982 Jan;17(1):75–77. doi: 10.1016/0378-1119(82)90102-0. [DOI] [PubMed] [Google Scholar]
- STEVENS B. J. THE FINE STRUCTURE OF THE NUCLEOLUS DURING MITOSIS IN THE GRASSHOPPER NEUROBLAST CELL. J Cell Biol. 1965 Mar;24:349–368. doi: 10.1083/jcb.24.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino R., Gerbi S. A. In vivo disruption of Xenopus U3 snRNA affects ribosomal RNA processing. EMBO J. 1990 Jul;9(7):2299–2308. doi: 10.1002/j.1460-2075.1990.tb07401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U., Benavente R. Functional and dynamic aspects of the mammalian nucleolus. Bioessays. 1990 Jan;12(1):14–21. doi: 10.1002/bies.950120104. [DOI] [PubMed] [Google Scholar]
- Scheer U., Rose K. M. Localization of RNA polymerase I in interphase cells and mitotic chromosomes by light and electron microscopic immunocytochemistry. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1431–1435. doi: 10.1073/pnas.81.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Zachmann M. S., Hügle-Dörr B., Franke W. W. A constitutive nucleolar protein identified as a member of the nucleoplasmin family. EMBO J. 1987 Jul;6(7):1881–1890. doi: 10.1002/j.1460-2075.1987.tb02447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semeshin V. F., Sherudilo A. I., Belyaeva E. S. Nucleoli formation under inhibited RNA synthesis. Exp Cell Res. 1975 Jul;93(2):458–467. doi: 10.1016/0014-4827(75)90472-3. [DOI] [PubMed] [Google Scholar]
- Spector D. L., Fu X. D., Maniatis T. Associations between distinct pre-mRNA splicing components and the cell nucleus. EMBO J. 1991 Nov;10(11):3467–3481. doi: 10.1002/j.1460-2075.1991.tb04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L., Lark G., Huang S. Differences in snRNP localization between transformed and nontransformed cells. Mol Biol Cell. 1992 May;3(5):555–569. doi: 10.1091/mbc.3.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L., Smith H. C. Redistribution of U-snRNPs during mitosis. Exp Cell Res. 1986 Mar;163(1):87–94. doi: 10.1016/0014-4827(86)90560-4. [DOI] [PubMed] [Google Scholar]
- Stockert J. C., Fernández-Gómez M. E., Giménez-Martín G., López-Sáez J. F. Organization of argyrophilic nucleolar material throughout the division cycle of meristematic cells. Protoplasma. 1970;69(2):265–278. doi: 10.1007/BF01280726. [DOI] [PubMed] [Google Scholar]
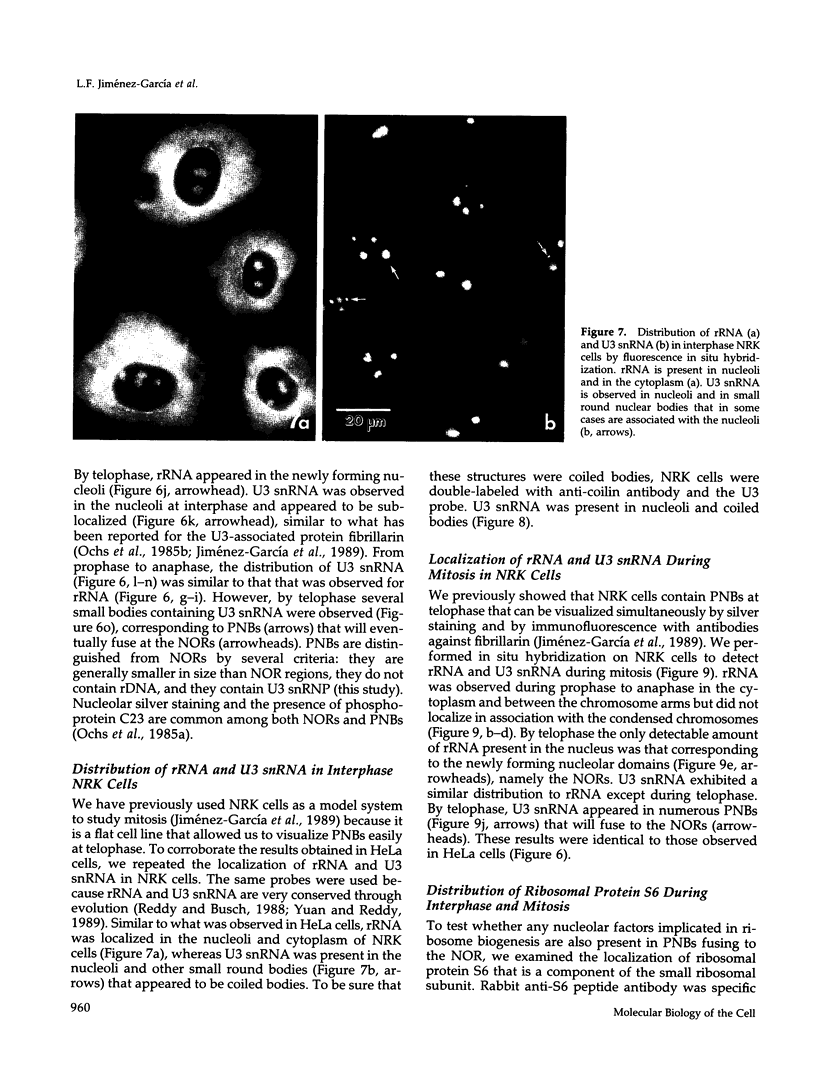
- Subrahmanyam C. S., Cassidy B., Busch H., Rothblum L. I. Nucleotide sequence of the region between the 18S rRNA sequence and the 28S rRNA sequence of rat ribosomal DNA. Nucleic Acids Res. 1982 Jun 25;10(12):3667–3680. doi: 10.1093/nar/10.12.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma R. S., Stolk J. A., Roth M. B. Identification and characterization of a sphere organelle protein. J Cell Biol. 1993 Aug;122(4):767–773. doi: 10.1083/jcb.122.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyc K., Steitz J. A. U3, U8 and U13 comprise a new class of mammalian snRNPs localized in the cell nucleolus. EMBO J. 1989 Oct;8(10):3113–3119. doi: 10.1002/j.1460-2075.1989.tb08463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. H., Gall J. G. U7 small nuclear RNA in C snurposomes of the Xenopus germinal vesicle. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6257–6259. doi: 10.1073/pnas.90.13.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Murphy C., Wu C. H., Tsvetkov A., Gall J. G. Snurposomes and coiled bodies. Cold Spring Harb Symp Quant Biol. 1993;58:747–754. doi: 10.1101/sqb.1993.058.01.082. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Reddy R. Genes for human U3 small nucleolar RNA contain highly conserved flanking sequences. Biochim Biophys Acta. 1989 Jun 1;1008(1):14–22. doi: 10.1016/0167-4781(89)90164-4. [DOI] [PubMed] [Google Scholar]
- de la Espina S. M., Sánchez-Pina M. A., Risueño M. C. Localization of acid phosphatase activity, phosphate ions and inorganic cations in plant nuclear coiled bodies. Cell Biol Int Rep. 1982 Jun;6(6):601–607. doi: 10.1016/0309-1651(82)90184-9. [DOI] [PubMed] [Google Scholar]
- de la Torre C., Fernandez-Gomez M. E., Gimenez-Martin G. Rate of nucleologenesis as a measure of gene activity. Nature. 1975 Aug 7;256(5517):503–505. doi: 10.1038/256503a0. [DOI] [PubMed] [Google Scholar]